Sex Differences under Vitamin D Supplementation in an Animal Model of Progressive Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Setup
2.3. Blood Withdrawal, Euthanasia and Tissue Harvesting
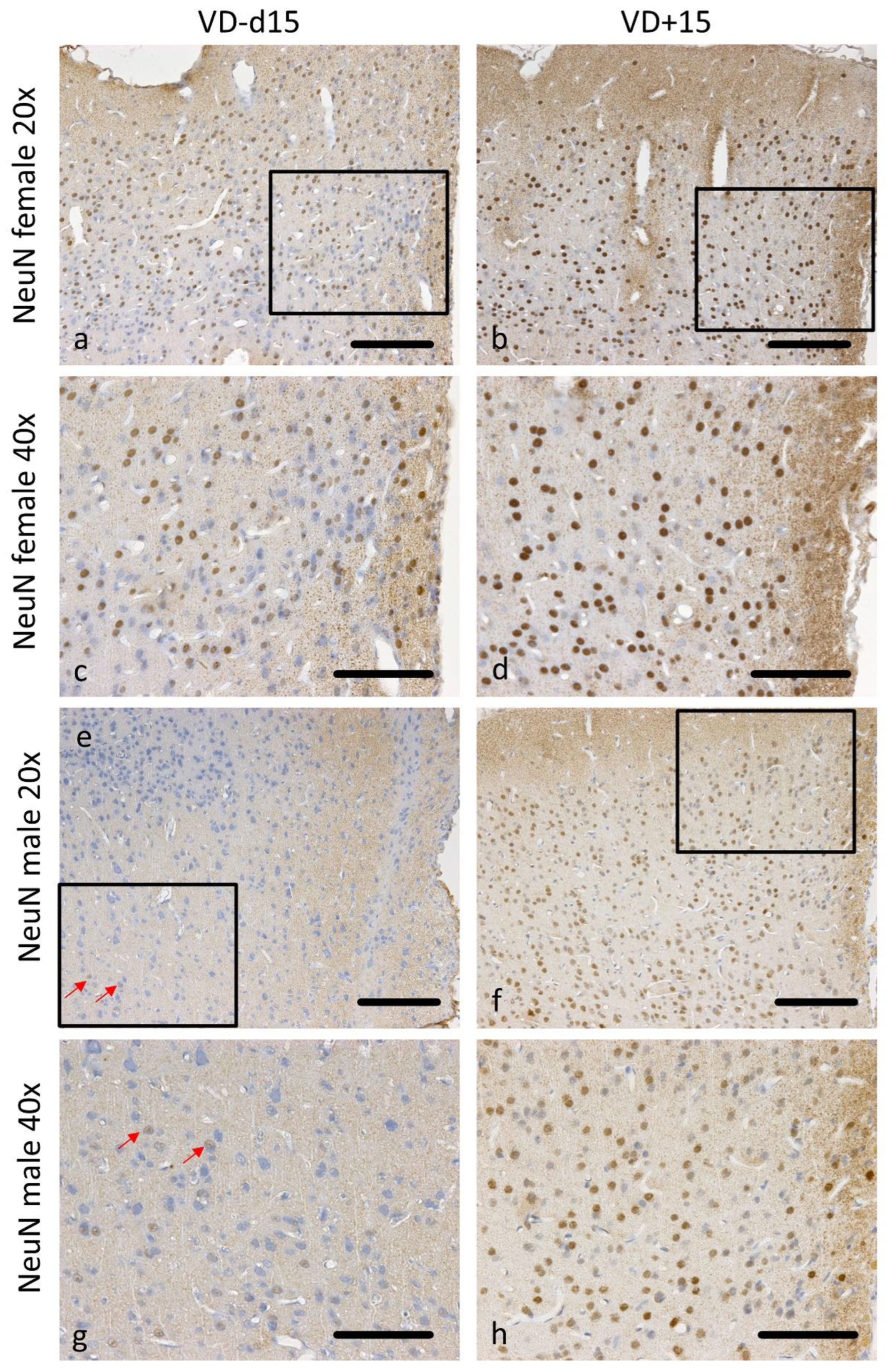
2.4. Histopathology and Immunohistochemistry
2.5. Quantitative Histopathological Evaluation
2.6. Colorimetric Methods for Oxidative Capacity and Polyphenols
2.7. NfL Measurement by Single-Molecule Array (SIMOA)
2.8. Statistical Analysis
3. Results
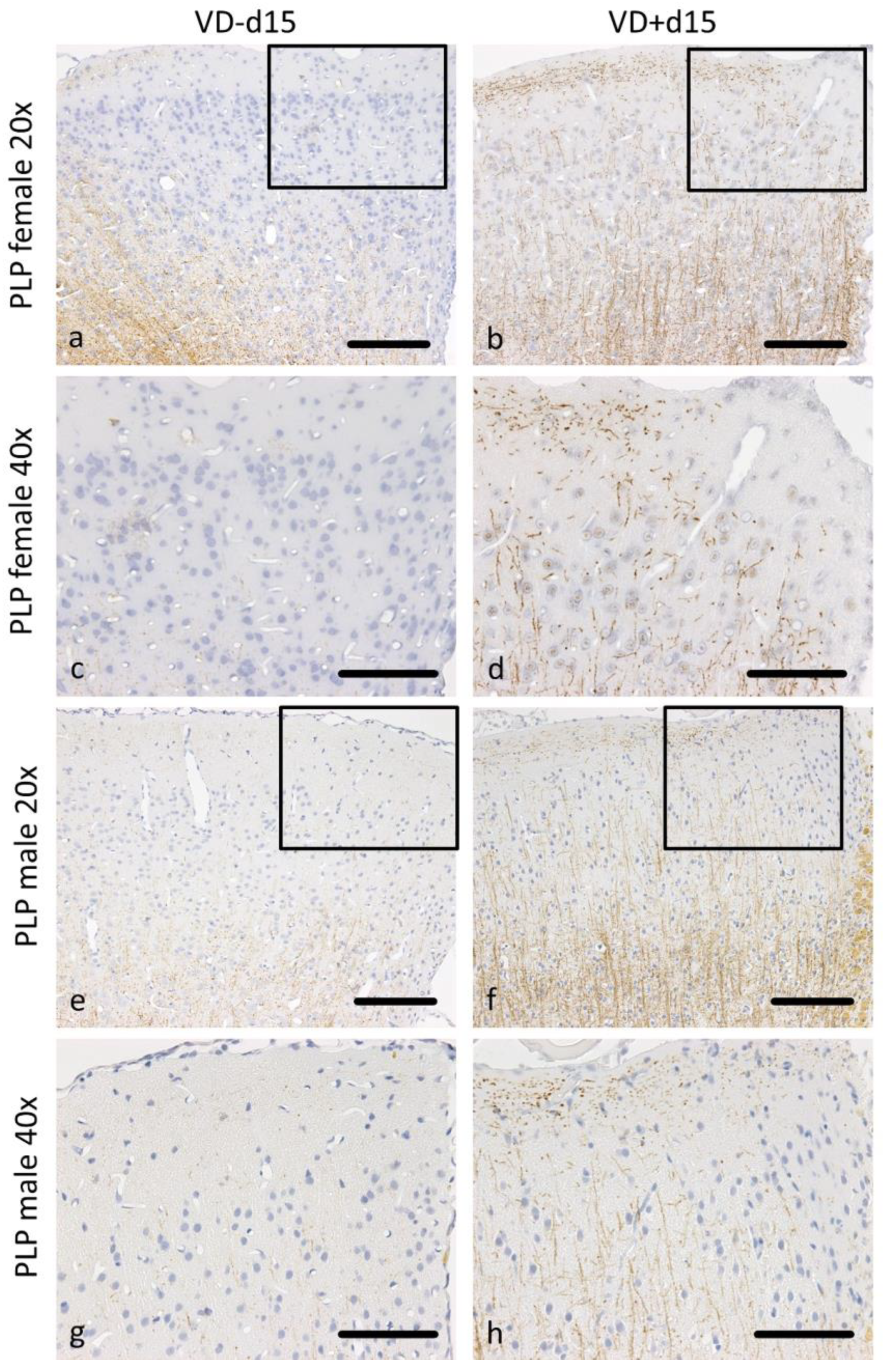
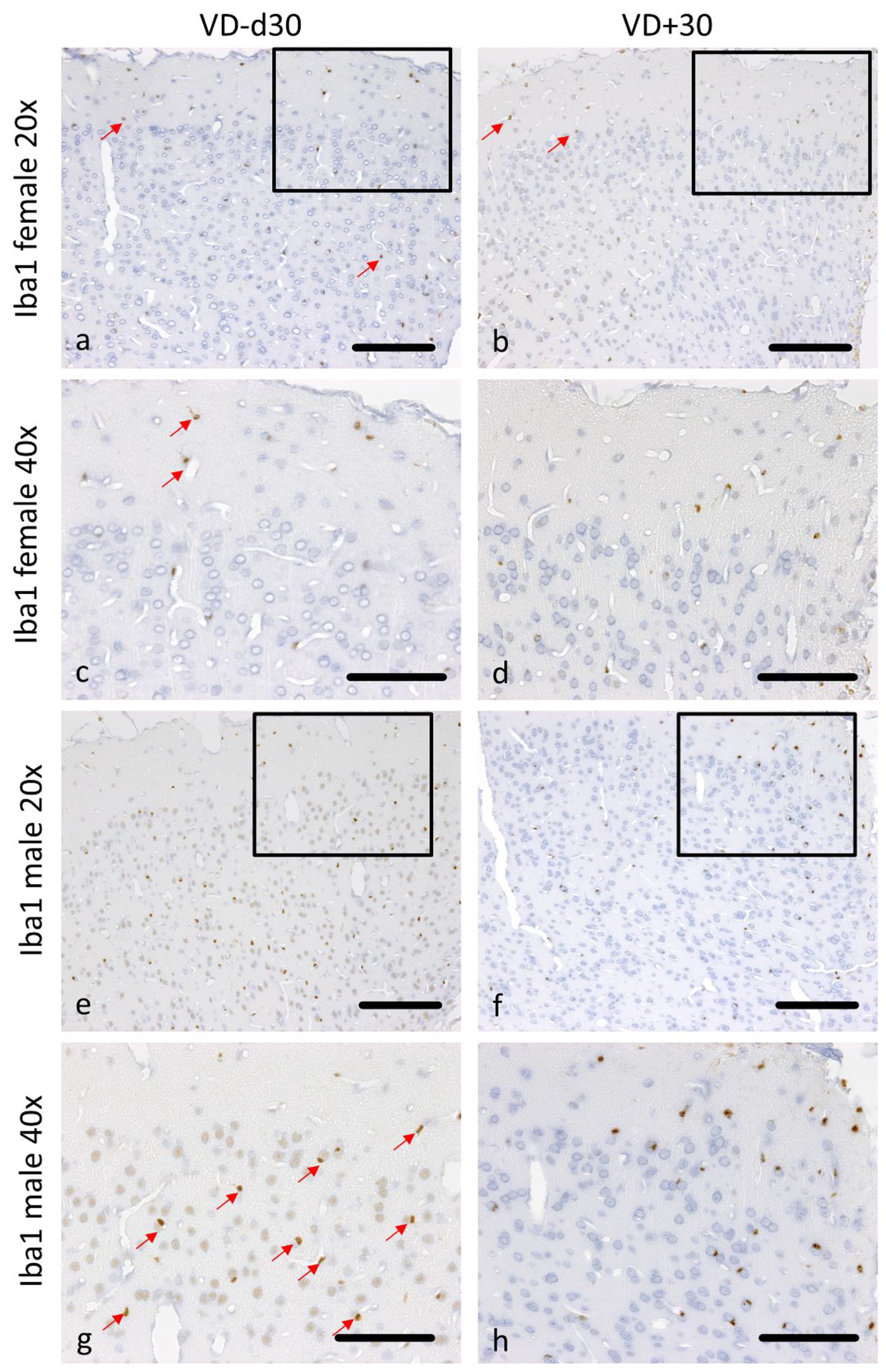
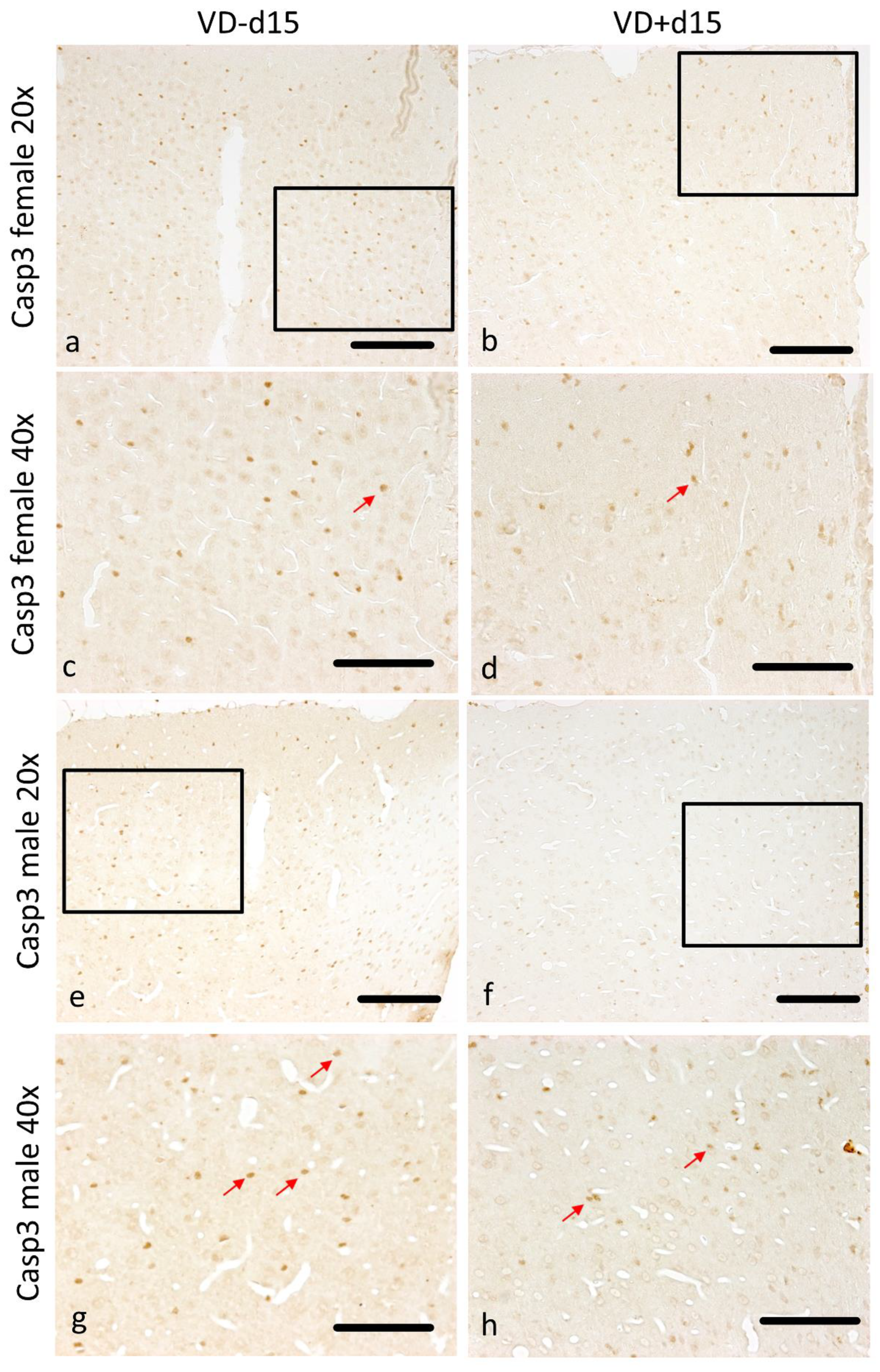
3.1. Histological Analysis Revealed Benefits of VD Supplementation and General Sex-Differences
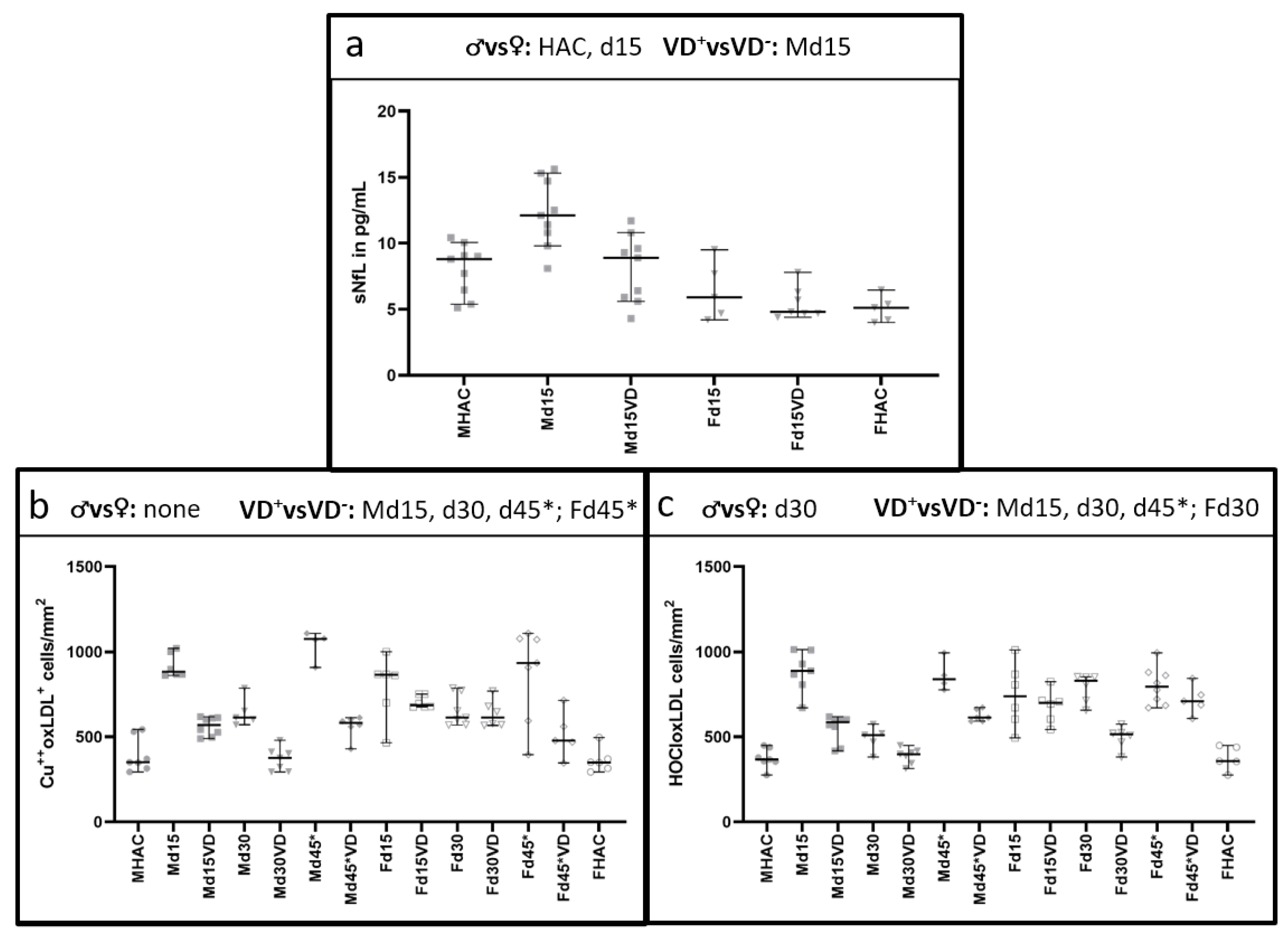
3.2. NfL Serum Level (sNfL) Is Lowered with VD Supplementation Only in Male VD+ Animals
3.3. Histological Oxidative Stress Markers Are Lower Mainly in Male VD+ Animals
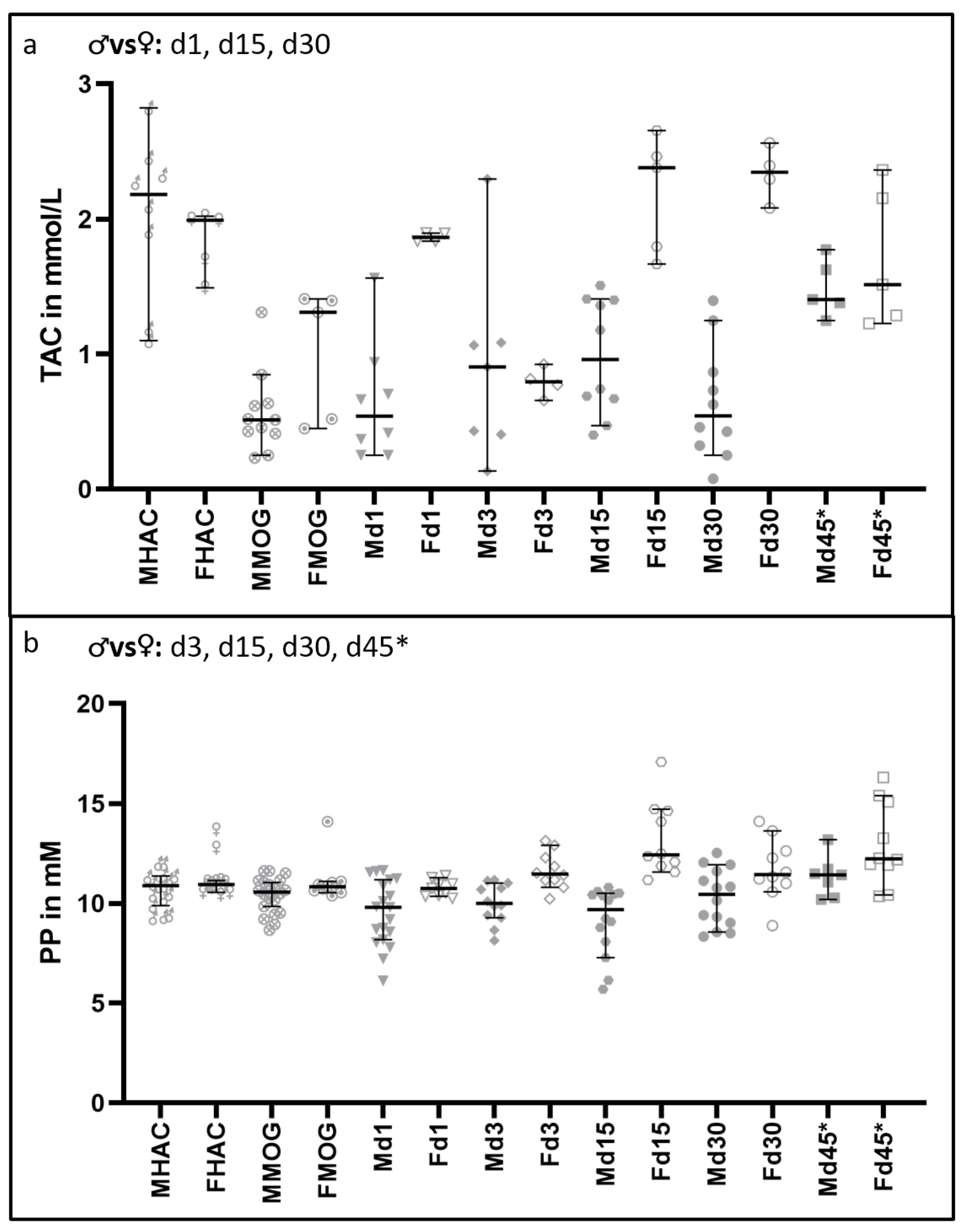
3.4. Total Antioxidant Capacity and Polyphenols Are Increased in Female Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, L.; Mills, K.H.G. Sex Differences Regulate Immune Responses in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Eur. J. Immunol. 2022, 52, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sanchez, N.; Dunn, S.E. Potential Biological Contributers to the Sex Difference in Multiple Sclerosis Progression. Front. Immunol. 2023, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Minisola, S. Vitamin D: Autoimmunity and Gender. Curr. Med. Chem. 2017, 24, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Magyari, M. Gender Differences in Multiple Sclerosis Epidemiology and Treatment Response. Dan. Med. J. 2016, 63, B5212. [Google Scholar]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The Role of Vitamin D in Autoimmune Diseases: Could Sex Make the Difference? Biol. Sex Differ. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Eikelenboom, M.J.; Killestein, J.; Kragt, J.J.; Uitdehaag, B.M.J.; Polman, C.H. Gender Differences in Multiple Sclerosis: Cytokines and Vitamin D. J. Neurol. Sci. 2009, 286, 40–42. [Google Scholar] [CrossRef]
- Avila, M.; Bansal, A.; Culberson, J.; Peiris, A.N. The Role of Sex Hormones in Multiple Sclerosis. Eur. Neurol. 2018, 80, 93–99. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative Stress in Multiple Sclerosis: Central and Peripheral Mode of Action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D Deficiency Accelerates Ageing and Age-Related Diseases: A Novel Hypothesis. J. Physiol. 2017, 595, 6825–6836. [Google Scholar] [CrossRef]
- Muris, A.H.; Rolf, L.; Broen, K.; Hupperts, R.; Damoiseaux, J.; Smolders, J. A Low Vitamin D Status at Diagnosis Is Associated with an Early Conversion to Secondary Progressive Multiple Sclerosis. J. Steroid Biochem. Mol. Biol. 2016, 164, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Abbatemarco, J.R.; Fox, R.J.; Li, H.; Ontaneda, D. Vitamin D and MRI Measures in Progressive Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 35, 276–282. [Google Scholar] [CrossRef]
- Ücal, M.; Haindl, M.T.; Adzemovic, M.Z.; Strasser, J.; Theisl, L.; Zeitelhofer, M.; Kraitsy, K.; Ropele, S.; Schäfer, U.; Fazekas, F.; et al. Widespread Cortical Demyelination of Both Hemispheres Can Be Induced by Injection of Pro-Inflammatory Cytokines via an Implanted Catheter in the Cortex of MOG-Immunized Rats. Exp. Neurol. 2017, 294, 32–44. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A Consensus Protocol for the Standardization of Cerebrospinal Fluid Collection and Biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Adzemovic, M.Z.; Zeitelhofer, M.; Hochmeister, S.; Gustafsson, S.A.; Jagodic, M. Efficacy of Vitamin D in Treating Multiple Sclerosis-like Neuroinflammation Depends on Developmental Stage. Exp. Neurol. 2013, 249, 39–48. [Google Scholar] [CrossRef]
- Tatzber, F.; Wonisch, W.; Lackner, S.; Lindschinger, M.; Pursch, W.; Resch, U.; Trummer, C.; Murkovic, M.; Zelzer, S.; Holasek, S.; et al. A Micromethod for Polyphenol High-Throughput Screening Saves 90 Percent Reagents and Sample Volume. Antioxidants 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Haindl, M.T.; Üçal, M.; Wonisch, W.; Lang, M.; Nowakowska, M.; Adzemovic, M.Z.; Khalil, M.; Enzinger, C.; Hochmeister, S. Vitamin D-An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis. Nutrients 2023, 15, 3309. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Ferri, C.; Alfiero, S.; Lombardo, I.; Laudisi, M.; Tecilla, G.; Boni, M.; Pizzicotti, S.; Fainardi, E.; Bellini, T.; et al. Sex-Related Differences in Cerebrospinal Fluid Plasma-Derived Proteins of Neurological Patients. Diagnostics 2021, 11, 884. [Google Scholar] [CrossRef]
- Sartoretti, T.; Wyss, M.; Sartoretti, E.; Reischauer, C.; Hainc, N.; Graf, N.; Binkert, C.; Najafi, A.; Sartoretti-Schefer, S. Sex and Age Dependencies of Aqueductal Cerebrospinal Fluid Dynamics Parameters in Healthy Subjects. Front. Aging Neurosci. 2019, 11, 199. [Google Scholar] [CrossRef]
- Vegeto, E.; Benedusi, V.; Maggi, A. Estrogen Anti-Inflammatory Activity in Brain: A Therapeutic Opportunity for Menopause and Neurodegenerative Diseases. Front. Neuroendocrinol. 2008, 29, 507–519. [Google Scholar] [CrossRef]
- Matías-Guíu, J.; Oreja-Guevara, C.; Matias-Guiu, J.A.; Gomez-Pinedo, U. Vitamin D and Remyelination in Multiple Sclerosis. Neurologia 2018, 33, 177–186. [Google Scholar] [CrossRef]
- Ko, P.; Burkert, R.; McGrath, J.; Eyles, D. Maternal Vitamin D3 Deprivation and the Regulation of Apoptosis and Cell Cycle during Rat Brain Development. Brain Res. Dev. Brain Res. 2004, 153, 61–68. [Google Scholar] [CrossRef]
- Şahin, S.; Gürgen, S.G.; Yazar, U.; İnce, İ.; Kamaşak, T.; Acar Arslan, E.; Diler Durgut, B.; Dilber, B.; Cansu, A. Vitamin D Protects against Hippocampal Apoptosis Related with Seizures Induced by Kainic Acid and Pentylenetetrazol in Rats. Epilepsy Res. 2019, 149, 107–116. [Google Scholar] [CrossRef]
- Lassmann, H. Cortical Lesions in Multiple Sclerosis: Inflammation versus Neurodegeneration. Brain 2012, 135, 2904–2905. [Google Scholar] [CrossRef]
- Martínez de Toda, I.; González-Sánchez, M.; Díaz-Del Cerro, E.; Valera, G.; Carracedo, J.; Guerra-Pérez, N. Sex Differences in Markers of Oxidation and Inflammation. Implications for Ageing. Mech. Ageing Dev. 2023, 211, 111797. [Google Scholar] [CrossRef]
- Ito, T.; Azuma, J. [Taurine is a possible anti-atherosclerotic agent]. Nihon Yakurigaku Zasshi. 2004, 123, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Vicca, S.; Hennequin, C.; Nguyen-Khoa, T.; Massy, Z.A.; Descamps-Latscha, B.; Drüeke, T.B.; Lacour, B. Caspase-Dependent Apoptosis in THP-1 Cells Exposed to Oxidized Low-Density Lipoproteins. Biochem. Biophys. Res. Commun. 2000, 273, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Ermak, N.; Lacour, B.; Goirand, F.; Drüeke, T.B.; Vicca, S. Differential Apoptotic Pathways Activated in Response to Cu-Induced or HOCl-Induced LDL Oxidation in U937 Monocytic Cell Line. Biochem. Biophys. Res. Commun. 2010, 393, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Healy, B.C.; Liu, Y.; Saxena, S.; Paul, A.; Polgar-Turcsanyi, M.; Guttmann, C.R.G.; Bakshi, R.; Kropshofer, H.; Weiner, H.L.; et al. Serum GFAP and NfL Levels Differentiate Subsequent Progression and Disease Activity in Patients With Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2023, 10, e200052. [Google Scholar] [CrossRef] [PubMed]
- Golden, L.C.; Voskuhl, R. The Importance of Studying Sex Differences in Disease: The Example of Multiple Sclerosis. J. Neurosci. Res. 2017, 95, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Li, R.; Sun, X.; Shu, Y.; Mao, Z.; Xiao, L.; Qiu, W.; Lu, Z.; Hu, X. Sex Differences in Outcomes of Disease-Modifying Treatments for Multiple Sclerosis: A Systematic Review. Mult. Scler. Relat. Disord. 2017, 12, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, M.; Wagenpfeil, S.; Schöpe, J.; Vieth, R.; Vogt, T.; Reichrath, J. Meta-Analysis of European Clinical Trials Characterizing the Healthy-Adult Serum 25-Hydroxyvitamin D Response to Vitamin D Supplementation. Nutrients 2023, 15, 3986. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
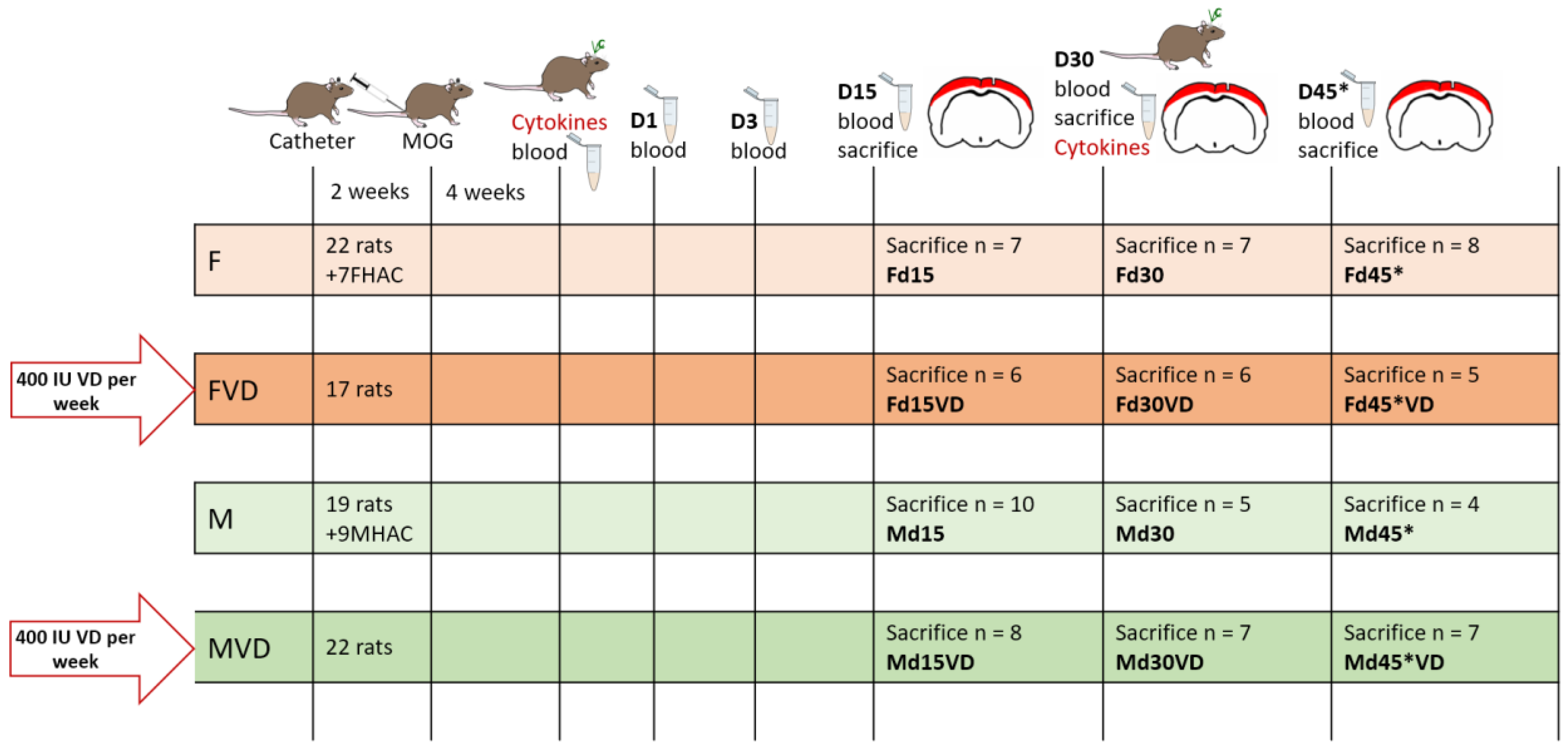

| Sacrifice | Number of Serum Samples | |||
|---|---|---|---|---|
| Tissue for IHC n = | Used for TAC n = | Used for PP n = | Used for NfL SIMOA n = | |
| FHAC | 7 | 5 | 14 | 5 |
| FMOG | No sacrifice | 5 | 10 | n.d. |
| Fd1 | No sacrifice | 4 | 9 | n.d. |
| Fd1VD | No sacrifice | n.d. | n.d. | n.d. |
| Fd3 | No sacrifice | 4 | 10 | n.d. |
| Fd3VD | No sacrifice | n.d. | n.d. | n.d. |
| Fd15 | 7 | 5 | 10 | 5 |
| Fd15VD | 6 | n.d. | n.d. | 7 |
| Fd30 | 7 | 4 | 10 | s.m. |
| Fd30VD | 6 | n.d. | n.d. | n.d. |
| Fd45 * | 8 | 5 | 10 | s.m. |
| Fd45 *VD | 5 | n.d. | n.d. | n.d. |
| MHAC | 9 | 8 | 16 | 9 |
| MMOG | No sacrifice | 11 | 20 | n.d. |
| Md1 | No sacrifice | 8 | 19 | n.d. |
| Md1VD | No sacrifice | n.d. | n.d. | n.d. |
| Md3 | No sacrifice | 7 | 12 | n.d. |
| Md3VD | No sacrifice | n.d. | n.d. | n.d. |
| Md15 | 10 | 10 | 14 | 9 |
| Md15VD | 8 | n.d. | n.d. | 9 |
| Md30 | 5 | 10 | 14 | n.d. |
| Md30VD | 7 | n.d. | n.d. | n.d. |
| Md45 * | 4 | 5 | 7 | n.d. |
| Md45 *VD | 7 | n.d. | n.d. | n.d. |
| TOTAL FEMALE | 46 | 32 | 73 | 17 |
| TOTAL MALE | 50 | 59 | 102 | 27 |
| TOTAL n = | 96 | 91 | 175 | 44 |
| PLP | Iba1 | Casp3 | NeuN | Cu++ oxLDL | HOCL oxLDL | |||
|---|---|---|---|---|---|---|---|---|
| Ipsi | Contra | Ipsi | Contra | Ipsi | Ipsi | Ipsi | Ipsi | |
| Shapiro–Wilk, Histogram, Q-Q-Plot | Most of the groups are not normally distributed | |||||||
| Kruskal–Wallis test p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.005 | <0.001 | <0.001 |
| Md15 vs. Md15VD | 0.142 | 0.064 | 0.197 | 0.197 | 0.002 | 0.019 | 0.002 | 0.001 |
| Md30 vs. Md30VD | 0.050 | 0.122 | 0.004 | 0.123 | 0.004 | 0.019 | 0.004 | 0.042 |
| Md45 * vs. Md45 *VD | 0.298 | 0.705 | 0.018 | 0.038 | 0.023 | 0.186 | 0.011 | 0.010 |
| Fd15 vs. Fd15VD | 0.122 | 0.027 | 0.109 | 0.005 | 0.003 | 0.022 | 0.108 | 0.688 |
| Fd30 vs. Fd30VD | 0.229 | 0.116 | 0.025 | 0.004 | 0.046 | 0.199 | 0.617 | 0.004 |
| Fd45 * vs. Fd45 * VD | 0.605 | 0.941 | 0.144 | 0.100 | 0.123 | 0.883 | 0.042 | 0.242 |
| Md15 vs. Fd15 | 0.022 | 0.003 | 0.828 | 0.828 | 0.001 | 0.042 | 0.335 | 0.199 |
| Md30 vs. Fd30 | 0.251 | 0.249 | 0.143 | 0.583 | 0.062 | 0.808 | 1.000 | 0.006 |
| Md45 * vs. Fd45 * | 0.496 | 1.000 | 0.050 | 0.010 | 0.186 | 0.496 | 0.450 | 0.396 |
| TAC | PP | NfL | |
|---|---|---|---|
| Shapiro–Wilk, Histogram, Q-Q-Plot | Most of the groups are not normally distributed | ||
| Kruskal–Wallis test p | <0.001 | <0.001 | <0.001 |
| MHAC vs. FHAC | 0.306 | 0.454 | 0.016 |
| MMOG vs. FMOG | 0.139 | 0.180 | x |
| Md1 vs. Fd1 | 0.014 | 0.065 | x |
| Md3 vs. Fd3 | 0.850 | 0.001 | x |
| Md15 vs. Fd15 | 0.002 | 0.001 | 0.004 |
| Md30 vs. Fd30 | 0.005 | 0.046 | x |
| Md45 * vs. Fd45 * | 0.754 | 0.040 | x |
| Md15 vs. Md15VD | x | x | 0.006 |
| Fd15 vs. Fd15VD | x | x | 0.683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haindl, M.T.; Üçal, M.; Tafrali, C.; Wonisch, W.; Erdogan, C.; Nowakowska, M.; Adzemovic, M.Z.; Enzinger, C.; Khalil, M.; Hochmeister, S. Sex Differences under Vitamin D Supplementation in an Animal Model of Progressive Multiple Sclerosis. Nutrients 2024, 16, 554. https://doi.org/10.3390/nu16040554
Haindl MT, Üçal M, Tafrali C, Wonisch W, Erdogan C, Nowakowska M, Adzemovic MZ, Enzinger C, Khalil M, Hochmeister S. Sex Differences under Vitamin D Supplementation in an Animal Model of Progressive Multiple Sclerosis. Nutrients. 2024; 16(4):554. https://doi.org/10.3390/nu16040554
Chicago/Turabian StyleHaindl, Michaela Tanja, Muammer Üçal, Cansu Tafrali, Willibald Wonisch, Cigdem Erdogan, Marta Nowakowska, Milena Z. Adzemovic, Christian Enzinger, Michael Khalil, and Sonja Hochmeister. 2024. "Sex Differences under Vitamin D Supplementation in an Animal Model of Progressive Multiple Sclerosis" Nutrients 16, no. 4: 554. https://doi.org/10.3390/nu16040554
APA StyleHaindl, M. T., Üçal, M., Tafrali, C., Wonisch, W., Erdogan, C., Nowakowska, M., Adzemovic, M. Z., Enzinger, C., Khalil, M., & Hochmeister, S. (2024). Sex Differences under Vitamin D Supplementation in an Animal Model of Progressive Multiple Sclerosis. Nutrients, 16(4), 554. https://doi.org/10.3390/nu16040554






