Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Literature Search Methods
2.3. Inclusion and Exclusion Criteria
2.4. Assessment of Quality
2.5. Data Collection Process
2.6. Statistical Analysis
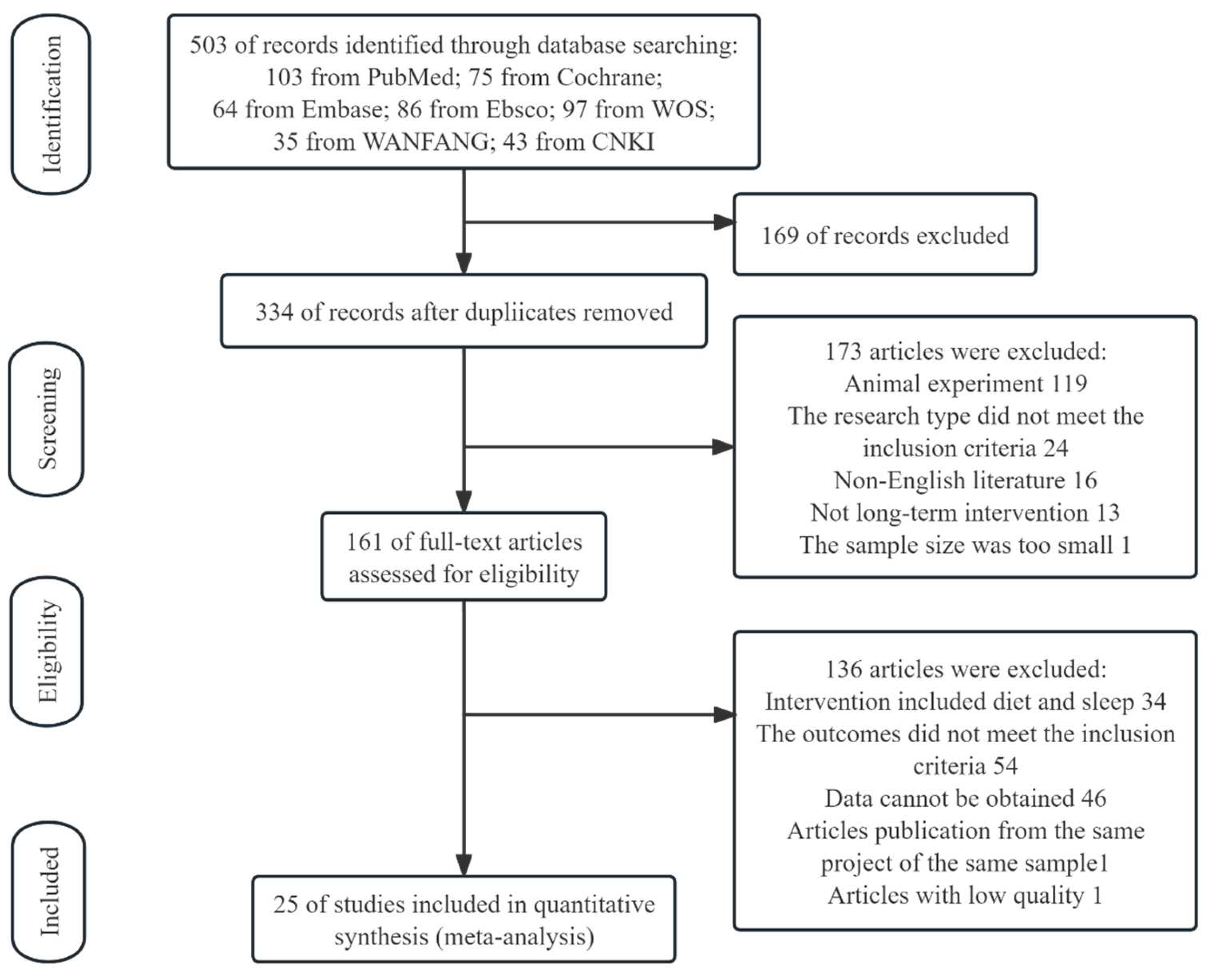
3. Results
3.1. Study Selection
3.2. Characteristics of Studies
3.3. Quality of the Evidence
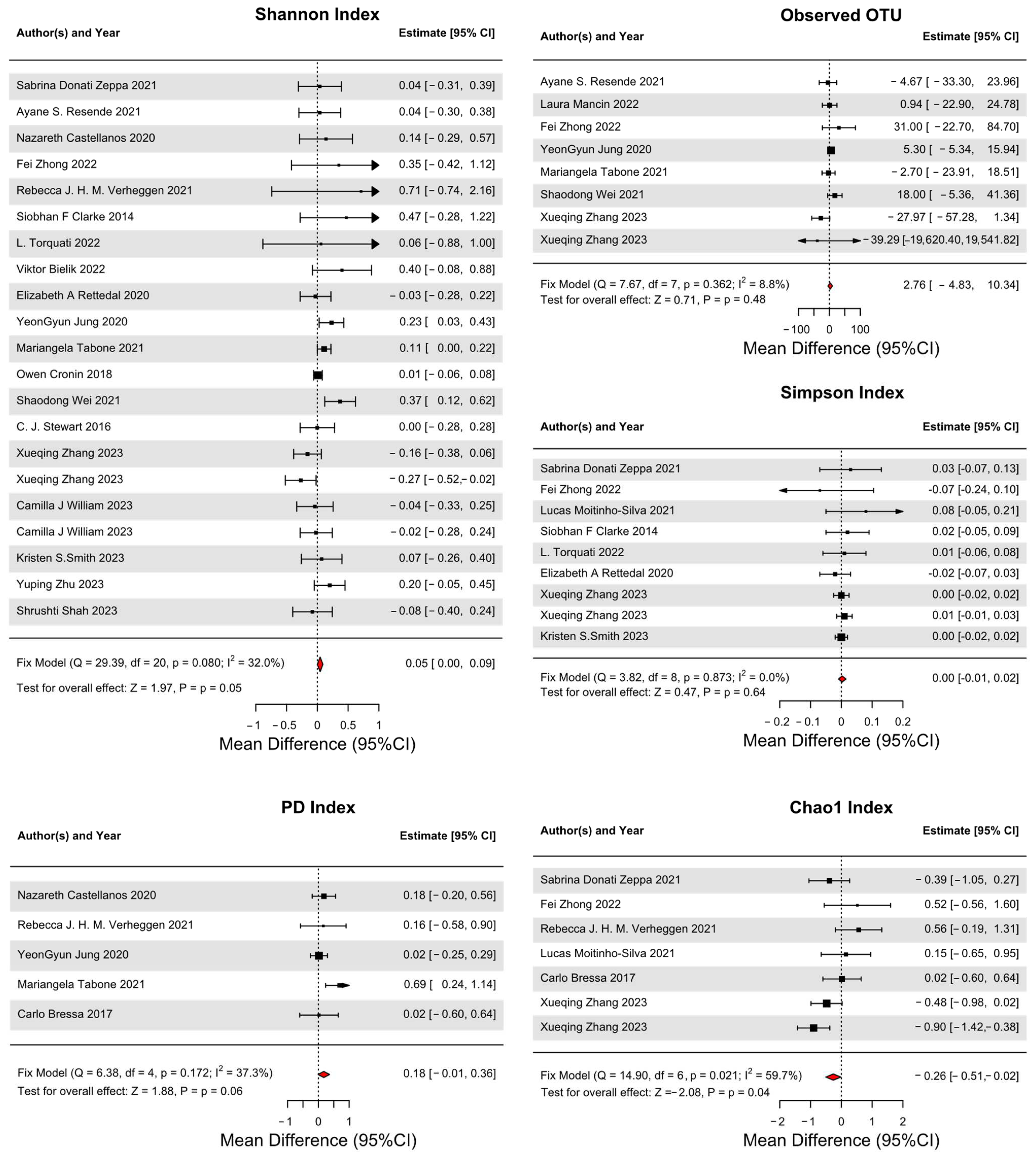
3.4. Primary Outcomes
3.5. Secondary Outcomes
- Significant Changes in Specific Bacterial Groups:
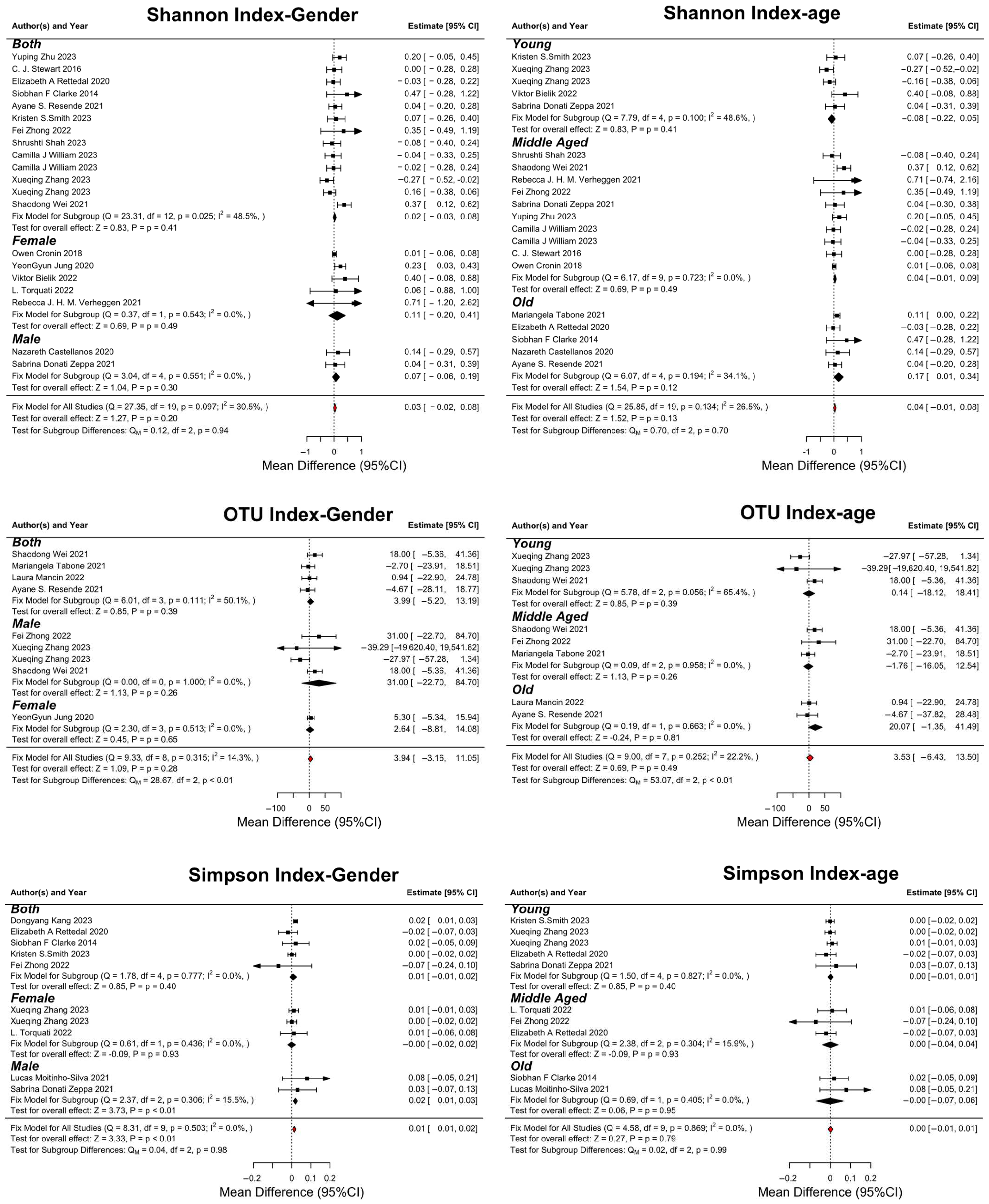
3.6. Subgroup Analysis
- Gender-Based Analysis:
- Age-Based Analysis:
4. Discussion
4.1. Findings and Novelty of This Review
4.2. Changes in Microbial Diversity Index
4.3. Changes in Specific Flora
4.3.1. The Two Most Abundant Phyla: Firmicutes and Bacteroidetes
4.3.2. SCFAs-Producing Bacteria
4.3.3. Other Representative Bacteria
4.4. Limitations
4.5. Future Direction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.-R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Azad, M.B. Quantifying and Interpreting the Association between Early-Life Gut Microbiota Composition and Childhood Obesity. mBio 2019, 10, e02787-18. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Tackling the effects of diet and exercise on the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Khan, A.A.; Tzora, A.; Voidarou, C.C.; Skoufos, I. Dietary Implications of the Bidirectional Relationship between the Gut Microflora and Inflammatory Diseases with Special Emphasis on Irritable Bowel Disease: Current and Future Perspective. Nutrients 2023, 15, 2956. [Google Scholar] [CrossRef] [PubMed]
- Koh, H. An adaptive microbiome α-diversity-based association analysis method. Sci. Rep. 2018, 8, 18026. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, X. Regulation of the gut microbiota by diet and exercise: Improvements in cognition and emotion. Future Foods 2023, 8, 100256. [Google Scholar] [CrossRef]
- Khan, K.; Qadir, A.; Trakman, G.; Aziz, T.; Khattak, M.I.; Nabi, G.; Alharbi, M.; Alshammari, A.; Shahzad, M. Sports and Energy Drink Consumption, Oral Health Problems and Performance Impact among Elite Athletes. Nutrients 2022, 14, 5089. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef] [PubMed]
- Aya, V.; Jimenez, P.; Muñoz, E.; Ramírez, J.D. Effects of exercise and physical activity on gut microbiota composition and function in older adults: A systematic review. BMC Geriatr. 2023, 23, 364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Sun, Y.; Zhang, X. Combined Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat of Metabolic Disease: A Review. Nutrients 2022, 14, 4774. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.R.G.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur. J. Sport Sci. 2022, 23, 530–541. [Google Scholar] [CrossRef]
- Lamprecht, M.; Frauwallner, A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Acute Top. Sport Nutr. 2012, 59, 47–56. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Amatori, S.; Sisti, D.; Gervasi, M.; Agostini, D.; Piccoli, G.; Pazienza, V.; Gobbi, P.; Rocchi, M.B.L.; Sestili, P.; et al. Nine weeks of high-intensity indoor cycling training induced changes in the microbiota composition in non-athlete healthy male college students. J. Int. Soc. Sports Nutr. 2021, 18, 74. [Google Scholar] [CrossRef]
- Resende, A.S.; Leite, G.S.F.; Lancha Junior, A.H. Changes in the Gut Bacteria Composition of Healthy Men with the Same Nutritional Profile Undergoing 10-Week Aerobic Exercise Training: A Randomized Controlled Trial. Nutrients 2021, 13, 2839. [Google Scholar] [CrossRef]
- Mancin, L.; Amatori, S.; Caprio, M.; Sattin, E.; Bertoldi, L.; Cenci, L.; Sisti, D.; Bianco, A.; Paoli, A. Effect of 30 days of ketogenic Mediterranean diet with phytoextracts on athletes’ gut microbiome composition. Front. Nutr. 2022, 9, 979651. [Google Scholar] [CrossRef]
- Castellanos, N.; Diez, G.G.; Antunez-Almagro, C.; Bailen, M.; Bressa, C.; Gonzalez Soltero, R.; Perez, M.; Larrosa, M. A Critical Mutualism—Competition Interplay Underlies the Loss of Microbial Diversity in Sedentary Lifestyle. Front. Microbiol. 2019, 10, 3142. [Google Scholar] [CrossRef]
- Zhong, F.; Xu, Y.; Lai, H.Y.; Yang, M.; Cheng, L.; Liu, X.; Sun, X.; Yang, Y.; Wang, J.; Lv, W.; et al. Effects of combined aerobic and resistance training on gut microbiota and cardiovascular risk factors in physically active elderly women: A randomized controlled trial. Front. Physiol. 2022, 13, 1004863. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, R.; Konstanti, P.; Smidt, H.; Hermus, A.; Thijssen, D.H.J.; Hopman, M.T.E. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Wegener, M.; May, S.; Schrinner, F.; Akhtar, A.; Boysen, T.J.; Schaeffer, E.; Hansen, C.; Schmidt, T.; Ruhlemann, M.C.; et al. Short-term physical exercise impacts on the human holobiont obtained by a randomised intervention study. BMC Microbiol. 2021, 21, 162. [Google Scholar] [CrossRef]
- Bielik, V.; Hric, I.; Ugrayova, S.; Kubanova, L.; Putala, M.; Grznar, L.; Penesova, A.; Havranova, A.; Sardzikova, S.; Grendar, M.; et al. Effect of High-intensity Training and Probiotics on Gut Microbiota Diversity in Competitive Swimmers: Randomized Controlled Trial. Sports Med. Open 2022, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, E.A.; Cree, J.M.E.; Adams, S.E.; MacRae, C.; Skidmore, P.M.L.; Cameron-Smith, D.; Gant, N.; Blenkiron, C.; Merry, T.L. Short-term high-intensity interval training exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Exp. Physiol. 2020, 105, 1268–1279. [Google Scholar] [CrossRef]
- Jung, Y.; Tagele, S.B.; Son, H.; Ibal, J.C.; Kerfahi, D.; Yun, H.; Lee, B.; Park, C.Y.; Kim, E.S.; Kim, S.J.; et al. Modulation of Gut Microbiota in Korean Navy Trainees following a Healthy Lifestyle Change. Microorganisms 2020, 8, 1265. [Google Scholar] [CrossRef] [PubMed]
- Tabone, M.; Bressa, C.; García-Merino, J.A.; Moreno-Pérez, D.; Van, E.C.; Castelli, F.A.; Fenaille, F.; Larrosa, M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci. Rep. 2021, 11, 3558. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbo, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietila, S.; Hollmen, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Wei, S.; Brejnrod, A.D.; Trivedi, U.; Mortensen, M.S.; Johansen, M.Y.; Karstoft, K.; Vaag, A.A.; Ried-Larsen, M.; Sorensen, S.J. Impact of intensive lifestyle intervention on gut microbiota composition in type 2 diabetes: A post-hoc analysis of a randomized clinical trial. Gut Microbes 2022, 14, 2005407. [Google Scholar] [CrossRef]
- Stewart, C.J.; Nelson, A.; Campbell, M.D.; Walker, M.; Stevenson, E.J.; Shaw, J.A.; Cummings, S.P.; West, D.J. Gut microbiota of Type 1 diabetes patients with good glycaemic control and high physical fitness is similar to people without diabetes: An observational study. Diabet. Med. 2017, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailen-Andrino, M.; Perez-Santiago, J.; Gonzalez-Soltero, R.; Perez, M.; Montalvo-Lominchar, M.G.; Mate-Munoz, J.L.; Dominguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zhang, K.; Zhang, J.; Lu, X.; Guo, H.; Yuan, G.; Zhu, Z.; Du, J.; Shi, H.; et al. Effects of exercise or tai chi on Internet addiction in college students and the potential role of gut microbiota: A randomized controlled trial. J. Affect. Disord. 2023, 327, 404–415. [Google Scholar] [CrossRef]
- Williams, C.J.; Torquati, L.; Li, Z.; Lea, R.A.; Croci, I.; Keating, E.; Little, J.P.; Eynon, N.; Coombes, J.S. Oligofructose-Enriched Inulin Intake, Gut Microbiome Characteristics, and the VO2 Peak Response to High-Intensity Interval Training in Healthy Inactive Adults. J. Nutr. 2022, 152, 680–689. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Morris, M.M.; Morrow, C.D.; Novak, J.R.; Roberts, M.D.; Fruge, A.D. Associations between Changes in Fat-Free Mass, Fecal Microbe Diversity, and Mood Disturbance in Young Adults after 10-Weeks of Resistance Training. Microorganisms 2022, 10, 2344. [Google Scholar] [CrossRef]
- Kang, D.; Wang, X.; Wang, J. Intervention study of tai chi training on the intestinal flora of college student basketball players. Medicine 2023, 102, e35044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, J.; Song, G. The impact of aerobic exercise training on cognitive function and gut microbiota in methamphetamine-dependent individuals in the community. Physiol. Behav. 2023, 270, 114302. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Mu, C.; Moossavi, S.; Shen-Tu, G.; Schlicht, K.; Rohmann, N.; Geisler, C.; Laudes, M.; Franke, A.; Zullig, T.; et al. Physical activity-induced alterations of the gut microbiota are BMI dependent. FASEB J. 2023, 37, e22882. [Google Scholar] [CrossRef]
- Özkurt, E.; Hildebrand, F. Lifelong sex-dependent trajectories of the human gut microbiota. Nat. Aging 2021, 1, 22–23. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.; Li, Y.; Shi, Z.; Ren, H.; Zhang, Z.; Zhou, X.; Tang, S.; Han, X.; Lin, Y.; et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging 2021, 1, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology 2022, 11, 479. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Dorelli, B.; Gallè, F.; De Vito, C.; Duranti, G.; Iachini, M.; Zaccarin, M.; Preziosi Standoli, J.; Ceci, R.; Romano, F.; Liguori, G.; et al. Can Physical Activity Influence Human Gut Microbiota Composition Independently of Diet? A Systematic Review. Nutrients 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cai, Y.; Lu, W.; Zhang, R.; Shao, R.; Yau, S.-Y.; Stubbs, B.; McIntyre, R.S.; Su, K.-P.; Xu, G.; et al. Exercise effect on the gut microbiota in young adolescents with subthreshold depression: A randomized psychoeducation-controlled Trial. Psychiatry Res. 2023, 319, 115005. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef]
- Moens, F.; Verce, M.; De Vuyst, L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int. J. Food Microbiol. 2017, 241, 225–236. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; De Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Rerksuppaphol, S. A Randomized Double-blind Controlled Trial of Lactobacillus acidophilus Plus Bifidobacterium bifidum versus Placebo in Patients with Hypercholesterolemia. J. Clin. Diagn. Res. 2015, 9, KC01. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Shelton, C.D.; Sing, E.; Mo, J.; Shealy, N.G.; Yoo, W.; Thomas, J.; Fitz, G.N.; Castro, P.R.; Hickman, T.T.; Torres, T.P.; et al. An early-life microbiota metabolite protects against obesity by regulating intestinal lipid metabolism. Cell Host Microbe 2023, 31, 1604–1619.e1610. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Naveed, M.; Makhdoom, S.I.; Ali, U.; Mughal, M.S.; Sarwar, A.; Khan, A.A.; Zhennai, Y.; Sameeh, M.Y.; Dablool, A.S.; et al. Genome Investigation and Functional Annotation of Lactiplantibacillus plantarum YW11 Revealing Streptin and Ruminococcin-A as Potent Nutritive Bacteriocins against Gut Symbiotic Pathogens. Molecules 2023, 28, 491. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Fahim, M.; Al Dalali, S.; Ud Din, Z.; Ud Din, J.; Xin, Z.; Jian, Z.; Pacheco Fill, T.; Zhennai, Y. In silico characterization of linoleic acid biotransformation to rumenic acid in food derived Lactobacillus plantarum YW11. Acta Biochim. Pol. 2020, 67, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Sarwar, A.; ud Din, J.; Al Dalali, S.; Khan, A.A.; Din, Z.U.; Yang, Z. Biotransformation of linoleic acid into different metabolites by food derived Lactobacillus plantarum 12-3 and in silico characterization of relevant reactions. Food Res. Int. 2021, 147, 110470. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Lun, H.; Yang, W.; Zhao, S.; Jiang, M.; Xu, M.; Liu, F.; Wang, Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. MicrobiologyOpen 2018, 8, e00678. [Google Scholar] [CrossRef] [PubMed]
| No. | Authors | Country | Sample (E/C) | Subjects Age (Years) | Sources of Participants | Gender | Exercise Protocol | Control Group | Main Outcomes | Region |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S. Donati Zeppa et al., 2021 [17] | Italy | 17 | 22 ± 2 | healthy college students | both | 36 indoor cycling training sessions during nine wks were performed. | self | Alpha diversity indexes (Chao1, Shannon and Simpson) | V3–V4 |
| 2 | Resende et al., 2021 [18] | Brazil | 12 | 25.18 ± 4.66 | healthy Brazilian men | male | 3 supervised training sessions per wk on non-consecutive days, lasting 50 min each, were performed over 10 wks | self | Alpha diversity index (Shannon) | V4 |
| 3 | Laura Mancin et al., 2022 [19] | Turkey | 16 | 25.5 ± 2.8 | semiprofessional soccer players | male | 8 h of training/wk | self | OTU & Shannon | V3–V4 |
| 4 | Nazareth Castellanos et al., 2019 [20] | Spain | 109 (64 + 45) | 32.17 ± 7.40 | Universidad Europea de Madrid | both | 3 days of exercise per wk for 30 min at a moderate intensity (bicycling at a regular pace, swimming or other fitness activities) | no intervention | Alpha diversity indexes (Chao1, Shannon and Simpson) | V3–V4 |
| 5 | Fei Zhong et al., 2022 [21] | China | 8 | 66.38 ± 4.07 | community | female | 4 times per wk. Each session lasted approximately 60 min of combined aerobic exercise (20 min) and resistance exercise (25 min) | self | Alpha diversity indexes (Chao1, Shannon and Simpson) | V3–V4 |
| 6 | Rebecca J. H. M. Verheggen et al., 2021 [22] | Netherlands | 14 | 51 ± 11 | inactive participants with obesity | 50% | 8 wks’ cycling exercise on an ergometer (Lode), starting with a 5-min warm-up, followed by 50 min of exercise at 65% to 85% of the individual HRR, and ending with a cooldown of 5 min. | self | Alpha diversity indexes (Chao1, Shannon and Simpson) | V5–V6 |
| 7 | Lucas Moitinho-Silva et al., 2021 [23] | Germany | 24 (13 + 11) | 30 ± 9.9 | healthy physically inactive German male and female | both | run 3 times per wk for at least 30 min for 6 wks | con | Alpha diversity indexes (Chao1, Shannon and Simpson) | V1–V2 |
| 8 | Siobhan F Clarke et al., 2014 [14] | Ireland | 63 (40 + 23) | 29 ± 4 | Male elite professional rugby players | male | NA | no intervention | Alpha diversity indexes (Chao1, Shannon and Simpson) | V4 |
| 9 | L. Torquati et al., 2022 [15] | Australia | 12 | 63.29 ± 6.625 | T2D | both | HIIT or MICT for 8 weeks | self | Alpha diversity indexes (Chao1, Shannon and Simpson) | NA |
| 10 | Viktor Bielik et al., 2022 [24] | Slovakia | 12 | 16–25 | swimmers competitive at the national level. The athletes came from two swimming clubs. | both | HIIT for 7 wks | self | Alpha diversity indexes (Chao1, Shannon and Simpson) | V1–V3 |
| 11 | Elizabeth A Rettedal et al., 2020 [25] | New Zealand | 29 | 20–45 | lean and overweight men | male | nine sessions of cycle ergometer HIIT on non-consecutive days over 3 wks | self | Alpha and Beta diversity | V3–V4 |
| 12 | YeonGyun Jung et al., 2020 [26] | Korea | 104 | N/A | navy trainees | both | through basic military training, and cardio and weight training performed together every day for 8 wks | self | Alpha and Beta diversity | V4–V5 |
| 13 | Mariangela Tabone et al., 2021 [27] | Spain | 40 | 35.79 ± 8.01 | different cross-country athletes’ teams in Madrid, Spain | male | ran with a slope of 1% at a speed of 10 km/h, with increments of 0.3 km/h every 30 s until volitional exhaustion. | self | Alpha and Beta diversity | V3–V4 |
| 14 | Owen Cronin et al., 2020 [28] | Ireland | 52 (25 + 27) | 35 (28, 38) | physically inactive for at least 3 months prior | both | an 8-wk (3 times per wk) mixed aerobic and resistance exercise training program with protein supplementation | protein supplementation | Alpha diversity indexes (Chao1, Shannon and Simpson) | NA |
| 15 | Eveliina Munukka et al., 2018 [29] | Finland | 17 | 36.8 ± 3.9 | sedentary overweight women | female | a 6 wks endurance exercise | self | Alpha diversity indexes (Chao1, Shannon and Simpson) | V4 |
| 16 | Shaodong Wei et al., 2022 [30] | Danish | 86 (60 + 26) | 54.3 ± 8.9 | above 18 years old, T2D diagnosis for less than 10 years | both | five to six weekly aerobic sessions whereof two to three sessions were combined with resistance training; 12 months | standard care group | Alpha and Beta diversity | V3–V4 |
| 17 | C. J. Stewart et al., 2017 [31] | UK | 20 (10 + 10) | 26.9 ± 5.06 | a duration of diabetes > 5 years | male | aerobic-based exercise for a minimum of 30 min at a time, at least three times per week | self | V4 | |
| 18 | Carlo Bressa et al., 2017 [32] | Spain | 40 (19 + 21) | 30.7 ± 5.9 | premenopausal women | female | at least 3 h of physical exercise per week | sedentary women | Alpha diversity indexes (Chao1, Shannon H and Simpson) | V3–V4 |
| 19 | Xueqing Zhang et al., 2023 [33] | China | 93 | 20.23 ± 0.62 | College Students with IDA | both | 8 wk of Tai Chi, 3 times a wk, 60 min, or 8-week conventional exercise, 60 min, 3 times per wk | no intervention | Alpha diversity (Shannon, Chao1, Simpson) & Beta diversity | V3–V4 |
| 20 | Camilla J Williams et al., 2022 [34] | Australia | 40 (20 + 20) | 30.4 ± 9.8 | sedentary and apparently healthy adults | both | 6 wk of supervised HIIT (4 × 4-min bouts at 85–95% HRmax, 3·wk–1) | self | Alpha diversity indexes (Shannon) | NA |
| 21 | Runtan Cheng et al., 2022 [35] | China | 40 (22 + 18) | 59.4 ± 0.5 | nonalcoholic fatty liver disease | both | progressive aerobic exercise training program, 2–3 times a wk, 30–60 min per session with 60–75% of the VO2Max. | no intervention | Alpha diversity indexes (Shannon) | V3–V4 |
| 22 | Kristen S.Smith et al., 2022 [36] | USA | 24 | 20.8 ± 1.7 | young female adults | female | 10 wks of Resistance T raining | self | Alpha diversity indexes (Shannon and Simpson) | V4 |
| 23 | Dongyang Kang et al., 2023 [37] | China | 30 (15 + 15) | N/A | basketball players from university | male | 90 min Tai Chi, 6 days per week, 5 months | no intervention | OUT & Alpha diversity indexes (Chao1, Shannon, Faith’s PD) | V3–V4 |
| 24 | Yuping Zhu et al., 2023 [38] | China | 32 | 30.30 ± 5.28 | MA dependent individuals | both | aerobic exercise, 3 times per wk, 60 min, 8 wks. | self | Alpha diversity indexes (Shannon) | V3–V4 |
| 25 | Shrushti Shah et al., 2023 [39] | Canada | 110 | 56.23 ± 6.36 | People with normal BMI and High BMI | both | IPAQ | people with low physical activity | Alpha diversity indexes (Shannon) & Beta diversity (Bray–Curtis dissimilarity metrics) | V3–V4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, L.; Ablitip, A.; Wang, R.; Luciana, T.; Wei, M.; Ma, X. Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1070. https://doi.org/10.3390/nu16071070
Min L, Ablitip A, Wang R, Luciana T, Wei M, Ma X. Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(7):1070. https://doi.org/10.3390/nu16071070
Chicago/Turabian StyleMin, Leizi, Alimjan Ablitip, Rui Wang, Torquati Luciana, Mengxian Wei, and Xindong Ma. 2024. "Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis" Nutrients 16, no. 7: 1070. https://doi.org/10.3390/nu16071070
APA StyleMin, L., Ablitip, A., Wang, R., Luciana, T., Wei, M., & Ma, X. (2024). Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients, 16(7), 1070. https://doi.org/10.3390/nu16071070






