Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets
Abstract
:1. Introduction
2. Bariatric Surgery and Changes in Gut Microbiota
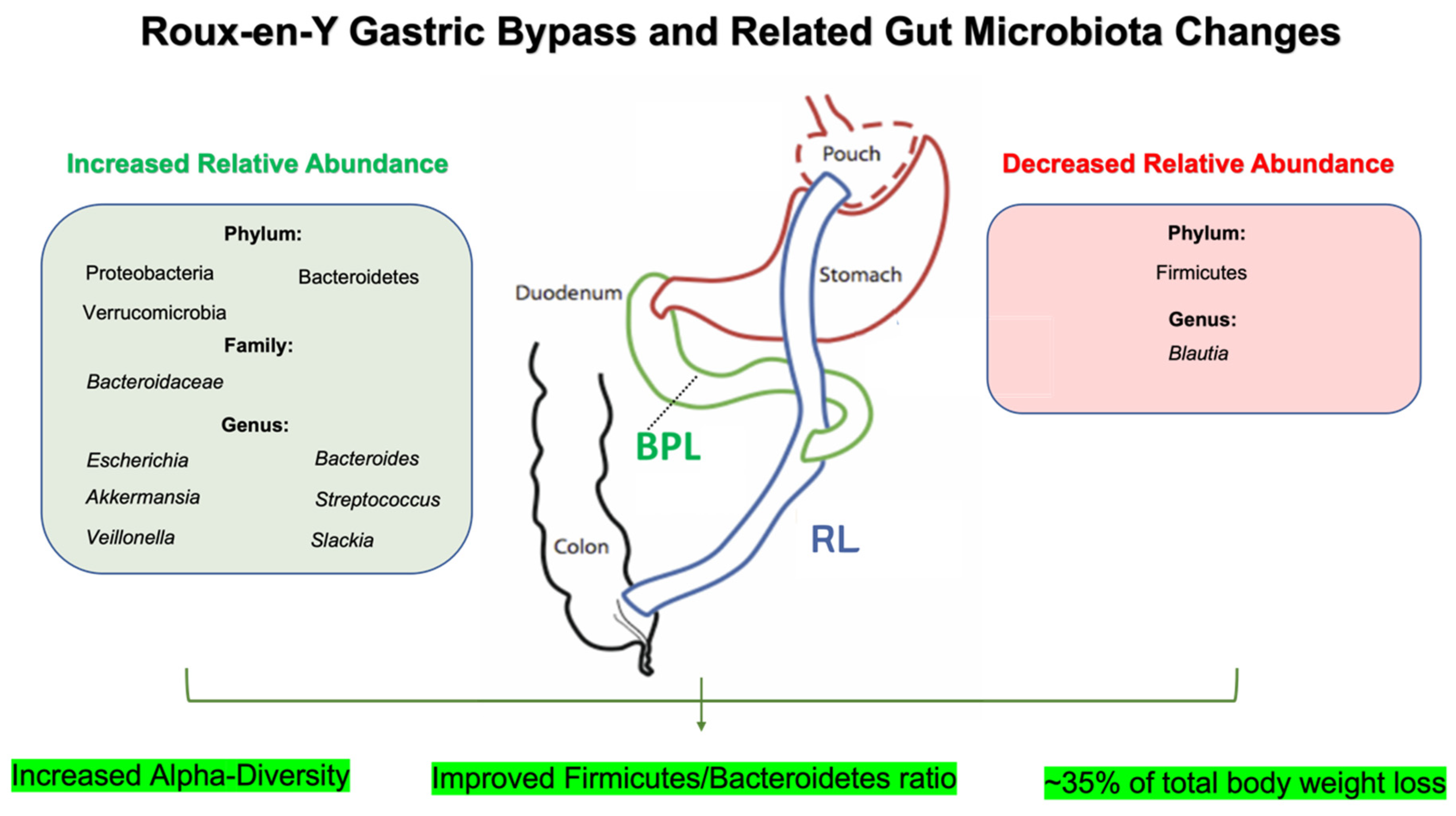
2.1. Roux-en-Y Gastric Bypass (RYGB) and Gut Microbiota
2.2. Sleeve Gastrectomy (SG) and Gut Microbiota
3. Bariatric Surgery, the Microbiota–Gut–Brain Axis, and Peripheral Targets
3.1. Bariatric Surgery, Gut Microbiota, and Vagal Contribution
3.2. Bariatric Surgery, Gut Microbiota, Cytokines, and Inflammation
3.3. Bariatric Surgery, Gut Microbiota, Nutrient-Sensing Receptors, and SCFA
3.4. Bariatric Surgery, Gut Microbiota, and Bile Acids
4. Bariatric Surgery, Gut Microbiota, and Regulatory Pathways Controlling Food Intake
4.1. Bariatric Surgery, Gut Microbiota, and Effects on Ghrelin and Leptin
4.2. Bariatric Surgery, CCK, and Gut Microbiota
4.3. Bariatric Surgery, Gut Microbiota, GLP-1, and GLP-2
4.4. Bariatric Surgery, Gut Microbiota, and PYY
4.5. Bariatric Surgery, Gut Microbiota, and Effects on Orexigenic/Anorexigenic Neuropeptides
5. Bariatric Surgery, Gut Microbiota, Dopamine, and Reward Pathways
5.1. Bariatric Surgery, Gut Microbiota, Mesolimbic Pathway, and Striatal Dopamine
5.2. Bariatric Surgery, Gut Microbiota, Dopamine, and Gut Hormones
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Busebee, B.; Ghusn, W.; Cifuentes, L.; Acosta, A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin. Proc. 2023, 98, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Public Health 2020, 53, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Alfaris, N.; Alqahtani, A.M.; Alamuddin, N.; Rigas, G. Global Impact of Obesity. Gastroenterol. Clin. N. Am. 2023, 52, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Overduin, J.; Foster-Schubert, K.E. Gastric bypass for obesity: Mechanisms of weight loss and diabetes resolution. J. Clin. Endocrinol. Metab. 2004, 89, 2608–2615. [Google Scholar] [CrossRef]
- Mulla, C.M.; Middelbeek, R.J.W.; Patti, M.E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 2018, 1411, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson’s Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar] [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.M., Jr.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra141. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Iatcu, O.C.; Covasa, M. Nutrition at the Intersection between Gut Microbiota Eubiosis and Effective Management of Type 2 Diabetes. Nutrients 2024, 16, 269. [Google Scholar] [CrossRef]
- Chae, Y.R.; Lee, Y.R.; Kim, Y.S.; Park, H.Y. Diet-induced gut dysbiosis and leaky gut syndrome. J. Microbiol. Biotechnol. 2024, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Baek, G.H.; Yoo, K.M.; Kim, S.Y.; Lee, D.H.; Chung, H.; Jung, S.C.; Park, S.K.; Kim, J.S. Collagen Peptide Exerts an Anti-Obesity Effect by Influencing the Firmicutes/Bacteroidetes Ratio in the Gut. Nutrients 2023, 15, 2610. [Google Scholar] [CrossRef]
- Özdemir, A.; Yozgat, A.; Işgın-Atıcı, K.; Avcı, E.; Yıldız, B.D.; Gündoğdu, A.; Nalbantoğlu, U.; Turhan, T.; Doğruman-Al, F.; Büyüktuncer, Z. Potential associations between alterations in gut microbiome and obesity-related traits after the bariatric surgery. J. Hum. Nutr. Diet. 2023, 36, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Garrido-Sánchez, L.; Alcaide-Torres, J.; Cornejo-Pareja, I.; Ocaña-Wilhelmi, L.; García-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Predictive Role of Gut Microbiota in Weight Loss Achievement after Bariatric Surgery. J. Am. Coll. Surg. 2022, 234, 861–871. [Google Scholar] [CrossRef]
- Frick, L.D.; Hankir, M.K.; Borner, T.; Malagola, E.; File, B.; Gero, D. Novel Insights into the Physiology of Nutrient Sensing and Gut-Brain Communication in Surgical and Experimental Obesity Therapy. Obes. Surg. 2023, 33, 2906–2916. [Google Scholar] [CrossRef]
- Hamamah, S.; Amin, A.; Al-Kassir, A.L.; Chuang, J.; Covasa, M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients 2023, 15, 3365. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Pizarroso, N.A.; Fuciños, P.; Gonçalves, C.; Pastrana, L.; Amado, I.R. A Review on the Role of Food-Derived Bioactive Molecules and the Microbiota-Gut-Brain Axis in Satiety Regulation. Nutrients 2021, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Posovszky, C.; Wabitsch, M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: Characteristics of enteroendocrine cells and their capability of weight regulation. Horm. Res. Paediatr. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Clapp, B.; Ponce, J.; DeMaria, E.; Ghanem, O.; Hutter, M.; Kothari, S.; LaMasters, T.; Kurian, M.; English, W. American Society for Metabolic and Bariatric Surgery 2020 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2022, 18, 1134–1140. [Google Scholar] [CrossRef]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Palma, R.; Kow, L.; Prager, G.; Ramos, A.; Shikora, S. IFSO Worldwide Survey 2020–2021: Current Trends for Bariatric and Metabolic Procedures. Obes. Surg. 2024. [Google Scholar] [CrossRef]
- Stefura, T.; Zapała, B.; Gosiewski, T.; Skomarovska, O.; Pędziwiatr, M.; Major, P. Changes in the Composition of Oral and Intestinal Microbiota After Sleeve Gastrectomy and Roux-En-Y Gastric Bypass and Their Impact on Outcomes of Bariatric Surgery. Obes. Surg. 2022, 32, 1439–1450. [Google Scholar] [CrossRef]
- Farin, W.; Oñate, F.P.; Plassais, J.; Bonny, C.; Beglinger, C.; Woelnerhanssen, B.; Nocca, D.; Magoules, F.; Le Chatelier, E.; Pons, N.; et al. Impact of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy on gut microbiota: A metagenomic comparative analysis. Surg. Obes. Relat. Dis. 2020, 16, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef]
- Ulker, İ.; Yildiran, H. The effects of bariatric surgery on gut microbiota in patients with obesity: A review of the literature. Biosci. Microbiota Food Health 2019, 38, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.G.; Gupta, N. Roux-en-Y Gastric Bypass. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Eghbali, F.; Bahardoust, M.; Pazouki, A.; Barahman, G.; Tizmaghz, A.; Hajmohammadi, A.; Karami, R.; Hosseini-Baharanchi, F.S. Predictors for weight loss after Roux-en-Y gastric bypass: The trend and associated factors for weight loss. BMC Surg. 2022, 22, 310. [Google Scholar] [CrossRef] [PubMed]
- Lager, C.J.; Esfandiari, N.H.; Subauste, A.R.; Kraftson, A.T.; Brown, M.B.; Cassidy, R.B.; Nay, C.K.; Lockwood, A.L.; Varban, O.A.; Oral, E.A. Roux-En-Y Gastric Bypass Vs. Sleeve Gastrectomy: Balancing the Risks of Surgery with the Benefits of Weight Loss. Obes. Surg. 2017, 27, 154–161. [Google Scholar] [CrossRef]
- Salminen, P.; Grönroos, S.; Helmiö, M.; Hurme, S.; Juuti, A.; Juusela, R.; Peromaa-Haavisto, P.; Leivonen, M.; Nuutila, P.; Ovaska, J. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients with Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2022, 157, 656–666. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Shang, X.Y.; Chen, Y.L.; Zhao, S.S.; Jin, S.; Yang, J.; Liu, H.X.; Du, J. Roux-en-Y gastric bypass-induced perturbative changes in microbial communities and metabolic pathways in rats. Front. Microbiol. 2022, 13, 1034839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, R.; Xu, B.; Hua, R.; Shen, Q.; He, K.; Yao, Q. Alterations of Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes. Surg. 2017, 27, 295–302. [Google Scholar] [CrossRef]
- Kong, L.C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.L.; Zucker, J.D.; Doré, J.; Clément, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef]
- Faria, S.L.; Santos, A.; Magro, D.O.; Cazzo, E.; Assalin, H.B.; Guadagnini, D.; Vieira, F.T.; Dutra, E.S.; Saad, M.J.A.; Ito, M.K. Gut Microbiota Modifications and Weight Regain in Morbidly Obese Women After Roux-en-Y Gastric Bypass. Obes. Surg. 2020, 30, 4958–4966. [Google Scholar] [CrossRef]
- Chiantera, V.; Laganà, A.S.; Basciani, S.; Nordio, M.; Bizzarri, M. A Critical Perspective on the Supplementation of Akkermansia muciniphila: Benefits and Harms. Life 2023, 13, 1247. [Google Scholar] [CrossRef]
- Fouladi, F.; Carroll, I.M.; Sharpton, T.J.; Bulik-Sullivan, E.; Heinberg, L.; Steffen, K.J.; Fodor, A.A. A microbial signature following bariatric surgery is robustly consistent across multiple cohorts. Gut Microbes 2021, 13, 1930872. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; DiBaise, J.K.; Isern, N.G.; Hoyt, D.W.; Marcus, A.K.; Kang, D.W.; Crowell, M.D.; Rittmann, B.E.; Krajmalnik-Brown, R. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017, 11, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Ling, L.; Dinh, D.M.; DePaoli, A.M.; Lieu, H.D.; Harrison, S.A.; Sanyal, A.J. The Commensal Microbe Veillonella as a Marker for Response to an FGF19 Analog in NASH. Hepatology 2021, 73, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.V.; Ashrafian, H.; Sarafian, M.; Homola, D.; Rushton, L.; Barker, G.; Cabrera, P.M.; Lewis, M.R.; Darzi, A.; Lin, E.; et al. Roux-en-Y gastric bypass-induced bacterial perturbation contributes to altered host-bacterial co-metabolic phenotype. Microbiome 2021, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.Y.; Thompson, R.; Overby, D.W.; Duke, M.C.; Farrell, T.M. Sleeve Gastrectomy: Surgical Tips. J. Laparoendosc. Adv. Surg. Technol. A 2018, 28, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, C.; Yan, H.; Xia, M.; Zhu, X.; Sun, X.; Yang, X.; Jiao, H.; Wu, H.; Lou, W.; et al. Acute Effects of Sleeve Gastrectomy on Glucose Variability, Glucose Metabolism, and Ghrelin Response. Obes. Surg. 2021, 31, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, M.; Eybpoosh, S.; Siadat, S.D.; Elyasinia, F.; Soroush, A.; Bouzari, S. Modulation of the Gut Microbiota and Serum Biomarkers After Laparoscopic Sleeve Gastrectomy: A 1-Year Follow-Up Study. Obes. Surg. 2021, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Karami, R.; Kermansaravi, M.; Pishgahroudsari, M.; Talebi, M.; Mohammadzadeh, N.; Pazouki, A. Changes in gut microbial flora after Roux-en-Y gastric bypass and sleeve gastrectomy and their effects on post-operative weight loss. Updates Surg. 2021, 73, 1493–1499. [Google Scholar] [CrossRef]
- Shen, W.D.; Lin, X.; Liu, H.M.; Li, B.Y.; Qiu, X.; Lv, W.Q.; Zhu, X.Z.; Greenbaum, J.; Liu, R.K.; Shen, J.; et al. Gut microbiota accelerates obesity in peri-/post-menopausal women via Bacteroides fragilis and acetic acid. Int. J. Obes. 2022, 46, 1918–1924. [Google Scholar] [CrossRef]
- Shi, G.; Lin, Y.; Wu, Y.; Zhou, J.; Cao, L.; Chen, J.; Li, Y.; Tan, N.; Zhong, S. Bacteroides fragilis Supplementation Deteriorated Metabolic Dysfunction, Inflammation, and Aorta Atherosclerosis by Inducing Gut Microbiota Dysbiosis in Animal Model. Nutrients 2022, 14, 2199. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Zhao, X.; Zhang, X.; Wu, Q.; Wang, Z.; Lu, J. Changes in gut microbiota, metabolite SCFAs, and GPR43 expression in obese diabetic mice after sleeve gastrectomy. J. Appl. Microbiol. 2022, 133, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, Y.; Wang, Q.; Sun, Y.; Lin, H.; Ni, M.; Chen, Y.; Zhang, L.; Jin, J.; Ying, X.; et al. Fecal short chain fatty acids modify therapeutic effects of sleeve gastrectomy. Front. Endocrinol. 2023, 14, 1277035. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, A.; Ohta, M.; Endo, Y.; Nakanuma, H.; Kawamura, M.; Hirashita, Y.; Kawasaki, T.; Masuda, T.; Hirashita, T.; Gotoh, K.; et al. Changes of Short-Chain Fatty Acids and Their Receptors in an Obese Rat Model After Sleeve Gastrectomy. Obes. Surg. 2022, 32, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.W.; Li, Y.; Cheng, Y.G.; Liu, Q.R.; Hu, S.Y.; Zhang, G.Y. Effect of oligofructose on resistance to postoperative high-fat diet-induced damage of metabolism in diabetic rats after sleeve gastrectomy. World J. Diabetes 2021, 12, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pan, Y.; Shao, W.; Wang, C.; Wang, R.; He, Y.; Zhang, M.; Wang, Y.; Li, T.; Wang, Z.; et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome 2022, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Kenny, B.J.; Bordoni, B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). In StatPearls; StatPearls Publishing Copyright © 2024; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hao, Z.; Townsend, R.L.; Mumphrey, M.B.; Patterson, L.M.; Ye, J.; Berthoud, H.R. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes. Surg. 2014, 24, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Ballsmider, L.A.; Vaughn, A.C.; David, M.; Hajnal, A.; Di Lorenzo, P.M.; Czaja, K. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015, 2015, 601985. [Google Scholar] [CrossRef] [PubMed]
- Covasa, M.; Stephens, R.W.; Toderean, R.; Cobuz, C. Intestinal Sensing by Gut Microbiota: Targeting Gut Peptides. Front. Endocrinol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burgos, A.; Wang, B.; Mao, Y.K.; Mistry, B.; McVey Neufeld, K.A.; Bienenstock, J.; Kunze, W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G211–G220. [Google Scholar] [CrossRef]
- Hosoi, T.; Okuma, Y.; Matsuda, T.; Nomura, Y. Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Auton. Neurosci. 2005, 120, 104–107. [Google Scholar] [CrossRef]
- de La Serre, C.B.; de Lartigue, G.; Raybould, H.E. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol. Behav. 2015, 139, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Siopi, E.; Galerne, M.; Rivagorda, M.; Saha, S.; Moigneu, C.; Moriceau, S.; Bigot, M.; Oury, F.; Lledo, P.M. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol. Psychiatry 2023, 28, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 2021, 29, 179–196.e179. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhang, X.; Liu, Z.; Zheng, R.; Zhang, L.; Yu, D.; Shen, X. The Genus Parabacteroides Is a Potential Contributor to the Beneficial Effects of Truncal Vagotomy-Related Bariatric Surgery. Obes. Surg. 2022, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, P.B.; Ren, Z.Q.; Zhou, F.; Hu, H.H.; Zhang, H.; Xue, K.K.; Xu, P.; Shao, X.Q. Changes of serum lipopolysaccharide, inflammatory factors, and cecal microbiota in obese rats with type 2 diabetes induced by Roux-en-Y gastric bypass. Nutrition 2019, 67–68, 110565. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fu, X. Dendrobium officinale Polysaccharide Alleviates Type 2 Diabetes Mellitus by Restoring Gut Microbiota and Repairing Intestinal Barrier via the LPS/TLR4/TRIF/NF-kB Axis. J. Agric. Food Chem. 2023, 71, 11929–11940. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Q.; Zhong, H.; Li, D.; Yu, S.; Yang, H.; Wang, C.; Yin, Z. Roux-en-Y Gastric Bypass Improved Insulin Resistance via Alteration of the Human Gut Microbiome and Alleviation of Endotoxemia. BioMed Res. Int. 2021, 2021, 5554991. [Google Scholar] [CrossRef]
- Wu, D.; Yan, Z.B.; Cheng, Y.G.; Zhong, M.W.; Liu, S.Z.; Zhang, G.Y.; Hu, S.Y. Deactivation of the NLRP3 inflammasome in infiltrating macrophages by duodenal-jejunal bypass surgery mediates improvement of beta cell function in type 2 diabetes. Metabolism 2018, 81, 1–12. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, J.; Cheng, Y.; Jia, B.; He, Y.; Yu, L.; Yu, G.; Wang, Y. Changes in gut microbiota and cytokines following laparoscopic sleeve gastrectomy are associated with cognitive function improvement. Heliyon 2023, 9, e19245. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Ojima, T.; Hayata, K.; Katsuda, M.; Kitadani, J.; Takeuchi, A.; Goda, T.; Ueda, Y.; Iwakura, H.; Nishi, M.; et al. Laparoscopic sleeve gastrectomy for morbid obesity improves gut microbiota balance, increases colonic mucosal-associated invariant T cells and decreases circulating regulatory T cells. Surg. Endosc. 2022, 36, 7312–7324. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Zhang, X.W. MAIT Cell Activation and Functions. Front. Immunol. 2020, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Haase, N.; Haange, S.B.; Sucher, R.; Münzker, J.; Jäger, E.; Schischke, K.; Seyfried, F.; von Bergen, M.; Hankir, M.K.; et al. Roux-en-Y gastric bypass contributes to weight loss-independent improvement in hypothalamic inflammation and leptin sensitivity through gut-microglia-neuron-crosstalk. Mol. Metab. 2021, 48, 101214. [Google Scholar] [CrossRef] [PubMed]
- Sewaybricker, L.E.; Huang, A.; Chandrasekaran, S.; Melhorn, S.J.; Schur, E.A. The Significance of Hypothalamic Inflammation and Gliosis for the Pathogenesis of Obesity in Humans. Endocr. Rev. 2023, 44, 281–296. [Google Scholar] [CrossRef] [PubMed]
- de Git, K.C.; Adan, R.A. Leptin resistance in diet-induced obesity: The role of hypothalamic inflammation. Obes. Rev. 2015, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Ruck, L.; Wiegand, S.; Kühnen, P. Relevance and consequence of chronic inflammation for obesity development. Mol. Cell. Pediatr. 2023, 10, 16. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Lee, C.H.; Suk, K.; Yu, R.; Kim, M.S. Cellular Contributors to Hypothalamic Inflammation in Obesity. Mol. Cells 2020, 43, 431–437. [Google Scholar] [PubMed]
- Boutagouga Boudjadja, M.; Culotta, I.; De Paula, G.C.; Harno, E.; Hunter, J.; Cavalcanti-de-Albuquerque, J.P.; Luckman, S.M.; Hepworth, M.; White, A.; Aviello, G.; et al. Hypothalamic AgRP neurons exert top-down control on systemic TNF-α release during endotoxemia. Curr. Biol. 2022, 32, 4699–4706.e4694. [Google Scholar] [CrossRef] [PubMed]
- Herrick, M.K.; Favela, K.M.; Simerly, R.B.; Abumrad, N.N.; Bingham, N.C. Attenuation of diet-induced hypothalamic inflammation following bariatric surgery in female mice. Mol. Med. 2018, 24, 56. [Google Scholar] [CrossRef]
- Puertollano, E.; Kolida, S.; Yaqoob, P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Ecklu-Mensah, G.; Choo-Kang, C.; Maseng, M.G.; Donato, S.; Bovet, P.; Viswanathan, B.; Bedu-Addo, K.; Plange-Rhule, J.; Oti Boateng, P.; Forrester, T.E.; et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: The METS-microbiome study. Nat. Commun. 2023, 14, 5160. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.G.; Mezzaroma, E.; Toldo, S.; Melendez, G.C.; Franco, R.L.; Lesnefsky, E.J.; Abbate, A.; Hundley, W.G.; Salloum, F.N. NLRP3-mediated inflammation in cardio-oncology: Sterile yet harmful. Transl. Res. 2023, 252, 9–20. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation - Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Valeur, J. Changes in Faecal Short-Chain Fatty Acids after Weight-Loss Interventions in Subjects with Morbid Obesity. Nutrients 2020, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, M.A.; Balaguer-Román, A.; Fernández-Ruiz, V.E.; Almansa-Saura, S.; García-Zafra, V.; Ferrer-Gómez, M.; Frutos, M.D.; Queipo-Ortuño, M.I.; Ruiz-Alcaraz, A.J.; Núñez-Sánchez, M.; et al. Plasma short-chain fatty acid changes after bariatric surgery in patients with severe obesity. Surg. Obes. Relat. Dis. 2023, 19, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Sowah, S.A.; Riedl, L.; Damms-Machado, A.; Johnson, T.S.; Schübel, R.; Graf, M.; Kartal, E.; Zeller, G.; Schwingshackl, L.; Stangl, G.I.; et al. Effects of Weight-Loss Interventions on Short-Chain Fatty Acid Concentrations in Blood and Feces of Adults: A Systematic Review. Adv. Nutr. 2019, 10, 673–684. [Google Scholar] [CrossRef]
- Meijer, J.L.; Roderka, M.N.; Chinburg, E.L.; Renier, T.J.; McClure, A.C.; Rothstein, R.I.; Barry, E.L.; Billmeier, S.; Gilbert-Diamond, D. Alterations in Fecal Short-Chain Fatty Acids after Bariatric Surgery: Relationship with Dietary Intake and Weight Loss. Nutrients 2022, 14, 4243. [Google Scholar] [CrossRef]
- Mukorako, P.; Lemoine, N.; Biertho, L.; Lebel, S.; Roy, M.C.; Plamondon, J.; Tchernof, A.; Varin, T.V.; Anhê, F.F.; St-Pierre, D.H.; et al. Consistent gut bacterial and short-chain fatty acid signatures in hypoabsorptive bariatric surgeries correlate with metabolic benefits in rats. Int. J. Obes. 2022, 46, 297–306. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Banan, B.; Ajouz, H.; Abumrad, N.N.; Flynn, C.R. Bile acids and bariatric surgery. Mol. Aspects Med. 2017, 56, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, Y.; Jin, L.; Huang, W. Bile acids, gut microbiota and metabolic surgery. Front. Endocrinol. 2022, 13, 929530. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Qian, W.; Li, M.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis. Biomedicines 2023, 11, 1048. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.K.; Tremaroli, V.; Clemmensen, C.; Kovatcheva-Datchary, P.; Myronovych, A.; Karns, R.; Wilson-Pérez, H.E.; Sandoval, D.A.; Kohli, R.; Bäckhed, F.; et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014, 509, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Sousa, K.M.; Jin, L.; Dong, B.; Kim, B.W.; Ramirez, R.; Xiao, Z.; Gu, Y.; Yang, Q.; Wang, J.; et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology 2016, 64, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.; Macias, R.I.; Briz, O.; Banales, J.M.; Monte, M.J. Bile Acids in Physiology, Pathology and Pharmacology. Curr. Drug Metab. 2015, 17, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, H.; Shen, C.; Zhang, M.; Wang, X.; Yuan, M.; Yuan, M.; Jia, S.; Cao, Z.; Wu, C.; et al. Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes 2023, 15, 2274124. [Google Scholar] [CrossRef]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.A.; Smith, N.K.; Erreger, K.; Ghose, D.; Saunders, C.; Foster, D.J.; Turner, B.; Poe, A.; Albaugh, V.L.; McGuinness, O.; et al. Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLoS Biol. 2018, 16, e2006682. [Google Scholar] [CrossRef]
- Jahansouz, C.; Xu, H.; Hertzel, A.V.; Serrot, F.J.; Kvalheim, N.; Cole, A.; Abraham, A.; Luthra, G.; Ewing, K.; Leslie, D.B.; et al. Bile Acids Increase Independently From Hypocaloric Restriction After Bariatric Surgery. Ann. Surg. 2016, 264, 1022–1028. [Google Scholar] [CrossRef]
- Ocaña-Wilhelmi, L.; Martín-Núñez, G.M.; Ruiz-Limón, P.; Alcaide, J.; García-Fuentes, E.; Gutiérrez-Repiso, C.; Tinahones, F.J.; Moreno-Indias, I. Gut Microbiota Metabolism of Bile Acids Could Contribute to the Bariatric Surgery Improvements in Extreme Obesity. Metabolites 2021, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Aida, M.; Yoshida, Y.; Matsumoto, S.; Tanaka, M.; Nakayama, J.; Nagao, Y.; Nakata, R.; Oki, E.; Akahoshi, T.; et al. Alteration in faecal bile acids, gut microbial composition and diversity after laparoscopic sleeve gastrectomy. Br. J. Surg. 2020, 107, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Wang, Q.; Billington, C.; Connett, J.; Ahmed, L.; Inabnet, W.; Chua, S.; Ikramuddin, S.; Korner, J. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes. Surg. 2016, 26, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Zhou, Z. Changes in fasting bile acid profiles after Roux-en-Y gastric bypass and sleeve gastrectomy. Medicine 2021, 100, e23939. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Z.; Wang, Y.; Dai, Y.; Zhang, X.; Hu, S. Role of Bile Acids in Bariatric Surgery. Front. Physiol. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.T.; Faria, S.; Dutra, E.S.; Ito, M.K.; Reis, C.E.G.; da Costa, T.H.M.; de Carvalho, K.M.B. Perception of Hunger/Satiety and Nutrient Intake in Women Who Regain Weight in the Postoperative Period After Bariatric Surgery. Obes. Surg. 2019, 29, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.; Mercer, J.G. Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake. Curr. Obes. Rep. 2016, 5, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Suárez-Zamorano, N.; Fabbiano, S.; Chevalier, C.; Stojanović, O.; Colin, D.J.; Stevanović, A.; Veyrat-Durebex, C.; Tarallo, V.; Rigo, D.; Germain, S.; et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med. 2015, 21, 1497–1501. [Google Scholar] [CrossRef]
- Hamamah, S.; Covasa, M. Gut Microbiota Restores Central Neuropeptide Deficits in Germ-Free Mice. Int. J. Mol. Sci. 2022, 23, 11756. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Guan, M.; Mayer, E.A.; Stains, J.; Liu, C.; Vora, P.; Jacobs, J.P.; Lagishetty, V.; Chang, L.; Barry, R.L.; et al. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain’s reward center. Gut Microbes 2022, 14, 2051999. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Chen, H.; Zhou, J.J.; Pradhan, G.; Sun, Y.; Pan, H.L.; Li, D.P. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J. Neurochem. 2017, 142, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Dardeno, T.A.; Chou, S.H.; Moon, H.S.; Chamberland, J.P.; Fiorenza, C.G.; Mantzoros, C.S. Leptin in human physiology and therapeutics. Front. Neuroendocrinol. 2010, 31, 377–393. [Google Scholar] [CrossRef]
- Schéle, E.; Grahnemo, L.; Anesten, F.; Hallén, A.; Bäckhed, F.; Jansson, J.O. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 2013, 154, 3643–3651. [Google Scholar] [CrossRef]
- Yao, H.; Fan, C.; Fan, X.; Lu, Y.; Wang, Y.; Wang, R.; Tang, T.; Qi, K. Effects of gut microbiota on leptin expression and body weight are lessened by high-fat diet in mice. Br. J. Nutr. 2020, 124, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Woelnerhanssen, B.; Peterli, R.; Steinert, R.E.; Peters, T.; Borbély, Y.; Beglinger, C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: Comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg. Obes. Relat. Dis. 2011, 7, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Reinehr, T.; Schernthaner, G.H.; Kopp, H.P.; Kriwanek, S.; Schernthaner, G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes. Surg. 2009, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, P.; Paluszkiewicz, R.; Wróblewski, T.; Remiszewski, P.; Grodzicki, M.; Bartoszewicz, Z.; Krawczyk, M. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg. Obes. Relat. Dis. 2017, 13, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Y.; Kumral, Z.N.; Memi, G.; Çevik, Ö.D.; Yeğen, C.; Yeğen, B. Serum Leptin, Obestatin, and Ghrelin Levels and Gastric Emptying Rates of Liquid and Solid Meals in Non-obese Rats with Roux-en-Y Bypass Surgery or Prosthesis Placement: Implications for the Role of Vagal Afferents. Obes. Surg. 2017, 27, 1037–1046. [Google Scholar] [CrossRef]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Rullmann, M.; Seyfried, F.; Preusser, S.; Poppitz, S.; Heba, S.; Gousias, K.; Hoyer, J.; Schütz, T.; Dietrich, A.; et al. Roux-en-Y gastric bypass surgery progressively alters radiologic measures of hypothalamic inflammation in obese patients. JCI Insight 2019, 4, e131329. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Slomiany, A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: Modulatory effect of ghrelin. Inflammopharmacology 2017, 25, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Slade, E.; Williams, L.; Gagnon, J. Hydrogen sulfide suppresses ghrelin secretion in vitro and delays postprandial ghrelin secretion while reducing appetite in mice. Physiol. Rep. 2018, 6, e13870. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.H.; Olesen, S.C.; Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Worm, D.; Almdal, T.; Naver, L.S.; Hvolris, L.E.; et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg. 2012, 22, 1084–1096. [Google Scholar] [CrossRef]
- Guijarro, A.; Osei-Hyiaman, D.; Harvey-White, J.; Kunos, G.; Suzuki, S.; Nadtochiy, S.; Brookes, P.S.; Meguid, M.M. Sustained weight loss after Roux-en-Y gastric bypass is characterized by down regulation of endocannabinoids and mitochondrial function. Ann. Surg. 2008, 247, 779–790. [Google Scholar] [CrossRef]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Peterli, R.; Keller, S.; Meyer-Gerspach, A.C.; Drewe, J.; Peters, T.; Beglinger, C. Bile acids and gut peptide secretion after bariatric surgery: A 1-year prospective randomized pilot trial. Obesity 2013, 21, E660–E668. [Google Scholar] [CrossRef]
- Carvalho, C.; de Souza, A.L.; Batista, G.A.; Duran, L.F.T.; Fernandes, D.P.; Molina, V.B.C.; Gonçalves, R.; Giorgetti, J.S.; Chaim, E.A.; Alegre, S.M. GLP-1: 10-year follow-up after Roux-en-Y gastric bypass. Langenbecks Arch. Surg. 2022, 407, 559–568. [Google Scholar] [CrossRef]
- Hernández-Montoliu, L.; Rodríguez-Peña, M.M.; Puig, R.; Astiarraga, B.; Guerrero-Pérez, F.; Virgili, N.; López-Urdiales, R.; Osorio, J.; Monseny, R.; Lazzara, C.; et al. A specific gut microbiota signature is associated with an enhanced GLP-1 and GLP-2 secretion and improved metabolic control in patients with type 2 diabetes after metabolic Roux-en-Y gastric bypass. Front. Endocrinol. 2023, 14, 1181744. [Google Scholar] [CrossRef]
- Chaudhari, S.N.; Luo, J.N.; Harris, D.A.; Aliakbarian, H.; Yao, L.; Paik, D.; Subramaniam, R.; Adhikari, A.A.; Vernon, A.H.; Kiliç, A.; et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 2021, 29, 408–424.e407. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Song, Z.; Zhang, H.; Zhan, J.; Yu, H.; Huang, H.; Yang, B.; Xie, L.; Dai, X.; et al. Single-Anastomosis Duodenal Jejunal Bypass Improve Glucose Metabolism by Regulating Gut Microbiota and Short-Chain Fatty Acids in Goto-Kakisaki Rats. Front. Microbiol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Browning, K.N.; Fortna, S.R.; Hajnal, A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J. Physiol. 2013, 591, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- McGregor, M.; Hamilton, J.; Hajnal, A.; Thanos, P.K. Roux-en-Y gastric bypass increases GABA-A receptor levels in regions of the rat brain involved in object recognition memory and perceptual acuity. Physiol. Behav. 2020, 224, 113053. [Google Scholar] [CrossRef]
- Praxedes, D.R.; Silva-Júnior, A.E.; Macena, M.L.; Gearhardt, A.N.; Bueno, N.B. Prevalence of food addiction among patients undergoing metabolic/bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13529. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Gupta, A.; Jacobs, J.P.; Lagishetty, V.; Gallagher, E.; Bhatt, R.R.; Vora, P.; Osadchiy, V.; Stains, J.; Balioukova, A.; et al. Improvement in Uncontrolled Eating Behavior after Laparoscopic Sleeve Gastrectomy Is Associated with Alterations in the Brain-Gut-Microbiome Axis in Obese Women. Nutrients 2020, 12, 2924. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel, C.P.; Jacobs, J.; Gupta, A.; Ju, T.; Stains, J.; Coveleskie, K.; Lagishetty, V.; Balioukova, A.; Chen, Y.; Dutson, E.; et al. Surgically Induced Changes in Gut Microbiome and Hedonic Eating as Related to Weight Loss: Preliminary Findings in Obese Women Undergoing Bariatric Surgery. Psychosom. Med. 2017, 79, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Huwart, S.J.P.; de Wouters d’Oplinter, A.; Rastelli, M.; Van Hul, M.; de Vos, W.M.; Luquet, S.; Cani, P.D.; Everard, A. Food Reward Alterations during Obesity Are Associated with Inflammation in the Striatum in Mice: Beneficial Effects of Akkermansia muciniphila. Cells 2022, 11, 2534. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Northcutt, A.L.; Cochran, T.A.; Zhang, X.; Fabisiak, T.J.; Haas, M.E.; Amat, J.; Li, H.; Rice, K.C.; Maier, S.F.; et al. Methamphetamine Activates Toll-Like Receptor 4 to Induce Central Immune Signaling within the Ventral Tegmental Area and Contributes to Extracellular Dopamine Increase in the Nucleus Accumbens Shell. ACS Chem. Neurosci. 2019, 10, 3622–3634. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, S.; Richardson, B.D.; Lugo, J.M.; Rossi, D.J.; Davis, J.F. Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity 2017, 25, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Su, D.W.; Wei, W.W.; Yao, R.R.; Yang, C.J.; Tian, H. Research on sleeve gastrectomy for the treatment of rats with type 2 diabetes mellitus and the regulation of ghrelin and intestinal lesions. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10653–10662. [Google Scholar]
- Richard, J.E.; Anderberg, R.H.; Göteson, A.; Gribble, F.M.; Reimann, F.; Skibicka, K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS ONE 2015, 10, e0119034. [Google Scholar] [CrossRef]
- Cawthon, C.R.; Kirkland, R.A.; Pandya, S.; Brinson, N.A.; de La Serre, C.B. Non-neuronal crosstalk promotes an inflammatory response in nodose ganglia cultures after exposure to byproducts from gram positive, high-fat-diet-associated gut bacteria. Physiol. Behav. 2020, 226, 113124. [Google Scholar] [CrossRef]
- Dischinger, U.; Kötzner, L.; Kovatcheva-Datchary, P.; Kleinschmidt, H.; Haas, C.; Perez, J.; Presek, C.; Koschker, A.C.; Miras, A.D.; Hankir, M.K.; et al. Hypothalamic integrity is necessary for sustained weight loss after bariatric surgery: A prospective, cross-sectional study. Metabolism 2023, 138, 155341. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Engelstoft, M.S.; Schwartz, T.W. Opposite Regulation of Ghrelin and Glucagon-like Peptide-1 by Metabolite G-Protein-Coupled Receptors. Trends Endocrinol. Metab. 2016, 27, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Engelstoft, M.S.; Park, W.M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nøhr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.D.; Zhou, Y.; Barak, L.S.; Caron, M.G. Ghrelin receptor signaling in health and disease: A biased view. Trends Endocrinol. Metab. 2023, 34, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; Aidy, S.E.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chandra, R.; Samsa, L.A.; Gooch, B.; Fee, B.E.; Cook, J.M.; Vigna, S.R.; Grant, A.O.; Liddle, R.A. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G528–G537. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef] [PubMed]
- Wank, S.A.; Pisegna, J.R.; de Weerth, A. Brain and gastrointestinal cholecystokinin receptor family: Structure and functional expression. Proc. Natl. Acad. Sci. USA 1992, 89, 8691–8695. [Google Scholar] [CrossRef]
- Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Jacobsen, S.H.; Clausen, T.R.; Worm, D.; Hartmann, B.; Rehfeld, J.F.; Damgaard, M.; et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 2013, 37, 1452–1459. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Lutz, B.; Ruiz de Azua, I. The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism. Front. Cell. Neurosci. 2022, 16, 867267. [Google Scholar] [CrossRef] [PubMed]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [PubMed]
- Christie, S.; O’Rielly, R.; Li, H.; Wittert, G.A.; Page, A.J. High fat diet induced obesity alters endocannabinoid and ghrelin mediated regulation of components of the endocannabinoid system in nodose ganglia. Peptides 2020, 131, 170371. [Google Scholar] [CrossRef] [PubMed]
- Forte, N.; Boccella, S.; Tunisi, L.; Fernández-Rilo, A.C.; Imperatore, R.; Iannotti, F.A.; De Risi, M.; Iannotta, M.; Piscitelli, F.; Capasso, R.; et al. Orexin-A and endocannabinoids are involved in obesity-associated alteration of hippocampal neurogenesis, plasticity, and episodic memory in mice. Nat. Commun. 2021, 12, 6137. [Google Scholar] [CrossRef] [PubMed]
- Argueta, D.A.; Perez, P.A.; Makriyannis, A.; DiPatrizio, N.V. Cannabinoid CB(1) Receptors Inhibit Gut-Brain Satiation Signaling in Diet-Induced Obesity. Front. Physiol. 2019, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Solari, J.; Prestifilippo, J.P.; Ossola, C.A.; Rettori, V.; Elverdin, J.C. Participation of the endocannabinoid system in lipopolysaccharide-induced inhibition of salivary secretion. Arch. Oral Biol. 2010, 55, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.; Sherf-Dagan, S.; Nemirovski, A.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Shibolet, O.; Tam, J.; et al. Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obes. Surg. 2019, 29, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Mallipedhi, A.; Prior, S.L.; Dunseath, G.; Bracken, R.M.; Barry, J.; Caplin, S.; Eyre, N.; Morgan, J.; Baxter, J.N.; O’Sullivan, S.E.; et al. Changes in plasma levels of N-arachidonoyl ethanolamine and N-palmitoylethanolamine following bariatric surgery in morbidly obese females with impaired glucose homeostasis. J. Diabetes Res. 2015, 2015, 680867. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Abu El Haija, M.; Morgan, D.A.; Guo, D.; Song, Y.; Frank, A.; Tian, L.; Riedl, R.A.; Burnett, C.M.L.; Gao, Z.; et al. Endocannabinoid Receptor-1 and Sympathetic Nervous System Mediate the Beneficial Metabolic Effects of Gastric Bypass. Cell Rep. 2020, 33, 108270. [Google Scholar] [CrossRef]
- Abdalqadir, N.; Adeli, K. GLP-1 and GLP-2 Orchestrate Intestine Integrity, Gut Microbiota, and Immune System Crosstalk. Microorganisms 2022, 10, 2061. [Google Scholar] [CrossRef]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef] [PubMed]
- Perez-Montes, D.E.O.A.; Pellitero, S.; Puig-Domingo, M. Obesity and GLP-1. Minerva Endocrinol. 2021, 46, 168–176. [Google Scholar] [CrossRef]
- Cazzo, E.; Pareja, J.C.; Chaim, E.A.; Geloneze, B.; Barreto, M.R.; Magro, D.O. GLP-1 and GLP-2 Levels are Correlated with Satiety Regulation After Roux-en-Y Gastric Bypass: Results of an Exploratory Prospective Study. Obes. Surg. 2017, 27, 703–708. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Svane, M.S.; Bojsen-Møller, K.N.; Nielsen, S.; Jørgensen, N.B.; Dirksen, C.; Bendtsen, F.; Kristiansen, V.B.; Hartmann, B.; Holst, J.J.; Madsbad, S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E505–E514. [Google Scholar] [CrossRef] [PubMed]
- Hindsø, M.; Hedbäck, N.; Svane, M.S.; Møller, A.; Martinussen, C.; Jørgensen, N.B.; Dirksen, C.; Gasbjerg, L.S.; Kristiansen, V.B.; Hartmann, B.; et al. The Importance of Endogenously Secreted GLP-1 and GIP for Postprandial Glucose Tolerance and β-Cell Function After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy Surgery. Diabetes 2023, 72, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Flynn, C.R.; Albaugh, V.L.; Abumrad, N.N. Metabolic Effects of Bile Acids: Potential Role in Bariatric Surgery. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.N.; Harris, D.A.; Aliakbarian, H.; Luo, J.N.; Henke, M.T.; Subramaniam, R.; Vernon, A.H.; Tavakkoli, A.; Sheu, E.G.; Devlin, A.S. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol. 2021, 17, 20–29. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Banan, B.; Antoun, J.; Xiong, Y.; Guo, Y.; Ping, J.; Alikhan, M.; Clements, B.A.; Abumrad, N.N.; Flynn, C.R. Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology 2019, 156, 1041–1051.e1044. [Google Scholar] [CrossRef]
- Freeland, K.R.; Wolever, T.M. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br. J. Nutr. 2010, 103, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Lafferty, R.A.; Flatt, P.R.; Irwin, N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides 2018, 100, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; Wang, X.; Wang, C.; Yang, J.; Guan, B. Change in Adipokines and Gastrointestinal Hormones after Bariatric Surgery: A Meta-analysis. Obes. Surg. 2023, 33, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Morínigo, R.; Vidal, J.; Lacy, A.M.; Delgado, S.; Casamitjana, R.; Gomis, R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann. Surg. 2008, 247, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Svane, M.S.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Dirksen, C.; Nielsen, S.; Kristiansen, V.B.; Toräng, S.; Wewer Albrechtsen, N.J.; Rehfeld, J.F.; Hartmann, B.; et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. 2016, 40, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Guida, C.; Stephen, S.D.; Watson, M.; Dempster, N.; Larraufie, P.; Marjot, T.; Cargill, T.; Rickers, L.; Pavlides, M.; Tomlinson, J.; et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine 2019, 40, 67–76. [Google Scholar] [CrossRef]
- Nannipieri, M.; Baldi, S.; Mari, A.; Colligiani, D.; Guarino, D.; Camastra, S.; Barsotti, E.; Berta, R.; Moriconi, D.; Bellini, R.; et al. Roux-en-Y gastric bypass and sleeve gastrectomy: Mechanisms of diabetes remission and role of gut hormones. J. Clin. Endocrinol. Metab. 2013, 98, 4391–4399. [Google Scholar] [CrossRef]
- Ionut, V.; Burch, M.; Youdim, A.; Bergman, R.N. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity 2013, 21, 1093–1103. [Google Scholar] [CrossRef]
- Pierre, J.F.; Peters, B.M.; La Torre, D.; Sidebottom, A.M.; Tao, Y.; Zhu, X.; Cham, C.M.; Wang, L.; Kambal, A.; Harris, K.G.; et al. Peptide YY: A Paneth cell antimicrobial peptide that maintains Candida gut commensalism. Science 2023, 381, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Joly-Amado, A.; Cansell, C.; Denis, R.G.; Delbes, A.S.; Castel, J.; Martinez, S.; Luquet, S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Lee, N.J.; Ip, C.K.; Enriquez, R.; Tasan, R.; Zhang, L.; Herzog, H. NPY derived from AGRP neurons controls feeding via Y1 and energy expenditure and food foraging behaviour via Y2 signalling. Mol. Metab. 2022, 59, 101455. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Patkar, P.P.; Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Berthoud, H.R.; Shin, A.C. Unlike calorie restriction, Roux-en-Y gastric bypass surgery does not increase hypothalamic AgRP and NPY in mice on a high-fat diet. Int. J. Obes. 2019, 43, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Menachemi, N.; Mazurenko, O.; Kazley, A.S.; Diana, M.L.; Ford, E.W. Market factors and electronic medical record adoption in medical practices. Health Care Manag. Rev. 2012, 37, 14–22. [Google Scholar] [CrossRef]
- Suyama, S.; Yada, T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef]
- El Karim, I.A.; Linden, G.J.; Orr, D.F.; Lundy, F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008, 200, 11–16. [Google Scholar] [CrossRef]
- Chen, W.C.; Liu, Y.B.; Liu, W.F.; Zhou, Y.Y.; He, H.F.; Lin, S. Neuropeptide Y Is an Immunomodulatory Factor: Direct and Indirect. Front. Immunol. 2020, 11, 580378. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Date, Y.; Murakami, N.; Shimbara, T.; Hanada, T.; Toshinai, K.; Niijima, A.; Furuya, M.; Inomata, N.; Osuye, K.; et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 2005, 146, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Aukan, M.I.; Brandsaeter, I.; Skårvold, S.; Finlayson, G.; Nymo, S.; Coutinho, S.; Martins, C. Changes in hedonic hunger and food reward after a similar weight loss induced by a very low-energy diet or bariatric surgery. Obesity 2022, 30, 1963–1972. [Google Scholar] [CrossRef]
- Nance, K.; Acevedo, M.B.; Pepino, M.Y. Changes in taste function and ingestive behavior following bariatric surgery. Appetite 2020, 146, 104423. [Google Scholar] [CrossRef]
- Holsen, L.M.; Davidson, P.; Cerit, H.; Hye, T.; Moondra, P.; Haimovici, F.; Sogg, S.; Shikora, S.; Goldstein, J.M.; Evins, A.E.; et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int. J. Obes. 2018, 42, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Papantoni, A.; Veldhuizen, M.G.; Kamath, V.; Harris, C.; Moran, T.H.; Carnell, S.; Steele, K.E. Taste-related reward is associated with weight loss following bariatric surgery. J. Clin. Investig. 2020, 130, 4370–4381. [Google Scholar] [CrossRef]
- Kenny, P.J. Reward mechanisms in obesity: New insights and future directions. Neuron 2011, 69, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R.; Fordahl, S.C. Bingeing on High-Fat Food Enhances Evoked Dopamine Release and Reduces Dopamine Uptake in the Nucleus Accumbens. Obesity 2021, 29, 721–730. [Google Scholar] [CrossRef]
- Dong, T.S.; Mayer, E.A.; Osadchiy, V.; Chang, C.; Katzka, W.; Lagishetty, V.; Gonzalez, K.; Kalani, A.; Stains, J.; Jacobs, J.P.; et al. A Distinct Brain-Gut-Microbiome Profile Exists for Females with Obesity and Food Addiction. Obesity 2020, 28, 1477–1486. [Google Scholar] [CrossRef]
- Menni, C.; Hernandez, M.M.; Vital, M.; Mohney, R.P.; Spector, T.D.; Valdes, A.M. Circulating levels of the anti-oxidant indoleproprionic acid are associated with higher gut microbiome diversity. Gut Microbes 2019, 10, 688–695. [Google Scholar] [CrossRef]
- de Wouters d’Oplinter, A.; Rastelli, M.; Van Hul, M.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.M.; Benninghoff, A.D.; Aardema, N.D.J.; Phatak, S.; Hintze, K.J. Basal Diet Determined Long-Term Composition of the Gut Microbiome and Mouse Phenotype to a Greater Extent than Fecal Microbiome Transfer from Lean or Obese Human Donors. Nutrients 2019, 11, 1630. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.O.; Ramos, M.R.Z.; Wagner, N.R.F.; Freitas, L.A.C.; Felicidade, I.; Campos, A.C.L. Probiotic supplementation attenuates binge eating and food addiction 1 year after Roux-en-y gastric bypass: A randomized, double-blind, placebo-controlled trial. Arq. Bras. Cir. Dig. 2022, 35, e1659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, T.; Xu, C.; Yang, H.; Zhang, T.; Liu, Y. Probiotics Can Further Reduce Waist Circumference in Adults with Morbid Obesity after Bariatric Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2021, 2021, 5542626. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Lehne, C.; Weiland, A.; Archid, R.; Ritze, Y.; Bauer, K.; Zipfel, S.; Penders, J.; Enck, P.; Mack, I. Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery-A Systematic Review of Their Interrelation. Nutrients 2020, 12, 2396. [Google Scholar] [CrossRef] [PubMed]
- Sherf-Dagan, S.; Zelber-Sagi, S.; Zilberman-Schapira, G.; Webb, M.; Buch, A.; Keidar, A.; Raziel, A.; Sakran, N.; Goitein, D.; Goldenberg, N.; et al. Probiotics administration following sleeve gastrectomy surgery: A randomized double-blind trial. Int. J. Obes. 2018, 42, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Taleghani, F.; Abiri, B.; Zamanian, A.; Saidpour, A. Effects of probiotic supplementation with weight reducing intervention on anthropometric measures, body composition, eating behavior, and related hormone levels in patients with food addiction and weight regain after bariatric surgery: A study protocol for a randomized clinical trial. BMC Nutr. 2023, 9, 63. [Google Scholar]
- Naef, L.; Pitman, K.A.; Borgland, S.L. Mesolimbic dopamine and its neuromodulators in obesity and binge eating. CNS Spectr. 2015, 20, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Williams, K.C.; Kirkland, R.A.; Schade, R.; Freeman, K.G.; Cawthon, C.R.; Rautmann, A.W.; Smith, J.M.; Edwards, G.L.; Glenn, T.C.; et al. The gut-brain axis mediates bacterial driven modulation of reward signaling. Mol. Metab. 2023, 75, 101764. [Google Scholar] [CrossRef]
- Hamilton, J.; Swenson, S.; Hajnal, A.; Thanos, P.K. Roux-en-Y gastric bypass surgery normalizes dopamine D1, D2, and DAT levels. Synapse 2018, 72, e22058. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Décarie-Spain, L.; Sharma, S.; Hryhorczuk, C.; Issa-Garcia, V.; Barker, P.A.; Arbour, N.; Alquier, T.; Fulton, S. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol. Metab. 2018, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Iyer, S.; Razani, B.; Cheng, G. NF-κB and innate immunity. Curr. Top. Microbiol. Immunol. 2011, 349, 115–143. [Google Scholar] [PubMed]
- Giri, R.; Hoedt, E.C.; Khushi, S.; Salim, A.A.; Bergot, A.S.; Schreiber, V.; Thomas, R.; McGuckin, M.A.; Florin, T.H.; Morrison, M.; et al. Secreted NF-κB suppressive microbial metabolites modulate gut inflammation. Cell Rep. 2022, 39, 110646. [Google Scholar] [CrossRef]
- Hartstra, A.V.; Schüppel, V.; Imangaliyev, S.; Schrantee, A.; Prodan, A.; Collard, D.; Levin, E.; Dallinga-Thie, G.; Ackermans, M.T.; Winkelmeijer, M.; et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol. Metab. 2020, 42, 101076. [Google Scholar] [CrossRef] [PubMed]
- Leyrolle, Q.; Cserjesi, R.; Mulders, M.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Amadieu, C.; Leclercq, S.; et al. Specific gut microbial, biological, and psychiatric profiling related to binge eating disorders: A cross-sectional study in obese patients. Clin. Nutr. 2021, 40, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Hajnal, A.; Covasa, M. Reduced Striatal Dopamine Transporter Availability and Heightened Response to Natural and Pharmacological Stimulation in CCK-1R-Deficient Obese Rats. Int. J. Mol. Sci. 2023, 24, 9773. [Google Scholar] [CrossRef]
- Scholtz, S.; Miras, A.D.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014, 63, 891–902. [Google Scholar] [CrossRef]
- Abizaid, A.; Liu, Z.W.; Andrews, Z.B.; Shanabrough, M.; Borok, E.; Elsworth, J.D.; Roth, R.H.; Sleeman, M.W.; Picciotto, M.R.; Tschöp, M.H.; et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Investig. 2006, 116, 3229–3239. [Google Scholar] [CrossRef]
- Jerlhag, E.; Janson, A.C.; Waters, S.; Engel, J.A. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS ONE 2012, 7, e49557. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A. Ghrelin and dopamine: New insights on the peripheral regulation of appetite. J. Neuroendocrinol. 2009, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, R.H.; Hansson, C.; Fenander, M.; Richard, J.E.; Dickson, S.L.; Nissbrandt, H.; Bergquist, F.; Skibicka, K.P. The Stomach-Derived Hormone Ghrelin Increases Impulsive Behavior. Neuropsychopharmacology 2016, 41, 1199–1209. [Google Scholar] [CrossRef]
- Edvardsson, C.E.; Vestlund, J.; Jerlhag, E. A ghrelin receptor antagonist reduces the ability of ghrelin, alcohol or amphetamine to induce a dopamine release in the ventral tegmental area and in nucleus accumbens shell in rats. Eur. J. Pharmacol. 2021, 899, 174039. [Google Scholar] [CrossRef]
- Figlewicz, D.P.; Evans, S.B.; Murphy, J.; Hoen, M.; Baskin, D.G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003, 964, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.W.; Gao, X.B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef]
- Leinninger, G.M.; Jo, Y.H.; Leshan, R.L.; Louis, G.W.; Yang, H.; Barrera, J.G.; Wilson, H.; Opland, D.M.; Faouzi, M.A.; Gong, Y.; et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009, 10, 89–98. [Google Scholar] [CrossRef]
- Fernandes, M.F.; Matthys, D.; Hryhorczuk, C.; Sharma, S.; Mogra, S.; Alquier, T.; Fulton, S. Leptin Suppresses the Rewarding Effects of Running via STAT3 Signaling in Dopamine Neurons. Cell Metab. 2015, 22, 741–749. [Google Scholar] [CrossRef]
- Noye Tuplin, E.W.; Chleilat, F.; Alukic, E.; Reimer, R.A. The Effects of Human Milk Oligosaccharide Supplementation During Critical Periods of Development on the Mesolimbic Dopamine System. Neuroscience 2021, 459, 166–178. [Google Scholar] [CrossRef]
- Seridi, L.; Leo, G.C.; Dohm, G.L.; Pories, W.J.; Lenhard, J. Time course metabolome of Roux-en-Y gastric bypass confirms correlation between leptin, body weight and the microbiome. PLoS ONE 2018, 13, e0198156. [Google Scholar] [CrossRef]
- Li, X.; Shi, W.; Xiong, Q.; Hu, Y.; Qin, X.; Wan, G.; Zeng, Q. Leptin improves intestinal flora dysfunction in mice with high-fat diet-induced obesity. J. Int. Med. Res. 2020, 48, 300060520920062. [Google Scholar] [CrossRef] [PubMed]
- Gauffin Cano, P.; Santacruz, A.; Moya, Á.; Sanz, Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaal, E.M.; de Weijer, B.A.; van de Giessen, E.M.; Janssen, I.; Berends, F.J.; van de Laar, A.; Ackermans, M.T.; Fliers, E.; la Fleur, S.E.; Booij, J.; et al. Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss. Eur. Neuropsychopharmacol. 2016, 26, 1190–1200. [Google Scholar] [CrossRef]
- Hayes, M.R.; Schmidt, H.D. GLP-1 influences food and drug reward. Curr. Opin. Behav. Sci. 2016, 9, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, U.; Weber, E.; Langhans, W.; Meyer, U. The Y2 receptor agonist PYY(3-36) increases the behavioural response to novelty and acute dopaminergic drug challenge in mice. Int. J. Neuropsychopharmacol. 2014, 17, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bo, T.; Xi, L.; Xu, X.; He, N.; Zhan, Y.; Li, W.; Liang, P.; Chen, Y.; Shi, J.; et al. Reversal of Functional Brain Activity Related to Gut Microbiome and Hormones After VSG Surgery in Patients with Obesity. J. Clin. Endocrinol. Metab. 2021, 106, e3619–e3633. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tao, F.; Tan, C.; Xu, C.Y.; Zheng, Z.H.; Pang, Q.; He, X.A.; Cao, J.Q.; Duan, J.Y. Enhanced glucose homeostasis via Clostridium symbiosum-mediated glucagon-like peptide 1 inhibition of hepatic gluconeogenesis in mid-intestinal bypass surgery. World J. Gastroenterol. 2023, 29, 5471–5482. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, W.J.; Jin, R.; Xu, X.; Wei, J.; Huang, H.; Tang, Y.H.; Zou, C.W.; Chen, T.T. Engineered probiotics Clostridium butyricum-pMTL007-GLP-1 improves blood pressure via producing GLP-1 and modulating gut microbiota in spontaneous hypertension rat models. Microb. Biotechnol. 2023, 16, 799–812. [Google Scholar] [CrossRef]




| Satiety Hormone, Neuropeptide or Neurotransmitter | Species Involved/Outcome Measured | Results/Implications | Humans vs. Murine | References |
|---|---|---|---|---|
| Leptin | Serum SCFA | In SG, leptin levels are reduced in correlation with upregulation of SCFA and increased expression of colonic GPR43. | Mice | [52] |
| Inflammatory Markers | In obesity, LPS- or LTA-mediated low-grade inflammation contributes to diminished sensitivity of the NG to leptin activity. RYGB mitigates these effects. | Rats | [78] | |
| Serum Leptin | Bariatric surgery lowers serum leptin levels to alleviate leptin resistance. | Humans | [133] | |
| Escherichia Bacteroides | RYGB-mediated alterations correlate with serum leptin and fat mass. Increased Bacteroides is associated with lower serum leptin. | Humans | [137] | |
| Hypothalamic Inflammation | Reduction in leptin inhibitor, SOCS3, following bariatric surgery mitigates leptin resistance. Microbiota-related changes improve hypothalamic inflammation. | Humans | [138] | |
| Ghrelin | Serum Ghrelin | Immediately after surgery, ghrelin levels are decreased in SG and increased in RYGB. At one year, both surgeries decrease ghrelin concentrations. | Humans | [135] |
| Faecalibacterium | Elevated following RYGB and negatively correlated with ghrelin concentrations. SCFA-producing bacterium promotes downstream inhibitory effects on ghrelin secretion following GPR43 binding. | Humans | [137] | |
| Inflammatory Markers | LPS activation of TLR-4 converges with activity of the GHR1a receptor. States of metabolic endotoxemia may modulate ghrelinergic activity. Bariatric surgery mitigates metabolic endotoxemia. | Mice | [139] | |
| Hydrogen Sulfide Concentrations | H2S suppresses ghrelinergic signaling via GHR1a receptor. H2S prolongs post-prandial drop of serum ghrelin and reduced food intake. RYGB promotes increased abundance of Escherichia, Veillonella, Clostridium, Bacteroides, and Streptococcus, all of which have H2S-producing capabilities. | Mice | [140] | |
| Cholecystokinin | Serum CCK | Following bariatric surgery, CCK receptor expression is increased post-prandially. | Humans | [141] |
| Endocannabinoids | Endocannabinoid concentrations are characteristically decreased after bariatric surgery. Sustained weight loss in RYGB correlates with reduced endocannabinoids and related increases in serum CCK. | Rats | [142] | |
| SCFA | GPR41 expression in the NG is essential for CCK secretion. Improved SCFA profile following bariatric surgery enhances its secretion. | Mice | [143] | |
| Glucagon-Like Peptide 1 | GLP-1 Concentrations | GLP-1 concentrations are increased as early as 1-week post-surgical intervention; GLP-1 remains elevated 10 years post-surgery. | Humans | [144,145] |
| Gut Microbiota | Akkermansiacea, Rickenellaceae, Veillonellaceae, Enterobacteriaceae, and Rickenellaceae were positively correlated with GLP-1, while Lachnospiraceae and Rumminococcacae had an opposite effect. These changes are evident in RYGB. | Humans | [146] | |
| Bile Acids | Lithocholic acid is elevated following SG; acts as agonist for TGR5 to influence GLP-1 secretion. | Rodent | [147] | |
| Akkermansia | Akkermansia is elevated after SG and RYGB; the protein component, P9, interacts with ICAM-2 to induce thermogenesis and systemic GLP-1 release. | PCR | [148] | |
| SCFA | Acetate infusions trigger GLP-1 release through raising calcium concentrations. In bariatric surgery, GPR41 and GPR43 expression is increased, correlating with enhanced GLP-1 secretion. | Rodent | [149] | |
| Peptide YY | SCFA | SCFA profile changes in bariatric surgery; propionate, butyrate, isobutyrate and isovalerate correlate with increased PYY in both SG and RYGB. | Rodent | [98] |
| PYY Concentrations | SG and RYGB increase PYY concentrations. | Humans | [144] | |
| Orexigenic and Anorexigenic Neuropeptides | Hypothalamic Inflammation | Bariatric surgery reverses hypothalamic inflammation and normalizes NPY and AgRP. | Mice | [85] |
| Neuropeptides | RYGB improves vagal neuronal health in the DMV to restore neuropeptide response to gut hormones. | Rats | [150] | |
| NPY and AgRP | Acetate suppresses NPY and AgRP expression by attenuating GABA activity. GABA signaling ensues following RYGB-induced alterations in extrahypothalamic areas that integrate memory and perception, all influencing eating behaviors. | Mice | [151,152] | |
| Eating Behaviors | Food Addiction | Bariatric surgery reduces prevalence of food addiction by 17%. | Humans | [153] |
| Eating Behaviors | SG improved maladaptive eating behaviors, which are associated with altered concentrations of Bacteroides, Ruminococcus, and Holdemanella. Microbiota metabolite 1-palmitoyl-2-palmitoleoyl is associated with reduced resting-state connectivity in amygdala and putamen. | Humans | [154] | |
| Hedonic Eating | SG elevated Akkermansia in obesity; was correlated with reduced hedonic eating. Akkermansia improves dysregulated reward behaviors through reductions in striatal inflammation and blood–brain barrier permeability. | Humans | [155,156] | |
| Dopamine | Extracellular Dopamine | LPS-induced activation of TLR-4 contributes to microglial activity within the VTA. Extracellular DA increases in NAcc shell. Bariatric surgery attenuates LPS-mediated inflammation. | Rodent | [157] |
| DAT | Bacteroides uniformis is associated with increased DAT binding, while Prevotella spp. has an opposite effect. | Rodent | [158] | |
| Ghrelin/Dopamine | Post-RYGB murine models exhibit reduced tonic dopamine firing secondary to diminished GHSR1a activity. Decreased ghrelinergic signaling with concomitant improvements in the Firmicutes/Bacteroidetes ratio. | Rodent | [159,160] | |
| GLP-1/Dopamine | Activation of GLP-1 receptors in the NTS contributes to food reward suppressing behaviors through targeting mesolimbic DA. | Rats | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamamah, S.; Hajnal, A.; Covasa, M. Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients 2024, 16, 1071. https://doi.org/10.3390/nu16071071
Hamamah S, Hajnal A, Covasa M. Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients. 2024; 16(7):1071. https://doi.org/10.3390/nu16071071
Chicago/Turabian StyleHamamah, Sevag, Andras Hajnal, and Mihai Covasa. 2024. "Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets" Nutrients 16, no. 7: 1071. https://doi.org/10.3390/nu16071071
APA StyleHamamah, S., Hajnal, A., & Covasa, M. (2024). Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients, 16(7), 1071. https://doi.org/10.3390/nu16071071







