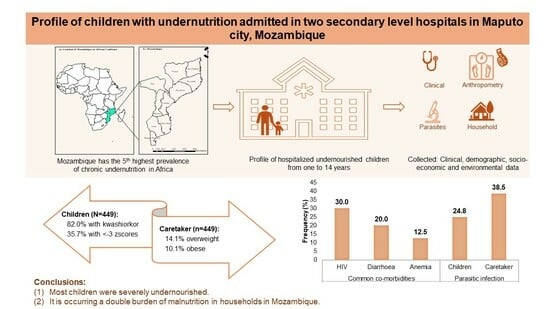
Profile of Children with Undernutrition Admitted in Two Secondary-Level Hospitals in Maputo City, Mozambique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Hospital: Clinical Data
2.3. Anthropometric Measurements
2.4. Stool Sample Collection and Parasitological Examinations
2.5. Household Environment Data
2.6. Data Analysis
2.7. Ethical Clearance
3. Results
3.1. Hospital: Clinical Data
3.2. Anthropometric Measurements of Children and Caretakers
3.3. Intestinal Parasite Infections of Children and Caretakers
3.4. Household Environment Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Fact Sheets—Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 9 March 2022).
- UNICEF; WHO; World Bank Group. Levels and Trends in Child Malnutrition; Joint Child Malnutrition Estimates; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- UNITLIFE. Why Mozambique? High Chronic Malnutrition Rates. Available online: https://www.unitlife.org/mozambique (accessed on 26 August 2022).
- Instituto Nacional de Estatística (INE). Inquérito Sobre Orçamento Familiar—IOF 2019/20. Estado Nutricional Das. Crianças Menores de 5 Anos e Percepção Dos Agregados Familiares Sobre a Segurança Alimentar. Suplemento Relatório Final; Instituto Nacional de Estatística—Moçambique: Maputo, Mozambique, 2021. [Google Scholar]
- De Onis, M.; Borghi, E.; Arimond, M.; Webb, P.; Croft, T.; Saha, K.; De-Regil, L.M.; Thuita, F.; Heidkamp, R.; Krasevec, J.; et al. Prevalence Thresholds for Wasting, Overweight and Stunting in Children under 5 Years. Public Health Nutr. 2019, 22, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde (MISAU); Instituto Nacional de Saúde (INE); ICF International (ICFI). Inquérito Demográfico e de Saúde 2011, MISAU, INE e ICFI: Calverton, MD, USA, 2011.
- Ministério da Saúde (MISAU); Instituto Nacional de Estatística (INE); ICF. Inquérito de Indicadores de Imunização, Malária e HIV/SIDA Em Moçambique 2015: Relatório Suplementar Incorporado Os Resultados de Biomarcadores de Antiretrovirais; INE: Maputo, Mozambique; Rockville, MA, USA, 2019. [Google Scholar]
- Sambo, J.; Cassocera, M.; Chissaque, A.; Bauhofer, A.F.L.; Roucher, C.; Chilaúle, J.; Cossa-Moiane, I.; Guimarães, E.L.; Manhique-Coutinho, L.; Anapakala, E.; et al. Characterizing Undernourished Children Under-Five Years Old with Diarrhoea in Mozambique: A Hospital Based Cross-Sectional Study, 2015–2019. Nutrients 2022, 14, 1164. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde (MISAU). Guia de Tratamento Antiretroviral e Infecções Oportunistas No Adulto, Criança, Adolescente e Grávida; MISAU: Maputo, Mozambique, 2013. [Google Scholar]
- World Health Organization (WHO). Nutrient Requirements for People Living with HIV/AIDS; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Augusto, G.; Nalá, R.; Casmo, V.; Sabonete, A.; Mapaco, L.; Monteiro, J. Geographic Distribution and Prevalence of Schistosomiasis and Soil-Transmitted Helminths among Schoolchildren in Mozambique. Am. J. Trop. Med. Hyg. 2009, 81, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Papier, K.; Williams, G.M.; Luceres-Catubig, R.; Ahmed, F.; Olveda, R.M.; McManus, D.P.; Chy, D.; Chau, T.N.P.; Gray, D.J.; Ross, A.G.P. Childhood Malnutrition and Parasitic Helminth Interactions. Clin. Infect. Dis. 2014, 59, 234–243. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Soil-Transmitted Helminth Infections; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Instituto Nacional de Estatística (INE). Inquérito Sobre Orçamento Familiar—IOF 2019/20—Relatório Final; INE: Maputo, Mozambique, 2021. [Google Scholar]
- Rey, L. Bases Da Parasitologia Médica, 3rd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Campos Ponce, M.; Incani, R.N.; Pinelli, E.; Ten Kulve, N.; Ramak, R.; Polman, K.; Doak, C.M. Are Intestinal Parasites Fuelling the Rise in Dual Burden Households in Venezuela? Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, L.S. Helminth Parasites, a Major Factor in Malnutrition. World Health Forum 1994, 15, 169–172. [Google Scholar] [PubMed]
- Fontes, F.; Damasceno, A.; Jessen, N.; Prista, A.; Silva-Matos, C.; Padrão, P.; Lunet, N. Prevalence of Overweight and Obesity in Mozambique in 2005 and 2015. Public Health Nutr. 2019, 22, 3118–3126. [Google Scholar] [CrossRef] [PubMed]
- Macicame, I.; Prista, A.; Parhofer, K.G.; Cavele, N.; Manhiça, C.; Nhachungue, S.; Saathoff, E.; Rehfuess, E. Social Determinants and Behaviors Associated with Overweight and Obesity among Youth and Adults in a Peri-Urban Area of Maputo City, Mozambique. J. Glob. Health 2021, 11, 04021. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the Double Burden of Malnutrition and the Changing Nutrition Reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Ministério da Saúde (MISAU). Manual de Tratamento e Reabilitação Nutricional: Tratamento Da Desnutrição Em Internamento (TDI); MISAU: Maputo, Mozambique, 2010; Volume 1. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- World Health Organization (WHO). WHO Anthro Survey Analyser; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization (WHO). WHO AnthroPlus for Personal Computers. Manual: Software for Assessing Growth of the World’s Children and Adolescents; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Cossa-Moiane, I.; Roucher, C.; Mac Quene, T.; Campos-Ponce, M.; de Deus, N.; Polman, K.; Doak, C. Association between Intestinal Parasite Infections and Proxies for Body Composition: A Scoping Review. Nutrients 2022, 14, 2229. [Google Scholar] [CrossRef]
- Instituto Nacional de Saúde (INS). Manual de Técnicas de Diagnóstico Microscópico de Parasitas Intestinais e Urinárias/Vesicais, 1st ed.; INS: Maputo, Mozambique, 2022; Volume 1. [Google Scholar]
- World Health Organization (WHO). Manual of Basic Techniques for a Health Laboratory, 2nd ed.; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Rutstein, S.O.; Johnson, K. The DHS Wealth Index. DHS Comparative Reports No. 6; ORC Macro: Calverton, MA, USA, 2004. [Google Scholar]
- Hjelm, A.M.L.; Miller, D.; Wadhwa, A. VAM Guidance Paper—Creation of a Wealth Index; World Food Programme (WFP): Rome, Italy, 2017. [Google Scholar]
- Callegari-Jacques, S.M. Bioestatística, Princípios e Aplicações, 1st ed.; Artmed Editora S.A: Porto Alegre, Brazil, 2008. [Google Scholar]
- Ministério da Saúde (MISAU). Manual Do Programa Alargado de Vacinação, 4th ed.; MISAU: Maputo, Mozambique, 2019. [Google Scholar]
- Ministério da Saúde (MISAU); Instituto Nacional de Saúde (INS). Sara 2018 Inventário Nacional; MISAU: Maputo, Mozambique, 2020. [Google Scholar]
- Schoenmaker, C.; Juffer, F.; van IJzendoorn, M.H.; van den Dries, L.; Linting, M.; van der Voort, A.; Bakermans-Kranenburg, M.J. Cognitive and Health-Related Outcomes after Exposure to Early Malnutrition: The Leiden Longitudinal Study of International Adoptees. Child. Youth Serv. Rev. 2015, 48, 80–86. [Google Scholar] [CrossRef]
- Christopher, S.; Centerles, M.D.; Günther, F.; Alfa, M.; David, B.; Honorati, M.; Emily, S.; Goodarz, D.; Majid, E.; Wafaie, F. Malnutrition and Its Determinants Are Associated with Suboptimal Cognitive, Communication, and Motor Development in Tanzanian Children. J. Nutr. 2015, 145, 2705–2714. [Google Scholar] [CrossRef]
- Sawaya, A.L.; Martins, P.; Hoffman, D.; Roberts, S.B. The Link between Childhood Undernutrition and Risk of Chronic Diseases in Adulthood: A Case Study of Brazil. Nutr. Rev. 2003, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lelijveld, N.; Seal, A.; Wells, J.C.; Kirkby, J.; Opondo, C.; Chimwezi, E.; Bunn, J.; Bandsma, R.; Heyderman, R.S.; Nyirenda, M.J.; et al. Chronic Disease Outcomes after Severe Acute Malnutrition in Malawian Children (ChroSAM): A Cohort Study. Lancet Glob. Health 2016, 4, e654–e662. [Google Scholar] [CrossRef] [PubMed]
- Antibiotics as Part of the Management of Severe Acute Malnutrition|NEJM. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1202851 (accessed on 8 April 2023).
- World Health Organization. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children’s Fund; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-159816-3. [Google Scholar]
- Bassat, Q.; Blau, D.M.; Ogbuanu, I.U.; Samura, S.; Kaluma, E.; Bassey, I.-A.; Sow, S.; Keita, A.M.; Tapia, M.D.; Mehta, A.; et al. Causes of Death among Infants and Children in the Child Health and Mortality Prevention Surveillance (CHAMPS) Network. JAMA Netw. Open 2023, 6, e2322494. [Google Scholar] [CrossRef]
- Kimani-Murage, E.W.; Muthuri, S.K.; Oti, S.O.; Mutua, M.K.; van de Vijver, S.; Kyobutungi, C. Evidence of a Double Burden of Malnutrition in Urban Poor Settings in Nairobi, Kenya. PLoS ONE 2015, 10, e0129943. [Google Scholar] [CrossRef] [PubMed]
- Modjadji, P.; Madiba, S. The Double Burden of Malnutrition in a Rural Health and Demographic Surveillance System Site in South Africa: A Study of Primary Schoolchildren and Their Mothers. BMC Public Health 2019, 19, 1087. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Romero, E.; Flórez-García, V.; Egede, L.E.; Yan, A. Factors Associated with the Double Burden of Malnutrition at the Household Level: A Scoping Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6961–6972. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shri, N.; Singh, A. Inequalities in the Prevalence of Double Burden of Malnutrition among Mother-Child Dyads in India. Sci. Rep. 2023, 13, 16923. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Double Burden of Malnutrition: Policy Brief. Available online: https://www.who.int/publications-detail-redirect/WHO-NMH-NHD-17.3 (accessed on 12 June 2023).
- Ministério da Saúde; Direcção Nacional de Saúde Pública; Departamento de Nutrição. Relatório Anual do Programa de Nutrição 2020; Ministério da Saúde: Maputo, Mozambique, 2020. [Google Scholar]
- Ministério da Saúde (MISAU). Relatório Anual do Programa de Nutrição 2021; MISAU: Maputo, Mozambique, 2022. [Google Scholar]
- Ministério da Saúde (MISAU). Relatório Anual de Balanço do Sector da Saúde 2021; MISAU: Maputo, Mozambique, 2022. [Google Scholar]
- Bashaka, P.J.; Sawe, H.R.; Mwafongo, V.; Mfinanga, J.A.; Runyon, M.S.; Murray, B.L. Undernourished Children Presenting to an Urban Emergency Department of a Tertiary Hospital in Tanzania: A Prospective Descriptive Study. BMC Pediatr. 2019, 19, 327. [Google Scholar] [CrossRef]
- Gavhi, F.; Kuonza, L.; Musekiwa, A.; Motaze, N.V. Factors Associated with Mortality in Children under Five Years Old Hospitalized for Severe Acute Malnutrition in Limpopo Province, South Africa, 2014–2018: A Cross-Sectional Analytic Study. PLoS ONE 2020, 15, e0232838. [Google Scholar] [CrossRef] [PubMed]
- Children and AIDS. Available online: https://www.unicef.org/tanzania/what-we-do/hiv-aids (accessed on 1 February 2023).
- Pedrini, M.; Moraleda, C.; Macete, E.; Gondo, K.; Brabin, B.J.; Menéndez, C. Clinical, Nutritional and Immunological Characteristics of HIV-Infected Children in an Area of High HIV Prevalence. J. Trop. Pediatr. 2015, 61, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Daminelli, E.N.; Tritinger, A.; Spada, C. Alterações hematológicas em pacientes infectados pelo vírus da imunodeficência humana submetidos à terapia antirretroviral com e sem inibidor de protease. Rev. Bras. Hematol. Hemoter. 2010, 32, 10–15. [Google Scholar] [CrossRef]
- Nhampossa, T.; Sigaúque, B.; Machevo, S.; Macete, E.; Alonso, P.; Bassat, Q.; Menéndez, C.; Fumadó, V. Severe Malnutrition among Children under the Age of 5 Years Admitted to a Rural District Hospital in Southern Mozambique. Public Health Nutr. 2013, 16, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, R.; Walker, D.; Weller, P. Tropical Infectious Diseases: Principles, Pathogens and Practice, 3rd ed.; Saunders: London, UK, 2011; ISBN 978-1-4377-3777-6. [Google Scholar]
- Sousa, S.; Gelormini, M.; Damasceno, A.; Lopes, S.A.; Maló, S.; Chongole, C.; Muholove, P.; Casal, S.; Pinho, O.; Moreira, P.; et al. Street Food in Maputo, Mozambique: Availability and Nutritional Value of Homemade Foods. Nutr. Health 2019, 25, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hinnig, P.D.F.; Monteiro, J.S.; de Assis, M.A.A.; Levy, R.B.; Peres, M.A.; Perazi, F.M.; Porporatti, A.L.; Canto, G.D.L. Dietary Patterns of Children and Adolescents from High, Medium and Low Human Development Countries and Associated Socioeconomic Factors: A Systematic Review. Nutrients 2018, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Wachira, L.J.; Uusi-Ranta, N.; Anono, E.L.; Walsh, H.M.; Erkkola, M.; Ochola, S.; Swindell, N.; Salmela, J.; Vepsäläinen, H.; et al. Wealth and Sedentary Time Are Associated with Dietary Patterns among Preadolescents in Nairobi City, Kenya. J. Nutr. Educ. Behav. 2023, 55, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Fewtrell, M.S. Is Body Composition Important for Paediatricians? Arch. Dis. Child. 2008, 93, 168–172. [Google Scholar] [CrossRef]
- Wells, J.C.K. Toward Body Composition Reference Data for Infants, Children, and Adolescents. Adv. Nutr. 2014, 5, 320S–329S. [Google Scholar] [CrossRef]
- Corkins, K.G.; Teague, E.E. Pediatric Nutrition Assessment. Available online: https://login.research4life.org/tacsgr1aspenjournals_onlinelibrary_wiley_com/doi/epdf/10.1177/0884533616679639 (accessed on 21 February 2023).
- The 17 Goals|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 29 November 2023).



| Characteristics of Study Population | Children (N = 449) | Caretaker (N = 449) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Age in years, median [Q1–Q3] | 1.0 [1.0–2.0] | 26 (22–33) | ||
| Sex | ||||
| Male | 242 | 53.9 | 16 | 3.6 |
| Female | 207 | 46.1 | 433 | 96.4 |
| Hospital | ||||
| HJM | 232 | 51.7 | NA | NA |
| HGM | 217 | 48.3 | NA | NA |
| Type of caretaker | ||||
| Mother | NA | NA | 413 | 92.0 |
| Father | NA | NA | 15 | 3.3 |
| Other | NA | NA | 21 | 4.7 |
| Literacy of caretaker (N = 444) | ||||
| Illiterate | NA | NA | 86 | 19.4 |
| Literate | NA | NA | 358 | 80.6 |
| Characteristics | n | % |
|---|---|---|
| Classification of undernutrition (N = 446) | ||
| Kwashiorkor | 159 | 35.7 |
| Marasmic-kwashiorkor | 102 | 22.9 |
| Marasmus | 151 | 33.9 |
| Other | 34 | 7.6 |
| Edema (N = 433) | ||
| No | 207 | 47.8 |
| Yes | 226 | 52.2 |
| Vaccination (N = 449) | ||
| Not vaccinated | 10 | 2.2 |
| Vaccinated | 439 | 97.8 |
| Vitamin A intake (N = 212) | ||
| No | 147 | 69.3 |
| Yes | 65 | 30.7 |
| Ever breastfed (N = 441) | ||
| No | 12 | 2.7 |
| Yes | 429 | 97.3 |
| Presence of infections/co-morbidities (N = 447) | ||
| No | 47 | 10.5 |
| Yes | 400 | 89.5 |
| Type of infections/co-morbidities (N = 400) | ||
| Anemia | 50 | 12.5 |
| Bronchopneumonia | 15 | 3.8 |
| Diarrhea | 80 | 20.0 |
| HIV | 120 | 30.0 |
| Malaria | 12 | 3.0 |
| Other(s) | 123 | 30.8 |
| Antiretroviral therapy (N = 119) | ||
| No | 15 | 12.6 |
| Yes | 104 | 87.4 |
| Measurement Mean (SD) | Children | Caretakers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 Years (N = 422) | 5–9 Years (N = 22) | 10–14 Years (N = 5) | Male (n = 16) | Female (n = 433) | p-Value | |||||||
| Boys (n = 227) | Girls (n = 195) | p-Value | Boys (n = 12) | Girls (n = 10) | p-Value | Boys (n = 3) | Girls (n = 2) | p-Value | ||||
| Height (N = 437 children; N = 410 caretaker) | 74.5 ± 5.5 | 74.1 ± 8.2 | 0.65 | 104.7 ± 10.6 | 108.2 ± 15.3 | 0.58 | 111.5 ± 16.6 | 139 ± 0.0 | NR | 166.4 ± 6.1 | 156.9 ± 6.4 | <0.01 |
| Weight (N = 206 children; N = 411 caretaker) | 7.6 ± 1.6 | 7.5 ± 2.3 | 0.18 | 14.0 ± 1.9 | 13.0 ± 3.0 | 0.40 | 20.4 ± 0.0 | 22.3 ± 0.0 | 0.99 | 59.6 ± 7.0 | 56.8 ± 11.5 | 0.40 |
| Presence of edema * (52.2%, 226/433, children) | 56.8 (125/220) | 48.4 (90/186) | 0.9 a | 41.7 (5/12) | 30.0 (3/10) | 0.68 b | 66.7 (2/3) | 50.0 (1/2) | 1.00b | NA | NA | NA |
| MUAC (N = 445 children; N = 408 caretaker) | 11.9 ± 1.6 | 12.0 ± 1.5 | 0.71 | 12.5 ± 1.3 | 11.9 ± 1.0 | 0.24 | 12.8 ± 1.2 | 13.0 ± 2.3 | 0.94 | 28.4 ± 3.6 | 26.9 ± 3.9 | 0.21 |
| WC (N = 443 children; N = 405 caretaker) | 39.8 ± 4.2 | 39.3 ± 4.6 | 0.29 | 48.3 ± 5.5 | 44.4 ± 4.3 | 0.08 | 47.2 ± 10.6 | 45.1 ± 8.6 | 0.83 | 74.3 ± 7.3 | 75.8 ± 11.2 | 0.65 |
| Skinfolds | ||||||||||||
| Triceps (N = 437 children; N = 407 caretaker) | 6.1 ± 2.1 | 6.4 ± 2.2 | 0.17 | 4.2 ± 1.7 | 4.0 ± 1.5 | 0.80 | 5.8 ± 0.1 | 4.3 ± 3.5 | 0.46 | 7.5 ± 4.8 | 17.8 ± 7.2 | <0.01 |
| Biceps (N = 436 children; N = 406 caretaker) | 5.6 ± 1.9 | 5.7 ± 2.0 | 0.33 | 3.5 ± 1.4 | 3.4 ± 0.9 | 0.75 | 4.3 ± 1.0 | 3.9 ± 3.2 | 0.81 | 6.1 ± 3.1 | 3.9 ± 6.1 | 0.04 |
| Subscapular (N = 429 children; N = 404 caretaker) | 4.1 ± 1.4 | 4.6 ± 2.0 | 0.00 | 3.2 ± 2.0 | 2.7 ± 0.7 | 0.52 | 3.0 ± 0.2 | 2.8 ± 0.3 | 0.41 | 7.6 ± 1.5 | 13.3 ± 7.3 | 0.01 |
| Suprailiac (N = 412 children; N = 230 caretaker) | 5.5 ± 2.5 | 5.9 ± 2.7 | 0.11 | 3.2 ± 1.0 | 3.2 ± 1.6 | 0.93 | 3.8 ± 0.4 | 3.4 ± 2.0 | 0.74 | 5.3 ± 2.5 | 7.6 ± 3.4 | 0.02 |
| Nutritional Status | Children | Caretakers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 Years (N = 422) | 5–9 Years (N = 22) | 10–14 Years (N = 5) | Male (n = 16) | Female (n = 433) | p-Value | |||||||
| Boys (n = 227) | Girls (n = 195) | p-Value | Boys (n = 12) | Girls (n = 10) | p-Value | Boys (n = 3) | Girls (n = 2) | p-Value | ||||
| BMI-for-age (BAZ) [Thinness—51.3% (106/207)] | ||||||||||||
| % (n) Normal (BAZ ≥ −2SD) | 44.7 (42/94) | 57.0 (57/100) | 0.23 | 14.3 (1/7) | 20.0 (1/5) | 1.00 | 0.0 (0/1) | 0.0 (0/0) | NR | NA | NA | NA |
| % (n) Thinness (−3SD ≤ BAZ < −2SD) | 25.3 (24/94) | 19.0 (19/100) | 0.0 (0/7) | 0.0 (0/5) | 0.0 (0/1) | 0.0 (0/0) | NA | NA | NA | |||
| % (n) Severe thinness (BAZ < −3SD) | 29.8 (28/94) | 24.0 (24/100) | 85.7 (6/7) | 80.0 (4/5) | 100 (1/1) | 0.0 (0/0) | NA | NA | NA | |||
| BMI [Malnutrition—33.0% (135/409)] | ||||||||||||
| % (n) Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | 91.7 (11/12) | 66.3 (263/397) | 0.26 |
| % (n) Underweight | NA | NA | NA | NA | NA | NA | NA | NA | NA | 8.3 (1/12) | 9.6 (38/397) | |
| % (n) Overweight | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.0 (0/12) | 10.1 (40/397) | |
| % (n) Obese | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.0 (0/12) | 14.1 (56/397) | |
| Wasting [Wasted—80.0% (336/420)] | ||||||||||||
| % (n) Normal (≥−2SD) | 16.4 (36/219) | 26.7 (50/187) | 0.03 | 0.0 (0/7) | 0.0 (0/5) | 1.00 | 0.0 (0/2) | 0.0 (0/2) | NR | NA | NA | NA |
| % (n) Wasted (−3DS ≤ WHZ < −2SD) | 9.1 (20/219) | 10.2 (19/187) | 14.3 (1/7) | 20.0 (1/5) | 0.0 (0/2) | 0.0 (0/2) | NA | NA | NA | |||
| % (n) Severe wasted (WHZ < −3SD) | 74.3 (163/219) | 63.1 (118/187) | 85.7 (6/7) | 80.0 (4/5) | 100 (2/2) | 100 (2/2) | NA | NA | NA | |||
| Stunting [Stunted—47.6% (195/410)] | ||||||||||||
| % (n) Normal (≥−2SD) | 51.2 (111/217) | 55.4 (102/184) | 0.66 | 63.6 (7/11) | 66.7 (4/6) | 0.79 | 50.0 (1/2) | 100 (1/1) | 1.00 | NA | NA | NA |
| % (n) Stunted (−3DS ≤ HAZ < −2SD) | 19.8 (43/217) | 19.0 (35/184) | 18.2 (2/11) | 0.0 (0/6) | 50.0 (1/2) | 0.0 (0/1) | NA | NA | NA | |||
| % (n) Severe stunted (HAZ < −3SD) | 29.0 (63/217) | 25.5 (47/184) | 18.2 (2/11) | 33.3 (2/6) | 0.0 (0/2) | 0.0 (0/1) | NA | NA | NA | |||
| % (n) More than one condition (BAZ, WHZ, HAZ) [93.7% (369/394)] | 96.0 (192/200) | 91.7 (154/168) | 0.20 | 100 (11/11) | 80.0 (8/10) | 0.49 | 100 (3/3) | 50.0 (1/2) | 0.60 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossa-Moiane, I.; Roucher, C.; Campos-Ponce, M.; Doak, C.; Bauhofer, A.; Chissaque, A.; Prista, A.; de Deus, N.; Polman, K. Profile of Children with Undernutrition Admitted in Two Secondary-Level Hospitals in Maputo City, Mozambique. Nutrients 2024, 16, 1056. https://doi.org/10.3390/nu16071056
Cossa-Moiane I, Roucher C, Campos-Ponce M, Doak C, Bauhofer A, Chissaque A, Prista A, de Deus N, Polman K. Profile of Children with Undernutrition Admitted in Two Secondary-Level Hospitals in Maputo City, Mozambique. Nutrients. 2024; 16(7):1056. https://doi.org/10.3390/nu16071056
Chicago/Turabian StyleCossa-Moiane, Idalécia, Clémentine Roucher, Maiza Campos-Ponce, Colleen Doak, Adilson Bauhofer, Assucênio Chissaque, António Prista, Nilsa de Deus, and Katja Polman. 2024. "Profile of Children with Undernutrition Admitted in Two Secondary-Level Hospitals in Maputo City, Mozambique" Nutrients 16, no. 7: 1056. https://doi.org/10.3390/nu16071056
APA StyleCossa-Moiane, I., Roucher, C., Campos-Ponce, M., Doak, C., Bauhofer, A., Chissaque, A., Prista, A., de Deus, N., & Polman, K. (2024). Profile of Children with Undernutrition Admitted in Two Secondary-Level Hospitals in Maputo City, Mozambique. Nutrients, 16(7), 1056. https://doi.org/10.3390/nu16071056