Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Selection and Housing Conditions
2.2. Tested Compounds and Dosages
2.3. Experimental Design and Group Allocation
2.4. Monitoring of Physiological Parameters
2.5. Hormones and Neurotransmitters Analysis
2.6. Statistical Analysis
3. Results
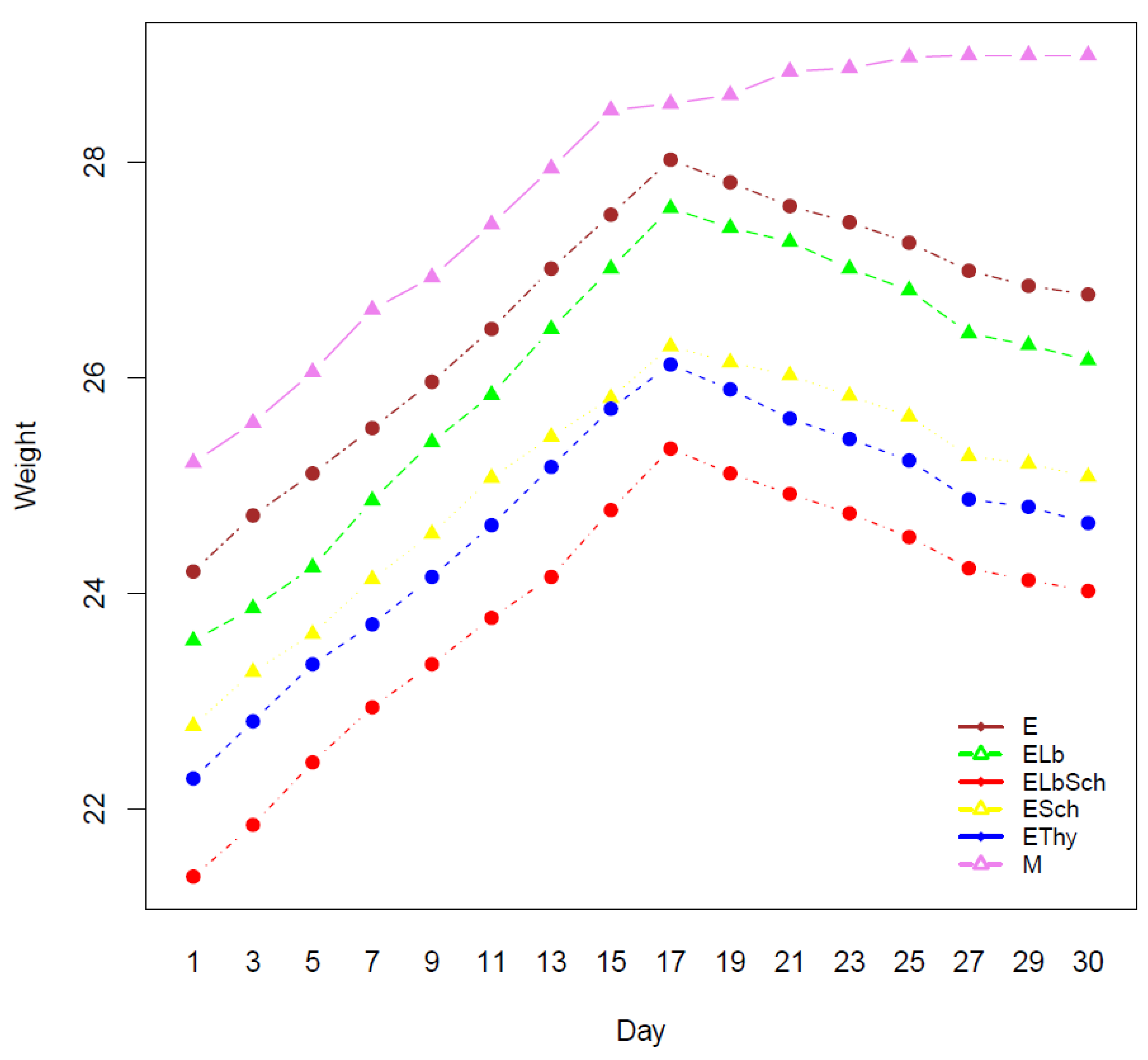
3.1. Monitoring of Physiological Parameters
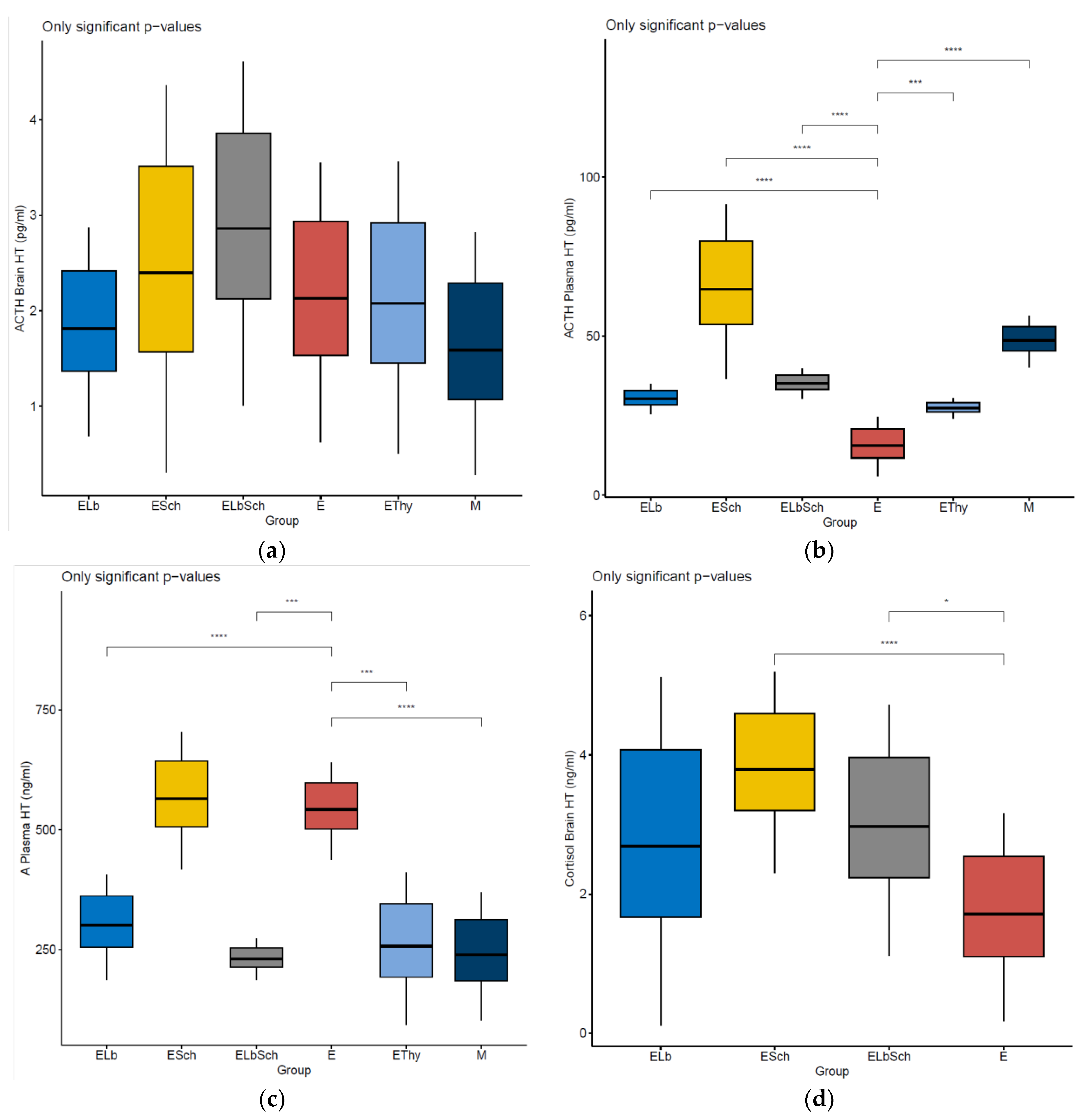
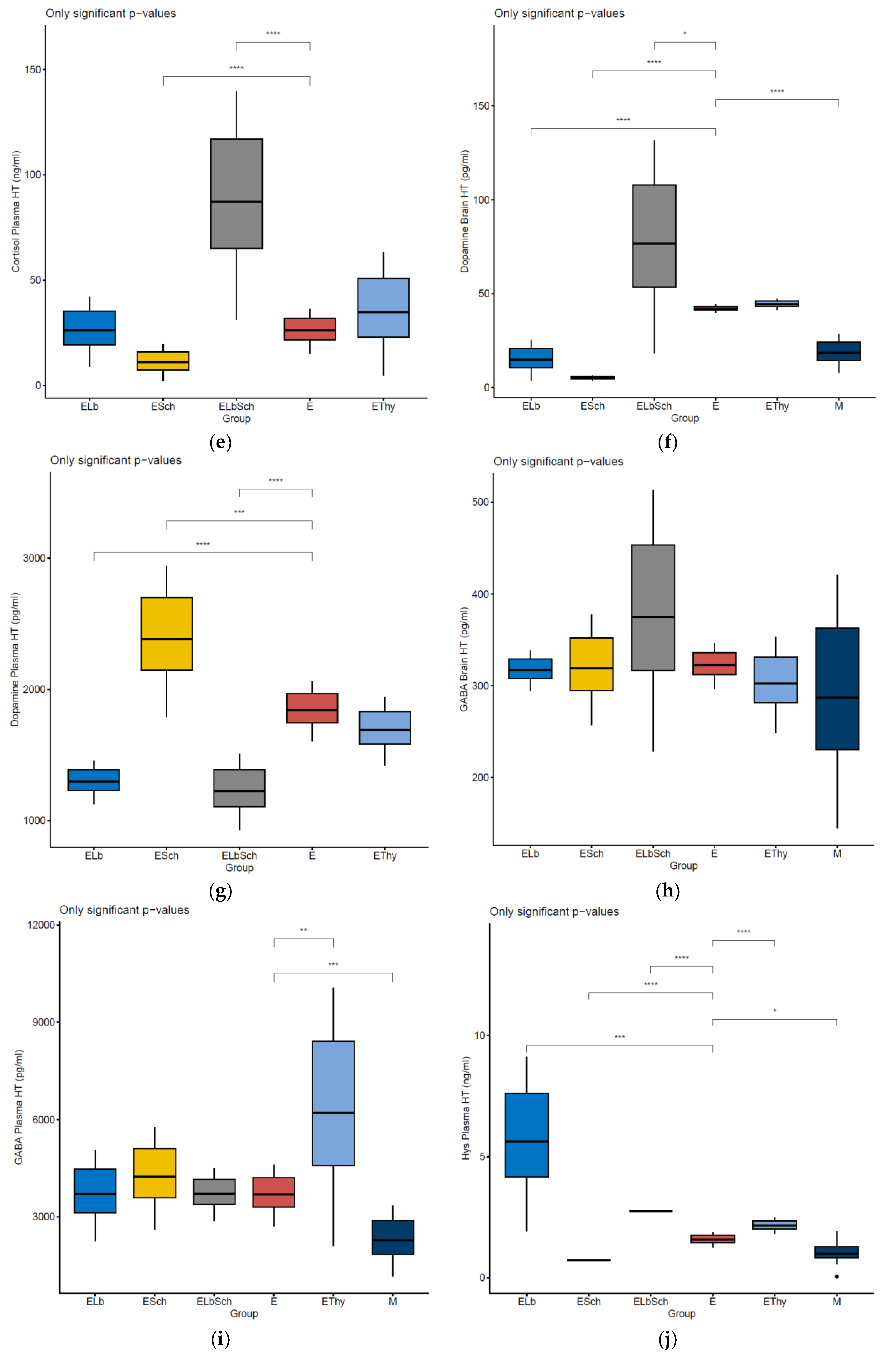
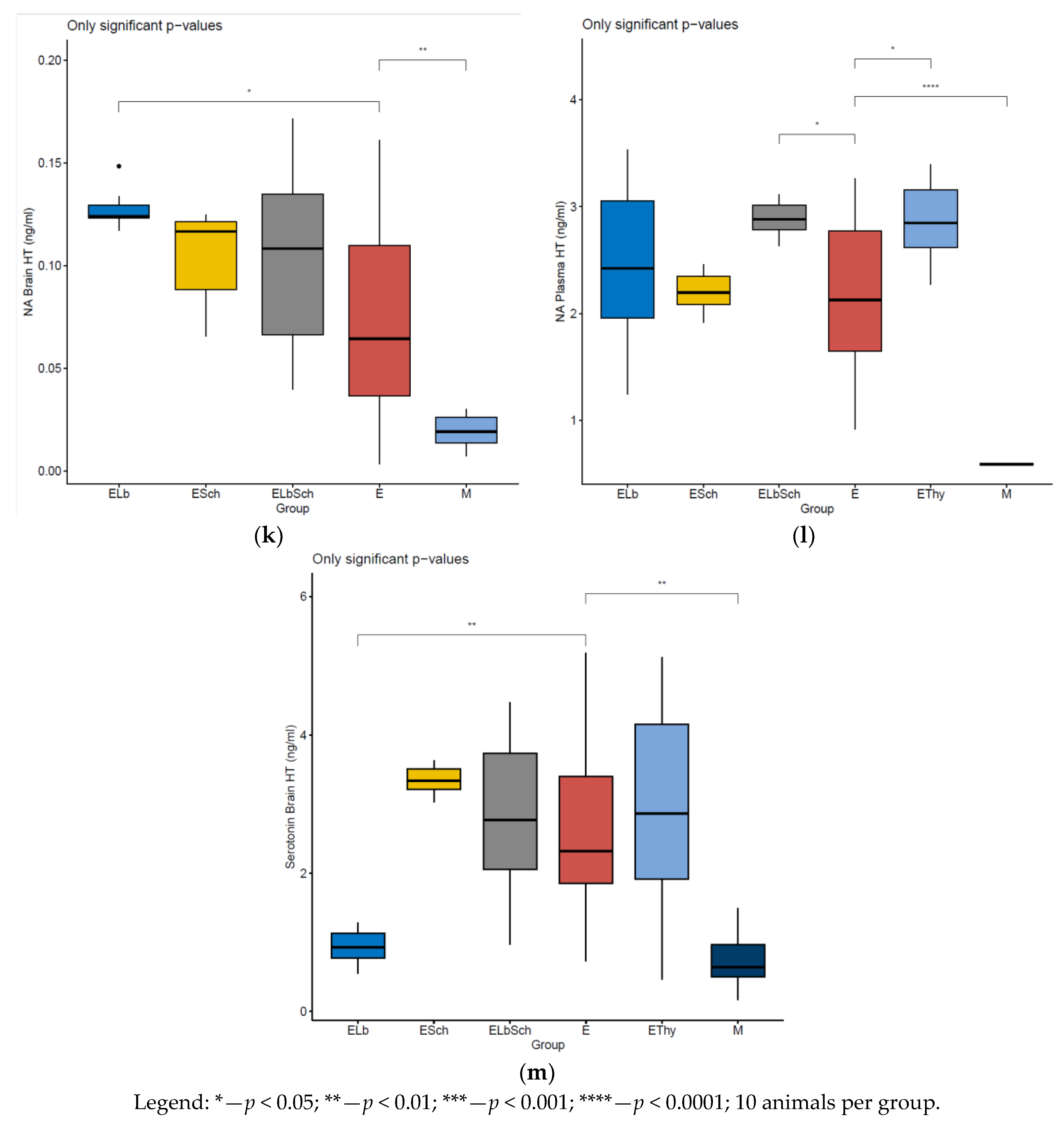
3.2. Variation in Dosed Parameters
3.3. Percentage Change in Dosed Parameter Effect (%) Compared with That in E, EThy and M Groups
4. Discussion
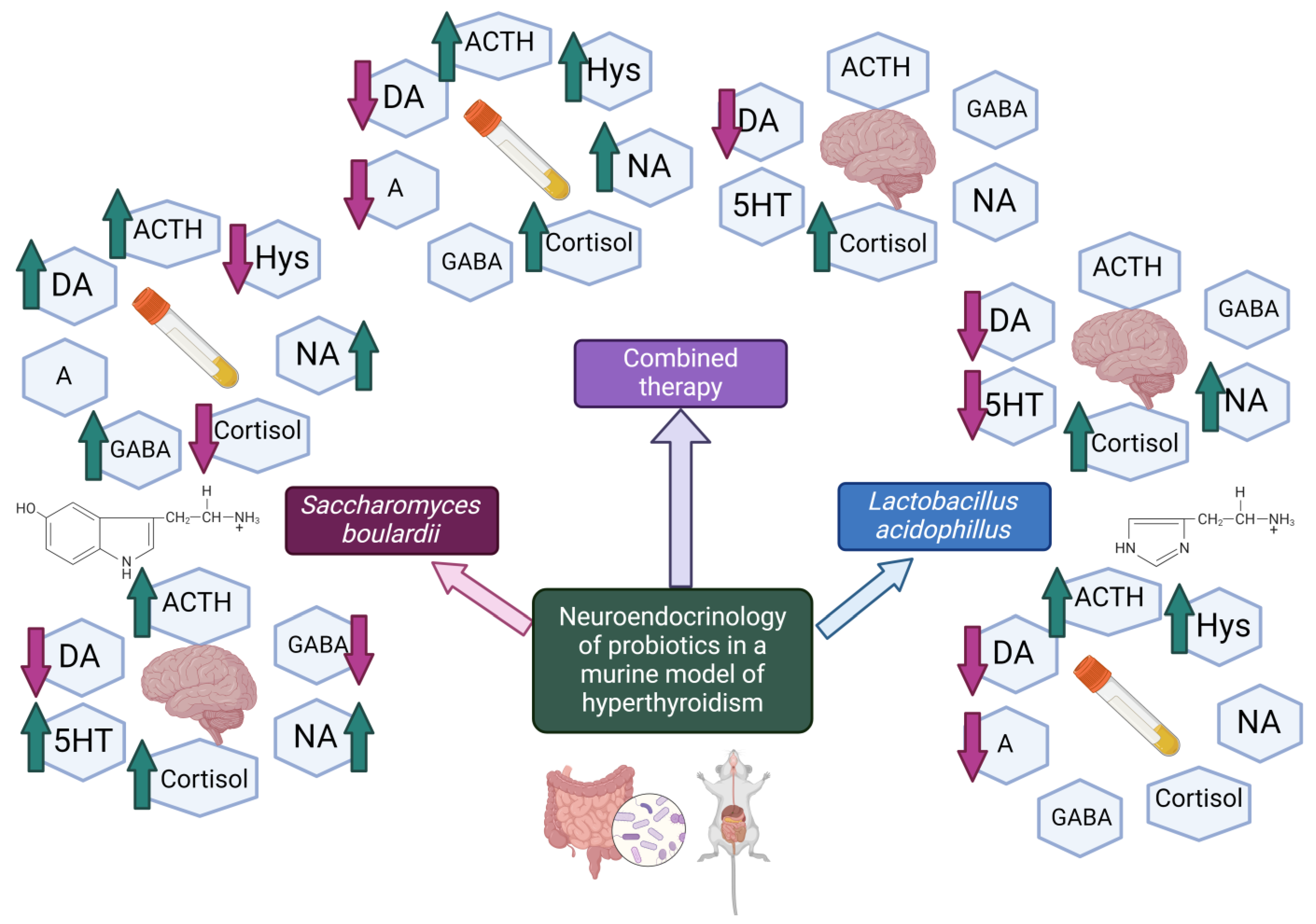
4.1. Evaluation of Neurohormonal Dynamics after Hyperthyroidism Induction
4.2. Effects of Lactobacillus acidophilus Administration in an Experimental Hyperthyroidism Model
4.3. Effects of Saccharomyces boulardii Administration in an Experimental Hyperthyroidism Model
4.4. Effects of Lactobacillus acidophilus and Saccharomyces boulardii Administration in an Experimental Hyperthyroidism Model
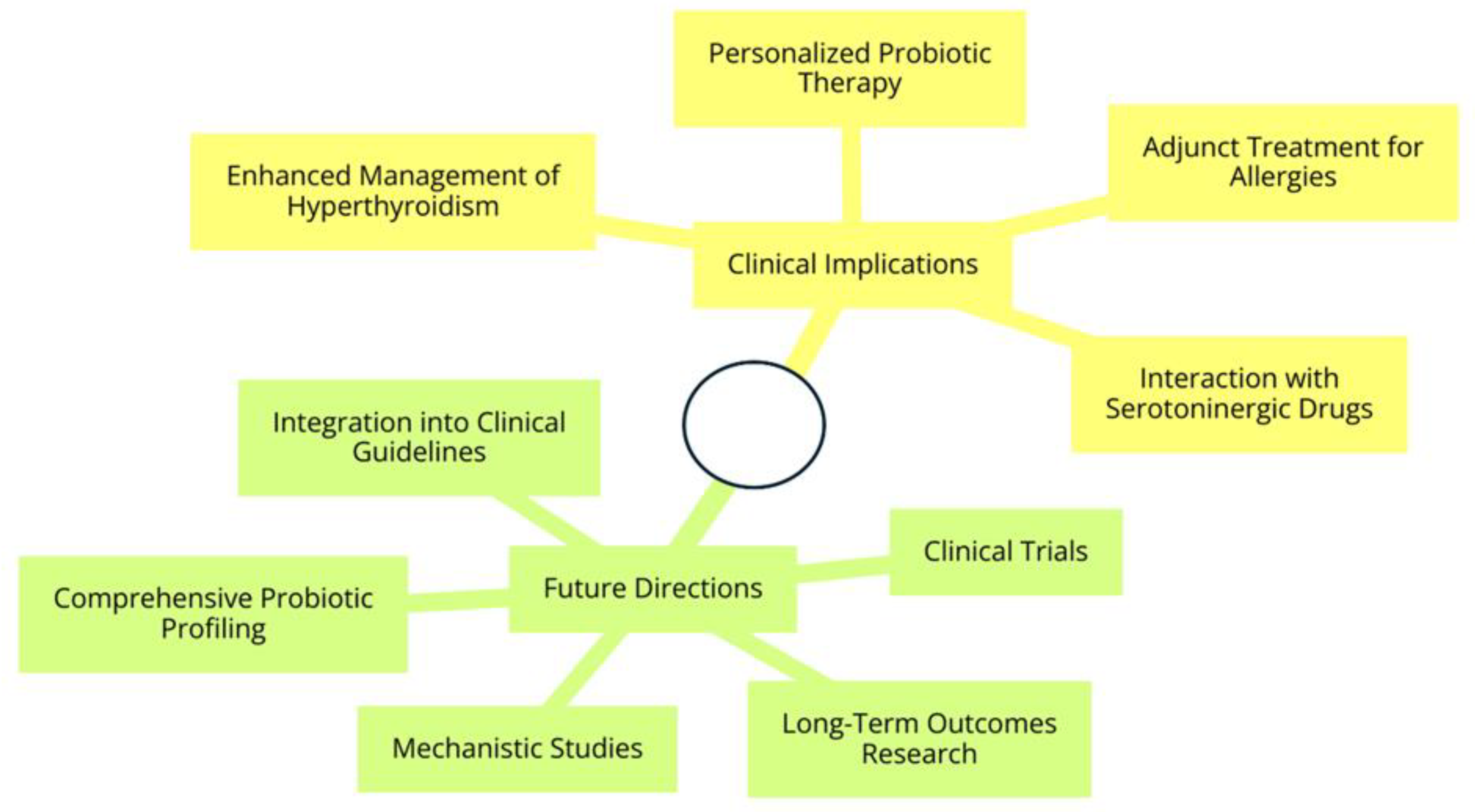
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Matei, M.-C. Evaluation of Endotoxemia, Soluble CD14 and IL-1β in Dogs with Intestinal Dysbiosis That Were Treated with Probiotics: A Prospective Study. Farmacia 2021, 69, 1153–1158. [Google Scholar] [CrossRef]
- Jandhyala, S.M. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Rusu, R.-N. Factors That Influence Treatment Adherence—Realities, Controversies, Perspectives. Farmacia 2023, 71, 638–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, D.-D.; Zhang, Y.-F.; Cheng, M.-D.; Zhu, W.-X.; Li, P.-F.; Zhang, Y.-F. The Emerging Function and Clinical Significance of CircRNAs in Thyroid Cancer and Autoimmune Thyroid Diseases. Int. J. Biol. Sci. 2021, 17, 1731–1741. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Zhao, L.; Li, H. The Gut Microbiota: Emerging Evidence in Autoimmune Diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef]
- Opazo, M.C.; Ortega-Rocha, E.M.; Coronado-Arrázola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.; Kalergis, A.M.; Riedel, C.A. Intestinal Microbiota Influences Non-Intestinal Related Autoimmune Diseases. Front. Microbiol. 2018, 9, 432. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Angoorani, P.; Soroush, A.-R.; Siadat, S.-D.; Shirzad, N.; Hasani-Ranjbar, S.; Larijani, B. Our Little Friends with Big Roles: Alterations of the Gut Microbiota in Thyroid Disorders. Endocr. Metab. Immune Disord.—Drug Targets 2020, 20, 344–350. [Google Scholar] [CrossRef]
- Talebi, S.; Karimifar, M.; Heidari, Z.; Mohammadi, H.; Askari, G. The Effects of Synbiotic Supplementation on Thyroid Function and Inflammation in Hypothyroid Patients: A Randomized, Double-blind, Placebo-controlled Trial. Complement. Ther. Med. 2020, 48, 102234. [Google Scholar] [CrossRef]
- Nakajima, H. About the Evaluation of Liver Disease by the Monitoring of Mahalanobis Distance: Examination for Acute Hepatic Failure. J. Community Med. Health Educ. 2013, 3, 220. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Zhao, C.; Xu, Q.; Liang, C.; Yang, Y.; Wang, H.; Shang, Y.; Wang, Y.; Mu, X.; et al. Dysbiosis of the Gut Microbiome Is Associated with Thyroid Cancer and Thyroid Nodules and Correlated with Clinical Index of Thyroid Function. Endocrine 2019, 64, 564–574. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The Function of Gut Microbiota in Immune-Related Neurological Disorders: A Review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6. [Google Scholar] [CrossRef]
- Amzăr, A.I. Signalling through the Microbiota-Gut-Brain Triade. Farmacia 2022, 70, 402–409. [Google Scholar] [CrossRef]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an Emerging Neurometabolic Facet of the Gut Microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, E.; Mikhailopulo, K.; Sviridov, O.; Novik, G.; Knirel, Y.; Szwajcer Dey, E. The Role of Components of Bifidobacterium and Lactobacillus in Pathogenesis and Serologic Diagnosis of Autoimmune Thyroid Diseases. Benef. Microbes 2011, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior. PLoS Pathog. 2013, 9, e1003726. [Google Scholar] [CrossRef] [PubMed]
- Casals-Pascual, C.; Vergara, A.; Vila, J. Intestinal Microbiota and Antibiotic Resistance: Perspectives and Solutions. Hum. Microbiome J. 2018, 9, 11–15. [Google Scholar] [CrossRef]
- Shizuma, T. Concomitant Thyroid Disorders and Inflammatory Bowel Disease: A Literature Review. Biomed Res. Int. 2016, 2016, 5187061. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, S.; Giral, M.; Ruiz de la Torre, J.L.; Russell, R.J.; Kramer, K. Acclimatization of Rats after Ground Transportation to a New Animal Facility. Lab. Anim. 2007, 41, 255–261. [Google Scholar] [CrossRef]
- Guidelines for Acclimation for Newly Received Laboratory Animals. Available online: https://www.research.uky.edu/division-laboratory-animal-resources/guidelines-acclimation-newly-received-laboratory-animals (accessed on 10 January 2024).
- Li, Z.; Ma, S.; Wang, X.; Wang, Y.; Yan, R.; Wang, J.; Xu, Z.; Wang, S.; Feng, Y.; Wang, J.; et al. Pharmacokinetic and Gut Microbiota Analyses Revealed the Effect of Lactobacillus Acidophilus on the Metabolism of Olsalazine in Ulcerative Colitis Rats. Eur. J. Pharm. Sci. 2022, 175, 106235. [Google Scholar] [CrossRef]
- Moorthy, G.; Murali, M.R.; Devaraj, S.N. Protective Role of Lactobacilli in Shigella Dysenteriae 1–Induced Diarrhea in Rats. Nutrition 2007, 23, 424–433. [Google Scholar] [CrossRef]
- Engels, K.; Rakov, H.; Zwanziger, D.; Hönes, G.S.; Rehders, M.; Brix, K.; Köhrle, J.; Möller, L.C.; Führer, D. Efficacy of Protocols for Induction of Chronic Hyperthyroidism in Male and Female Mice. Endocrine 2016, 54, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.; da Silva, A.E.; Serakides, R.; Gomes, M.G.; Cassali, G.D. Ehrlich Tumor as Model to Study Artificial Hyperthyroidism Influence on Breast Cancer. Pathol.—Res. Pract. 2007, 203, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Isman, C.; Yegen, B.; Alican, I. Methimazole-Induced Hypothyroidism in Rats Ameliorates Oxidative Injury in Experimental Colitis. J. Endocrinol. 2003, 177, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Han, Y.; Mail, W.; Liu, J.; Wang, H.; Feng, L.; Gao, L.; Zhao, J.-J. Different Doses and Routes of Administration of Methimazole Affect Thyroid Status in Methimazole-Induced Hypothyroidism in Rats. West Indian Med. J. 2015, 65, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the Effect of Probiotics Supplementation on Intestinal Microbiota of Food Allergic Mice. Am. J. Transl. Res. 2017, 9, 376–385. [Google Scholar] [PubMed]
- Sierra, S.; Lara-Villoslada, F.; Sempere, L.; Olivares, M.; Boza, J.; Xaus, J. Intestinal and Immunological Effects of Daily Oral Administration of Lactobacillus Salivarius CECT5713 to Healthy Adults. Anaerobe 2010, 16, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Lara-Villoslada, F.; Sierra, S.; Maldonado, J.A.; Martín, R.; López-Huertas, E.; Rodríguez, J.M.; Xaus, J. Oral Administration of Two Probiotic Strains, Lactobacillus Gasseri CECT5714 and Lactobacillus Coryniformis CECT5711, Enhances the Intestinal Function of Healthy Adults. Int. J. Food Microbiol. 2006, 107, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of Oral Consumption of Probiotic Lactobacillus Planatarum P-8 on Fecal Microbiota, SIgA, SCFAs, and TBAs of Adults of Different Ages. Nutrition 2014, 30, 776–783.e1. [Google Scholar] [CrossRef]
- Montero-Pedrazuela, A.; Fernández-Lamo, I.; Alieva, M.; Pereda-Pérez, I.; Venero, C.; Guadaño-Ferraz, A. Adult-Onset Hypothyroidism Enhances Fear Memory and Upregulates Mineralocorticoid and Glucocorticoid Receptors in the Amygdala. PLoS ONE 2011, 6, e26582. [Google Scholar] [CrossRef]
- Sánchez-Franco, F.; Fernández, L.; Fernández, G.; Cacicedo, L. Thyroid Hormone Action on ACTH Secretion. Horm. Metab. Res. 1989, 21, 550–552. [Google Scholar] [CrossRef]
- Hidal, J.T.; Kaplan, M.M. Inhibition of Thyroxine 5′-Deiodination Type II in Cultured Human Placental Cells by Cortisol, Insulin, 3′,5′-Cyclic Adenosine Monophosphate, and Butyrate. Metabolism 1988, 37, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Martinez-deMena, R.; Obregón, M.-J. Insulin Increases the Adrenergic Stimulation of 5′ Deiodinase Activity and MRNA Expression in Rat Brown Adipocytes; Role of MAPK and PI3K. J. Mol. Endocrinol. 2005, 34, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve Their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.; Beausoleil, N. Extending the ‘Five Domains’ Model for Animal Welfare Assessment to Incorporate Positive Welfare States. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Sherwin, C.M. Can Invertebrates Suffer? Or, How Robust Is Argument-By-Analogy? Anim. Welf. 2001, 10, S103–S118. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism. JAMA 2023, 330, 1472. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The Hypothalamus-Pituitary-Thyroid (HPT)-Axis and Its Role in Physiology and Pathophysiology of Other Hypothalamus-Pituitary Functions. Mol. Cell. Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef]
- Stanculescu, D.; Larsson, L.; Bergquist, J. Hypothesis: Mechanisms That Prevent Recovery in Prolonged ICU Patients Also Underlie Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Med. 2021, 8, 628029. [Google Scholar] [CrossRef]
- Lizcano, F.; Rodríguez, J.S. Thyroid Hormone Therapy Modulates Hypothalamo-Pituitary-Adrenal Axis. Endocr. J. 2011, 58, 137–142. [Google Scholar] [CrossRef]
- Hughes, K.; Eastman, C. Thyroid Disease: Long-Term Management of Hyperthyroidism and Hypothyroidism. Aust. J. Gen. Pract. 2021, 50, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Cen, C.; Chang, H.; Ou, Q.; Jiang, S.; Pan, Y.; Chen, K.; Zhang, J. Probiotic Bifidobacterium Longum Supplied with Methimazole Improved the Thyroid Function of Graves’ Disease Patients through the Gut-Thyroid Axis. Commun. Biol. 2021, 4, 1046. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Kamilaris, T.C.; Calogero, A.E.; Gold, P.W.; Chrousos, G.P. Experimentally-Induced Hyperthyroidism Is Associated with Activation of the Rat Hypothalamic–Pituitary–Adrenal Axis. Eur. J. Endocrinol. 2005, 153, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Heinz, A.; Whybrow, P.C. Thyroid Hormones, Serotonin and Mood: Of Synergy and Significance in the Adult Brain. Mol. Psychiatry 2002, 7, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, E.H.; Schultz, H.D. Hypothyroidism Stimulates D2 Receptor-Mediated Breathing in Response to Acute Hypoxia and Alters D2 Receptors Levels in Carotid Bodies and Brain. Respir. Physiol. Neurobiol. 2012, 180, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Crocker, A.D.; Overstreet, D.H.; Crocker, J.M. Hypothyroidism Leads to Incresed Dopamine Receptor Sensitivity and Concentration. Pharmacol. Biochem. Behav. 1986, 24, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, P.B.; Kirmaz, C.; Oz, D.; Bilgir, F.; Ozmen, B.; Degirmenci, M.; Colak, H.; Yilmaz, H.; Ozyurt, B. Allergic Rhinitis and Its Relationship with Autoimmune Thyroid Diseases. Am. J. Rhinol. Allergy 2015, 29, 257–261. [Google Scholar] [CrossRef]
- Erzsebet Kelemen, I.M. The Prevalence and Characteristics of Allergy in Autoimmune Thyroid Diseases. J. Clin. Cell. Immunol. 2015, 6, 306. [Google Scholar] [CrossRef]
- Wiens, S.C.; Trudeau, V.L. Thyroid Hormone and γ-Aminobutyric Acid (GABA) Interactions in Neuroendocrine Systems. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 332–344. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Morelli, M.B.; Gambardella, J.; Lombardi, A.; Santulli, G. Thyroid Hormones Regulate Both Cardiovascular and Renal Mechanisms Underlying Hypertension. J. Clin. Hypertens. 2021, 23, 373–381. [Google Scholar] [CrossRef]
- Hage, M.P.; Azar, S.T. The Link between Thyroid Function and Depression. J. Thyroid Res. 2012, 2012, 590648. [Google Scholar] [CrossRef]
- Sabit, H.; Kassab, A.; Alaa, D.; Mohamed, S.; Abdel-Ghany, S.; Mansy, M.; Said, O.A.; Khalifa, M.A.; Hafiz, H.; Abushady, A.M. The Effect of Probiotic Supplementation on the Gut–Brain Axis in Psychiatric Patients. Curr. Issues Mol. Biol. 2023, 45, 4080–4099. [Google Scholar] [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr. Rev. 2020, 41, bnaa002. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 117864691985299. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Ranuh, R.; Athiyyah, A.F.; Darma, A.; Risky, V.P.; Riawan, W.; Surono, I.S.; Sudarmo, S.M. Effect of the Probiotic Lactobacillus Plantarum IS-10506 on BDNF and 5HT Stimulation: Role of Intestinal Microbiota on the Gut-Brain Axis. Iran. J. Microbiol. 2019, 11, 145–150. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of Regulating Gut Microbiota on the Serotonin Metabolism in the Chronic Unpredictable Mild Stress Rat Model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Picca, A.; Lo Monaco, M.R.; Landi, F.; Bernabei, R.; Marzetti, E. Of Microbes and Minds: A Narrative Review on the Second Brain Aging. Front. Med. 2018, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Winiarska, E.; Globinska, A.; Jutel, M. Histamine: A Mediator of Intestinal Disorders—A Review. Metabolites 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Major, A.; Rendon, D.; Lugo, M.; Jackson, V.; Shi, Z.; Mori-Akiyama, Y.; Versalovic, J. Histamine H2 Receptor-Mediated Suppression of Intestinal Inflammation by Probiotic Lactobacillus Reuteri. MBio 2015, 6, e01358-15. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine Derived from Probiotic Lactobacillus Reuteri Suppresses TNF via Modulation of PKA and ERK Signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Hariu, M.; Nakashima, Y.; Watanabe, K.; Yasuda, S.; Igoshi, K. Lactic Acid Bacterial Exopolysaccharides Strongly Bind Histamine and Can Potentially Be Used to Remove Histamine Contamination in Food. Microbiology 2021, 167. [Google Scholar] [CrossRef]
- Levey, G.S.; Klein, I. Catecholamine-Thyroid Hormone Interactions and the Cardiovascular Manifestations of Hyperthyroidism. Am. J. Med. 1990, 88, 642–646. [Google Scholar] [CrossRef]
- Bode, H.; Ivens, B.; Bschor, T.; Schwarzer, G.; Henssler, J.; Baethge, C. Hyperthyroidism and Clinical Depression: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2022, 12, 362. [Google Scholar] [CrossRef]
- Hasler, G. Pathophysiology of Depression: Do We Have Any Solid Evidence of Interest to Clinicians? World Psychiatry 2010, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.D.N.; Wanner, S.P.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.S.; Cardoso, V.N. Supplementation with Saccharomyces Boulardii Increases the Maximal Oxygen Consumption and Maximal Aerobic Speed Attained by Rats Subjected to an Incremental-Speed Exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, C.; Qin, X.; Zhou, B.; Liu, X.; Liu, T.; Xie, R.; Liu, J.; Wang, B.; Cao, H. Saccharomyces Boulardii, a Yeast Probiotic, Inhibits Gut Motility through Upregulating Intestinal Serotonin Transporter and Modulating Gut Microbiota. Pharmacol. Res. 2022, 181, 106291. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell. Infect. Microbiol. 2021, 11, 760076. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Pothoulakis, C. Review Article: Anti-inflammatory Mechanisms of Action of Saccharomyces Boulardii. Aliment. Pharmacol. Ther. 2009, 30, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Takemura, Y.; Yamada, T.; Ohtsuka, H.; Sakai, H.; Miyahara, Y.; Aizawa, T.; Terao, A.; Onuma, S.; Junen, K.; et al. A Possible Role of Immunoglobulin E in Patients with Hyperthyroid Graves’ Disease. J. Clin. Endocrinol. Metab. 1999, 84, 3602–3605. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, N.; Ertel, N. Urticaria and Pruritus: Uncommon Manifestations of Hyperthyroidism. J. Allergy Clin. Immunol. 1971, 48, 73–81. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef]
- Akram, N.; Faisal, Z.; Irfan, R.; Shah, Y.A.; Batool, S.A.; Zahid, T.; Zulfiqar, A.; Fatima, A.; Jahan, Q.; Tariq, H.; et al. Exploring the serotonin-probiotics-gut Health Axis: A Review of Current Evidence and Potential Mechanisms. Food Sci. Nutr. 2024, 12, 694–706. [Google Scholar] [CrossRef]

| Score | Parameters |
|---|---|
| Fur | |
| [0] | Clean and smooth; no signs of neglect or damage. |
| [1] | Slightly unkempt; may have some areas that are more matted. |
| [2] | Matted or with missing portions; visible signs of neglect. |
| [3] | Visible lesions, extensive areas without fur, signs of self-mutilation. |
| Eyes | |
| [0] | Clear and open; no discharge or redness. |
| [1] | Slight discharge or slight redness. |
| [2] | Obvious discharge or redness; signs of irritation. |
| [3] | Closed, inflamed, or showing signs of infection or injury. |
| Activity | |
| [0] | Normal, active, moving freely. |
| [1] | Slightly lethargic; some changes in mobility. |
| [2] | Lethargic, with significant difficulty moving. |
| [3] | Immobility; unable or unwilling to move. |
| Food and water consumption | |
| [0] | Normal, consumes usual amounts of food and water. |
| [1] | Slightly reduced; we observe a decrease in interest in food and water. |
| [2] | Significantly reduced; consumes very little food and water. |
| [3] | Complete refusal; does not consume food or water. |
| Aggressiveness | |
| [0] | Calm, normal behaviour: the animal interacts peacefully; there are no signs of unwarranted aggressive or defensive behaviour. |
| [1] | Easily irritable: shows minor signs of irritability when provoked or under stress. These may include avoiding contact or sudden movements, but without resorting to aggression. |
| [2] | Moderately aggressive: shows sporadic aggressive behaviours such as biting, scratching, or pushing other animals, especially in situations of competition for resources or space. |
| [3] | Highly aggressive: frequently exhibits aggressive behaviours, even in the absence of direct provocation. It can be dangerous for congeners or for people who take care of the animals. Aggressiveness is evident even without the pressure of external stressors. |
| Interpretation of the final score | |
| Score 0–0.5 | Excellent state of health, without signs of discomfort or stress. |
| Score 0.6–1.5 | Slight concern; needs further monitoring. |
| Score 1.6–2.5 | Moderate health problems may require interventions. |
| Score 2.6–3.0 | Severe health problems, requiring immediate intervention. |
| Mean ± SD | ELb | ESch | ELbSch | E | EThy | M |
|---|---|---|---|---|---|---|
| Cortisol Brain HT (ng/mL) | 1.63 ± 1.56 | 3.18 ± 0.90 | 2.21 ± 1.12 | 1.08 ± 0.93 | N/A | N/A |
| Cortisol Plasma HT (ng/mL) | 19.03 ± 10.31 | 7.29 ± 5.42 | 64.2 ± 33.68 | 21.49 ± 6.65 | 22.47 ± 18.11 | N/A |
| ACTH Brain HT (pg/mL) | 1.35 ± 0.68 | 1.54 ± 1.26 | 2.1 ± 1.12 | 1.51 ± 0.91 | 1.43 ± 0.95 | 1.05 ± 0.79 |
| ACTH Plasma HT (pg/mL) | 28.27 ± 2.96 | 53.18 ± 17.06 | 33.13 ± 2.98 | 11.55 ± 5.84 | 26.03 ± 1.96 | 45.14 ± 5.03 |
| Serotonin Brain HT (ng/mL) | 0.77 ± 0.23 | 3.21 ± 0.19 | 2.03 ± 1.09 | 1.55 ± 2.04 | 1.88 ± 1.45 | 0.41 ± 0.61 |
| Dopamine Brain HT (pg/mL) | 10.35 ± 6.69 | 4.54 ± 0.92 | 52.66 ± 35.20 | 41.23 ± 1.33 | 43.19 ± 1.89 | 14.18 ± 6.41 |
| Dopamine Plasma HT (pg/mL) | 1228 ± 102.5 | 2140 ± 357.5 | 1103 ± 180.8 | 1743 ± 142.6 | 1578 ± 161.8 | N/A |
| Hys Plasma HT (ng/mL) | 4.11 ± 2.24 | 0.73 | 2.76 | 1.44 ± 0.20 | 2.02 ± 0.21 | 0.73 ± 0.80 |
| GABA Brain HT (pg/mL) | 307.8 ± 13.65 | 293.8 ± 37.31 | 314.8 ± 88.55 | 311.8 ± 15.48 | 280.6 ± 32.38 | 228.4 ± 85.84 |
| GABA Plasma HT (pg/mL) | 3106 ± 873.3 | 3569 ± 978.2 | 3363 ± 507.9 | 3287 ± 590.2 | 4522 ± 2477 | 1819 ± 676.6 |
| A Plasma HT (ng/mL) | 254.4 ± 68.35 | 504.4 ± 88.93 | 212.8 ± 26.59 | 499.8 ± 62.37 | 190.2 ± 98.74 | 183.7 ± 82.70 |
| NA Brain HT (ng/mL) | 0.13 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.05 | 0.08 ± 0.05 | 0.09 | 0.02 ± 0.01 |
| NA Plasma HT (ng/mL) | 1.94 ± 0.71 | 2.08 ± 0.17 | 2.78 ± 0.15 | 1.63 ± 0.73 | 2.61 ± 0.35 | 0.59 |
| Effect (%) vs. E | Elb vs. E (%) | ESch vs. E (%) | ELbSch vs. E (%) | EThy vs. E (%) | M vs. E (%) |
|---|---|---|---|---|---|
| Cortisol Brain HT (ng/mL) | 50.93 | 194.44 | 104.63 | N/A | N/A |
| Cortisol Plasma HT (ng/mL) | −11.45 | −66.08 | 198.74 | 4.56 | N/A |
| ACTH Brain HT (pg/mL) | −10.60 | 1.99 | 39.07 | −5.30 | −30.46 |
| ACTH Plasma HT (pg/mL) | 144.76 | 360.43 | 186.84 | 125.37 | 290.82 |
| Serotonin Brain HT (ng/mL) | −50.32 | 107.10 | 30.97 | 21.29 | −73.55 |
| Dopamine Brain HT (pg/mL) | −74.90 | −88.99 | 27.72 | 4.75 | −65.61 |
| Dopamine Plasma HT (pg/mL) | −29.55 | 22.78 | −36.72 | −9.47 | N/A |
| Hys Plasma HT (ng/mL) | 185.42 | −49.31 | 91.67 | 40.28 | −49.31 |
| GABA Brain HT (pg/mL) | −1.28 | −5.77 | 0.96 | −10.01 | −26.75 |
| GABA Plasma HT (pg/mL) | −5.51 | 8.58 | 2.31 | 37.57 | −44.66 |
| A Plasma HT (ng/mL) | −49.10 | 0.92 | −57.42 | −61.94 | −63.25 |
| NA Brain HT (ng/mL) | 62.50 | 37.50 | 25.00 | 12.50 | −75.00 |
| NA Plasma HT (ng/mL) | 19.02 | 27.61 | 70.55 | 60.12 | −63.80 |
| Effect (%) vs. EThy | ELb vs. EThy (%) | ESch vs. EThy (%) | ELbSch vs. EThy (%) | E vs. EThy (%) | M vs. EThy (%) |
| Cortisol Brain HT (ng/mL) | N/A | N/A | N/A | N/A | N/A |
| Cortisol Plasma HT (ng/mL) | −15.31 | −67.56 | 185.71 | −4.36 | N/A |
| ACTH Brain HT (pg/mL) | −5.59 | 7.69 | 46.85 | 5.59 | −26.57 |
| ACTH Plasma HT (pg/mL) | 8.61 | 104.30 | 27.28 | −55.63 | 73.42 |
| Serotonin Brain HT (ng/mL) | −59.04 | 70.74 | 7.98 | −17.55 | −78.19 |
| Dopamine Brain HT (pg/mL) | −76.04 | −89.49 | 21.93 | −4.54 | −67.17 |
| Dopamine Plasma HT (pg/mL) | −22.18 | 35.61 | −30.10 | 10.46 | N/A |
| Hys Plasma HT (ng/mL) | 103.47 | −63.86 | 36.63 | −28.71 | −63.86 |
| GABA Brain HT (pg/mL) | 9.69 | 4.70 | 12.19 | 11.12 | −18.60 |
| GABA Plasma HT (pg/mL) | −31.31 | −21.07 | −25.63 | −27.31 | −59.77 |
| A Plasma HT (ng/mL) | 33.75 | 165.19 | 11.88 | 162.78 | −3.42 |
| NA Brain HT (ng/mL) | 44.44 | 22.22 | 11.11 | −11.11 | −77.78 |
| NA Plasma HT (ng/mL) | −25.67 | −20.31 | 6.51 | −37.55 | −77.39 |
| Effect (%) vs. M | E vs. M (%) | ||||
| Cortisol Brain HT (ng/mL) | N/A | ||||
| Cortisol Plasma HT (ng/mL) | N/A | ||||
| ACTH Brain HT (pg/mL) | 43.81 | ||||
| ACTH Plasma HT (pg/mL) | −74.41 | ||||
| Serotonin Brain HT (ng/mL) | 278.05 | ||||
| Dopamine Brain HT (pg/mL) | 190.76 | ||||
| Dopamine Plasma HT (pg/mL) | N/A | ||||
| Hys Plasma HT (ng/mL) | 97.26 | ||||
| GABA Brain HT (pg/mL) | 36.51 | ||||
| GABA Plasma HT (pg/mL) | 80.70 | ||||
| A Plasma HT (ng/mL) | 172.07 | ||||
| NA Brain HT (ng/mL) | 300.00 | ||||
| NA Plasma HT (ng/mL) | 176.27 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, S.N.; Scărlătescu, A.I.; Apetroaei, M.-M.; Nedea, M.I.; Blejan, I.E.; Udeanu, D.I.; Velescu, B.Ș.; Ghica, M.; Nedea, O.A.; Cobelschi, C.P.; et al. Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism. Nutrients 2024, 16, 1077. https://doi.org/10.3390/nu16071077
Voicu SN, Scărlătescu AI, Apetroaei M-M, Nedea MI, Blejan IE, Udeanu DI, Velescu BȘ, Ghica M, Nedea OA, Cobelschi CP, et al. Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism. Nutrients. 2024; 16(7):1077. https://doi.org/10.3390/nu16071077
Chicago/Turabian StyleVoicu, Sorina Nicoleta, Anca Ioana (Amzăr) Scărlătescu, Miruna-Maria Apetroaei, Marina Ionela (Ilie) Nedea, Ionuț Emilian Blejan, Denisa Ioana Udeanu, Bruno Ștefan Velescu, Manuela Ghica, Octavian Alexandru Nedea, Călin Pavel Cobelschi, and et al. 2024. "Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism" Nutrients 16, no. 7: 1077. https://doi.org/10.3390/nu16071077
APA StyleVoicu, S. N., Scărlătescu, A. I., Apetroaei, M.-M., Nedea, M. I., Blejan, I. E., Udeanu, D. I., Velescu, B. Ș., Ghica, M., Nedea, O. A., Cobelschi, C. P., & Arsene, A. L. (2024). Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism. Nutrients, 16(7), 1077. https://doi.org/10.3390/nu16071077










