Fresh Parent’s Own Milk for Preterm Infants: Barriers and Future Opportunities
Abstract
:1. Introduction and Overview of NICU Practices and Implications for Parent’s Own Milk Feeding
2. Potential Benefits of Fresh Parent’s Own Milk to Infant Health and Development
3. Future Research and Directions
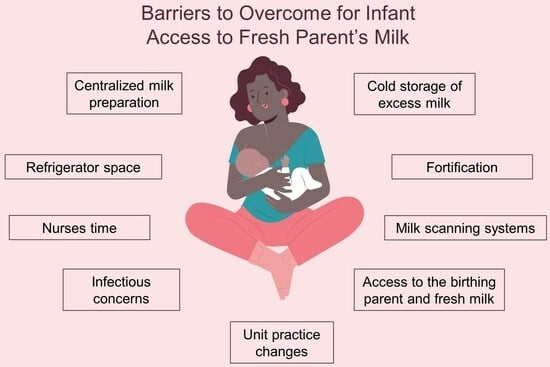
4. What Can We Do Today?
5. Summary
- Standardized Definitions: Develop standardized definitions and classifications of different types of human milk feeding (e.g., fresh, refrigerated, frozen) to facilitate consistent reporting and analysis in clinical care and research.
- Healthcare Economics: Investigate the healthcare cost implications of feeding fresh human milk versus frozen milk, considering short-term and long-term outcomes, which may inform policy decisions.
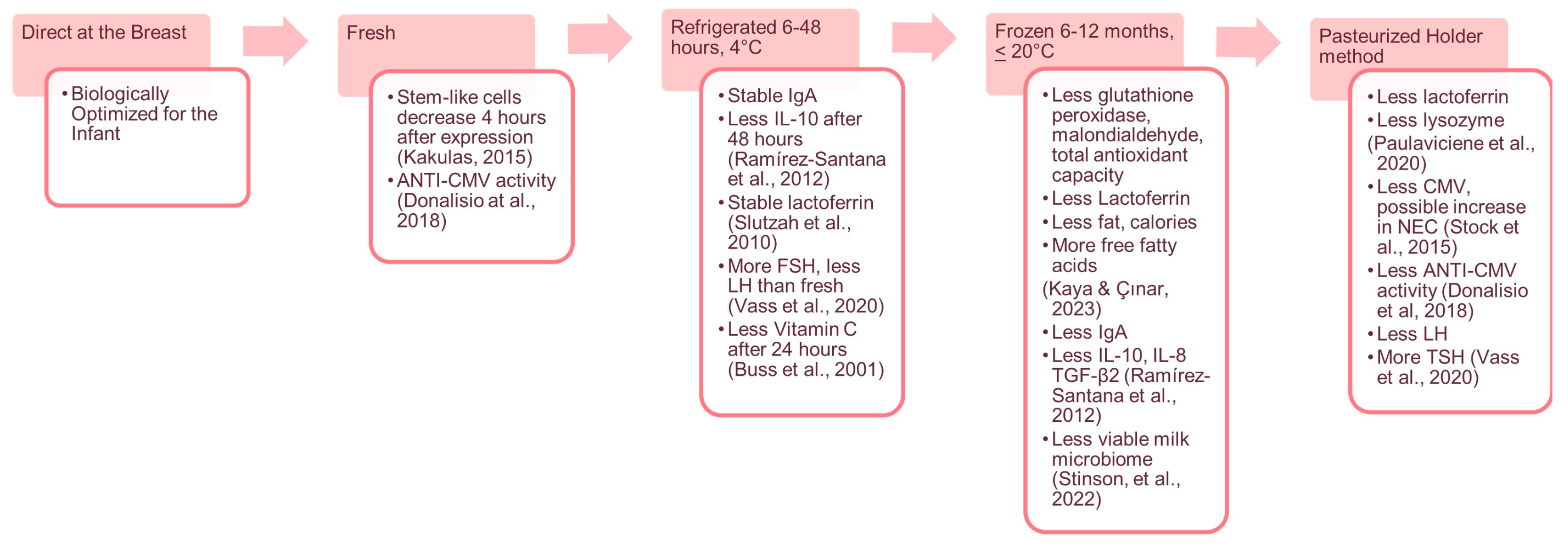
- Biological Impact: Continue to explore the biological changes in human milk over time and during storage and assess how these changes impact the health and development of preterm infants.
- Long-Term Outcomes: Conduct long-term follow-up studies to understand the potential lifelong benefits of fresh milk feeding on preterm infants’ health, growth, and neurodevelopment.
- Parental Education and Support: Develop and implement educational programs to inform parents about the benefits of fresh milk and empower them to participate in the decision-making process regarding their infant’s nutrition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steele, C.; Short, R. Centralized Infant Formula Preparation Room in the Neonatal Intensive Care Unit Reduces Incidence of Microbial Contamination. J. Am. Diet. Assoc. 2008, 108, 1700–1703. [Google Scholar] [CrossRef]
- White, R.D.; Smith, J.A.; Shepley, M.M.; on behalf of the Committee to Establish Recommended Standards for Newborn ICU Design. Recommended Standards for Newborn ICU Design, Eighth Edition. J. Perinatol. 2013, 33, S2–S16. [Google Scholar] [CrossRef]
- Spatz, D.L.; Schmidt, K.J.; Kinzler, S. Implementation of a Human Milk Management Center. Adv. Neonatal Care 2014, 14, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Steele, C. Best Practices for Handling and Administration of Expressed Human Milk and Donor Human Milk for Hospitalized Preterm Infants. Front. Nutr. 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Brock, W.W.; Cunningham, C.A.; Brandon, D.H.; Hoehn, V.; Carter, B. Improving the Process of Enteral Nutrition Preparation With Milk Technicians: Perceptions of Cost, Time, and Quality. Adv. Neonatal. Care 2016, 16, 124–134. [Google Scholar] [CrossRef]
- Sun, H.; Cao, Y.; Han, S.; Cheng, R.; Liu, L.; Liu, J.; Xia, S.; Zhang, J.; Li, Z.; Cheng, X.; et al. A Randomized Controlled Trial Protocol Comparing the Feeds of Fresh versus Frozen Mother’s Own Milk for Preterm Infants in the NICU. Trials 2020, 21, 170. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H. Breastfeeding in Hospitalised Preterm Infants: A Survey from 18 Tertiary Neonatal Intensive Care Units across Mainland China. J Paediatr. Child Health 2020, 56, 1432–1437. [Google Scholar] [CrossRef]
- Andersson, Y.; Sävman, K.; Bläckberg, L.; Hernell, O. Pasteurization of Mother’s Own Milk Reduces Fat Absorption and Growth in Preterm Infants. Acta Paediatr. 2007, 96, 1445–1449. [Google Scholar] [CrossRef]
- Aceti, A.; Cavallarin, L.; Martini, S.; Giribaldi, M.; Vitali, F.; Ambretti, S.; Zambrini, V.; Corvaglia, L. Effect of Alternative Pasteurization Techniques on Human Milk’s Bioactive Proteins. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Akinbi, H.; Meinzen-Derr, J.; Auer, C.; Ma, Y.; Pullum, D.; Kusano, R.; Reszka, K.J.; Zimmerly, K. Alterations in the Host Defense Properties of Human Milk Following Prolonged Storage or Pasteurization. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wemelle, E.; Marousez, L.; Lesage, J.; De Lamballerie, M.; Knauf, C.; Carneiro, L. In Vivo Assessment of Antioxidant Potential of Human Milk Treated by Holder Pasteurization or High Hydrostatic Pressure Processing: A Preliminary Study on Intestinal and Hepatic Markers in Adult Mice. Antioxidants 2022, 11, 1091. [Google Scholar] [CrossRef]
- Hassiotou, F.; Savigni, D.; Hartmann, P.; Geddes, D. Optimization of Cell Isolation from Human Milk. FASEB J. 2015, 29, 582.7. [Google Scholar] [CrossRef]
- Sun, H.; Han, S.; Cheng, R.; Hei, M.; Kakulas, F.; Lee, S.K. Testing the Feasibility and Safety of Feeding Preterm Infants Fresh Mother’s Own Milk in the NICU: A Pilot Study. Sci. Rep. 2019, 9, 941. [Google Scholar] [CrossRef]
- Kaya, Ö.; Çınar, N. The Effects of Freezing and Thawing on Mature Human Milk’s Contains: A Systematic Review. Midwifery 2023, 118, 103519. [Google Scholar] [CrossRef]
- Donalisio, M.; Rittà, M.; Tonetto, P.; Civra, A.; Coscia, A.; Giribaldi, M.; Cavallarin, L.; Moro, G.E.; Bertino, E.; Lembo, D. Anti-Cytomegalovirus Activity in Human Milk and Colostrum from Mothers of Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 654–659. [Google Scholar] [CrossRef]
- Ramírez-Santana, C.; Pérez-Cano, F.J.; Audí, C.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Castellote, C.; Franch, A. Effects of Cooling and Freezing Storage on the Stability of Bioactive Factors in Human Colostrum. J. Dairy Sci. 2012, 95, 2319–2325. [Google Scholar] [CrossRef]
- Slutzah, M.; Codipilly, C.N.; Potak, D.; Clark, R.M.; Schanler, R.J. Refrigerator Storage of Expressed Human Milk in the Neonatal Intensive Care Unit. J. Pediatr. 2010, 156, 26–28. [Google Scholar] [CrossRef]
- Vass, R.A.; Roghair, R.D.; Bell, E.F.; Colaizy, T.T.; Johnson, K.J.; Schmelzel, M.L.; Walker, J.R.; Ertl, T. Pituitary Glycoprotein Hormones in Human Milk before and after Pasteurization or Refrigeration. Nutrients 2020, 12, 687. [Google Scholar] [CrossRef]
- Buss, I.; McGill, F.; Winterbourn, C. Vitamin C Is Reduced in Human Milk after Storage. Acta Paediatr. 2001, 90, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Trevenen, M.L.; Geddes, D.T. Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk. Nutrients 2022, 14, 1875. [Google Scholar] [CrossRef] [PubMed]
- Paulaviciene, I.J.; Liubsys, A.; Eidukaite, A.; Molyte, A.; Tamuliene, L.; Usonis, V. The Effect of Prolonged Freezing and Holder Pasteurization on the Macronutrient and Bioactive Protein Compositions of Human Milk. Breastfeed. Med. 2020, 15, 583–588. [Google Scholar] [CrossRef]
- Stock, K.; Griesmaier, E.; Brunner, B.; Neubauer, V.; Kiechl-Kohlendorfer, U.; Trawöger, R. Pasteurization of Breastmilk Decreases the Rate of Postnatally Acquired Cytomegalovirus Infections, but Shows a Nonsignificant Trend to an Increased Rate of Necrotizing Enterocolitis in Very Preterm Infants—A Preliminary Study. Breastfeed. Med. 2015, 10, 113–117. [Google Scholar] [CrossRef]
- Briere, C.-E.; McGrath, J.M.; Jensen, T.; Matson, A.; Finck, C. Breast Milk Stem Cells: Current Science and Implications for Preterm Infants. Adv. Neonatal Care 2016, 16, 410–419. [Google Scholar] [CrossRef]
- Briere, C.-E.; Jensen, T.; McGrath, J.M.; Young, E.E.; Finck, C. Stem-like Cell Characteristics from Breast Milk of Mothers with Preterm Infants as Compared to Mothers with Term Infants. Breastfeed. Med. 2017, 12, 174–179. [Google Scholar] [CrossRef]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s Alive: Microbes and Cells in Human Milk and Their Potential Benefits to Mother and Infant. Adv. Nutr. 2014, 5, 571–573. [Google Scholar] [CrossRef]
- Gomez, J.; Abela, K.; LoBiondo-Wood, G. A Systemic Review of the Difference Between Diets for Preterm Infants Containing Raw Mother’s Own Milk and Frozen or Pasteurized Mother’s Own Milk. J. Hum. Lact. 2024. [Google Scholar] [CrossRef]
- Boone, K.M.; Geraghty, S.R.; Keim, S.A. Feeding at the Breast and Expressed Milk Feeding: Associations with Otitis Media and Diarrhea in Infants. J. Pediatr. 2016, 174, 118–125. [Google Scholar] [CrossRef]
- Soto-Ramírez, N.; Karmaus, W.; Zhang, H.; Davis, S.; Agarwal, S.; Albergottie, A. Modes of Infant Feeding and the Occurrence of Coughing/Wheezing in the First Year of Life. J. Hum. Lact. 2013, 29, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Vehling, L.; Chan, D.; Klopp, A.; Nickel, N.C.; McGavock, J.M.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food. Pediatrics 2018, 142, e20181092. [Google Scholar] [CrossRef]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Azad, M.B.; Daley, D.; et al. Modes of Infant Feeding and the Risk of Childhood Asthma: A Prospective Birth Cohort Study. J. Pediatr. 2017, 190, 192–199.e2. [Google Scholar] [CrossRef]
- Dicky, O.; Ehlinger, V.; Montjaux, N.; Gremmo-Féger, G.; Sizun, J.; Rozé, J.-C.; Arnaud, C.; Casper, C.; the EPIPAGE 2 Nutrition, S.G.; the EPINUTRI, S.G. Policy of Feeding Very Preterm Infants with Their Mother’s Own Fresh Expressed Milk Was Associated with a Reduced Risk of Bronchopulmonary Dysplasia. Acta Paediatr. 2017, 106, 755–762. [Google Scholar] [CrossRef]
- Martínez-Herrero, S.; Martínez, A. Adrenomedullin: Not Just Another Gastrointestinal Peptide. Biomolecules 2022, 12, 156. [Google Scholar] [CrossRef]
- Peila, C. Effects of Pasteurization and Refrigerated Storage on Human Milk Neurobiomarkers Concentrations. Ph.D. Thesis, Maastricht University, Maastricht, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Ahrabi, A.F.; Handa, D.; Codipilly, C.N.; Shah, S.; Williams, J.E.; McGuire, M.A.; Potak, D.; Aharon, G.G.; Schanler, R.J. Effects of Extended Freezer Storage on the Integrity of Human Milk. J. Pediatr. 2016, 177, 140–143. [Google Scholar] [CrossRef]
- Pandya, S.; Doshi, H.; Codipilly, C.; Fireizen, Y.; Potak, D.; Schanler, R. Bacterial Stability with Freezer Storage of Human Milk. J. Perinat. Med. 2021, 49, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Coni, P.; Piras, M.; Piludu, M.; Lachowicz, J.I.; Matteddu, A.; Coni, S.; Reali, A.; Fanos, V.; Jaremko, M.; Faa, G.; et al. Exploring Cell Surface Markers and Cell-Cell Interactions of Human Breast Milk Stem Cells. J. Public Health Res. 2023, 12, 227990362211503. [Google Scholar] [CrossRef] [PubMed]
- Van Haastert, P.J.M. How Cells Use Pseudopods for Persistent Movement and Navigation. Sci. Signal. 2011, 4, pe6. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Khalid, N.; Azimpouran, M. Necrosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Huang, J.; Zheng, Z.; Zhao, X.-Y.; Huang, L.-H.; Wang, L.; Zhang, X.-L.; Lin, X.-Z. Short-Term Effects of Fresh Mother’s Own Milk in Very Preterm Infants. Matern. Child Nutr. 2023, 19, e13430. [Google Scholar] [CrossRef]
- Binder, C.; Baumgartner-Parzer, S.; Gard, L.-I.; Berger, A.; Thajer, A. Human Milk Processing and Its Effect on Protein and Leptin Concentrations. Nutrients 2023, 15, 347. [Google Scholar] [CrossRef]
- Johnson, T.J.; Patra, K.; Greene, M.M.; Hamilton, M.; Dabrowski, E.; Meier, P.P.; Patel, A.L. NICU Human Milk Dose and Health Care Use after NICU Discharge in Very Low Birth Weight Infants. J. Perinatol. 2019, 39, 120–128. [Google Scholar] [CrossRef]
- Cortez, R.V.; Fernandes, A.; Sparvoli, L.G.; Padilha, M.; Feferbaum, R.; Neto, C.M.; Taddei, C.R. Impact of Oropharyngeal Administration of Colostrum in Preterm Newborns’ Oral Microbiome. Nutrients 2021, 13, 4224. [Google Scholar] [CrossRef]
- Granger, C.L.; Lamb, C.A.; Embleton, N.D.; Beck, L.C.; Masi, A.C.; Palmer, J.M.; Stewart, C.J.; Berrington, J.E. Secretory Immunoglobulin A in Preterm Infants: Determination of Normal Values in Breast Milk and Stool. Pediatr. Res. 2022, 92, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Lanzieri, T.M.; Dollard, S.C.; Josephson, C.D.; Schmid, D.S.; Bialek, S.R. Breast Milk–Acquired Cytomegalovirus Infection and Disease in VLBW and Premature Infants. Pediatrics 2013, 131, e1937–e1945. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.S. Viral Infections and the Neonatal Brain. Semin. Pediatr. Neurol. 2019, 32, 100769. [Google Scholar] [CrossRef]
- Brecht, K.F.; Goelz, R.; Bevot, A.; Krägeloh-Mann, I.; Wilke, M.; Lidzba, K. Postnatal Human Cytomegalovirus Infection in Preterm Infants Has Long-Term Neuropsychological Sequelae. J. Pediatr. 2015, 166, 834–839. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L.; Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Meier, P.P. More Evidence: Mothers’ Own Milk Is Personalized Medicine for Very Low Birthweight Infants. Cell Rep. Med. 2022, 3, 100710. [Google Scholar] [CrossRef]
- Parker, L.A.; Cacho, N.; Bendixen, M.M.; Sullivan, S.; Magalhaes, M.; Krueger, C.; Mueller, M. Measures of Lactation Outcomes in Women Delivering Preterm Infants. Nurs. Res. 2021, 70, 193–199. [Google Scholar] [CrossRef]
- Bujold, M.; Feeley, N.; Axelin, A.; Cinquino, C. Expressing Human Milk in the NICU: Coping Mechanisms and Challenges Shape the Complex Experience of Closeness and Separation. Adv. Neonatal Care 2018, 18, 38–48. [Google Scholar] [CrossRef]
- Treherne, S.C.; Feeley, N.; Charbonneau, L.; Axelin, A. Parents’ Perspectives of Closeness and Separation with Their Preterm Infants in the NICU. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 737–747. [Google Scholar] [CrossRef]
- Acuna-Muga, J.; Ureta-Velasco, N.; de la Cruz-Bertolo, J.; Ballesteros-Lopez, R.; Sanchez-Martinez, R.; Miranda-Casabona, E.; Miguel-Trigoso, A.; Garcia-San Jose, L.; Pallas-Alonso, C. Volume of Milk Obtained in Relation to Location and Circumstances of Expression in Mothers of Very Low Birth Weight Infants. J. Hum. Lact. 2014, 30, 41–46. [Google Scholar] [CrossRef]
- Kumar, J.; Meena, J.; Ranjan, A.; Kumar, P. Oropharyngeal Application of Colostrum or Mother’s Own Milk in Preterm Infants: A Systematic Review and Meta-Analysis. Nutr. Rev. 2023, 81, 1254–1266. [Google Scholar] [CrossRef]
- Maffei, D.; Brewer, M.; Codipilly, C.; Weinberger, B.; Schanler, R.J. Early Oral Colostrum Administration in Preterm Infants. J. Perinatol. 2020, 40, 284–287. [Google Scholar] [CrossRef]
- Parker, M.G.; Melvin, P.; Graham, D.A.; Gupta, M.; Burnham, L.A.; Lopera, A.M.; Zera, C.A.; Belfort, M.B. Timing of First Milk Expression to Maximize Breastfeeding Continuation Among Mothers of Very Low-Birth-Weight Infants. Obstet. Gynecol. 2019, 133, 1208–1215. [Google Scholar] [CrossRef]
- Parker, L.A.; Sullivan, S.; Kruger, C.; Mueller, M. Timing of Milk Expression Following Delivery in Mothers Delivering Preterm Very Low Birth Weight Infants: A Randomized Trial. J. Perinatol. 2020, 40, 1236–1245. [Google Scholar] [CrossRef]
- Briere, C.-E.; McGrath, J.M.; Cong, X.; Brownell, E.; Cusson, R. Direct-Breastfeeding in the Neonatal Intensive Care Unit and Breastfeeding Duration for Premature Infants. Appl. Nurs. Res. 2016, 32, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Son, H.; Byun, S.Y.; Han, G. Effect of Direct Breastfeeding Program for Premature Infants in Neonatal Intensive Care Unit. J. Korean Acad. Nurs. 2021, 51, 119. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Girma, B. The Impact of Exclusive Breastfeeding on Malocclusion: A Systematic Review. SN Compr. Clin. Med. 2021, 3, 95–103. [Google Scholar] [CrossRef]
- Meier, P.P. Human Milk and Clinical Outcomes in Preterm Infants. Hum. Milk Compos. Clin. Benefits Future Oppor. 2019, 90, 163–174. [Google Scholar] [CrossRef]
- Meier, P.; Patel, A.; Janes, J. Sharing the Science of Lactation and Mothers’ Own Milk with NICU Families and Staff. Breastfeed. Med. 2020, 15, A14. [Google Scholar] [CrossRef]
- Parish, O.; Williams, D.; Odd, D.; Joseph-Williams, N. Barriers and Facilitators to Shared Decision-Making in Neonatal Medicine: A Systematic Review and Thematic Synthesis of Parental Perceptions. Patient Educ. Couns. 2022, 105, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briere, C.-E.; Gomez, J. Fresh Parent’s Own Milk for Preterm Infants: Barriers and Future Opportunities. Nutrients 2024, 16, 362. https://doi.org/10.3390/nu16030362
Briere C-E, Gomez J. Fresh Parent’s Own Milk for Preterm Infants: Barriers and Future Opportunities. Nutrients. 2024; 16(3):362. https://doi.org/10.3390/nu16030362
Chicago/Turabian StyleBriere, Carrie-Ellen, and Jessica Gomez. 2024. "Fresh Parent’s Own Milk for Preterm Infants: Barriers and Future Opportunities" Nutrients 16, no. 3: 362. https://doi.org/10.3390/nu16030362