Scoping Review of Available Culinary Nutrition Interventions for People with Neurological Conditions
Abstract
:1. Introduction
2. Materials and Method
2.1. Methodological Framework
2.2. Eligibility Criteria
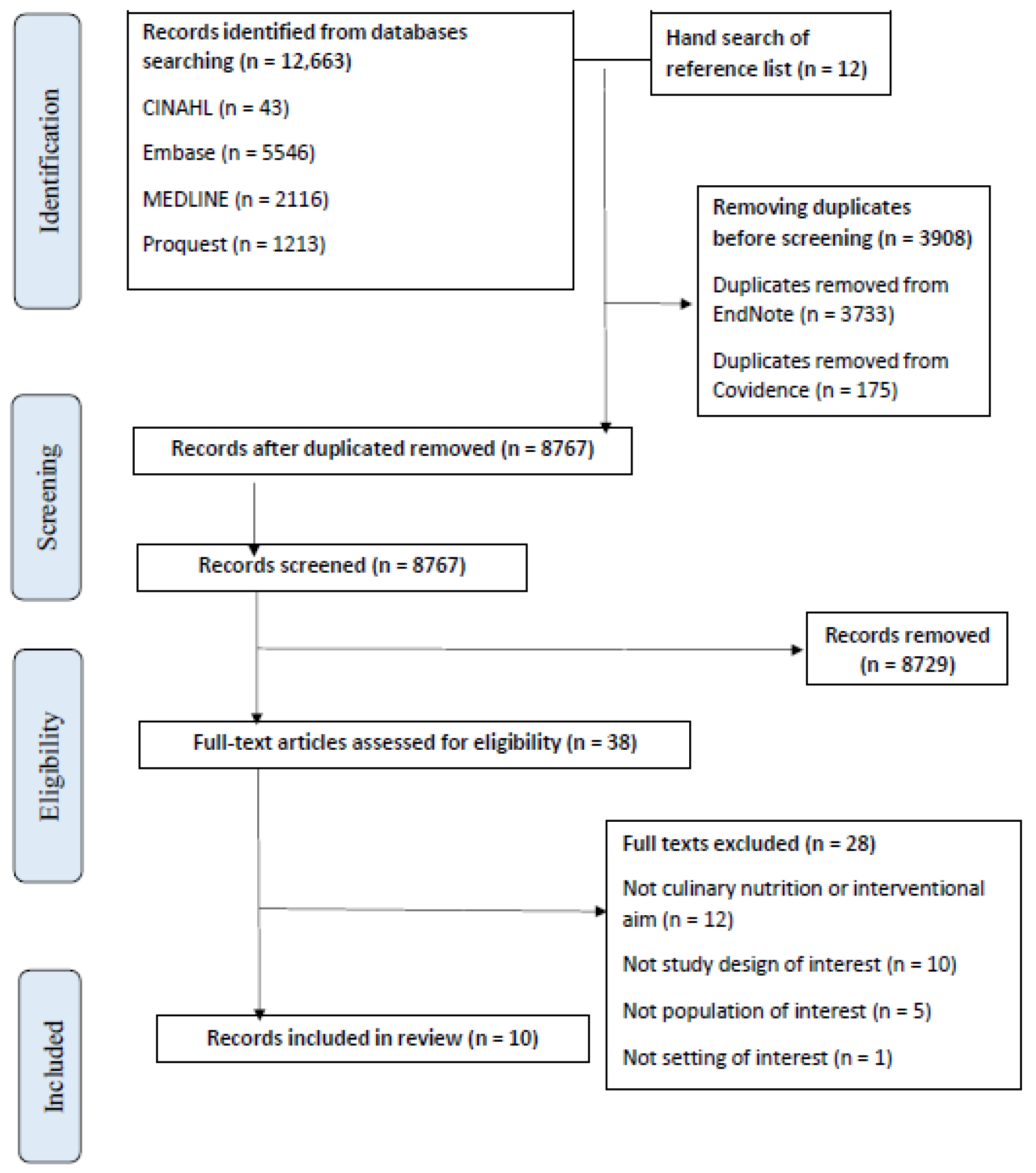
2.3. Information Sources and Search
2.4. Selection of Sources of Evidence
2.5. Data-Charting Process and Data Items
2.6. Synthesis of Results
3. Results
3.1. Included Studies
3.2. Study Characteristics (Table 2)
| No. | Authors, Year | Study Characteristics | Neurological Condition | Participants’ Characteristics | |||
|---|---|---|---|---|---|---|---|
| Journal, Country | Study Design | Total Number of Participants | Age in Years (Mean ± SD or Range) | %Female; Other Characteristics | |||
| 1 | Lin, Shu-Chi et al., 2021 [19] | Medicine Journal, Taiwan | Pilot, RCT, single-blinded | Stroke (dysphagia) | n = 22 | 78 ± 8 | 36; caregivers involved |
| 2 | Amytis Towfighi et al., 2020 [20] | Journal of Stroke and Cerebrovascular Diseases, US | Pilot, RCT | Stroke and TIA | n = 100 | 58 ± 9 | 38; 60% spanish-speaking |
| 3 | Valerie A. Hill et al., 2017 [21] | Journal of Stroke and Cerebrovascular Diseases, US | |||||
| 4 | James H. Rimmer et al., 2020 [22] | American Journal of Preventive Medicine, US | Pilot, pre–post | Stroke | n = 62 | 53 ± 8 | 75; predominantly urban African-American population, 37% had hemiplegia and 74% used a cane to ambulate |
| 5 | Ilana Katz Sanda et al., 2019 [23] | Multiple Sclerosis and Related Disorders, US | Pilot, RCT | Multiple Sclerosis | n = 36 | 43 (32–51) | 100 |
| 6 | Mary J. Doidge, 1993 [24] | Journal of Human Nutrition and Dietetics, UK | Pilot, pre–post | Multiple Sclerosis | n = 48 | 47 ± 10 | 60 |
| 7 | Rebecca D. Russell et al., 2023 [25] | Disability and Rehabilitation, Australia | Pilot, mixed-method, co-design | Multiple Sclerosis | Phase 1: n = 114; Phase 2: n = 16; Phase 3: n = 8 | Phase 1: 52 ± 12; Phase 2: NR; Phase 3: 39 ± 11 | Phase 1: 20, Phase 2: NR; stakeholders included, Phase 3: 22; caregivers included |
| 8 | M. McGraw-Hunter et al., 2009 [26] | Brain Injury, US | Pilot, pre–post | Traumatic brain injury (TBI) | n = 4 | 27 | 25 (All Caucasian) |
| 9 | Min-Soo Cho et al., 2019 [27] | Journal of Exercise Rehabilitation, Korea | Pilot, pre–post | Mild dementia | n = 23 | 84 ± 5 | NR; caregivers involved |
| 10 | Priscilla Brenes et al., 2021 [28] | Thesis Dissertation, US | Pilot, mixed-method, pre–post | Parkinson’s Disease | n = 27 | 67 | NR; caregivers involved |
3.3. Characteristics of Included Interventions (Table 3)
| No. | Authors, Year | Characteristics of Intervention | |||||
|---|---|---|---|---|---|---|---|
| Format | Group Size | Educator(s) | Duration | Tailoring | Motivational Experiences | ||
| 1 | Lin, Shu-Chi et al., 2021 [19] | Food Preparation Program | 3 to 4 | Dietitian | 6 weeks (frequency NR) | Food and drink texture modification; disease-specific content | Challenge, Palate Development, Recipe Concept, Skill Building, Success |
| 2 | Valerie A. Hill et al., 2017 [21] | Lifestyle Education Program (Embedded Nutrition Education) | 3 to 8 | Occupational therapist | 6 weeks (weekly 2 h session) | Personal learning goals; disease-specific content | Challenge, Celebration, Collaboration, Home Environment, Palate Development, Peer Support, Recipe Concept, Skill Building, Skill Reinforcement, Success |
| 3 | Amytis Towfighi et al., 2020 [20] | ||||||
| 4 | James H. Rimmer et al., 2000 [22] | Health Promotion Intervention (Embedded Nutrition Education) | NR | Dietitian (nutrition classes); other relevant allied health professional for other classes | 12 weeks (3 days a week) | Socioeconomic situation; disease-specific content | Challenge, Home Environment, Palate Development, Recipe Concept, Skill Building, Skill Reinforcement, Success |
| 5 | Ilana Katz Sanda et al., 2019 [23] | Dietary Education | 5 | Dietitian | 6 months (frequency NR) | Disease-specific content | Challenge, Palate Development, Recipe Concept, Skill Building, Skill Reinforcement, Success |
| 6 | Mary J. Doidge, 1993 [24] | Nutrition Education Programme | 8 to 11 | Dietitian (nutrition session); physiotherapist (exercise session) | >8 weeks (8 weekly 90 min sessions) | Co-designed; disease-specific content | Challenge, Palate Development, Recipe Concept, Skill Building, Success |
| 7 | Rebecca D. Russell et al., 2023 [25] | Online Nutrition Education Modules | N/A; self-learning | People with MS and health professionals (dietitian, professor, etc.) | 1 year (self-paced) | Co-designed; disease-specific content | Challenge, Collaboration, Palate Development, Peer Support, Recipe Concept, Skill Building, Skill Reinforcement, Success |
| 8 | M. McGraw-Hunter et al., 2009 [26] | Video Self-Modelling | N/A; self-learning | Researcher | 4 weeks (4 training sessions) | Providing verbal prompts individualised to each participant | Challenge, Home Environment, Skill Building, Skill Reinforcement, Success |
| 9 | Min-Soo Cho et al., 2019 [27] | Exercise and nutrition education program | NR | NR | 16 weeks (16 nutrition education sessions, 20 min each) | Ongoing check-in with health and nutrition issues; disease-specific content | Challenge, Recipe Concept, Skill Building |
| 10 | Priscilla Brenes et al., 2021 [28] | Online Nutrition Education Modules | NR | Instructor | 8 weeks (6 modules) | Disease-specific content | Challenge, Recipe Concept, Skill Building, Success |
3.4. Culinary Nutrition Components and Intervention Outcome (Table 4)
| No. | Authors, Year | Targeted Culinary Nutrition Components | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Culinary Nutrition Content | Knowledge Provision/Skills Training | Cooking Skills/Food Skills | Culinary Nutrition Literacy | Evaluation Tool(s) | Comparator | Intervention Outcome | ||
| 1 | Lin, Shu-Chi et al., 2021 [19] | Oral motor exercises, food texture and thickener recognition, hands-on food preparation, nutrition education. Choosing healthy food, maintaining a balanced diet, preparing ingredients, natural thickeners, energy-boosting foods, techniques of reshaping food, soft food recipes, a balanced and nutritious texture-modified diet. | Both | Both | Plan & manage, Select, Prepare, Eat | Dysphagia self-detection tool (EAT-10), Dietary Well-Being Questionnaire (brief version of the WHO QoL, Swallowing QoL Questionnaire and MNA) Health-related QoL Scale (WHOQOL-BREF) | Usual Care | Positive effects on patients’ self-perceived diet quality and well-being/QoL. Potential improvements in health-related QoL; QoL associated with the process of swallowing, and nutritional status. |
| 2 | Amytis Towfighi et al., 2020 [20] | Nutrition Education (‘Eating Healthy—introducing Mediterranean and DASH diet’, ‘Avoid Dietary Pitfalls’) Implementation practice (e.g., making green smoothies, preparing healthy salads, taking field trips to a local grocery store and restaurant with a small budget) | Both | Both | Plan & manage, Select, Prepare, Eat | Related body measures, dietary intake, program adherence, focus groups | Usual Care | Intervention shown to be feasible and efficacious. Insignificant outcome in dietary and biomedical measures. |
| 3 | Valerie A. Hill et al., 2017 [21] | |||||||
| 4 | James H. Rimmer et al., 2000 [22] | The classes included ‘hands-on’ cooking instruction that focused on low-fat, low-cholesterol food items. Participants were taught how to cook healthy meals during the first two classes of the week and then cooked their own healthy meal during the third class. Group discussion in ‘Health Behaviour’ class. | Both | Both | Plan & manage, Select, Prepare, Eat | Biomedical, fitness, nutritional and psychosocial measures. Nutrition-related measures: dietary fat intake, LSQ22, SCL-90R. | Control Group | Treatment group reduced total cholesterol, weight, social isolation; increased cardiovascular fitness, strength, flexibility, life satisfaction and ability to manage self-care needs. |
| 5 | Ilana Katz Sanda et al., 2019 [23] | Nutrition education regarding healthy Mediterranean-style eating pattern for Americans (tips for grocery shopping, sample menu plan, reading food labels, eating in restaurants and travel) with access to registered dietitian’s guidance (meetings or emails). | Both | Both | Plan & manage, Select, Prepare, Eat | Various, including: dietary measure, e.g., Food Frequency Questionnaire, 3 dietary recalls, adherence to US-style Mediterranean diet score. Symptom evaluation, e.g., Neurological Fatigue Index-MS score, MS Impact Scale, Expanded Disability Status Scale. Program self-adherence | Non-Interventional Group | Excellent program self-adherence. The intervention group showed significant decline in fatigue and MS disease impact and disability status. |
| 6 | Mary J. Doidge, 1993 [24] | Topics in the program are Introductory Diet Analysis, Fat, Healthy Eating, Preparing Food at Home, Choosing Food Sensibly, Vitamins and Minerals, Lifestyles, Recipe Tasting. | Both | Both | Plan & manage, Select, Prepare, Eat | Seven-day weighed food and drink record. Participant’s dietary attitude assessment, subjective questionnaires (participants and dietitians) | No | (1) Dietary analysis: significant positive improvements in nutrient intakes. (2) Attitude change towards meal preparation: small increase because the majority of participants already had positive attitudes prior to the program. (3) Dietitians’ and participants’ subjective evaluation: All the dietitians felt the programme had gone well and all had enjoyed it themselves. Session aims and objectives had generally been met. Suggestions were made on several program topics. |
| 7 | Rebecca D. Russell et al., 2023 [25] | Topics in the intervention are Diet, MS Progression, MS Symptoms (Managing Fatigue in kitchen), Healthy Eating for MS, Assessing evidence, Putting into practice—meal planning and managing MS, Inflammation, Gut health, Depression, Future dietary research. Diet content, video, discussion board, workbook activities. | Both | Both | Plan & manage, Select, Prepare, Eat | Qualitative methods (survey, focus group, interviews) | No | Identified recommendations to the intervention; developed a full program prototype for feasibility study. |
| 8 | M. McGraw-Hunter et al., 2009 [26] | Participants received instruction in cooking stovetop meals (i.e., a boxed rice meal, stovetop noodles) at own home. They watched videotapes of themselves cooking and practiced that skill while receiving prompts and feedback. | Skill Training | Cooking Skills | Prepare | Ability to complete a 25-step recipe (percentage of completion) | No | Three of the four individuals achieved criterion performance (stovetop food preparation) within four training sessions; substantially maintained their skills 2- and 4-weeks following training and generalised their skills to a novel food item. |
| 9 | Min-Soo Cho et al., 2019 [27] | The main contents of nutrition education were divided into four fields: the concept of health, proper eating habits, nutrition and nutrients, and the problems of hypernutrition and nutrient deficiency. | Knowledge Provision | Food Skills | Select, Eat | MNA | No | Significant increase in MNA score (reduced risk of malnutrition) |
| 10 | Priscilla Brenes et al., 2021 [28] | General nutrition knowledge, Label reading, Parkinson’s Disease–diet relationship and tips. | Knowledge Provision | Food Skills | Plan & manage, Select, Prepare, Eat | Bowel health (BHQ), diet history (DHQ3), MNA, Nutrition Knowledge and Program Evaluation, Disease QoL (PDQ-39, UPDRS, CHAMPS, TSRQ) | No | Participant’s total consumption of macro- and micronutrients increased. A total of 50% of participants improved QoL scores. Participants more aware of healthy eating, gut health, hydration, food–medication interaction and constipation. |
3.5. Summary of Program Design, Delivery and Evaluation for Included Interventions (Table 5)
| No. | Culinary Nutrition Intervention | Program Design | Program Delivery | Program Evaluation (Process, Impact, Outcome) | Positive Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease-Specific Content | Side Effects Accommodation | Skills/Knowledge | Food/Cooking Skills | Culinary Nutrition Literacy | Attendance Flexibility | Co-Design | Appropriate Educator | Motivational Experiences | ||||
| 1 | Lin, Shu-Chi et al., 2021 [19] | √ | √ (Dysphagia) | √ (Both) | √ (Both) | √ (All) | X | X | √ (Dietitian) | √ (Lacking Celebration, Collaboration, Peer Support, Skill Reinforcement) | √ (Impact, Outcome) | √ (Dietary quality, QoL, side effect management, nutritional status) |
| 2 | Valerie A. Hill et al., 2017 [20] | √ | X | √ (Both) | √ (Both) | √ (All) | X | X | √ (Occupational therapist) | √ (All) | √ (Process, Impact) | √ (Feasibility and efficacy of program) |
| 3 | Amytis Towfighi et al., 2020 [21] | |||||||||||
| 4 | James H. Rimmer et al., 2000 [22] | √ | X | √ (Both) | √ (Both) | √ (All) | X | X | √ (Dietitian) | √ (Lacking Celebration, Collaboration) | √ (Impact, Outcome) | √ (Biomedical measures related to cardiovascular health, life satisfaction, self-management ability) |
| 5 | Ilana Katz Sanda et al., 2019 [23] | √ | √ (Fatigue) | √ (Both) | √ (Both) | √ (All) | X | X | √ (Dietitian) | √ (Lacking Celebration, Collaboration, Peer Support) | √ (All) | √ (Program self-adherence, fatigue, MS disease impact and disability status) |
| 6 | Mary J. Doidge, 1993 [24] | √ | X | √ (Both) | √ (Both) | √ (All) | X | X | √ (Dietitian) | √ (Lacking Celebration, Collaboration, Peer Support, Skill Reinforcement) | √ (All) | √ (Nutrient intakes, attitudes towards diet, program design) |
| 7 | Rebecca D. Russell, 2023 [25] | √ | X | √ (Both) | √ (Both) | √ (All) | √ | √ | √ (Dietitian, people with MS) | √ (Lacking Celebration, Home Environment) | √ (Process) | NR (Developed a full program prototype) |
| 8 | M. McGraw-Hunter et al., 2009 [26] | √ | X | √ (Skills training) | √ (Cooking) | √ (Prepare) | X | X | NR (Researcher) | √ (Lacking Celebration, Collaboration, Palate Development, Peer Support) | √ (Impact) | √ (Sustainable stovetop food preparation skills) |
| 9 | Min-Soo Cho et al., 2019 [27] | √ | X | √ (Knowledge provision) | √ (Food) | √ (Select, Eat) | X | X | NR | √ (Lacking Celebration, Collaboration, Home Environment, Palate Development, Peer Support, Skill Reinforcement, Success) | √ (Impact) | √ (Nutrition condition) |
| 10 | Priscilla Brenes, 2021 [28] | √ | √ (Gut health, inflammation) | √ (Knowledge Provision) | √ (Food) | √ (All) | √ | X | NR (Instructor) | √ (Lacking Celebration, Collaboration, Palate Development, Peer Support, Skill Reinforcement) | √ (All) | √ (Diet quality, QoL, awareness of food and disease relationship) |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef]
- Australia Government Department of Health and Aged Care. What We’re Doing about Neurological Conditions 2020. Available online: https://www.health.gov.au/topics/chronic-conditions/what-were-doing-about-chronic-conditions/what-were-doing-about-neurological-conditions (accessed on 1 May 2023).
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef] [PubMed]
- English, C.; MacDonald-Wicks, L.; Patterson, A.; Attia, J.; Hankey, G.J. The role of diet in secondary stroke prevention. Lancet Neurol. 2021, 20, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Ranson, J.M.; Gregory, S.; Macpherson, H.; Milte, C.; Lentjes, M.; Mulligan, A.; McEvoy, C.; Griffiths, A.; Matu, J.; et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: Findings from the UK Biobank prospective cohort study. BMC Med. 2023, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.D.; Black, L.J.; Begley, A. Nutrition Education Programs for Adults with Neurological Diseases Are Lacking: A Scoping Review. Nutrients 2022, 14, 1577. [Google Scholar] [CrossRef] [PubMed]
- Asher, R.C.; Shrewsbury, V.A.; Bucher, T.; Collins, C.E. Culinary medicine and culinary nutrition education for individuals with the capacity to influence health related behaviour change: A scoping review. J. Hum. Nutr. Diet. 2022, 35, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Pritlove, C.; Capone, G.; Kita, H.; Gladman, S.; Maganti, M.; Jones, J.M. Cooking for Vitality: Pilot Study of an Innovative Culinary Nutrition Intervention for Cancer-Related Fatigue in Cancer Survivors. Nutrients 2020, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Loftin, W.; Barnett, S.; Sullivan, P.; Bunn, P.S.; Tavakoli, A. Culturally competent dietary education for southern rural African Americans with diabetes. Diabetes Educ. 2002, 28, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Flego, A.; Gibbs, L.; Waters, E.; Swinburn, B.; Reynolds, J.; Moodie, M. Wider impacts of a 10-week community cooking skills program—Jamie’s Ministry of Food, Australia. BMC Public. Health 2014, 14, 1161. [Google Scholar] [CrossRef]
- Foley, W.; Spurr, S.; Lenoy, L.; De Jong, M.; Fichera, R. Cooking skills are important competencies for promoting healthy eating in an urban Indigenous health service. Nutr. Diet. 2011, 68, 291–296. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O'Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Arksey, H.; O'Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Vidgen, H.A.; Gallegos, D. Defining food literacy and its components. Appetite 2014, 76, 50–59. [Google Scholar] [CrossRef]
- Lavelle, F.; McGowan, L.; Hollywood, L.; Surgenor, D.; McCloat, A.; Mooney, E.; Caraher, M.; Raats, M.; Dean, M. The development and validation of measures to assess cooking skills and food skills. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Clarivate. EndNote 20.2.1. Available online: https://clarivate.libguides.com/endnote_training/endnote_online (accessed on 1 May 2023).
- Veritas Health Innovation Ltd. Covidence. Available online: https://www.covidence.org/ (accessed on 1 May 2023).
- Fredericks, L.; Koch, P.A.; Liu, A.; Galitzdorfer, L.; Costa, A.; Utter, J. Experiential Features of Culinary Nutrition Education That Drive Behavior Change: Frameworks for Research and Practice. Health Promot. Pract. 2020, 21, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Lin, K.-H.; Tsai, Y.-C.; Chiu, E.-C. Effects of a food preparation program on dietary well-being for stroke patients with dysphagia: A pilot study. Medicine 2021, 100, e26479. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, A.; Cheng, E.M.; Hill, V.A.; Barry, F.; Lee, M.; Valle, N.P.; Mittman, B.; Ayala-Rivera, M.; Moreno, L.; Espinosa, A.; et al. Results of a Pilot Trial of a Lifestyle Intervention for Stroke Survivors: Healthy Eating and Lifestyle after Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105323. [Google Scholar] [CrossRef] [PubMed]
- Hill, V.A.; Vickrey, B.G.; Cheng, E.M.; Valle, N.P.; Ayala-Rivera, M.; Moreno, L.; Munoz, C.; Dombish, H.; Espinosa, A.; Wang, D.; et al. A Pilot Trial of a Lifestyle Intervention for Stroke Survivors: Design of Healthy Eating and Lifestyle after Stroke (HEALS). J. Stroke Cerebrovasc. Dis. 2017, 26, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, J.H.; Braunschweig, C.; Silverman, K.; Riley, B.; Creviston, T.; Nicola, T. Effects of a short-term health promotion intervention for a predominantly African-American group of stroke survivors. Am. J. Prev. Med. 2000, 18, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I.; Benn, E.K.T.; Fabian, M.; Fitzgerald, K.C.; Digga, E.; Deshpande, R.; Miller, A.; Gallo, S.; Arab, L. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2019, 36, 101403. [Google Scholar] [CrossRef] [PubMed]
- Doidge, M.J. Evaluation of a nutrition education programme for people with multiple sclerosis. J. Hum. Nutr. Diet. 1993, 6, 131–147. [Google Scholar] [CrossRef]
- Russell, R.D.; Black, L.J.; Purdue, J.; Daly, A.; Begley, A. A collaborative approach to designing an online nutrition education program for people with multiple sclerosis. Disabil. Rehabil. 2023, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- McGraw-Hunter, M.; Faw, G.D.; Davis, P.K. The use of video self-modelling and feedback to teach cooking skills to individuals with traumatic brain injury: A pilot study. Brain Inj. 2006, 20, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Kim, J.Y. Effects of exercise and nutrition education programs on motor function and eating habit in mild dementia patients. J. Exerc. Rehabil. 2019, 15, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.H.P. Virtual Nutrition Education for People Affected by Parkinson’s Disease. Master’s Degree, Kansas State University, Manhattan, KS, USA, 2021. [Google Scholar]
- Meloncelli, N.; Young, A.; Christoffersen, A.; Rushton, A.; Zhelnov, P.; Wilkinson, S.A.; Scott, A.M.; de Jersey, S. Co-designing nutrition interventions with consumers: A scoping review. J. Hum. Nutr. Diet. 2023, 36, 1045–1067. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.; Gabiatti, M.P.; Moreira, J.D.; Ribeiro, L.C.; Lunardi, M.d.S.; Lin, K.; Venske, D.K. Ketogenic diet, epilepsy and cognition: What do we know so far? A systematic review. Nutr. Rev. 2022, 80, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Tyrlikova, I.; Mathews, G.C. Dietary treatment in adults with refractory epilepsy: A review. Neurology 2014, 83, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Żukiewicz-Sobczak, W.; Król, R.; Wróblewska, P.; Piątek, J.; Gibas-Dorna, M. Huntington Disease–Principles and practice of nutritional management. Neurol. I Neurochir. Pol. 2014, 48, 442–448. [Google Scholar] [CrossRef]
- Salvioni, C.C.d.S.; Stanich, P.; Almeida, C.S.; Oliveira, A.S.B. Nutritional care in motor neurone disease/amyotrophic lateral sclerosis. Arq. Neuro-Psiquiatr. 2014, 72, 157–163. [Google Scholar] [CrossRef]
- Aterman, S.; Ghahari, S.; Kessler, D. Characteristics of peer-based interventions for individuals with neurological conditions: A scoping review. Disabil. Rehabil. 2023, 45, 344–375. [Google Scholar] [CrossRef]
- González-Fernández, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after Stroke: An Overview. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Trimble, B.; Morgenstern, L.B. Stroke in minorities. Neurol. Clin. 2008, 26, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Sacco, R.L.; Rundek, T.; Battistella, V.; Cheung, Y.K.; Elkind, M.S.V. Race and Ethnic Disparities in Stroke Incidence in the Nothern Manhattan Study. Stroke 2020, 51, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Kissela, B.M.; Kleindorfer, D.O.; McClure, L.A.; Soliman, E.Z.; Judd, S.E.; Rhodes, J.D.; Cushman, M.; Moy, C.S.; Sands, K.A.; et al. Differences in the role of black race and stroke risk factors for first vs. recurrent stroke. Neurology 2016, 86, 637–638. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.; Kim, B.; Lee, K.H. Effect of the PRECEDE-PROCEED model on health programs: A systematic review and meta-analysis. Syst. Rev. 2022, 11, 213. [Google Scholar] [CrossRef]
| Motivational Experiences | Definition |
|---|---|
| Challenge | Encourage participants to move out of their ‘comfort zone’ by exploring new foods/flavours/skills via their taste, smell, texture and sound |
| Celebration | Create a fun, enjoyable and special atmosphere; create deliciousness from nutritious meals to encourage participants to enjoy the taste and try new things |
| Collaboration | Generate a positive group dynamic, values group accomplishments, sharing positive feelings about food with peers; create a feeling of being a part of something bigger |
| Home Environment | Actively addressing home dynamics, facilities and access to nutritious meals; create solutions and strategies tailored to own home environment |
| Palate Development | Explore, investigate and taste a wide range of flavours from fresh ingredients, spices and condiments; build anticipation and excitement in new combinations |
| Peer Support | Create a supportive environment among peers with similar experiences; normalise and accept new behaviours |
| Recipe Concept | Move beyond recipe-driven cooking, encourage participants to utilise recipe concepts; swap ingredients according to availability |
| Skill Building | Build culinary nutrition-related skills, motivate participants to share their learnings with others |
| Skill Reinforcement | Reinforce learnt skills via repetitive prompt or performance assessments over multiple sessions |
| Success | Create activities with small steps, increase participants’ confidence and competency, develop a sense of accomplishment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, C.T.; MacDonald-Wicks, L.; English, C.; Lannin, N.A.; Patterson, A. Scoping Review of Available Culinary Nutrition Interventions for People with Neurological Conditions. Nutrients 2024, 16, 462. https://doi.org/10.3390/nu16030462
Chun CT, MacDonald-Wicks L, English C, Lannin NA, Patterson A. Scoping Review of Available Culinary Nutrition Interventions for People with Neurological Conditions. Nutrients. 2024; 16(3):462. https://doi.org/10.3390/nu16030462
Chicago/Turabian StyleChun, Chian Thong (Nicole), Lesley MacDonald-Wicks, Coralie English, Natasha A. Lannin, and Amanda Patterson. 2024. "Scoping Review of Available Culinary Nutrition Interventions for People with Neurological Conditions" Nutrients 16, no. 3: 462. https://doi.org/10.3390/nu16030462
APA StyleChun, C. T., MacDonald-Wicks, L., English, C., Lannin, N. A., & Patterson, A. (2024). Scoping Review of Available Culinary Nutrition Interventions for People with Neurological Conditions. Nutrients, 16(3), 462. https://doi.org/10.3390/nu16030462








