Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies
Abstract
:1. Introduction
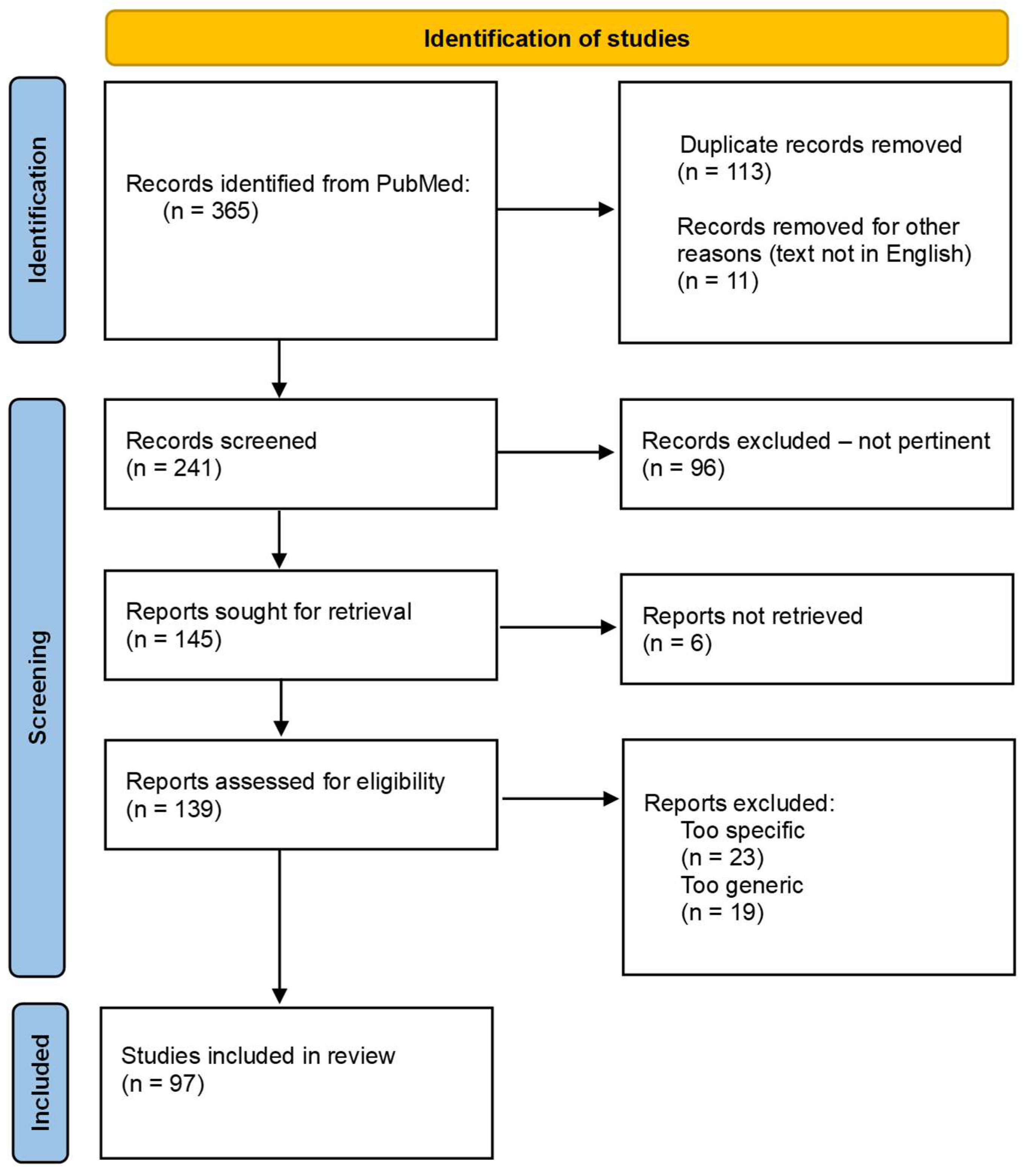
2. Materials and Methods
3. Results
3.1. Gluten-Free Diet: When to Use?
3.2. Adherence to GFD
3.3. Monitoring Adherence to the GFD
3.3.1. Clinical Assessment
3.3.2. Questionnaires
3.3.3. Serology
3.3.4. Endoscopy
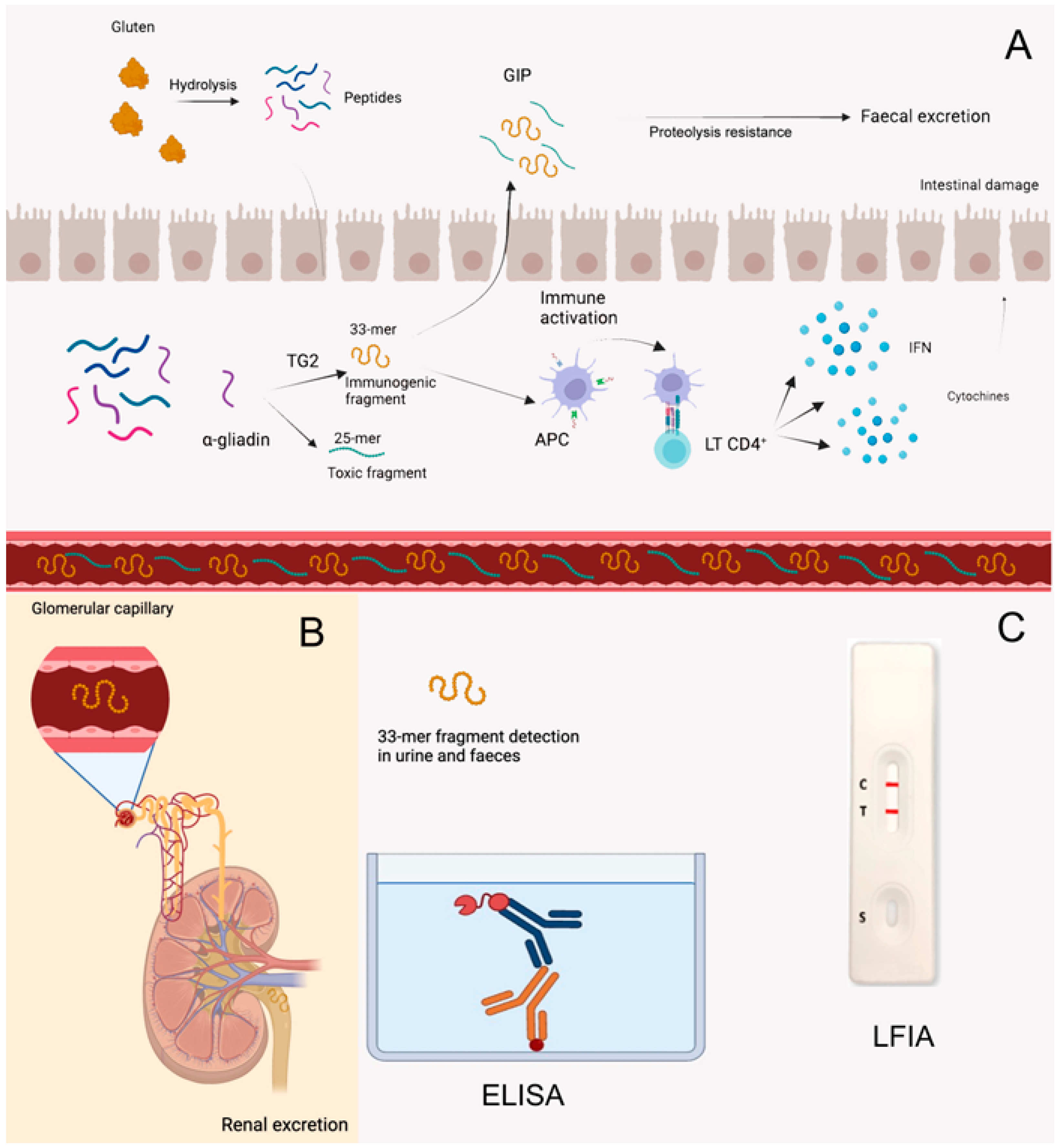
3.3.5. Detection of Gluten Immunogenic Peptides (GIPs) in Faeces and Urine
3.3.6. miRNAs
3.4. Gluten Contaminations
3.5. Nutritional Imbalances
3.6. Psychosocial Quality of Life in Patients on GFD
3.7. Current and Future Perspectives on GFDs
3.7.1. New Ingredients for New Gluten-Free Products
3.7.2. New Therapies on the Horizon beyond the Gluten-Free Diet
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sabença, C.; Ribeiro, M.; Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Malalgoda, M.; Ohm, J.B.; Simsek, S. Celiac Antigenicity of Ancient Wheat Species. Foods 2019, 12, 675. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Keller, A. Gluten cross contact in oats: Retrospective database analysis 2011 to 2023. Front. Nutr. 2023, 22, 1284636. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; DeBruyn, J.; Ronksley, P.E.; Shaheen, A.A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, S.; Najafimehr, H.; Pourhoseingholi, M.A.; Rostami, K.; Asadzadeh-Aghdaei, H.; Rostami-Nejad, M.; Tavirani, M.R.; Olfatifar, M.; Makharia, G.K.; Zali, M.R. Prevalence of celiac disease in low and high risk population in Asia-Pacific region: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Shiha, M.G.; Zammit, S.C.; Elli, L.; Sanders, D.S.; Sidhu, R. Updates in the diagnosis and management of coeliac disease. Best. Pract. Res. Clin. Gastroenterol. 2023, 64–65, 101843. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Saito, A.; Yanagida, N.; Sato, S.; Ebisawa, M. Cross-reactivity of each fraction among cereals in children with wheat allergy. Pediatr. Allergy Immunol. 2022, 33, e13831. [Google Scholar] [CrossRef]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef]
- Czaja-Bulsa, G.; Bulsa, M. What Do We Know Now about IgE-Mediated Wheat Allergy in Children? Nutrients 2017, 9, 35. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 18, 4966–4977. [Google Scholar] [CrossRef]
- Zanini, B.; Marullo, M.; Villanacci, V.; Salemme, M.; Lanzarotto, F.; Ricci, C.; Lanzini, A. Persistent Intraepithelial Lymphocytosis in Celiac Patients Adhering to Gluten-Free Diet Is Not Abolished Despite a Gluten Contamination Elimination Diet. Nutrients 2016, 8, 525. [Google Scholar] [CrossRef]
- Lamacchia, C.; Camarca, A.; Picascia, S.; Di Luccia, A.; Gianfrani, C. Cereal-Based Gluten-Free Food: How to Reconcile Nutritional and Technological Properties of Wheat Proteins with Safety for Celiac Disease Patients. Nutrients 2014, 6, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martinez, M.I.; Alegre-Martínez, A.; García-Ibánez, J.; Cauli, O. Quality of Life in People with Coeliac Disease: Psychological and Socio- Economic Aspects. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Niewinski, M.M. Advances in Celiac Disease and Gluten-Free Diet. J. Am. Diet. Assoc. 2008, 108, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Sbravati, F.; Pagano, S.; Retetangos, C.; Spisni, E.; Bolasco, G.; Labriola, F.; Filardi, M.C.; Grondona, A.G.; Alvisi, P. Adherence to Gluten-free Diet in a Celiac Pediatric Population Referred to the General Pediatrician After Remission. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 78–82. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.T.; Murray, J.A. Mucosal Recovery and Mortality in Adults With Celiac Disease After Treatment With a Gluten-Free Diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M.; Elisei, W.; Inchingolo, C.D.; Monardo, E.; Aiello, F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: A 2-year prospective study. Endoscopy 2006, 38, 702–707. [Google Scholar] [CrossRef]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef]
- Zanini, B.; Villanacci, V.; Marullo, M.; Cadei, M.; Lanzarotto, F.; Bozzola, A.; Ricci, C. Duodenal histological features in suspected non-celiac gluten sensitivity: New insights into a still undefined condition. Virchows Arch. 2018, 473, 229–234. [Google Scholar] [CrossRef]
- Tio, M.; Cox, M.R.; Eslick, G.D. Meta-analysis: Coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment. Pharmacol. Ther. 2012, 35, 540–551. [Google Scholar] [CrossRef]
- Emilsson, L.; Semrad, C.; Lebwohl, B.; Green, P.H.R.; Ludvigsson, J.F. Risk of Small Bowel Adenocarcinoma, Adenomas, and Carcinoids in a Nationwide Cohort of Individuals With Celiac Disease. Gastroenterology 2020, 159, 1686–1694.e2. [Google Scholar] [CrossRef]
- Ahmed, A.; Dixit, K.; Singh, A.; Agarwal, A.; Mehtab, W.; Prasad, S.; Rajput, M.S.; Chauhan, A.; Agarwal, A.; Mehta, S.; et al. Sieving out non-celiac gluten sensitivity amongst patients with irritable bowel syndrome. Dig. Liver Dis. 2023, 56, 451–457. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef]
- Lerner, A.; O’Bryan, T.; Matthias, T. Navigating the Gluten-Free Boom: The Dark Side of Gluten Free Diet. Front. Pediatr. 2019, 7, 414. [Google Scholar] [CrossRef]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Hao, J.; Zeng, J.; Yin, D.; Yi, Y.; Zhu, M.; Mandal, A.; Hua, Y.; Ng, C.K.; et al. Consumption of the Fish Oil High-Fat Diet Uncouples Obesity and Mammary Tumor Growth through Induction of Reactive Oxygen Species in Protumor Macrophages. Cancer Res. 2020, 80, 2564–2574. [Google Scholar] [CrossRef]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 2, 357. [Google Scholar] [CrossRef]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac disease and obesity: Need for nutritional follow-up after diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients 2021, 13, 2274. [Google Scholar] [CrossRef]
- Muhammad, H.; Reeves, S.; Ishaq, S.; Mayberry, J.; Jeanes, Y. Adherence to a Gluten Free Diet Is Associated with Receiving Gluten Free Foods on Prescription and Understanding Food Labelling. Nutrients 2017, 9, 705. [Google Scholar] [CrossRef]
- Comino, I.; Fernández-Bañares, F.; Esteve, M.; Ortigosa, L.; Castillejo, G.; Fambuena, B.; Ribes-Koninckx, C.; Sierra, C.; Rodríguez-Herrera, A.; Salazar, J.C.; et al. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am. J. Gastroenterol. 2016, 111, 1456–1465. [Google Scholar] [CrossRef]
- Porcelli, B.; Ferretti, F.; Cinci, F.; Biviano, I.; Santini, A.; Grande, E.; Quagliarella, F.; Terzuoli, L.; Bacarelli, M.R.; Bizzaro, N.; et al. Fecal gluten immunogenic peptides as indicators of dietary compliance in celiac patients. Minerva Gastroenterol. Dietol. 2020, 66, 201–207. [Google Scholar] [CrossRef]
- Poslt Königová, M.; Sebalo Vňuková, M.; Řehořková, P.; Anders, M.; Ptáček, R. The effectiveness of gluten-free dietary interventions: A systematic review. Front. Psychol. 2023, 4, 1107022. [Google Scholar] [CrossRef]
- Chaudrey, K.H. ACG Guideline: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 23. [Google Scholar] [CrossRef]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic review: Adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef]
- Tye-Din, J.A. Review article: Follow-up of coeliac disease. Aliment. Pharmacol. Ther. 2022, 56, S49–S63. [Google Scholar] [CrossRef]
- Leffler, D.A.; Edwards-George, J.; Dennis, M.; Schuppan, D.; Cook, F.; Franko, D.L.; Blom-Hoffman, J.; Kelly, C.P. Factors that Influence Adherence to a Gluten-Free Diet in Adults with Celiac Disease. Dig. Dis. Sci. 2008, 53, 1573–1581. [Google Scholar] [CrossRef]
- Moreno, M.; Rodríguez-Herrera, A.; Sousa, C.; Comino, I. Biomarkers to Monitor Gluten-Free Diet Compliance in Celiac Patients. Nutrients 2017, 9, 46. [Google Scholar] [CrossRef]
- Rodrigo, L.; Pérez-Martinez, I.; Lauret-Braña, E.; Suárez-González, A. Descriptive Study of the Different Tools Used to Evaluate the Adherence to a Gluten-Free Diet in Celiac Disease Patients. Nutrients 2018, 10, 1777. [Google Scholar] [CrossRef]
- Frossi, B.; De Carli, M.; Calabrò, A. Coeliac Disease and Mast Cells. Int. J. Mol. Sci. 2019, 20, 3400. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef]
- Coto, L.; Mendia, I.; Sousa, C.; Bai, J.C.; Cebolla, A. Determination of gluten immunogenic peptides for the management of the treatment adherence of celiac disease: A systematic review. World J. Gastroenterol. 2021, 27, 6306–6321. [Google Scholar] [CrossRef]
- Silvester, J.A.; Comino, I.; Rigaux, L.N.; Segura, V.; Green, K.H.; Cebolla, A.; Weiten, D.; Dominguez, R.; Leffler, D.A.; Leon, F.; et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2020, 52, 1469–1479. [Google Scholar] [CrossRef]
- Silvester, J.A.; Comino, I.; Kelly, C.P.; Sousa, C.; Duerksen, D.R.; DOGGIE BAG Study Group. Most Patients With Celiac Disease on Gluten-Free Diets Consume Measurable Amounts of Gluten. Gastroenterology 2020, 158, 1497–1499.e1. [Google Scholar] [CrossRef]
- Moreno, M.L.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef]
- Laserna-Mendieta, E.J.; Casanova, M.J.; Arias, Á.; Arias-González, L.; Majano, P.; Mate, L.A.; Gordillo-Vélez, C.H.; Jiménez, M.; Angueira, T.; Tébar-Romero, E.; et al. Poor Sensitivity of Fecal Gluten Immunogenic Peptides and Serum Antibodies to Detect Duodenal Mucosal Damage in Celiac Disease Monitoring. Nutrients 2020, 13, 98. [Google Scholar] [CrossRef]
- Delić, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS ONE 2016, 11, e0150154. [Google Scholar] [CrossRef]
- Felli, C.; Baldassarre, A.; Uva, P.; Alisi, A.; Cangelosi, D.; Ancinelli, M.; Caruso, M.; Paolini, A.; Montano, A.; Silano, M.; et al. Circulating microRNAs as novel non-invasive biomarkers of paediatric celiac disease and adherence to gluten-free diet. eBioMedicine 2022, 76, 103851. [Google Scholar] [CrossRef]
- Paolini, A.; Sarshar, M.; Felli, C.; Bruno, S.P.; Rostami-Nejad, M.; Ferretti, F.; Masotti, A.; Baldassarre, A. Biomarkers to Monitor Adherence to Gluten-Free Diet by Celiac Disease Patients: Gluten Immunogenic Peptides and Urinary miRNAs. Foods 2022, 11, 1380. [Google Scholar] [CrossRef]
- Tan, I.L.; Coutinho de Almeida, R.; Modderman, R.; Stachurska, A.; Dekens, J.; Barisani, D.; Meijer, C.R.; Roca, M.; Martinez-Ojinaga, E.; Shamir, R.; et al. Circulating miRNAs as Potential Biomarkers for Celiac Disease Development. Front. Immunol. 2021, 12, 734763. [Google Scholar] [CrossRef]
- Guz, M.; Dworzański, T.; Jeleniewicz, W.; Cybulski, M.; Kozicka, J.; Stepulak, A.; Celiński, K. Elevated miRNA Inversely Correlates with E-cadherin Gene Expression in Tissue Biopsies from Crohn Disease Patients in contrast to Ulcerative Colitis Patients. Biomed. Res. Int. 2020, 2020, 4250329. [Google Scholar] [CrossRef]
- Codex Alimentarius-European Commission. Available online: https://european-union.europa.eu/index_en (accessed on 30 January 2024).
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef]
- Lähdeaho, M.-L.; Mäki, M.; Laurila, K.; Huhtala, H.; Kaukinen, K. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011, 11, 129. [Google Scholar] [CrossRef]
- Verma, A.K.; Gatti, S.; Galeazzi, T.; Monachesi, C.; Padella, L.; Baldo, G.D.; Annibali, R.; Lionetti, E.; Catassi, C. Gluten Contamination in Naturally or Labeled Gluten-Free Products Marketed in Italy. Nutrients 2017, 9, 115. [Google Scholar] [CrossRef]
- Mehtab, W.; Sachdev, V.; Singh, A.; Agarwal, S.; Singh, N.; Malik, R.; Malhotra, A.; Ahuja, V.; Makharia, G. Gluten content in labeled and unlabeled gluten-free food products used by patients with celiac disease. Eur. J. Clin. Nutr. 2021, 75, 1245–1253. [Google Scholar] [CrossRef]
- Gibert, A.; Kruizinga, A.G.; Neuhold, S.; Houben, G.F.; Canela, M.A.; Fasano, A.; Catassi, C. Might gluten traces in wheat substitutes pose a risk in patients with celiac disease? A population-based probabilistic approach to risk estimation. Am. J. Clin. Nutr. 2013, 97, 109–116. [Google Scholar] [CrossRef]
- Lerner, B.A.; Phan Vo, L.T.; Yates, S.; Rundle, A.G.; Green, P.H.R.; Lebwohl, B. Detection of Gluten in Gluten-Free Labeled Restaurant Food: Analysis of Crowd-Sourced Data. Am. J. Gastroenterol. 2019, 114, 792–797. [Google Scholar] [CrossRef]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef]
- Rashid, M.; Butzner, D.; Burrows, V.; Zarkadas, M.; Case, S.; Molloy, M.; Warren, R.; Pulido, O.; Switzer, C. Consumption of Pure Oats by Individuals with Celiac Disease: A Position Statement by the Canadian Celiac Association. Can. J. Gastroenterol. 2007, 21, 649–651. [Google Scholar] [CrossRef]
- Spector Cohen, I.; Day, A.S.; Shaoul, R. To Be Oats or Not to Be? An Update on the Ongoing Debate on Oats for Patients with Celiac Disease. Front. Pediatr. 2019, 7, 384. [Google Scholar] [CrossRef]
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345. [Google Scholar] [CrossRef]
- Fritz, R.D.; Chen, Y. Oat safety for celiac disease patients: Theoretical analysis correlates adverse symptoms in clinical studies to contaminated study oats. Nutr. Res. 2018, 60, 54–67. [Google Scholar] [CrossRef]
- Rostami-Nejad, M.; Asri, N.; Olfatifar, M.; Khorsand, B.; Houri, H.; Rostami, K. Systematic Review and Dose-Response Meta-Analysis on the Relationship between Different Gluten Doses and Risk of Coeliac Disease Relapse. Nutrients 2023, 15, 1390. [Google Scholar] [CrossRef]
- Hollon, J.R.; Cureton, P.A.; Martin, M.L.; Puppa, E.L.L.; Fasano, A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- Lebwohl, B.; Green, P.H.R.; Söderling, J.; Roelstraete, B.; Ludvigsson, J.F. Association Between Celiac Disease and Mortality Risk in a Swedish Population. JAMA 2020, 323, 1277. [Google Scholar] [CrossRef]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef]
- Mariani, P.; Viti, M.G.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The Gluten-Free Diet: A Nutritional Risk Factor for Adolescents with Celiac Disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar]
- Ballestero-Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac. Nutrients 2021, 13, 1626. [Google Scholar] [CrossRef]
- Lionetti, E.; Antonucci, N.; Marinelli, M.; Bartolomei, B.; Franceschini, E.; Gatti, S.; Catassi, G.N.; Verma, A.K.; Monachesi, C.; Catassi, C. Nutritional Status, Dietary Intake, and Adherence to the Mediterranean Diet of Children with Celiac Disease on a Gluten-Free Diet: A Case-Control Prospective Study. Nutrients 2020, 12, 143. [Google Scholar] [CrossRef]
- de la Calle, I.; Ros, G.; Peñalver Miras, R.; Nieto, G. Celiac disease: Causes, pathology, and nutritional assessment of gluten-free diet. A review. Nutr. Hosp. 2020, 37, 1043. [Google Scholar]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and Celiac Disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef]
- Lee, A.R.; Ng, D.L.; Diamond, B.; Ciaccio, E.J.; Green, P.H.R. Living with coeliac disease: Survey results from the USA. J. Hum. Nutr. Diet. 2012, 25, 233–238. [Google Scholar] [CrossRef]
- Hallert, C.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Living with Coeliac Disease: Controlled Study of the Burden of Illness. Scand. J. Gastroenterol. 2002, 37, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Zarkadas, M.; Cranney, A.; Case, S.; Molloy, M.; Switzer, C.; Graham, I.D.; Butzner, J.D.; Rashid, M.; Warren, R.E.; Burrows, V. The impact of a gluten-free diet on adults with coeliac disease: Results of a national survey. J. Hum. Nutr. Diet. 2006, 19, 41–49. [Google Scholar] [CrossRef]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Abenavoli, L.; Vonghia, L.; Cardone, S.; Leso, V.; Cossari, A.; et al. State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008, 62, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Nachman, F.; del Campo, M.P.; González, A.; Corzo, L.; Vázquez, H.; Sfoggia, C.; Smecuol, E.; Sánchez, M.I.; Niveloni, S.; Sugai, E.; et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig. Liver Dis. 2010, 42, 685–691. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Card, T.; Ciclitira, P.J.; Swift, G.L.; Nasr, I.; Sanders, D.S.; Ciacci, C. Support for patients with celiac disease: A literature review. United Eur. Gastroenterol. J. 2015, 3, 146–159. [Google Scholar] [CrossRef]
- Queiroz, V.A.V.; Dizlek, H.; de Barros, F.A.R.; Tardin, F.D.; Figueiredo, J.E.F.; Awika, J.M. Baking Process Effects and Combined Cowpea Flour and Sorghum Bran on Functional Properties of Gluten-Free Cookies. Plant Foods Hum. Nutr. 2022, 77, 552–559. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Soltanizadeh, N.; Mirmoghtadaee, P.; Banavand, P.; Mirmoghtadaie, L.; Shojaee-Aliabadi, S. Gluten-free products in celiac disease: Nutritional and technological challenges and solutions. J. Res. Med. Sci. 2018, 23, 109. [Google Scholar]
- Li, Y.; Shi, R.; Qin, C.; Zhang, Y.; Liu, L.; Wu, Z. Gluten-free and prebiotic oat bread: Optimization formulation by transglutaminase improvement dough structure. J. Food Process Preserv. 2021, 45, e15684. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef]
- Greco, L.; Gobbetti, M.; Auricchio, R.; Di Mase, R.; Landolfo, F.; Paparo, F.; Di Cagno, R.; De Angelis, M.; Rizzello, C.G.; Cassone, A.; et al. Safety for Patients with Celiac Disease of Baked Goods Made of Wheat Flour Hydrolyzed During Food Processing. Clin. Gastroenterol. Hepatol. 2011, 9, 24–29. [Google Scholar] [CrossRef]
- Ribeiro, M.; Sousa, T.; de Poeta, P.; Bagulho, A.S.; Igrejas, G. Review of Structural Features and Binding Capacity of Polyphenols to Gluten Proteins and Peptides In Vitro: Relevance to Celiac Disease. Antioxidants 2020, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential Effects of Flavonoids on Barrier Integrity in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- Nyembwe, P.M.; de Kock, H.L.; Taylor, J.R.N. Potential of defatted marama flour-cassava starch composites to produce functional gluten-free bread-type dough. LWT 2018, 92, 429–434. [Google Scholar] [CrossRef]
- Bernardi, C.; Sánchez, H.; Freyre, M.; Osella, C. Gluten-free bread formulated with Prosopis ruscifolia (vinal) seed and corn flours. Int. J. Food Sci. Nutr. 2010, 61, 245–255. [Google Scholar]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, M.D.; Palmisano, F.; Maurano, F.; et al. Highly Efficient Gluten Degradation by Lactobacilli and Fungal Proteases during Food Processing: New Perspectives for Celiac Disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef]
- Gianfrani, C.; Siciliano, R.A.; Facchiano, A.M.; Camarca, A.; Mazzeo, M.F.; Costantini, S.; Salvati, V.M.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; et al. Transamidation of Wheat Flour Inhibits the Response to Gliadin of Intestinal T Cells in Celiac Disease. Gastroenterology 2007, 133, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Lehmann, J.W.; Peterson, D.M. Amaranth and its oil inhibit cholesterol biosynthesis in 6-week-old female chickens. J. Nutr. 1996, 126, 1972–1978. [Google Scholar]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten–free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef]
- Coțovanu, I.; Stroe, S.G.; Ursachi, F.; Mironeasa, S. Addition of Amaranth Flour of Different Particle Sizes at Established Doses in Wheat Flour to Achieve a Nutritional Improved Wheat Bread. Foods 2022, 12, 133. [Google Scholar] [CrossRef]
- Bergamo, P.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; Vocca, I.; Rivelli, A.R.; De Falco, E.; Gianfrani, C.; Rossi, M. Immunological evaluation of the alcohol-soluble protein fraction from gluten-free grains in relation to celiac disease. Mol. Nutr. Food Res. 2011, 55, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Lamacchia, C.; Chillo, S.; Lamparelli, S.; Suriano, N.; Notte, E.; Nobile, M.A. Amaranth, quinoa and oat doughs: Mechanical and rheological behaviour, polymeric protein size distribution and extractability. J. Food Eng. 2010, 96, 97–106. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; de Morais, E.C.; Steel, C.J.; Bolini, H.M.A. Sensory characterisation of gluten-free bread with addition of quinoa, amaranth flour and sweeteners as an alternative for coeliac patients. Int. J. Food Sci. Technol. 2017, 52, 872–879. [Google Scholar] [CrossRef]
- Machado Alencar, N.M.; Steel, C.J.; Alvim, I.D.; de Morais, E.C.; Andre Bolini, H.M. Addition of quinoa and amaranth flour in gluten-free breads: Temporal profile and instrumental analysis. LWT Food Sci. Technol. 2015, 62, 1011–1018. [Google Scholar] [CrossRef]
- Hamzehpour, R.; Dastgerdi, A.A. The Effects of Quinoa and Amaranth Flour on the Qualitative Characteristics of Gluten-Free Cakes. Int. J. Food Sci. 2023, 2023, 6042636. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chickpea and tiger nut flours as alternatives to emulsifier and shortening in gluten-free bread. LWT Food Sci. Technol. 2015, 62, 225–232. [Google Scholar] [CrossRef]
- Dogruer, I.; Coban, B.; Baser, F.; Gulec, S.; Ozen, B. Techno-Functional and In Vitro Digestibility Properties of Gluten-Free Cookies Made from Raw, Pre-Cooked, and Germinated Chickpea Flours. Foods 2023, 12, 2829. [Google Scholar] [CrossRef] [PubMed]
- Sarawong, C.; Gutiérrez, Z.R.; Berghofer, E.; Schoenlechner, R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int. J. Food Sci. Technol. 2014, 49, 1825–1833. [Google Scholar] [CrossRef]
- Sandri, L.T.B.; Santos, F.G.; Fratelli, C.; Capriles, V.D. Development of gluten-free bread formulations containing whole chia flour with acceptable sensory properties. Food Sci. Nutr. 2017, 5, 1021–1028. [Google Scholar] [CrossRef]
- Smith, B.M.; Bean, S.R.; Herald, T.J.; Aramouni, F.M. Effect of HPMC on the Quality of Wheat-Free Bread Made from Carob Germ Flour-Starch Mixtures. J. Food Sci. 2012, 77, C684–C689. [Google Scholar] [CrossRef] [PubMed]
- Norsa, L. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J. Gastroenterol. 2013, 19, 5658. [Google Scholar] [CrossRef] [PubMed]
- Martín-Esparza, M.E.; Raigón, M.D.; García-Martínez, M.D.; Albors, A. Toward the Development of Potentially Healthy Low-Energy-Density Snacks for Children Based on Pseudocereal and Pulse Flours. Foods 2023, 12, 2873. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Jarmakiewicz Czaja, S.; Piątek, D.; Filip, R. The impact of selected food additives on the gastrointestinal tract in the example of nonspecific inflammatory bowel diseases. Arch. Med. Sci. 2021, 18, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, K.; Camilleri, M. Effects of dietary components on intestinal permeability in health and disease. Am. J. Physiol. Liver Physiol. 2020, 319, G589–G608. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.; Gocmen, D. Use of almond flour and stevia in rice-based gluten-free cookie production. J. Food Sci. Technol. 2021, 58, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Raczyk, M.; Kruszewski, B.; Michałowska, D. Effect of Coconut and Chestnut Flour Supplementations on Texture, Nutritional and Sensory Properties of Baked Wheat Based Bread. Molecules 2021, 26, 4641. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.L.; Wang, L.J.; Li, T.T.; Wang, F.; Quan, Z.Y.; Zhou, M.; Huo, Z.Y.; Qian, J.Y. Pre-Gelatinisation of Rice Flour and Its Effect on the Properties of Gluten Free Rice Bread and Its Batter. Foods 2021, 10, 2648. [Google Scholar] [CrossRef]
- Pereira, J.; Hu, H.; Xing, L.; Zhang, W.; Zhou, G. Influence of Rice Flour, Glutinous Rice Flour, and Tapioca Starch on the Functional Properties and Quality of an Emulsion-Type Cooked Sausage. Foods 2019, 9, 9. [Google Scholar] [CrossRef]
- Coţovanu, I.; Mironeasa, C.; Mironeasa, S. Incorporation of Buckwheat Flour at Different Particle Sizes and Distinctive Doses in Wheat Flour to Manufacture an Improved Wheat Bread. Foods 2023, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Ari Akin, P.; Demirkesen, I.; Bean, S.R.; Aramouni, F.; Boyaci, I.H. Sorghum Flour Application in Bread: Technological Challenges and Opportunities. Foods 2022, 11, 2466. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Abdo Qasem, A.A.; Serag El-Din, M.F.; Almaiman, S.A.M. Wheat-millet flour cookies: Physical, textural, sensory attributes and antioxidant potentia. Wheat–millet flour cookies: Physical, textural, sensory attributes and antioxidant potential. Food Sci. Technol. Int. 2020, 26, 311–320. [Google Scholar] [CrossRef]
- Lu, H.; Guo, L.; Zhang, L.; Xie, C.; Li, W.; Gu, B.; Li, K. Study on quality characteristics of cassava flour and cassava flour short biscuits. Food Sci. Nutr. 2020, 8, 521–533. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in research and applications of cassava flour and starch: A review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Taghdir, M.; Mazloomi, S.M.; Honar, N.; Sepandi, M.; Ashourpour, M.; Salehi, M. Effect of soy flour on nutritional, physicochemical, and sensory characteristics of gluten-free bread. Food Sci. Nutr. 2017, 5, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, H.; Huang, B.; Hao, S.; Li, S.; Li, P.; Yu, H. Studying the Role of Potato Powder on the Physicochemical Properties and Dough Characteristics of Wheat Flour. Gels 2023, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Chochkov, R.; Savova-Stoyanova, D.; Papageorgiou, M.; Rocha, J.M.; Gotcheva, V.; Angelov, A. Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality. Foods 2022, 11, 1012. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Song, Y.; Huang, X.; Wu, T.; Xu, Z.Z.; Lu, H. Green Banana Flour Contributes to Gut Microbiota Recovery and Improves Colonic Barrier Integrity in Mice Following Antibiotic Perturbation. Front. Nutr. 2022, 9, 832848. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, M.; Chen, L.; Zheng, B. Impact of using whole chestnut flour as a substitute for cake flour on digestion, functional and storage properties of chiffon cake: A potential application study. Food Chem. 2024, 432, 137016. [Google Scholar] [CrossRef] [PubMed]
- Lucini Mas, A.; Brigante, F.I.; Salvucci, E.; Ribotta, P.; Martinez, M.L.; Wunderlin, D.A.; Baroni, M.V. Novel cookie formulation with defatted sesame flour: Evaluation of its technological and sensory properties. Changes in phenolic profile, antioxidant activity, and gut microbiota after simulated gastrointestinal digestion. Food Chem. 2022, 389, 133122. [Google Scholar] [CrossRef] [PubMed]
- Pycia, K.; Juszczak, L. The Effect of the Addition of Hazelnut or Walnut Flour on the Rheological Characteristics of Wheat Dough. Materials 2022, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Razzaghi, M.; Asadzadeh-Aghdaei, H.; Zali, M.R. Novel Therapeutic Strategies for Celiac Disease. Middle East. J. Dig. Dis. 2020, 12, 229–237. [Google Scholar] [PubMed]
- Syage, J.A.; Murray, J.A.; Green, P.H.R.; Khosla, C. Latiglutenase Improves Symptoms in Seropositive Celiac Disease Patients While on a Gluten-Free Diet. Dig. Dis. Sci. 2017, 62, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Dieckman, T.; Koning, F.; Bouma, G. Celiac disease: New therapies on the horizon. Curr. Opin. Pharmacol. 2022, 66, 102268. [Google Scholar] [CrossRef] [PubMed]
- Pultz, I.S.; Hill, M.; Vitanza, J.M.; Wolf, C.; Saaby, L.; Liu, T.; Winkle, P.; Leffler, D.A. Gluten Degradation, Pharmacokinetics, Safety, and Tolerability of TAK-062, an Engineered Enzyme to Treat Celiac Disease. Gastroenterology 2021, 161, 81–93.e3. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Durai, M.; Kitchens, K.; Tamiz, A.P.; Somerville, R.; Ginski, M.; Paterson, B.M.; Murray, J.A.; Verdu, E.F.; Alkan, S.S.; et al. B Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides 2012, 35, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Mäki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, Ø.; et al. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Lenti, M.V.; Corazza, G.R.; Gianfrani, C. Vaccine Immunotherapy for Celiac Disease. Front. Med. 2018, 5, 187. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Jhaveri, V.M.; Kay, S.S.; Debs, L.H.; Parrish, J.M.; Pan, D.R.; Nguyen, D.; Mittal, J.; Jayant, R.D. Recent advancements in nanoparticle based drug delivery for gastrointestinal disorders. Expert. Opin. Drug Deliv. 2018, 15, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, S.; Abbasi, A.; Heravi, F.S.; Somi, M.H.; Nikniaz, Z.; Moaddab, S.Y.; Ebrahimzadeh Leylabadlo, H. The gut microbiota and celiac disease: Pathophysiology, current perspective and new therapeutic approaches. Crit. Rev. Food Sci. Nutr. 2024, 64, 2176–2196. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Disadvantages | |
|---|---|---|
| Clinical Assessment | Non-invasive Rapid Cheap | Low sensitivity and specificity |
| Validated questionnaires | Non-invasive Rapid Cheap Good information about patient’s diet | Subjective Poor correlation with symptoms and histological findings |
| Serology | High sensitivity and specificity | Different available testing platform Poor correlation with symptoms and histological findings Unable to detect occasional transgression |
| Biopsies | High sensitivity and specificity | Invasive Expensive Unsuitable for frequent monitoring |
| GIP Detection | Non-invasive Rapid Cheap Able to detect occasional transgression | Not yet clear correlation with mucosal damages and serology |
| Flour Type | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Almond flour | Rich in protein and dietary fibre Good sensory properties Cholesterol-lowering effect | Can be dense High in calories | [118] |
| Coconut flour | High fibre content Mild sweetness | Absorbs a lot of moisture and requires more liquid | [119] |
| Rice flour | Neutral flavour Digestibility Hypoallergenic proteins | May result in a gritty texture if not finely ground | [120] |
| Tapioca flour (from the cassava root) | Starchy flour used as thickener Light texture Neutral taste | Lacks significant nutritional value compared to other flours | [121] |
| Chickpea flour | High protein content High water retention capacity Good acceptability | Strong flavour | [109] |
| Quinoa flour | Excellent protein source Mild flavour Increased loaf volume More uniform crumb structure | Can be expensive compared to other flours | [103] |
| Buckwheat flour | High nutritional value Antioxidant activity Reduced glycaemic index | Strong, bitter flavour | [122,123] |
| Sorghum flour | Neutral taste Good texture | May require additional binders | [124] |
| Millet flour | Mild flavour Good texture High contents of antioxidants and health-promoting polyphenols | May require blending with other flours | [125] |
| Amaranth and quinoa flour | High in fat, fibre content and complete protein source Minimal impact on texture and volume | Can have a strong flavour | [107] |
| Cassava flour | Starchy and neutral taste High nutritional value Good texture | May result in denser baked goods | [126,127] |
| Soy flour | Rich in protein Good texture Increased binding properties | Strong soy taste can be overpowering | [128] |
| Potato flour | Provides moisture and a soft texture in baked foods High fibre content | Limited nutritional value and flavour | [129] |
| Teff flour | Mild flavour High nutritional value and complete protein source Low glycaemic index | Not widely available | [130] |
| Green Banana flour | High in resistant starch for gut health Low glycaemic index | Limited flavour and may require recipe adjustments. | [131] |
| Chestnut flour | Sweet flavour High nutritional value and antioxidant properties | Limited availability and can be expensive | [132] |
| Sesame flour | Rich in healthy fats and protein Antioxidant properties. Beneficial effect on gut microbiota Good sensorial acceptance | Modified surface appearance (colour and cracking) | [133] |
| Hazelnut flour | High nutritional value and health benefits Good sensory characteristics | Low water absorption of the flour Increased dough development time Reduced dough stability during kneading | [134] |
| Green plantain flour | Its incorporation in a flour blend of rice flour and GF wheat starch showed good potential for improving the quality of GF bread | [110] | |
| Chia flour | If added to rice flour, the reduction in loaf volume, crumb firmness and crumb moisture is negligible | Chia flour alone is not suitable for bread production | [111] |
| Carob germ proteins | Good viscoelastic properties, high nutritional value | Not widely available | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzola, A.M.; Zammarchi, I.; Valerii, M.C.; Spisni, E.; Saracino, I.M.; Lanzarotto, F.; Ricci, C. Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies. Nutrients 2024, 16, 1006. https://doi.org/10.3390/nu16071006
Mazzola AM, Zammarchi I, Valerii MC, Spisni E, Saracino IM, Lanzarotto F, Ricci C. Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies. Nutrients. 2024; 16(7):1006. https://doi.org/10.3390/nu16071006
Chicago/Turabian StyleMazzola, Anna Maria, Irene Zammarchi, Maria Chiara Valerii, Enzo Spisni, Ilaria Maria Saracino, Francesco Lanzarotto, and Chiara Ricci. 2024. "Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies" Nutrients 16, no. 7: 1006. https://doi.org/10.3390/nu16071006
APA StyleMazzola, A. M., Zammarchi, I., Valerii, M. C., Spisni, E., Saracino, I. M., Lanzarotto, F., & Ricci, C. (2024). Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies. Nutrients, 16(7), 1006. https://doi.org/10.3390/nu16071006










