The Effect of Creatine Nitrate and Caffeine Individually or Combined on Exercise Performance and Cognitive Function: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview and Ethical Considerations
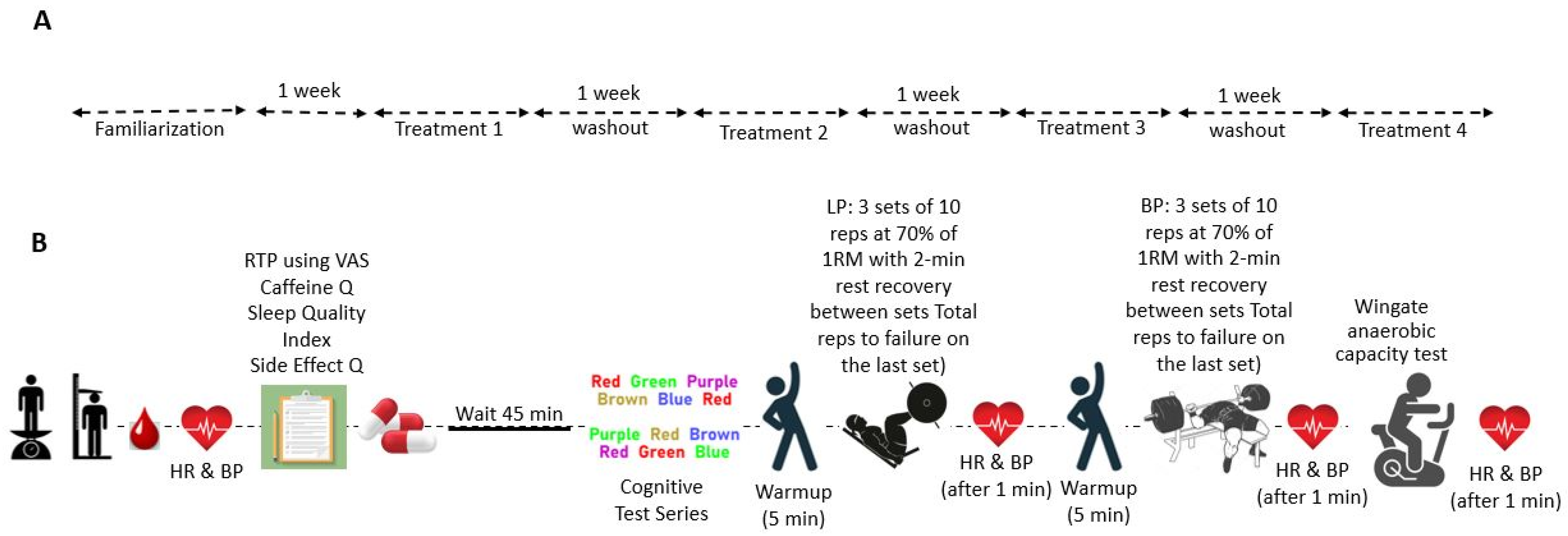
2.2. Study Protocol
2.2.1. Recruitment and Familiarization
2.2.2. Eligibility Criteria
2.2.3. Performance Testing Protocol
2.2.4. Supplementation
2.2.5. Anthropometry
2.2.6. Dietary Monitoring
2.2.7. Stroop Word–Color Test
2.2.8. Readiness to Perform Exercise
2.2.9. Muscular Strength and Wingate Anaerobic Power
2.2.10. Blood Chemistry Assessment
2.2.11. Sleep Quality, Caffeine Tolerance Assessment, and Side Effect Questionnaires
2.3. Statistical Analysis
3. Results
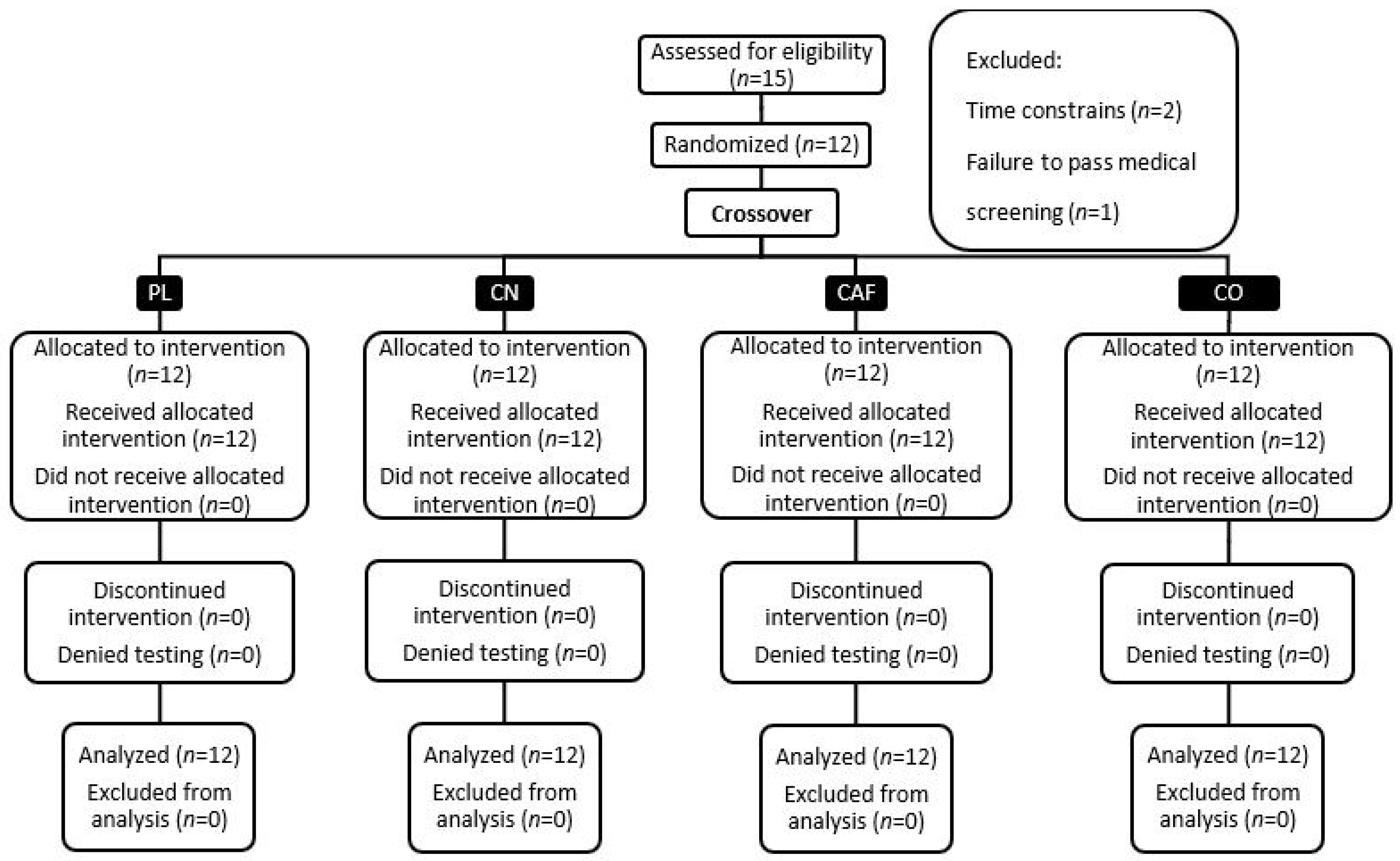
3.1. Participants
3.2. Primary Outcomes
3.2.1. Exercise Performance
Bench Press
Leg Press
Wingate Testing
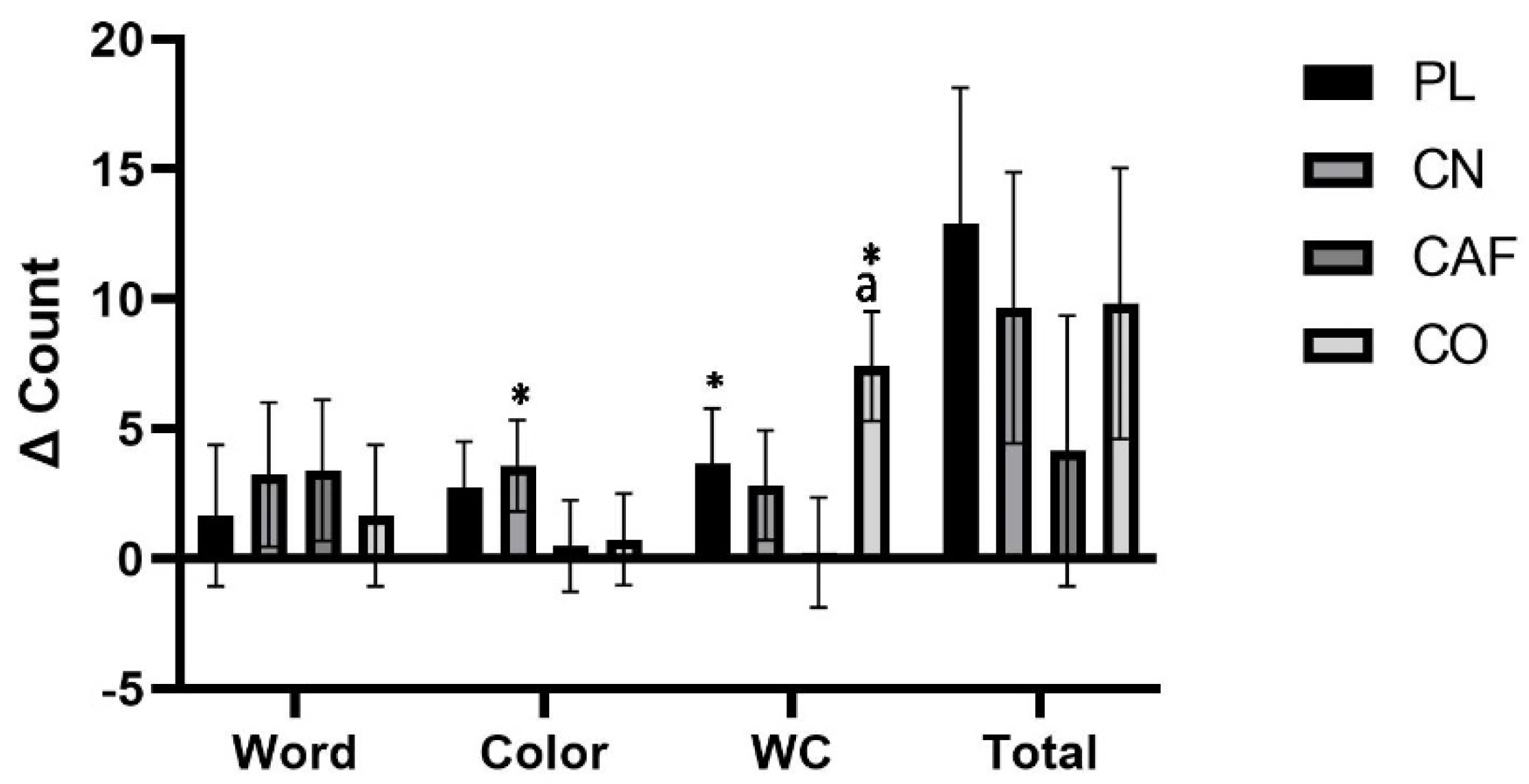
3.2.2. Stroop Testing and Readiness to Perform
3.3. Secondary Outcomes
3.3.1. Cardiovascular Hemodynamics
3.3.2. Blood Chemistry
3.3.3. Safety Profiles
Caffeine Questionnaire
Assessment of Sleep Quality
Side Effects Questionnaire
4. Discussion
4.1. Primary Outcomes: Exercise Performance and Cognitive Function
4.2. Secondary Outcomes: Cardiovascular Function and Blood Chemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H. IOC consensus statement: Dietary supplements and the high-performance athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol. Toxicol. 1995, 76, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.H.; Gonçalves, J.S.; Araújo, P.K.; Lima-Silva, A.E.; Ataide-Silva, T.; de Araujo, G.G. Effects of creatine and caffeine ingestion in combination on exercise performance: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Earnest, C.P.; Lowery, R.P.; Wilson, J.M.; Willems, M.E.T. Co-ingestion of Nutritional Ergogenic Aids and High-Intensity Exercise Performance. Sports Med. 2016, 46, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J. Effects of Caffeine on Resistance Exercise: A Review of Recent Research. Sports Med. 2021, 51, 2281–2298. [Google Scholar] [CrossRef]
- San Juan, A.F.; López-Samanes, Á.; Jodra, P.; Valenzuela, P.L.; Rueda, J.; Veiga-Herreros, P.; Pérez-López, A.; Domínguez, R. Caffeine Supplementation Improves Anaerobic Performance and Neuromuscular Efficiency and Fatigue in Olympic-Level Boxers. Nutrients 2019, 11, 2120. [Google Scholar] [CrossRef]
- Barreto, G.; Esteves, G.P.; Marticorena, F.; Oliveira, T.N.; Grgic, J.; Saunders, B. Caffeine, CYP1A2 Genotype and Exercise Performance: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2023, 56, 328–339. [Google Scholar] [CrossRef]
- James, J.E. Critical review of dietary caffeine and blood pressure: A relationship that should be taken more seriously. Psychosom. Med. 2004, 66, 63–71. [Google Scholar] [CrossRef]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. In Cochrane Database of Systematic Reviews; John Wiley & Sons Ltd.: Chichester, UK, 2010. [Google Scholar]
- Tomporowski, P.; Beasman, K.; Ganio, M.; Cureton, K. Effects of dehydration and fluid ingestion on cognition. Int. J. Sports Med. 2007, 28, 891–896. [Google Scholar] [CrossRef]
- Jagim, A.R.; Stecker, R.A.; Harty, P.S.; Erickson, J.L.; Kerksick, C.M. Safety of Creatine Supplementation in Active Adolescents and Youth: A Brief Review. Front. Nutr. 2018, 5, 115. [Google Scholar] [CrossRef]
- Racette, S.B. Creatine supplementation and athletic performance. J. Orthop. Sports Phys. Ther. 2003, 33, 615–621. [Google Scholar] [CrossRef]
- Volek, J.S.; Rawson, E.S. Scientific basis and practical aspects of creatine supplementation for athletes. Nutrition 2004, 20, 609–614. [Google Scholar] [CrossRef]
- Jacobs, I. Dietary creatine monohydrate supplementation. Can. J. Appl. Physiol. = Rev. Can. Physiol. Appl. 1999, 24, 503–514. [Google Scholar]
- Mujika, I.; Padilla, S. Creatine supplementation as an ergogenic aid for sports performance in highly trained athletes: A critical review. Int. J. Sports Med. 1997, 18, 491–496. [Google Scholar] [CrossRef]
- Besco, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.M.; Lowery, R.P.; Falcone, P.H.; Mosman, M.M.; Vogel, R.M.; Carson, L.R.; Tai, C.Y.; Choate, D.; Kimber, D.; Ormes, J.A.; et al. 28 days of creatine nitrate supplementation is apparently safe in healthy individuals. J. Int. Soc. Sports Nutr. 2014, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Nikolaidis, A. Amino Acid Compounds. 2017. Available online: http://www.google.com/patents/US20090076110 (accessed on 12 December 2023).
- Ostojic, S.M.; Stajer, V.; Vranes, M.; Ostojic, J. Searching for a better formulation to enhance muscle bioenergetics: A randomized controlled trial of creatine nitrate plus creatinine vs. creatine nitrate vs. creatine monohydrate in healthy men. Food Sci. Nutr. 2019, 7, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Galvan, E.; Walker, D.K.; Simbo, S.Y.; Dalton, R.; Levers, K.; O’Connor, A.; Goodenough, C.; Barringer, N.D.; Greenwood, M.; Rasmussen, C.; et al. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R.L.; Sowinski, R.J.; Grubic, T.J.; Collins, P.B.; Coletta, A.M.; Reyes, A.G.; Sanchez, B.; Koozehchian, M.; Jung, Y.P.; Rasmussen, C.; et al. Hematological and Hemodynamic Responses to Acute and Short-Term Creatine Nitrate Supplementation. Nutrients 2017, 9, 1359. [Google Scholar] [CrossRef]
- Hespel, P.; Op‘t Eijnde, B.; Van Leemputte, M. Opposite actions of caffeine and creatine on muscle relaxation time in humans. J. Appl. Physiol. 2002, 92, 513–518. [Google Scholar] [CrossRef]
- Harris, R.C.; Sale, C.; Delves, S.K. Modification of the Ergogenic Effects of Creatine Loading by Caffeine: 1835 2:30 PM–2:45 PM. Med. Sci. Sports Exerc. 2005, 37, S348–S349. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Roelofs, E.J.; Hirsch, K.R.; Persky, A.M.; Mock, M.G. Effects of Coffee and Caffeine Anhydrous Intake during Creatine Loading. J. Strength Cond. Res. 2016, 30, 1438–1446. [Google Scholar] [CrossRef]
- Elosegui, S.; López-Seoane, J.; Martínez-Ferrán, M.; Pareja-Galeano, H. Interaction between Caffeine and Creatine When Used as Concurrent Ergogenic Supplements: A Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Pakulak, A.; Candow, D.G.; Totosy de Zepetnek, J.; Forbes, S.C.; Basta, D. Effects of Creatine and Caffeine Supplementation during Resistance Training on Body Composition, Strength, Endurance, Rating of Perceived Exertion and Fatigue in Trained Young Adults. J. Diet. Suppl. 2022, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Smith-Ryan, A.E. Creatine and Caffeine: Considerations for Concurrent Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 607–623. [Google Scholar] [CrossRef]
- Baechle, T.R.; Earle, R.W. (Eds.) Essentials of Strength and Conditioning; Human Kinetics: Champaign, IL USA, 2008. [Google Scholar]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Longobardi, I.; Perim, P.; Duarte, B.; Ferreira, P.; Gualano, B.; Roschel, H.; Saunders, B. Timing of Creatine Supplementation around Exercise: A Real Concern? Nutrients 2021, 13, 2844. [Google Scholar] [CrossRef]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef]
- Koozehchian, M.S.; Earnest, C.P.; Jung, Y.P.; Collins, P.B.; O’Connor, A.; Dalton, R.; Shin, S.Y.; Sowinski, R.; Rasmussen, C.; Murano, P.S. Dose Response to One Week of Supplementation of a Multi-Ingredient Preworkout Supplement Containing Caffeine Before Exercise. J. Caffeine Res. 2017, 7, 81–94. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; Kliszczewicz, B.; Garner, D.M.; Cavalcante, T.C.F.; da Silva, A.A.M.; Santana, M.D.R.; Valenti, V.E. Is Caffeine Recommended before Exercise? A Systematic Review to Investigate Its Impact on Cardiac Autonomic Control via Heart Rate and Its Variability. J. Am. Coll. Nutr. 2020, 39, 563–573. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643. [Google Scholar] [CrossRef]
- Stroop, J.R. Factors affecting speed in serial verbal reactions. Psychol. Monogr. 1938, 50, 38. [Google Scholar] [CrossRef]
- Golden, C.J. A group version of the Stroop Color and Word Test. J. Personal. Assess. 1975, 39, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.R.; Rohwer, W.D., Jr. The Stroop color-word test: A review. Acta Psychol. 1966, 25, 36–93. [Google Scholar] [CrossRef] [PubMed]
- Franzen, M.D.; Tishelman, A.C.; Sharp, B.H.; Friedman, A.G. An investigation of the test-retest reliability of the stroop colorword test across two intervals. Arch. Clin. Neuropsychol. 1987, 2, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Campbell, B.I.; Roberts, M.D.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Early-phase adaptations to a split-body, linear periodization resistance training program in college-aged and middle-aged men. J. Strength Cond. Res. 2009, 23, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Jamaly, S.; Ramberg, C.; Olsen, R.; Latysheva, N.; Webster, P.; Sovershaev, T.; Brækkan, S.K.; Hansen, J.-B. Impact of preanalytical conditions on plasma concentration and size distribution of extracellular vesicles using Nanoparticle Tracking Analysis. Sci. Rep. 2018, 8, 17216. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Burgower, R.R. The Effects of Energy Drinks on Sleep and Daily Functioning. Doctoral Dissertation, American University, Washington, DC, USA, 2015. [Google Scholar]
- Morava, A. The Effects of Acute Aerobic Exercise and Caffeine on Working Memory and Caffeine Withdrawal. Doctoral Dissertation, The University of Western Ontario, London, ON, Canada, 2019. [Google Scholar]
- Erdogmus, T.N.; Aras, D.; Gulu, M.; Aldhahi, M.I.; Dahesh, A.M.; Badri, S. Combination of High-Intensity Interval Training and Creatine Intake Enhances Leg Strength and Anaerobic Power without Changes in Body Composition in Physically Active Adult Men. 2023. Available online: https://www.researchsquare.com/article/rs-3304243/v1 (accessed on 15 December 2023).
- Jagim, A.R.; Jones, M.T.; Wright, G.A.; St. Antoine, C.; Kovacs, A.; Oliver, J.M. The acute effects of multi-ingredient pre-workout ingestion on strength performance, lower body power, and anaerobic capacity. J. Int. Soc. Sports Nutr. 2016, 13, 11. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Triantafyllidis, K.K.; Kechagias, K.S.; Forbes, S.C.; Candow, D.G. Effects of creatine supplementation on memory in healthy individuals: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2023, 81, 416–427. [Google Scholar] [CrossRef]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Sandkühler, J.F.; Kersting, X.; Faust, A.; Königs, E.K.; Altman, G.; Ettinger, U.; Lux, S.; Philipsen, A.; Müller, H.; Brauner, J. The effects of creatine supplementation on cognitive performance—A randomised controlled study. BMC Med. 2023, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.; Bourbeau, K.; Dorman, K.; Runyon, L.; Glaser, N.; Brandt, J.; Hoodjer, M.; Forbes, S.C.; Candow, D.G. Dose–Response of Creatine Supplementation on Cognitive Function in Healthy Young Adults. Brain Sci. 2023, 13, 1276. [Google Scholar] [PubMed]
- Lutsch, D.J.; Camic, C.L.; Jagim, A.R.; Johnston, N.J.; Musgjerd, T.L. Acute Effects of a Multi-Ingredient Pre-Workout Supplement On 5-KM Running Performance in Recreationally-Trained Athletes. Int. J. Exerc. Sci. 2019, 12, 1045–1056. [Google Scholar] [PubMed]
- Kedia, A.; Sandrock, J.; Habowski, S.; Ferrando, A.; Gothard, M.; Lopez, M.D.C.F.H. Effects of a Pre-workout Supplement on Lean Mass, Muscular Performance, Subjective Workout Experience and Biomarkers of Safety. Int. J. Med. Sci. 2014, 11, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J. Caffeine ingestion enhances Wingate performance: A meta-analysis. Eur. J. Sport Sci. 2018, 18, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Marques-Jiménez, D.; Refoyo, I.; Del Coso, J.; León-Guereño, P.; Calleja-González, J. Effect of Caffeine Supplementation on Sports Performance Based on Differences Between Sexes: A Systematic Review. Nutrients 2019, 11, 2313. [Google Scholar] [CrossRef]
- Hall, M.; Manetta, E.; Tupper, K. Creatine Supplementation: An Update. Curr. Sports Med. Rep. 2021, 20, 338–344. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Caffeine and creatine use in sport. Ann. Nutr. Metab. 2011, 57 (Suppl. 2), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.S.C.; Costa, N.M.B.; Ferreira, S.A.; Carneiro-Junior, M.A.; Natali, A.J. The effects of a high dosage of creatine and caffeine supplementation on the lean body mass composition of rats submitted to vertical jumping training. J. Int. Soc. Sports Nutr. 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Jäger, R.; Purpura, M. Bioavailability, efficacy, safety, and regulatory status of creatine and related compounds: A critical review. Nutrients 2022, 14, 1035. [Google Scholar] [CrossRef] [PubMed]

| Measurement | Mean SD |
|---|---|
| Age (year) | 21.9 ± 0.79 |
| Height (cm) | 179.9 ± 9.53 |
| Body Mass Index (kg/m2) | 26.6 ± 5.11 |
| Bench press 1RM (kg) | 101.1 ± 27.8 |
| Leg press 1RM (kg) | 371.4 ± 64.7 |
| Variable | Treatment | Mean SD | p-Value |
|---|---|---|---|
| Energy Intake (kcal/d/kg) | PL | 24.1 ± 9.15 | 0.07 |
| CN | 21.9 ± 7.22 | ||
| CAF | 23.2 ± 8.27 | ||
| CO | 20.4 ± 5.12 | ||
| Protein (g/d/kg) | PL | 1.34 ± 0.52 | 0.08 |
| CN | 1.26 ± 0.50 | ||
| CAF | 1.32 ± 0.35 | ||
| CO | 1.10 ± 0.37 | ||
| Carbohydrate (g/d/kg) | PL | 2.24 ± 1.24 | 0.43 |
| CN | 2.16 ± 0.90 | ||
| CAF | 2.34 ± 1.38 | ||
| CO | 1.99 ± 0.61 | ||
| Fat (g/d/kg) | PL | 1.01 ± 0.42 | 0.09 |
| CN | 0.86 ± 0.41 | ||
| CAF | 0.83 ± 0.45 | ||
| CO | 0.79 ± 0.35 |
| Variable | Treatment | Day 0 | Day 7 | Treatment | ||
|---|---|---|---|---|---|---|
| Mean SD | Mean SD | Mean SE | p-Value | |||
| Lifting repetitions and volume | ||||||
| BP Repetitions to failure at 70% 1RM | PL | 10.3 ± 2.71 | 10.7 ± 2.34 c,d | 10.5 ± 0.51 | Time | 0.02 |
| CN | 10.5 ± 2.93 | 11.9 ± 3.55 * | 11.2 ± 0.66 | Trt | 0.01 | |
| CAF | 11.5 ± 3.84 | 13.7 ± 3.76 a,* | 12.6 ± 0.79 | T×T | 0.43 | |
| CO | 11.7 ± 3.64 | 13.9 ± 4.64 a,* | 12.8 ± 0.86 | |||
| BP third set lifting volume (kg) | PL | 711.6± 239.1 | 734.5 ± 219.7 | 723.1 ± 45.9 | Time | 0.007 |
| CN | 695.3 ± 168.2 | 804.4 ± 284.8 * | 749.9 ± 48.1 | Trt | 0.02 | |
| CAF | 789.8 ± 284.2 | 941.9 ± 289.6 * | 865.9 ± 59.4 | T×T | 0.50 | |
| CO | 793.4 ± 249.8 | 949.1 ± 332.7 * | 871.3 ± 60.9 | |||
| LP Repetitions to failure at 70% 1RM | PL | 28.1 ± 9.57 | 29.4 ± 8.96 | 28.7 ± 1.85 | Time | 0.06 |
| CN | 28.2 ± 9.71 | 32.7 ± 10.8 | 30.5 ± 2.11 | Trt | 0.16 | |
| CAF | 33.4 ± 16.4 | 32.4 ± 16.9 | 32.9 ± 3.33 | T×T | 0.86 | |
| CO | 33.9 ± 14.8 | 38.5 ± 15.8 | 36.2 ± 3.11 | |||
| LP third set lifting volume (kg) | PL | 7935.1 ± 4139.5 | 7715.7 ± 3394.4 | 7825.4 ± 756.1 | Time | 0.12 |
| CN | 7328.9 ± 2923.5 | 8473.8 ± 3236.8 | 7901.4 ± 627.2 | Trt | 0.28 | |
| CAF | 8735.1 ± 4474.6 | 8435.8 ± 4675.6 | 8585.4 ± 914.1 | T×T | 0.79 | |
| CO | 8906.7 ± 4401.7 | 10,024.3 ± 4623.8 | 9465.5 ± 908.7 | |||
| Combined lifting volume (kg) | PL | 14,443.4 ± 4684.9 | 14,966.5 ± 4463.0 | 14,705.0 ± 915.0 | Time | 0.07 |
| CN | 14,540.5 ± 3922.7 | 15,794.5 ± 4247.7 | 15,167.5 ± 826.6 | Trt | 0.12 | |
| CAF | 15,669.9 ± 6258.7 | 15,727.7 ± 5706.3 | 15,698.8 ± 1195.6 | T×T | 0.80 | |
| CO | 16,216.4 ± 5355.6 | 17,489.7 ± 5489.4 | 16,853.1 ± 1090.7 | |||
| Anaerobic capacity test Wingate test | ||||||
| Total work (J) | PL | 19,394.1 ± 3032.1 | 19,742.7 ± 2791.9 c | 19,568.4 ± 582.9 | Time | 0.40 |
| CN | 19,555.6 ± 2639.2 | 19,999.3 ± 2812.3 c | 19,777.5 ±546.4 | Trt | 0.54 | |
| CAF | 20,039.2 ± 2368.4 | 19,341.1 ± 2257.1 a,b,d | 19,690.1 ± 467.5 | T×T | 0.20 | |
| CO | 19,111.5 ± 2710.1 | 19,373.4 ± 2883.6 c | 19,242.5 ± 559.3 | |||
| Mean power (W) | PL | 671.7 ± 99.7 | 677.7 ± 87.9 c | 674.7 ± 18.7 | Time | 0.31 |
| CN | 681.2 ± 89.7 | 698.1 ± 98.2 c | 689.6 ± 18.8 | Trt | 0.35 | |
| CAF | 688.5 ± 82.7 | 663.4 ± 78.5 a,b,d | 675.9 ± 16.3 | T×T | 0.15 | |
| CO | 659.8 ± 92.3 | 673.3 ± 105.5 c | 666.5 ± 19.8 | |||
| Peak power (W) | PL | 1021.3 ± 174.1 | 1025.0 ± 173.5 | 1023.2 ± 34.7 | Time | 0.01 |
| CN | 1026.6 ± 186.4 | 1041.2 ± 180.4 | 1033.9 ± 36.6 | Trt | 0.78 | |
| CAF | 991.8 ± 189.0 | 956.7 ± 176.1 | 974.2 ± 36.6 | T×T | 0.67 | |
| CO | 941.3 ± 136.1 | 969.0 ± 180.7 | 962.8 ± 35.6 | |||
| Minimum power (W) | PL | 389.1 ± 69.5 | 396.5 ± 95.3 | 392.8 ± 16.6 | Time | 0.09 |
| CN | 356.4 ± 91.3 | 366.4 ± 88.6 | 361.4 ± 18.0 | Trt | 0.09 | |
| CAF | 410.2 ± 75.9 | 413.6 ± 70.6 | 411.9 ± 14.6 | T×T | 0.75 | |
| CO | 396.7 ± 70.5 | 380.1 ± 97.9 | 388.4 ± 17.1 | |||
| Fatigue index (%) | PL | 61.6 ± 6.41 | 59.7 ± 12.1 | 60.7 ± 1.94 | Time | 0.01 |
| CN | 64.9 ± 8.63 | 64.4 ± 8.66 | 64.6 ± 1.72 | Trt | 0.21 | |
| CAF | 57.6 ± 9.52 | 55.7 ± 10.9 | 56.6 ± 2.05 | T×T | 0.74 | |
| CO | 57.3 ± 9.06 | 59.3 ± 14.2 | 58.3 ± 2.39 | |||
| Variable | Treatment | Day 0 | Day 7 | Treatment | ||
|---|---|---|---|---|---|---|
| Mean SD | Mean SD | Mean SE | p-Value | |||
| Word (counts) | PL | 109.3 ± 17.7 | 111.0 ± 13.4 | 110.1 ± 3.14 | Time | 0.006 |
| CN | 113.2 ± 12.7 | 116.0 ± 14.2 | 114.8 ± 2.72 | Trt | 0.15 | |
| CAF | 116.1 ± 14.5 | 119.5 ± 17.3 | 117.7 ± 3.21 | T×T | 0.55 | |
| CO | 118.1 ± 20.3 | 119.7 ± 16.4 | 118.9 ± 3.69 | |||
| Color (counts) | PL | 83.6 ± 16.2 | 86.41 ± 15.1 | 85.0 ± 3.14 | Time | 0.003 |
| CN | 83.4 ± 13.4 | 87.0 ± 13.8 * | 85.2 ± 2.74 | Trt | 0.26 | |
| CAF | 92.1 ± 15.5 | 92.5 ± 16.6 | 92.3 ± 3.21 | T×T | 0.64 | |
| CO | 90.6 ± 17.3 | 91.4 ± 17.2 | 91.0 ± 3.45 | |||
| Word–Color (counts) | PL | 51.3 ± 18.2 | 59.8 ± 14.3 b,* | 55.5 ± 3.39 | Time | 0.01 |
| CN | 54.0 ± 11.2 | 56.8 ± 10.8 | 55.4 ± 2.22 | Trt | 0.03 | |
| CAF | 62.5 ± 14.8 | 62.7 ± 14.8 a,c | 62.6 ± 2.95 | T×T | 0.04 † | |
| CO | 58.5 ± 18.2 | 65.9 ± 17.3 b,* | 62.2 ± 3.63 | |||
| Total Stroop Results (counts) | PL | 244.3 ± 48.1 | 257.2 ± 40.6 * | 250.7 ± 8.98 | Time | 0.003 |
| CN | 250.6 ± 35.5 | 260.3 ± 35.1 | 255.5 ± 7.12 | Trt | 0.03 | |
| CAF | 270.6 ± 38.7 | 274.8 ± 46.2 | 272.7 ± 8.52 | T×T | 0.67 | |
| CO | 267.2 ± 53.8 | 277.1 ± 46.9 | 273.1 ± 10.2 | |||
| Variable | Treatment | Day 0 | Day 7 | ||
|---|---|---|---|---|---|
| Mean SD | Mean SD | p-Value | |||
| ALP (U/L) | PL | 69.1 ± 25.6 | 69.0 ± 22.2 | Time | 0.63 |
| CN | 67.8 ± 22.6 | 70.7 ± 25.8 | Trt | 0.32 | |
| CAF | 69.4 ± 24.2 | 67.7 ± 24.4 | T×T | 0.86 | |
| CO | 68.1 ± 23.2 | 69.5 ± 25.7 | |||
| ALT (U/L) | PL | 21.5 ± 9.98 | 22.5 ± 11.7 | Time | 0.48 |
| CN | 21.9 ± 14.3 | 21.0 ± 11.1 | Trt | 0.62 | |
| CAF | 23.4 ± 16.0 | 20.9 ± 10.2 | T×T | 0.51 | |
| CO | 24.2 ± 11.2 | 22.4 ± 9.27 | |||
| AST (U/L) | PL | 24.3 ± 7.41 | 25.7 ± 6.56 | Time | 0.48 |
| CN | 24.1 ± 6.88 | 23.1 ± 5.49 | Trt | 0.63 | |
| CAF | 24.8 ± 7.04 | 27.0 ± 16.7 | T×T | 0.58 | |
| CO | 25.4 ± 9.69 | 27.8 ± 14.1 | |||
| CK (U/L) | PL | 294.7 ± 203.4 | 300.5 ± 162.9 | Time | 0.67 |
| CN | 280.1 ± 192.5 | 273.3 ± 265.2 | Trt | 0.94 | |
| CAF | 270.1 ± 174.3 | 273.3 ± 151.6 | T×T | 0.89 | |
| CO | 243.3 ± 224.3 | 301.8 ± 226.4 | |||
| LDH (U/L) | PL | 165.1 ± 34.2 | 164.5 ± 29.1 | Time | 0.70 |
| CN | 168.7 ± 27.9 | 170.3 ± 30.4 | Trt | 0.39 | |
| CAF | 160.5 ± 26.5 | 169.9 ± 32.6 | T×T | 0.33 | |
| CO | 161.5 ± 26.4 | 169.2 ± 26.7 | |||
| BUN (mg/dL) | PL | 17.5 ± 3.59 | 17.9 ± 4.82 | Time | 0.67 |
| CN | 17.1 ± 5.07 | 18.1 ± 2.57 | Trt | 0.69 | |
| CAF | 18.8 ± 3.05 | 17.3 ± 3.08 | T×T | 0.37 | |
| CO | 16.2 ± 3.70 | 17.2 ± 3.86 | |||
| Creatinine (mg/dL) | PL | 1.39 ± 0.24 | 1.42 ± 0.30 | Time | 0.13 |
| CN | 1.32 ± 0.24 | 1.33 ± 0.25 | Trt | 0.62 | |
| CAF | 1.23 ± 0.21 | 1.30 ± 0.17 | T×T | 0.29 | |
| CO | 1.43 ± 0.31 | 1.25 ± 0.13 | |||
| BUN/Creatinine | PL | 13.1 ± 4.44 | 13.1 ± 4.42 | Time | 0.56 |
| CN | 13.4 ± 4.67 | 14.1 ± 3.11 | Trt | 0.51 | |
| CAF | 15.7 ± 3.79 | 13.3 ± 2.33 | T×T | 0.14 | |
| CO | 11.8 ± 3.81 | 13.9 ± 3.68 | |||
| Variable | Treatment | Day 0 | Day 7 | ||
|---|---|---|---|---|---|
| Mean SD | Mean SD | p-Value | |||
| Cholesterol (mg/dL) | PL | 172.5 ± 51.1 | 163.0 ± 38.5 | Time | 0.72 |
| CN | 172.8 ± 52.4 | 172.1 ± 46.2 | Trt | 0.19 | |
| CAF | 167.5 ± 45.1 | 165.6 ± 42.5 | T×T | 0.56 | |
| CO | 173.6 ± 41.7 | 169.9 ± 48.2 | |||
| HDL-c (mg/dL) | PL | 52.8 ± 12.7 | 51.0 ± 11.6 c | Time | 0.27 |
| CN | 52.2 ± 10.3 | 52.8 ± 10.4 | Trt | 0.10 | |
| CAF | 50.9 ± 11.7 | 49.8 ± 13.1 c | T×T | 0.36 | |
| CO | 50.6 ± 12.4 | 53.8 ± 11.6 *,a | |||
| TC: HDL-c | PL | 3.43 ± 1.26 | 3.35 ± 1.08 | Time | 0.14 |
| CN | 3.44 ± 1.22 | 3.37 ± 1.05 | Trt | 0.19 | |
| CAF | 3.51 ± 1.48 | 3.52 ± 1.25 c | T×T | 0.13 | |
| CO | 3.69 ± 1.48 | 3.37 ± 1.11 b | |||
| LDL-c (mg/dL) | PL | 104.6 ± 48.2 | 97.7 ± 37.3 | Time | 0.43 |
| CN | 107.4 ± 50.4 | 104.9 ± 44.5 | Trt | 0.06 | |
| CAF | 97.5 ± 42.1 | 101.3 ± 40.8 | T×T | 0.21 | |
| CO | 107.0 ± 40.1 | 101.2 ± 44.9 | |||
| TG (mg/dL) | PL | 78.7 ± 29.1 | 82.1 ± 34.3 | Time | 0.43 |
| CN | 81.1 ± 32.3 | 76.2 ± 26.3 | Trt | 0.29 | |
| CAF | 102.1 ± 76.7 | 75.8 ± 40.6 | T×T | 0.31 | |
| CO | 83.1 ± 28.3 | 77.0 ± 36.4 | |||
| Variable | Treatment | Day 0 | Day 7 | ||
|---|---|---|---|---|---|
| Mean SD | Mean SD | p-Value | |||
| WBC Count (×103/μL) | PL | 6.20 ± 1.71 | 6.15 ± 1.51 | Time | 0.35 |
| CN | 5.96 ± 1.39 | 6.46 ± 1.83 | Trt | 0.30 | |
| CAF | 6.29 ± 1.45 | 6.11 ± 1.74 | T×T | 0.89 | |
| CO | 6.45 ± 1.45 | 6.33 ± 1.61 | |||
| RBC Count (×106/μL) | PL | 4.99 ± 0.92 | 5.19 ± 0.21 | Time | 0.92 |
| CN | 5.25 ± 0.26 | 5.22 ± 0.21 | Trt | 0.45 | |
| CAF | 5.19 ± 0.27 | 5.14 ± 0.18 | T×T | 0.41 | |
| CO | 5.22 ± 0.23 | 5.13 ± 0.24 | |||
| Hemoglobin (g/dL) | PL | 16.2 ± 1.73 | 15.7 ± 0.41 | Time | 0.09 |
| CN | 15.9 ± 0.74 | 15.9 ± 0.71 | Trt | 0.33 | |
| CAF | 15.9 ± 0.67 | 15.7 ± 0.73 | T×T | 0.78 | |
| CO | 15.7 ± 0.65 | 15.6 ± 0.61 | |||
| Hematocrit (%) | PL | 49.1 ± 4.72 | 47.9 ± 1.61 | Time | 0.04 |
| CN | 48.3 ± 1.91 | 48.4 ± 2.11 | Trt | 0.42 | |
| CAF | 48.1 ± 1.79 | 47.4 ± 1.96 | T×T | 0.92 | |
| CO | 48.0 ± 1.61 | 47.5 ± 1.84 | |||
| MCV (fL) | PL | 92.7 ± 3.98 | 92.6 ± 3.85 b | Time | 0.34 |
| CN | 92.3 ± 3.91 | 92.7 ± 3.80 a,c | Trt | 0.07 | |
| CAF | 92.7 ± 3.87 | 92.3 ± 3.59 b,d | T×T | 0.64 | |
| CO | 92.2 ± 3.68 | 92.6 ± 3.55 c | |||
| MCH (pg/cell) | PL | 30.7 ± 1.61 | 30.3 ± 1.53 b,d | Time | 0.61 |
| CN | 30.4 ± 1.37 | 30.5 ± 1.41 a | Trt | 0.01 | |
| CAF | 30.7 ± 1.57 | 30.6 ± 1.48 | T×T | 0.60 | |
| CO | 30.3 ± 1.51 | 30.6 ± 1.42 a | |||
| MCHC (g/dL) | PL | 33.1 ± 0.57 | 32.7 ± 0.61 | Time | 0.28 |
| CN | 32.9 ± 0.48 | 32.9 ± 0.50 | Trt | 0.34 | |
| CAF | 33.1 ± 0.71 | 33.1 ± 0.62 | T×T | 0.39 | |
| CO | 32.9 ± 0.58 | 33.0 ± 0.51 | |||
| RDW (%) | PL | 12.8 ± 0.69 | 12.6 ± 0.66 | Time | 0.77 |
| CN | 12.7 ± 0.47 | 12.6 ± 0.68 | Trt | 0.64 | |
| CAF | 12.7 ± 0.81 | 12.6 ± 0.95 | T×T | 0.91 | |
| CO | 12.7 ± 0.51 | 12.7 ± 0.64 | |||
| Platelet Count (×103/μL) | PL | 241.7 ± 55.5 | 234.1 ± 46.5 | Time | 0.33 |
| CN | 262.3 ± 43.3 | 257.6 ± 42.8 | Trt | 0.002 | |
| CAF | 252.5 ± 45.5 | 244.1 ± 41.4 | T×T | 0.47 | |
| CO | 261.9 ± 38.6 | 254.1 ± 40.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabrey, G.; Koozehchian, M.S.; Newton, A.T.; Naderi, A.; Forbes, S.C.; Haddad, M. The Effect of Creatine Nitrate and Caffeine Individually or Combined on Exercise Performance and Cognitive Function: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 766. https://doi.org/10.3390/nu16060766
Mabrey G, Koozehchian MS, Newton AT, Naderi A, Forbes SC, Haddad M. The Effect of Creatine Nitrate and Caffeine Individually or Combined on Exercise Performance and Cognitive Function: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(6):766. https://doi.org/10.3390/nu16060766
Chicago/Turabian StyleMabrey, Gina, Majid S. Koozehchian, Andrew T. Newton, Alireza Naderi, Scott C. Forbes, and Monoem Haddad. 2024. "The Effect of Creatine Nitrate and Caffeine Individually or Combined on Exercise Performance and Cognitive Function: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 6: 766. https://doi.org/10.3390/nu16060766
APA StyleMabrey, G., Koozehchian, M. S., Newton, A. T., Naderi, A., Forbes, S. C., & Haddad, M. (2024). The Effect of Creatine Nitrate and Caffeine Individually or Combined on Exercise Performance and Cognitive Function: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial. Nutrients, 16(6), 766. https://doi.org/10.3390/nu16060766








