Herbal Extracts Mixed with Essential Oils: A Network Approach for Gastric and Intestinal Motility Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. HEMEO Chemical Composition
2.1.1. Identification of Essential Oils Components
2.1.2. Determination of Total Phenolic Content (TPC)
2.2. Ex Vivo Studies
2.2.1. Animals
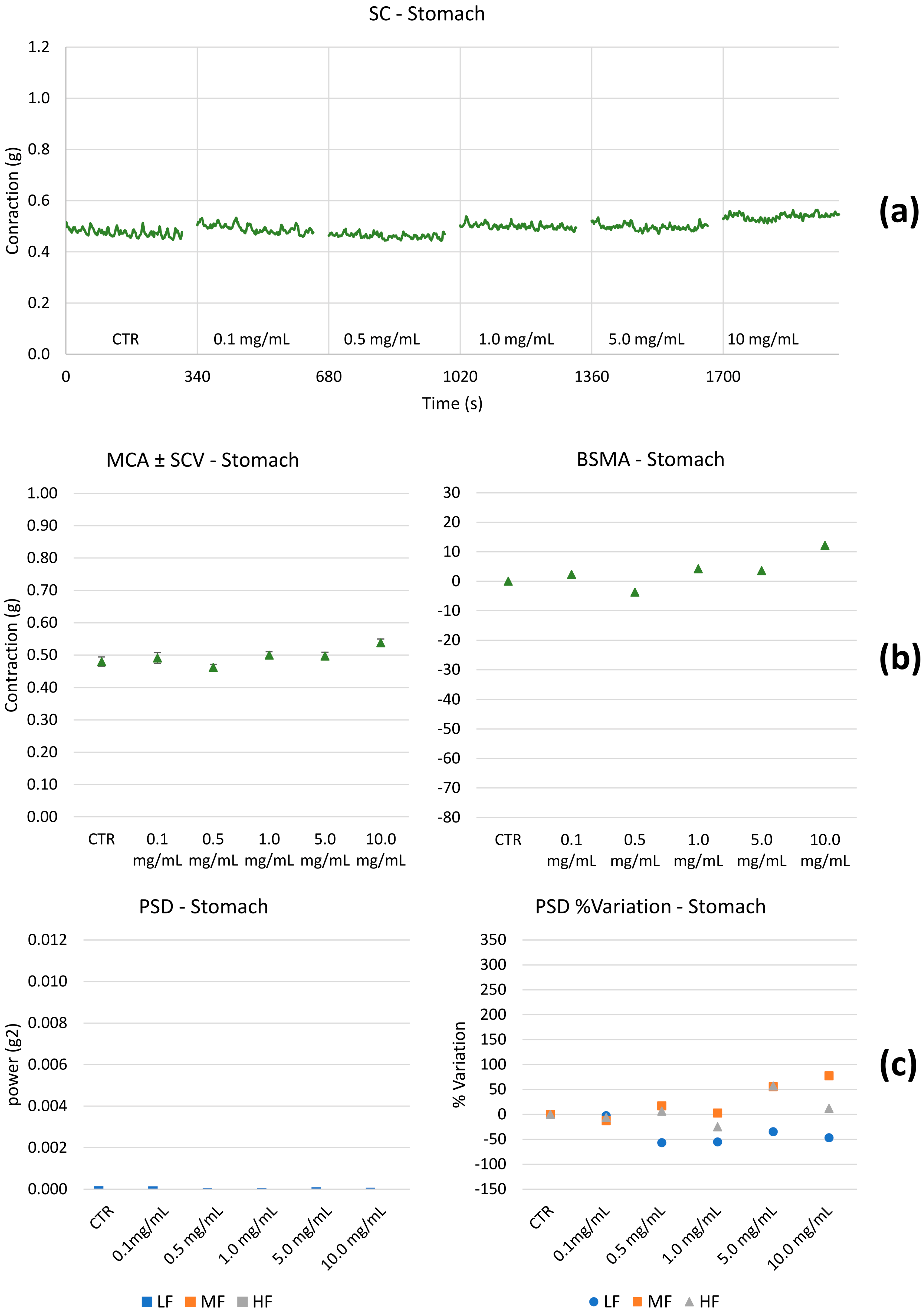
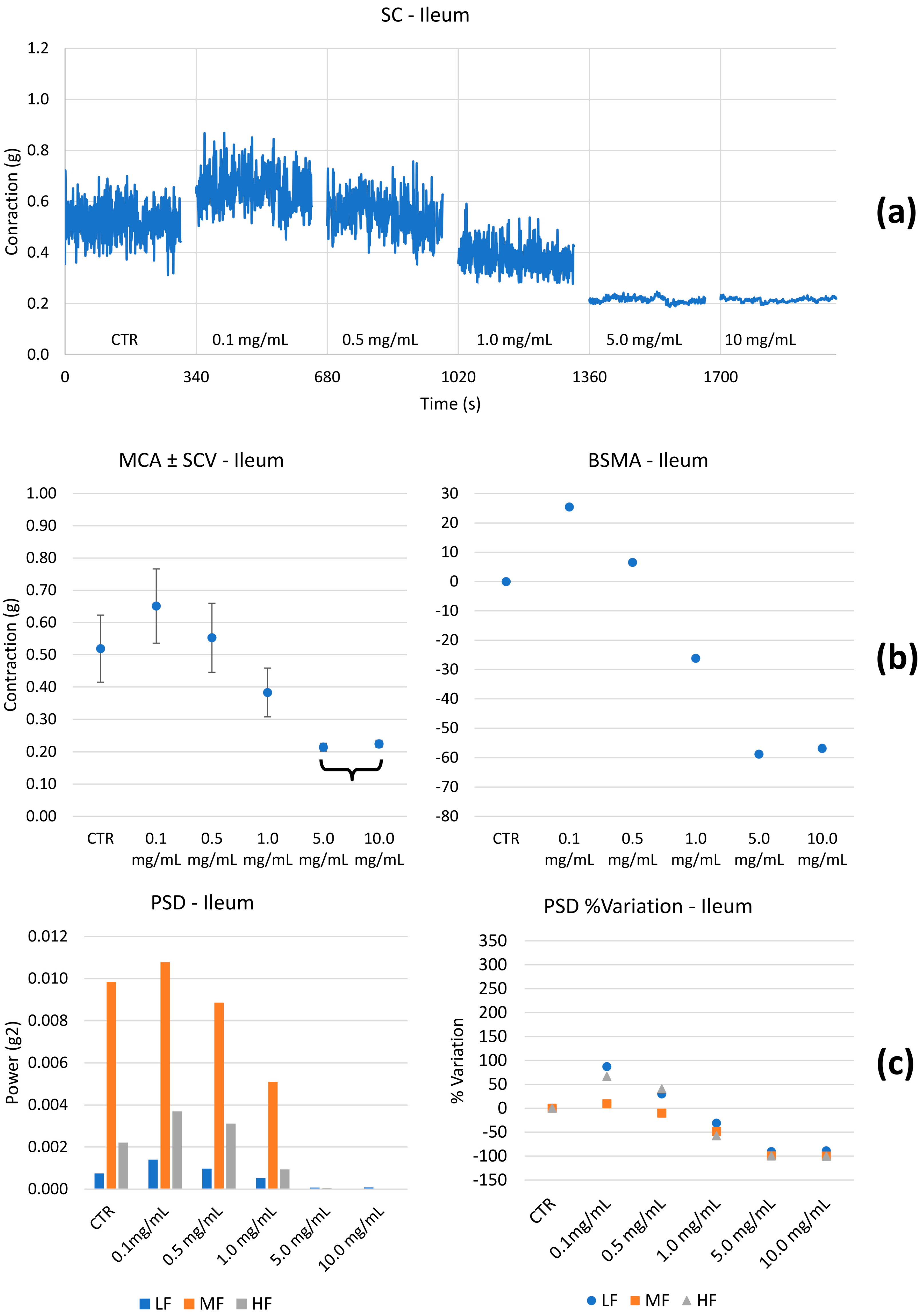
2.2.2. Spontaneous Contractility on the Ileum and Colon
- Mean contraction amplitude (MCA), evaluated as the mean force value (g);
- Standard deviations of the force values over the period as an index of the spontaneous contraction variability (SCV);
- Basal spontaneous motor activity (BSMA), as the percentage (%) variation in each mean force value (g) for the control period;
- Spontaneous contractions were investigated in the frequency domain through a standard FFT analysis and a subsequent power spectral density (PSD) plot. The absolute powers of the following frequency bands of interest: low [0.0, 0.2] Hz (LF), medium [0.2, 0.6] Hz (MF), and high [0.6, 1.0] Hz (HF) [49], were then calculated.
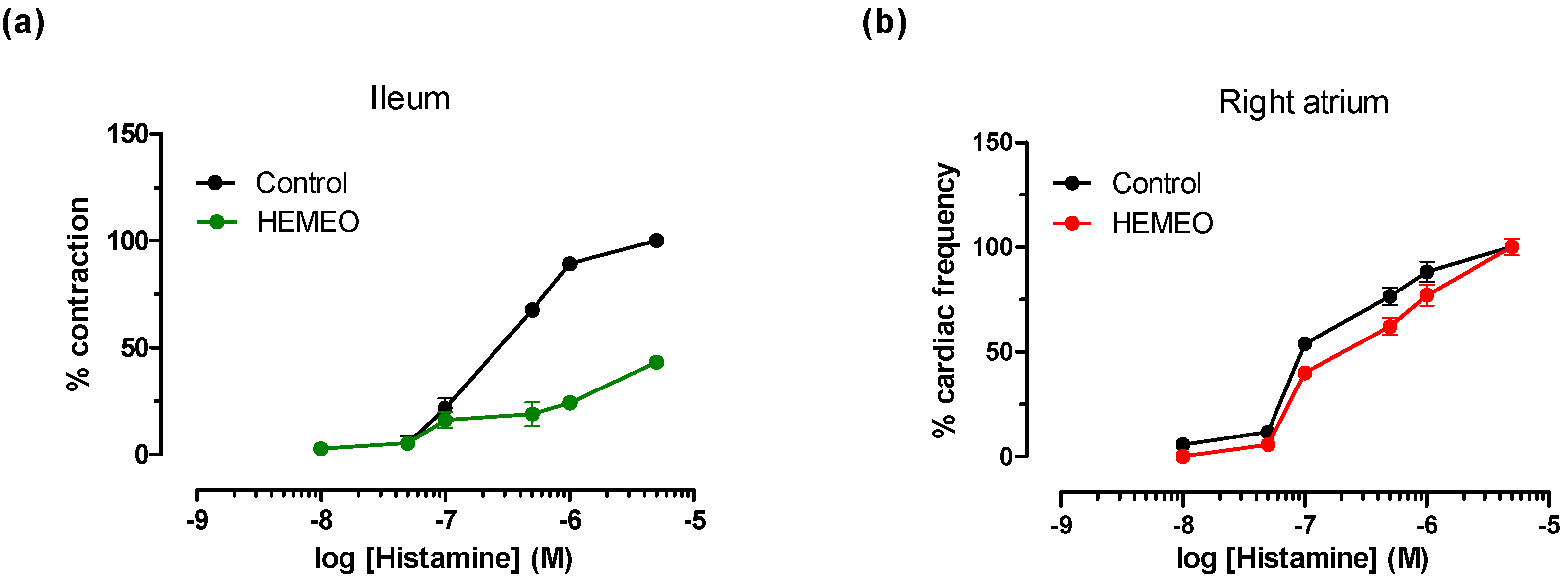
2.2.3. Induced Contractility
2.2.4. Cardiovascular Guinea Pig Preparations
2.2.5. Statistical Analysis
2.3. Antimicrobial Disc Diffusion Test
2.4. Antioxidant Capacity Assay
3. Results
3.1. HEMEO Essential Oils Components Qualitative Profile
3.2. Total Phenolic Content (TPC) of HEMEO
3.3. Ex Vivo Studies
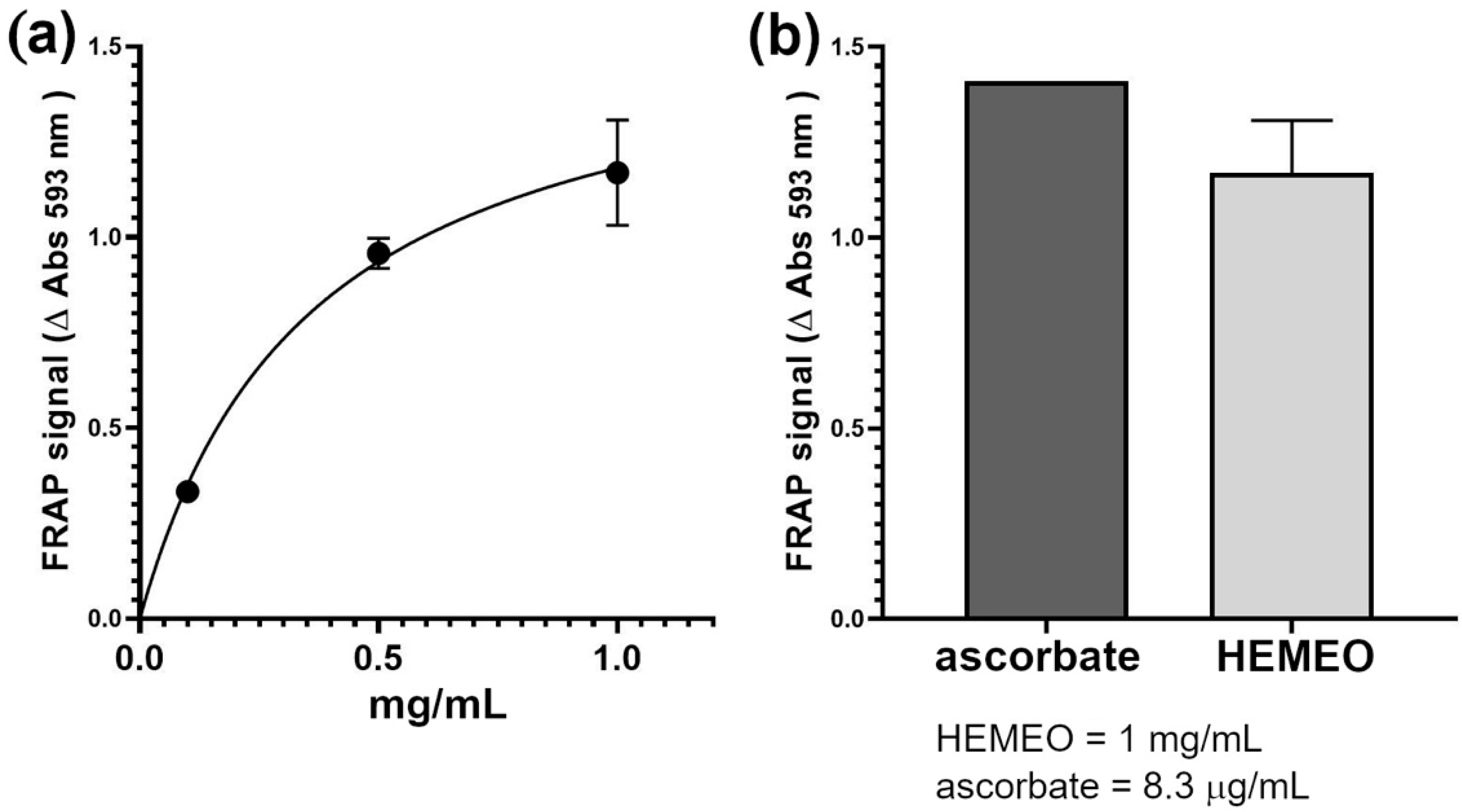
3.4. Antioxidant Activity
3.5. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, L.K.; O’Grady, G.; Du, P.; Egbuji, J.U.; Windsor, J.A.; Pullan, A.J. Gastrointestinal System. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, M.R.; Athavale, O.N.; Wang, X.; Du, P.; Cheng, L.K.; Liu, Z.; Furness, J.B. Functional and Anatomical Gastric Regions and Their Relations to Motility Control. Neurogastroenterol. Motil. 2023, 35, e14560. [Google Scholar] [CrossRef] [PubMed]
- Van Den Abeele, J.; Rubbens, J.; Brouwers, J.; Augustijns, P. The Dynamic Gastric Environment and Its Impact on Drug and Formulation Behaviour. Eur. J. Pharm. Sci. 2017, 96, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Abell, T.L.; Werkman, R.F. Gastrointestinal Motility Disorders. Am. Fam. Physician 1996, 53, 895–902. [Google Scholar] [PubMed]
- Malagelada, J.R. The Brain-Gut Team. Dig. Dis. 2020, 38, 293–298. [Google Scholar] [CrossRef]
- Tack, J.; Janssen, P. Gastroduodenal Motility. Curr. Opin. Gastroenterol. 2010, 26, 647–655. [Google Scholar] [CrossRef]
- Camilleri, M. Management Options for Irritable Bowel Syndrome. Mayo Clin. Proc. 2018, 93, 1858–1872. [Google Scholar] [CrossRef]
- Hunt, R.H.; Camilleri, M.; Crowe, S.E.; El-Omar, E.M.; Fox, J.G.; Kuipers, E.J.; Malfertheiner, P.; McColl, K.E.L.; Pritchard, D.M.; Rugge, M.; et al. The Stomach in Health and Disease. Gut 2015, 64, 1650–1668. [Google Scholar] [CrossRef]
- Ghoshal, U.C. Marshall and Warren Lecture 2019: A Paradigm Shift in Pathophysiological Basis of Irritable Bowel Syndrome and Its Implication on Treatment. J. Gastroenterol. Hepatol. 2020, 35, 712–721. [Google Scholar] [CrossRef]
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter Pylori Infection. Helicobacter 2017, 22 (Suppl. 1), e12403. [Google Scholar] [CrossRef]
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter Pylori and Extragastric Diseases: A Review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Polyzos, S.A.; Doulberis, M.; Zeglinas, C.; Artemaki, F.; Vardaka, E.; Deretzi, G.; Giartza-Taxidou, E.; Tzivras, D.; Vlachaki, E.; et al. Potential Impact of Helicobacter Pylori-Related Metabolic Syndrome on Upper and Lower Gastrointestinal Tract Oncogenesis. Metabolism 2018, 87, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Feilstrecker Balani, G.; Dos Santos Cortez, M.; Picasky da Silveira Freitas, J.E.; Freire de Melo, F.; Zarpelon-Schutz, A.C.; Teixeira, K.N. Immune Response Modulation in Inflammatory Bowel Diseases by Helicobacter Pylori Infection. World J. Gastroenterol. 2023, 29, 4604–4615. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.T.; Anju, K.A.; Anandan, E.; Balachandran, I. Synergistic Interactions of Phytochemicals in Polyherbal Formulation Enhance the Chemical Transformations of Active Constituents. J. Appl. Spectrosc. 2021, 88, 181–186. [Google Scholar] [CrossRef]
- Mattioli, L.B.; Frosini, M.; Corazza, I.; Fiorino, S.; Zippi, M.; Micucci, M.; Budriesi, R. Long COVID-19 Gastrointestinal Related Disorders and Traditional Chinese Medicine: A Network Target-Based Approach. Phytother. Res. 2024, 38, 2323–2346. [Google Scholar] [CrossRef]
- Mobeen, A.; Moazzam, S.W. Jawarish Shahi: A Special Dosage Form of Herbal Formulations for Functional Gastrointestinal Disorders in Unani Medicine—A Comprehensive Review. J. Ethnopharmacol. 2022, 293, 115319. [Google Scholar] [CrossRef]
- Dubey, R.K.; Shukla, S. Exploring Novel Herbal Compounds and Formulations for Inflammatory Bowel Disease (IBD) Management. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2023, 39, e20230003. [Google Scholar] [CrossRef]
- Lashgari, N.-A.; Roudsari, N.M.; Momtaz, S.; Niazi Shahraki, F.; Zandi, N.; Pazoki, B.; Farzaei, M.H.; Ghasemi, M.; Abdollahi, M.; Abdolghaffari, A.H. Systematic Review on Herbal Preparations for Controlling Visceral Hypersensitivity in Functional Gastrointestinal Disorders. Curr. Pharm. Biotechnol. 2024, 25, 1632–1650. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Ghoshal, U.C.; Ro, S. Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders. Front. Pharmacol. 2022, 13, 808195. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Narayanan, A.S.; Raja, S.S.S.; Ponmurugan, K.; Kandekar, S.C.; Natarajaseenivasan, K.; Maripandi, A.; Mandeel, Q.A. Antibacterial Activity of Selected Medicinal Plants against Multiple Antibiotic Resistant Uropathogens: A Study from Kolli Hills, Tamil Nadu, India. Benef. Microbes 2011, 2, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bopana, N.; Saxena, S. Asparagus Racemosus--Ethnopharmacological Evaluation and Conservation Needs. J. Ethnopharmacol. 2007, 110, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.C.; Nandy, A.; Pal, M.; Saha, B.P. Evaluation of Antibacterial Activity of Asparagus Racemosus Willd. Root. Phytother. Res. 2000, 14, 118–119. [Google Scholar] [CrossRef]
- Datta, G.K.; Sairam, K.; Priyambada, S.; Debnath, P.K.; Goel, R.K. Antiulcerogenic Activity of Satavari Mandur--an Ayurvedic Herbo-Mineral Preparation. Indian. J. Exp. Biol. 2002, 40, 1173–1177. [Google Scholar]
- Bhatnagar, M.; Sisodia, S.S. Antisecretory and Antiulcer Activity of Asparagus Racemosus Willd. against Indomethacin plus Phyloric Ligation-Induced Gastric Ulcer in Rats. J. Herb. Pharmacother. 2006, 6, 13–20. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Sisodia, S.S.; Bhatnagar, R. Antiulcer and Antioxidant Activity of Asparagus Racemosus Willd and Withania Somnifera Dunal in Rats. Ann. N. Y. Acad. Sci. 2005, 1056, 261–278. [Google Scholar] [CrossRef]
- Venkatesan, N.; Thiyagarajan, V.; Narayanan, S.; Arul, A.; Raja, S.; Vijaya Kumar, S.G.; Rajarajan, T.; Perianayagam, J.B. Anti-Diarrhoeal Potential of Asparagus Racemosus Wild Root Extracts in Laboratory Animals. J. Pharm. Pharm. Sci. 2005, 8, 39–46. [Google Scholar]
- Twardowschy, A.; Freitas, C.S.; Baggio, C.H.; Mayer, B.; dos Santos, A.C.; Pizzolatti, M.G.; Zacarias, A.A.; dos Santos, E.P.; Otuki, M.F.; Marques, M.C.A. Antiulcerogenic Activity of Bark Extract of Tabebuia Avellanedae, Lorentz Ex Griseb. J. Ethnopharmacol. 2008, 118, 455–459. [Google Scholar] [CrossRef]
- Goel, R.K.; Pathak, N.K.; Biswas, M.; Pandey, V.B.; Sanyal, A.K. Effect of Lapachol, a Naphthaquinone Isolated from Tectona Grandis, on Experimental Peptic Ulcer and Gastric Secretion. J. Pharm. Pharmacol. 1987, 39, 138–140. [Google Scholar] [CrossRef]
- van Uum, S.H. Liquorice and Hypertension. Neth. J. Med. 2005, 63, 119–120. [Google Scholar]
- Chen, G.; Zhu, L.; Liu, Y.; Zhou, Q.; Chen, H.; Yang, J. Isoliquiritigenin, a Flavonoid from Licorice, Plays a Dual Role in Regulating Gastrointestinal Motility in Vitro and in Vivo. Phytother. Res. 2009, 23, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gan, R.-Y.; Zhang, J.-R.; Farha, A.K.; Li, H.-B.; Zhu, F.; Wang, X.-H.; Corke, H. Antivirulence Properties and Related Mechanisms of Spice Essential Oils: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1018–1055. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of Pathogenic and Commensal Bacteria from the Human Colon to Essential Oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum Vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Brković, D.L.; Čomić, L.; Solujić, S.S. Antibacterial Activity of Some Plants from Family Apiaceae in Relation to Selected Phytopathogenic Bacteria. Kragujev. J. Sci. 2006, 28, 65–72. [Google Scholar]
- Gulfraz, M.; Mehmood, S.; Minhas, N.; Jabeen, N.; Kausar, R.; Jabeen, K.; Arshad, G. Composition and Antimicrobial Properties of Essential Oil of Foeniculum Vulgare. Afr. J. Biotechnol. 2008, 7, 4364–4368. [Google Scholar]
- Ozbek, H.; Uğraş, S.; Dülger, H.; Bayram, I.; Tuncer, I.; Oztürk, G.; Oztürk, A. Hepatoprotective Effect of Foeniculum Vulgare Essential Oil. Fitoterapia 2003, 74, 317–319. [Google Scholar] [CrossRef]
- Asano, T.; Aida, S.; Suemasu, S.; Mizushima, T. Anethole Restores Delayed Gastric Emptying and Impaired Gastric Accommodation in Rodents. Biochem. Biophys. Res. Commun. 2016, 472, 125–130. [Google Scholar] [CrossRef]
- Carrillo, E.; Ramírez-Rivera, S.; Bernal, G.; Aquea, G.; Tessini, C.; Thomet, F.A. Water-Soluble Ru(II)-Anethole Compounds with Promising Cytotoxicity toward the Human Gastric Cancer Cell Line AGS. Life Sci. 2019, 217, 193–201. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Acker, B.W.; Cash, B.D. Medicinal Foods for Functional GI Disorders. Curr. Gastroenterol. Rep. 2017, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella Anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants 2023, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Minden, A. Current Molecular Combination Therapies Used for the Treatment of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 11046. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, L.B.; Corazza, I.; Budriesi, R.; Hrelia, S.; Malaguti, M.; Caliceti, C.; Amoroso, R.; Maccallini, C.; Crupi, P.; Clodoveo, M.L.; et al. From Waste to Health: Olive Mill Wastewater for Cardiovascular Disease Prevention. Nutrients 2024, 16, 2986. [Google Scholar] [CrossRef]
- Cavalluzzi, M.M.; Lamonaca, A.; Rotondo, N.P.; Miniero, D.V.; Muraglia, M.; Gabriele, P.; Corbo, F.; De Palma, A.; Budriesi, R.; De Angelis, E.; et al. Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization. Molecules 2022, 27, 7471. [Google Scholar] [CrossRef]
- Micucci, M.; Angeletti, A.; Cont, M.; Corazza, I.; Aldini, R.; Donadio, E.; Chiarini, A.; Budriesi, R. Hibiscus Sabdariffa L. Flowers and Olea Europea L. Leaves Extract-Based Formulation for Hypertension Care: In Vitro Efficacy and Toxicological Profile. J. Med. Food 2016, 19, 504–512. [Google Scholar] [CrossRef]
- Roselli, M.; Carocci, A.; Budriesi, R.; Micucci, M.; Toma, M.; Di Cesare Mannelli, L.; Lovece, A.; Catalano, A.; Cavalluzzi, M.M.; Bruno, C.; et al. Synthesis, Antiarrhythmic Activity, and Toxicological Evaluation of Mexiletine Analogues. Eur. J. Med. Chem. 2016, 121, 300–307. [Google Scholar] [CrossRef]
- Milani, G.; Budriesi, R.; Tavazzani, E.; Cavalluzzi, M.M.; Mattioli, L.B.; Miniero, D.V.; Delre, P.; Belviso, B.D.; Denegri, M.; Cuocci, C.; et al. hERG Stereoselective Modulation by Mexiletine-Derived Ureas: Molecular Docking Study, Synthesis, and Biological Evaluation. Arch. Pharm. 2023, 356, e2300116. [Google Scholar] [CrossRef]
- Mattioli, L.B.; Frosini, M.; Amoroso, R.; Maccallini, C.; Chiano, E.; Aldini, R.; Urso, F.; Corazza, I.; Micucci, M.; Budriesi, R. Olea Europea L. Leaves and Hibiscus Sabdariffa L. Petals Extracts: Herbal Mix from Cardiovascular Network Target to Gut Motility Dysfunction Application. Nutrients 2022, 14, 463. [Google Scholar] [CrossRef]
- Saheera, S.; Potnuri, A.G.; Guha, A.; Palaniyandi, S.S.; Thandavarayan, R.A. Histamine 2 Receptors in Cardiovascular Biology: A Friend for the Heart. Drug Discov. Today 2022, 27, 234–245. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Mean, Standard Deviation, and Confidence Limits. In Manual of Pharmacologic Calculations: With Computer Programs; Tallarida, R.J., Murray, R.B., Eds.; Springer: New York, NY, USA, 1987; pp. 7–10. ISBN 978-1-4612-4974-0. [Google Scholar]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: New York, NY, USA, 2004; ISBN 978-0-19-517179-2. [Google Scholar]
- Motulsky, H.J. Prism 5 Statistics Guide; GraphPad Software Inc.: San Diego, CA, USA, 2007. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Raal, A.; Arak, E. Essential Oil Composition of Pimpinella Anisum L. Fruits from Various European Countries. Nat. Prod. Res. 2008, 22, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A.; Arak, E. Essential Oil Composition of Foeniculum Vulgare Mill. Fruits from Pharmacies in Different Countries. Nat. Prod. Res. 2012, 26, 1173–1178. [Google Scholar] [CrossRef]
- Basaglia, G.; Fiori, J.; Leoni, A.; Gotti, R. Determination of Estragole in Fennel Herbal Teas by HS-SPME and GC–MS. Anal. Lett. 2014, 47, 368–379. [Google Scholar] [CrossRef]
- Fiori, J.; Naldi, M.; Gotti, R. HS–SPME–GC–MS for the Quantitation and Chiral Characterization of Camphor and Menthol in Creams. Chromatographia 2010, 72, 941–947. [Google Scholar] [CrossRef]
- Curci, F.; Corbo, F.; Clodoveo, M.L.; Salvagno, L.; Rosato, A.; Corazza, I.; Budriesi, R.; Micucci, M.; Mattioli, L.B. Polyphenols from Olive-Mill Wastewater and Biological Activity: Focus on Irritable Bowel Syndrome. Nutrients 2022, 14, 1264. [Google Scholar] [CrossRef]
- Chiarini, A.; Minarini, A.; Budriesi, R.; Melchiorre, C. Molecular Properties of the Histamine H2-Receptor. Covalent Inhibition by Tetraamine Disulfides. Farmaco 1990, 45, 1001–1011. [Google Scholar]
- Hajimahmoodi, M.; Shams-Ardakani, M.; Saniee, P.; Siavoshi, F.; Mehrabani, M.; Hosseinzadeh, H.; Foroumadi, P.; Safavi, M.; Khanavi, M.; Akbarzadeh, T.; et al. In Vitro Antibacterial Activity of Some Iranian Medicinal Plant Extracts against Helicobacter Pylori. Nat. Prod. Res. 2011, 25, 1059–1066. [Google Scholar] [CrossRef]
- Tabak, M.; Armon, R.; Neeman, I. Cinnamon Extracts’ Inhibitory Effect on Helicobacter Pylori. J. Ethnopharmacol. 1999, 67, 269–277. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alkofahi, A.S.; Alzoubi, K.H.; Tumah, H.N.; Bani-Hani, K. Anti-Helicobactor Pylori Activity of Some Jordanian Medicinal Plants. Pharm. Biol. 2014, 52, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Ngnameko, C.R.; Marchetti, L.; Zambelli, B.; Quotadamo, A.; Roncarati, D.; Bertelli, D.; Njayou, F.N.; Smith, S.I.; Moundipa, P.F.; Costi, M.P.; et al. New Insights into Bioactive Compounds from the Medicinal Plant Spathodea Campanulata P. Beauv. and Their Activity against Helicobacter Pylori. Antibiotics 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Anti-Helicobacter Pylori Flavonoids from Licorice Extract. Life Sci. 2002, 71, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Hosaka, H.; Kawada, A.; Kuribayashi, S.; Shimoyama, Y.; Zai, H.; Kawamura, O.; Yamada, M. Gastrointestinal Motility and Functional Gastrointestinal Diseases. Curr. Pharm. Des. 2014, 20, 2775–2782. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ro, S.; Ward, S.M. Regulation of Gastrointestinal Motility—Insights from Smooth Muscle Biology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 633–645. [Google Scholar] [CrossRef]
- Hildebrand, F.; Ebersbach, T.; Nielsen, H.B.; Li, X.; Sonne, S.B.; Bertalan, M.; Dimitrov, P.; Madsen, L.; Qin, J.; Wang, J.; et al. A Comparative Analysis of the Intestinal Metagenomes Present in Guinea Pigs (Cavia porcellus) and Humans (Homo sapiens). BMC Genom. 2012, 13, 514. [Google Scholar] [CrossRef]
- Poitras, P.; Honde, C.; Havrankova, J.; Lahaie, R.G.; Trudel, L.; Goyer, R.; Junien, J.L.; Pascaud, X. Effect of Trimebutine on Intestinal Motility and Plasma Motilin in the Dog. Am. J. Physiol. 1986, 251, G349–G353. [Google Scholar] [CrossRef]
- Carocci, A.; Roselli, M.; Budriesi, R.; Micucci, M.; Desaphy, J.-F.; Altamura, C.; Cavalluzzi, M.M.; Toma, M.; Passeri, G.I.; Milani, G.; et al. Synthesis and Evaluation of Voltage-Gated Sodium Channel Blocking Pyrroline Derivatives Endowed with Both Antiarrhythmic and Antioxidant Activities. ChemMedChem 2021, 16, 578–588. [Google Scholar] [CrossRef]
- Ioan, P.; Carosati, E.; Micucci, M.; Cruciani, G.; Broccatelli, F.; Zhorov, B.S.; Chiarini, A.; Budriesi, R. 1,4-Dihydropyridine Scaffold in Medicinal Chemistry, the Story so Far and Perspectives (Part 1): Action in Ion Channels and GPCRs. Curr. Med. Chem. 2011, 18, 4901–4922. [Google Scholar] [CrossRef]
- Evangelista, S. Otilonium Bromide: A Selective Spasmolytic for the Gastrointestinal Tract. J. Int. Med. Res. 1999, 27, 207–222. [Google Scholar] [CrossRef]
- Strege, P.R.; Evangelista, S.; Lyford, G.L.; Sarr, M.G.; Farrugia, G. Otilonium Bromide Inhibits Calcium Entry through L-Type Calcium Channels in Human Intestinal Smooth Muscle. Neurogastroenterol. Motil. 2004, 16, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.; Corazziari, E.S.; Mearin, F.; Tack, J. IBS and the Role of Otilonium Bromide. Int. J. Colorectal Dis. 2013, 28, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Korecka, A.; Arulampalam, V. The Gut Microbiome: Scourge, Sentinel or Spectator? J. Oral. Microbiol. 2012, 4, 9367. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, T.C.; Bergh, K.; Waldum, H.L. Gastric Juice: A Barrier against Infectious Diseases. Basic. Clin. Pharmacol. Toxicol. 2005, 96, 94–102. [Google Scholar] [CrossRef]
- Naylor, G.; Axon, A. Role of Bacterial Overgrowth in the Stomach as an Additional Risk Factor for Gastritis. Can. J. Gastroenterol. 2003, 17 (Suppl. B), 13B–17B. [Google Scholar] [CrossRef]
- Reyes, V.E. Helicobacter Pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef]
- Huang, J.; Sridhar, S.; Hunt, R. Role of Helicobacter Pylori Infection and Non-Steroidal Anti-Inflammatory Drugs in Peptic-Ulcer Disease: A Meta-Analysis. Lancet 2002, 359, 14–22. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, N. Review of Atrophic Gastritis and Intestinal Metaplasia as a Premalignant Lesion of Gastric Cancer. J. Cancer Prev. 2015, 20, 25–40. [Google Scholar] [CrossRef]
- Yang, J.-C.; Lu, C.-W.; Lin, C.-J. Treatment of Helicobacter Pylori Infection: Current Status and Future Concepts. World J. Gastroenterol. 2014, 20, 5283–5293. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Grande, R.; Cannatelli, M.A.; Marzio, L.; Alonzo, V. Antibacterial Effect of Plant Extracts against Helicobacter Pylori. Phytother. Res. 2005, 19, 198–202. [Google Scholar] [CrossRef]
- Imai, H.; Osawa, K.; Yasuda, H.; Hamashima, H.; Arai, T.; Sasatsu, M. Inhibition by the Essential Oils of Peppermint and Spearmint of the Growth of Pathogenic Bacteria. Microbios 2001, 106 (Suppl. 1), 31–39. [Google Scholar] [PubMed]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African Peppermint (Mentha Piperita) from Morocco: Chemical Composition and Antimicrobial Properties of Essential Oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Alomar, H.A.; Fathallah, N.; Abdel-Aziz, M.M.; Ibrahim, T.A.; Elkady, W.M. GC-MS Profiling, Anti-Helicobacter Pylori, and Anti-Inflammatory Activities of Three Apiaceous Fruits’ Essential Oils. Plants 2022, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Sathianarayanan, S.; Ammanath, A.V.; Biswas, R.; Anita, B.; Sukumaran, S.; Venkidasamy, B. A New Approach against Helicobacter Pylori Using Plants and Its Constituents: A Review Study. Microb. Pathog. 2022, 168, 105594. [Google Scholar] [CrossRef]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Glycyrrhiza Glabra (Licorice): A Review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- do Nascimento, M.H.M.; de Araújo, D.R. Exploring the Pharmacological Potential of Glycyrrhizic Acid: From Therapeutic Applications to Trends in Nanomedicine. Future Pharmacol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Li, B.; Cheung, K.S.; Wong, I.Y.-H.; Leung, W.K.; Law, S. Calcium Channel Blockers Are Associated with Lower Gastric Cancer Risk: A Territory-Wide Study with Propensity Score Analysis. Int. J. Cancer 2021, 148, 2148–2157. [Google Scholar] [CrossRef]
- Carosati, E.; Ioan, P.; Micucci, M.; Broccatelli, F.; Cruciani, G.; Zhorov, B.S.; Chiarini, A.; Budriesi, R. 1,4-Dihydropyridine Scaffold in Medicinal Chemistry, The Story So Far And Perspectives (Part 2): Action in Other Targets and Antitargets. Curr. Med. Chem. 2012, 19, 4306–4323. [Google Scholar] [CrossRef]
- Salem, M.S.E.-D.; Mahfouz, A.Y.; Fathy, R.M. The Antibacterial and Antihemolytic Activities Assessment of Zinc Oxide Nanoparticles Synthesized Using Plant Extracts and Gamma Irradiation against the Uro-Pathogenic Multidrug Resistant Proteus Vulgaris. Biometals 2021, 34, 175–196. [Google Scholar] [CrossRef]
- Onlom, C.; Khanthawong, S.; Waranuch, N.; Ingkaninan, K. In vitro anti-Malassezia activity and potential use in anti-dandruff formulation of Asparagus racemosus. Int. J. Cosmet. Sci. 2014, 36, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Shaha, P.; Bellankimath, A. Pharmacological Profile of Asparagus Racemosus: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1215–1223. [Google Scholar] [CrossRef]
- Singh, R. Asparagus Racemosus: A Review on Its Phytochemical and Therapeutic Potential. Nat. Prod. Res. 2016, 30, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Chakŭrski, I.; Matev, M.; Koĭchev, A.; Angelova, I.; Stefanov, G. Treatment of chronic colitis with an herbal combination of Taraxacum officinale, Hipericum perforatum, Melissa officinaliss, Calendula officinalis and Foeniculum vulgare. Vutr. Boles. 1981, 20, 51–54. [Google Scholar]
- Pradhan, M.; Sribhuwaneswari, S.; Karthikeyan, D.; Minz, S.; Sure, P.; Chandu, A.N.; Mishra, U.; Kamalakannan, K.; Saravanakumar, A.; Sivakumar, T. In-Vitro Cytoprotection Activity of Foeniculum Vulgare and Helicteres Isora in Cultured Human Blood Lymphocytes and Antitumour Activity against B16F10 Melanoma Cell Line. Res. J. Pharm. Technol. 2008, 1, 450–452. [Google Scholar]
- Tabebuia—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/tabebuia (accessed on 30 October 2024).
- Kalpoutzakis, E.; Chatzimitakos, T.; Athanasiadis, V.; Mitakou, S.; Aligiannis, N.; Bozinou, E.; Gortzi, O.; Skaltsounis, L.A.; Lalas, S.I. Determination of the Total Phenolics Content and Antioxidant Activity of Extracts from Parts of Plants from the Greek Island of Crete. Plants 2023, 12, 1092. [Google Scholar] [CrossRef]
- Tovar, R.T.; Petzel, R.M. Herbal Toxicity. Dis. Mon. 2009, 55, 592–641. [Google Scholar] [CrossRef]
- Heinrich, M. Quality and Safety of Herbal Medical Products: Regulation and the Need for Quality Assurance along the Value Chains. Br. J. Clin. Pharmacol. 2015, 80, 62–66. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. North. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Roe, A.L.; Paine, M.F.; Gurley, B.J.; Brouwer, K.R.; Jordan, S.; Griffiths, J.C. Assessing Natural Product-Drug Interactions: An End-to-End Safety Framework. Regul. Toxicol. Pharmacol. 2016, 76, 1–6. [Google Scholar] [CrossRef]

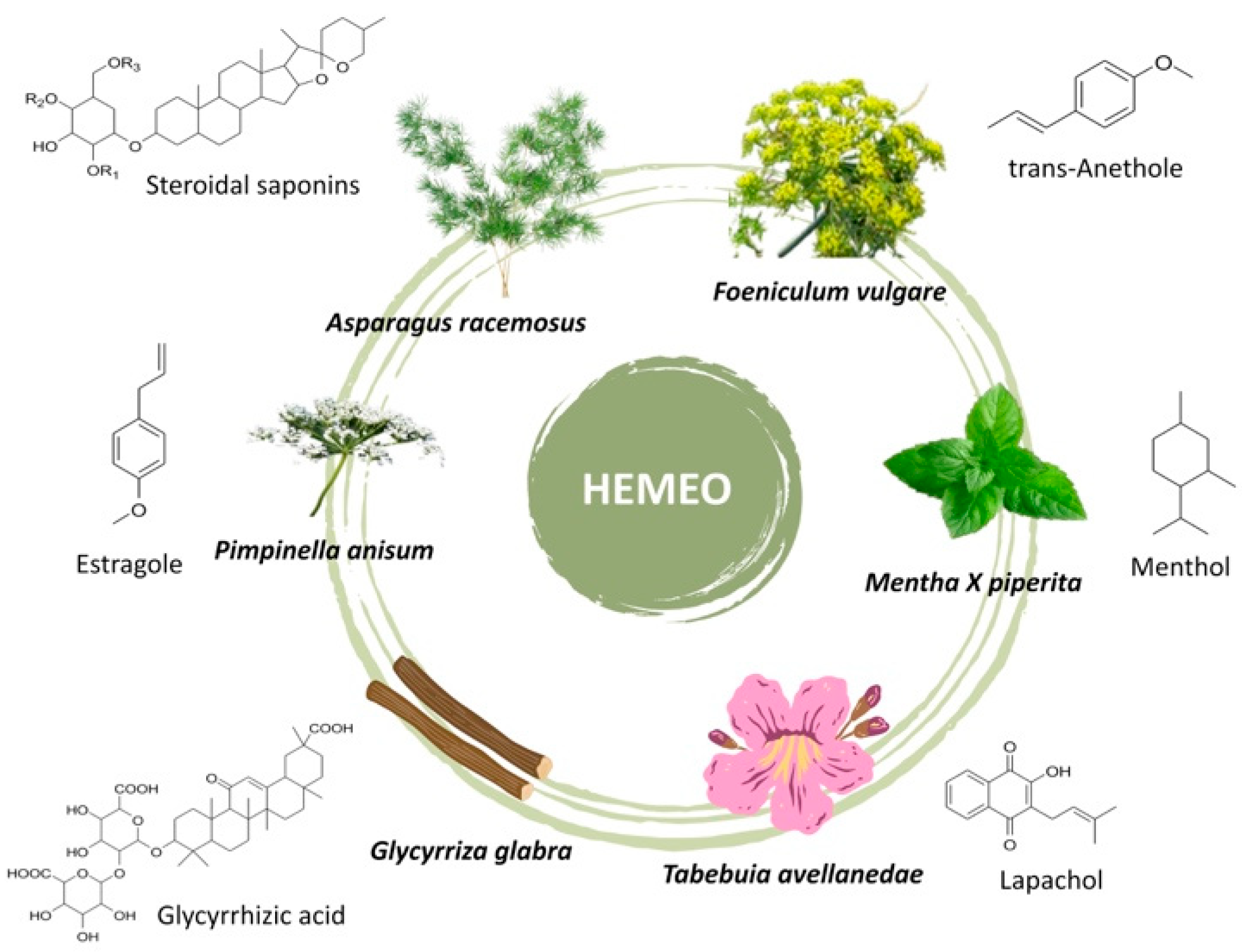


| Concentration | TPC a |
|---|---|
| HEMEO | 9.925 ± 0.42 |
| Stomach | Ileum | Colon | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/mL | 0.1 | 0.5 | 1.0 | 5.0 | 10.0 | 0.1 | 0.5 | 1.0 | 5.0 | 10.0 | 0.1 | 0.5 | 1.0 | 5.0 | 10.0 |
| Transit speed variation% | = | - | - | = | = | + | = | = | - | - | + | = | - | - | - |
| Pain% | = | = | = | + | + | + | = | - | - | - | + | = | = | - | - |
| Longitudinal contraction variation% | = | = | = | = | = | ++ | + | -- | --- | --- | + | - | -- | ---- | ---- |
| Ileum a | Right Atrium b | ||
|---|---|---|---|
| IC50 c | 95% Conf-Lim. | IC50 c | 95% Conf-Lim. |
| 0.78 | 0.66–0.87 | # | |
| Tissue | IA a | IC50 b | 95% Conf-Lim. |
|---|---|---|---|
| Gastric Fundus | 24.0 ± 2.6 | ||
| Ileum | 63.0 ± 3.7 | 1.33 | 1.11–1.60 |
| Colon | 98.0 ± 1.3 | 5.31 | 4.84–5.84 |
| Aorta | 12.0 ± 0.3 c |
| Left Atrium | Right Atrium | |
|---|---|---|
| Negative Inotropy a | Negative Inotropy b | Negative Chronotropy |
| IA a | IA b | IA c |
| * | * | * |
| Sample | Concentration | |||
|---|---|---|---|---|
| 2.5 mg/mL a | 20.0 mg/mL a | 100 mg/mL a | MAQ b | |
| HEMEO | NT | 12 ± 2 | 22 ± 4 | 20.0 |
| Karnamicyn | 35 ± 2 | NT | NT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budriesi, R.; Corazza, I.; Roncioni, S.; Scanferlato, R.; De Luca, D.; Marzetti, C.; Gotti, R.; Rizzardi, N.; Bergamini, C.; Micucci, M.; et al. Herbal Extracts Mixed with Essential Oils: A Network Approach for Gastric and Intestinal Motility Disorders. Nutrients 2024, 16, 4357. https://doi.org/10.3390/nu16244357
Budriesi R, Corazza I, Roncioni S, Scanferlato R, De Luca D, Marzetti C, Gotti R, Rizzardi N, Bergamini C, Micucci M, et al. Herbal Extracts Mixed with Essential Oils: A Network Approach for Gastric and Intestinal Motility Disorders. Nutrients. 2024; 16(24):4357. https://doi.org/10.3390/nu16244357
Chicago/Turabian StyleBudriesi, Roberta, Ivan Corazza, Simone Roncioni, Roberta Scanferlato, Dalila De Luca, Carla Marzetti, Roberto Gotti, Nicola Rizzardi, Christian Bergamini, Matteo Micucci, and et al. 2024. "Herbal Extracts Mixed with Essential Oils: A Network Approach for Gastric and Intestinal Motility Disorders" Nutrients 16, no. 24: 4357. https://doi.org/10.3390/nu16244357
APA StyleBudriesi, R., Corazza, I., Roncioni, S., Scanferlato, R., De Luca, D., Marzetti, C., Gotti, R., Rizzardi, N., Bergamini, C., Micucci, M., Roncarati, D., & Mattioli, L. B. (2024). Herbal Extracts Mixed with Essential Oils: A Network Approach for Gastric and Intestinal Motility Disorders. Nutrients, 16(24), 4357. https://doi.org/10.3390/nu16244357








