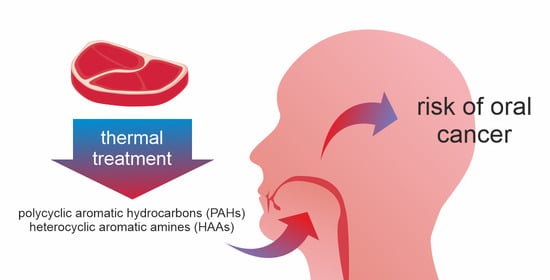
The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study and Control Groups
2.2. Source of Diet Information: Food Frequency Questionnaire (FFQ)
2.3. Method Limitations
2.4. Statistical Analysis
- A dependent variable as a grouping variable (membership in the study group (1) and control group (0));
- An independent variable, referred to as a predictor (the individual answers in the FFQ survey covering the frequency of consumption of specific products).
3. Results
3.1. High Total Meat Consumption as a Risk Factor for Oral Cancer
3.2. High Consumption of Red Meat as a Risk Factor for Oral Cancer
3.3. High Consumption of Thermally Processed Meat Products as a Risk Factor for Oral Cancer
3.4. The Impact of Fruit and Vegetable Consumption on the Risk of Oral Cancer
4. Discussion
4.1. Consumption of Meat and Meat Products as Risk Factors in the Etiopathology of Oral Cancer
4.2. Consumption of Thermally Processed Meat and the Risk of Oral Cancer
4.3. Fruit and Vegetable Consumption and the Risk of Oral Cancer
5. Conclusions
- The high consumption of thermally processed meat, especially smoked, fried, roasted, and boiled, increases the risk of oral cancer. Such processes lead to the formation of carcinogenic and mutagenic polycyclic aromatic hydrocarbons and heterocyclic aromatic amines.
- The high consumption of red meat, which includes pork and beef often consumed in Poland, is a risk factor for oral cancer.
- The consumption of vegetables is a protective factor against oral cancer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dybing, E.; O’Brien, J.; Renwick, A.; Sanner, T. Risk assessment of dietary exposures to compounds that are genotoxic and carcinogenic—An overview. Toxicol. Lett. 2008, 180, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chiavarini, M.; Bertarelli, G.; Minelli, L.; Fabiani, R. Dietary Intake of Meat Cooking-Related Mutagens (HCAs) and Risk of Colorectal Adenoma and Cancer: A Systematic Review and Meta-Analysis. Nutrients 2017, 18, 514. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Red Meat and Processed Meat; Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Publication: Lyon, France, 2018; Volume 114. [Google Scholar]
- Bulanda, S.; Janoszka, B. Polycyclic Aromatic Hydrocarbons (PAHs) in Roasted Pork Meat and the Effect of Dried Fruits on PAH Content. Int. J. Environ. Res. Public Health 2023, 20, 4922. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.d.C.; Puente-Gutiérrez, C.; Delgado-Somolinos, E.; Carreras-Presas, C.M.; Fernández-Farhall, J.; López-Sánchez, A.F. Association between oral cancer and diet: An update. Nutrients 2021, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Lella, A.; Borgiel-Marek, P. Oral Cancer Prevention (Polish Redaction); Polish Dental Society. ELset: Olsztyn, Poland, 2021. [Google Scholar]
- Wunschel, M.; Neumeier, M.; Utpatel, K.; Reichert, T.E.; Ettl, T.; Spanier, G. Staging more important than grading? Evaluation of malignancy grading, depth of invasion and resection margins in oral squamous cell carcinoma. Clin. Oral Investig. 2021, 25, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Barańska, K.; Michałek, I.; Olasek, P.; Miklewska, M.; Didkowska, J. Cancer in Poland in 2020; Polish National Cancer Registry: Warszawa, Poland, 2022. [Google Scholar]
- Kumar, M.; Nanavati, R.; Modi, T.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Chan, W.G.; Hajaligol, M.R. Product compositions from pyrolysis of some aliphatic α-amino acids. J. Anal. Appl. Pyrolysis 2006, 75, 69–81. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M. Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.; Taborelli, M.; Montella, M.; Libra, M.; Zucchetto, A.; Crispo, A.; Grimaldi, M.; La Vecchia, C.; Serraino, D.; et al. Dietary inflammatory index and non-hodgkin lymphoma risk in an Italian case-control study. Cancer Causes Control 2017, 28, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Capelas, S.; Delerue-Matos, C.; Morais, S. Grill Workers Exposure to Polycyclic Aromatic Hydrocarbons: Levels and Excretion Profiles of the Urinary Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Varshney, J.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Duedahl-Olesen, L.; Aaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.; Binderup, M.-L. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control 2015, 57, 169–176. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–302. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Formation of heterocyclic aromatic amines with the structure of aminoimidazoazarenes in food products. Food Chem. 2020, 313, 126–128. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Jägerstad, M.; Skog, K.; Arvidsson, P.; Solyakov, A. Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Z. Leb. Und-Forsch. 1998, 207, 419–427. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, Y.; Ren, X.; Zhang, Y.; Peng, Z.; Zhou, G. Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat. Foods 2020, 9, 572. [Google Scholar] [CrossRef]
- Polak, M.L.; Demšar, L.; Zahija, I.; Polak, T. Influence of temperature on the formation of heterocyclic aromatic amines in pork steaks. Czech J. Food Sci. 2020, 38, 248–254. [Google Scholar] [CrossRef]
- Knize, M.G.; Dolbeare, F.A.; Carroll, K.L.; Moore, D.H.; Felton, J.S. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem. Toxicol. 1994, 32, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Linghu, Z.; Karim, F.; Taghvaei, M.; Albashabsheh, Z.; Houser, T.; Smith, S. Amino acids effects on heterocyclic amines formation and physicochemical properties in pan-fried beef patties. J. Food Sci. 2020, 85, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Szterk, A.; Waszkiewicz-Robak, B. Influence of selected quality factors of beef on the profile and the quantity of heterocyclic aromatic amines during processing at high temperature. Meat Sci. 2014, 96, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Meurillon, M.; Angénieux, M.; Mercier, F.; Blinet, P.; Chaloin, L.; Chevolleau, S.; Debrauwer, L.; Engel, E. Mitigation of heterocyclic aromatic amines in cooked meat. Part. I: Informed selection of antioxidants based on molecula. Food Chem. 2020, 331, 127264. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Consumption of Thermally processed meat containing carcinogenic compounds (polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) versus a risk of some cancers in humans and the possibility of reducing their formation by natural food additives—A literature review. Int. J. Environ. Res. Public Health 2022, 19, 4781. [Google Scholar] [CrossRef]
- Wadolowska, L. Validation of food frequency questionnaire [FFQ]. Reproducibility assessment. Bromatol. Chem. Toksykol. 2005, 38, 27–33. [Google Scholar]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Wu, Y.; Li, X.; Bai, B. Meat consumption and risk of oral cavity and oropharynx cancer: A meta-analysis of observational studies. PLoS ONE 2014, 9, e95048. [Google Scholar] [CrossRef]
- Diggs, D.; Huderson, A.; Harris, K.; Myers, J.; Banks, L.; Rekhadevi, P.; Niaz, M.; Ramesh, A. Polycyclic aromatic hydrocarbons and digestive tract cancers: A perspective. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2011, 29, 324–357. [Google Scholar] [CrossRef]
- Boldo, E.; de Larrea, N.F.; Pollán, M.; Martín, V.; Obón-Santacana, M.; Guevara, M.; Castaño-Vinyals, G.; Canga, J.M.; Pérez-Gómez, B.; Gómez-Acebo, I.; et al. Meat intake, cooking methods, doneness preferences and risk of gastric adenocarcinoma in the MCC-spain study. Nutrients 2022, 14, 4852. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 2017, 152, 1944–1953. [Google Scholar] [CrossRef]
- Li, F.; Duan, F.; Zhao, X.; Song, C.; Cui, S.; Dai, L. Red meat and processed meat consumption and nasopharyngeal carcinoma risk: A dose-response meta-analysis of observational studies. Nutr. Cancer 2016, 68, 1034–1043. [Google Scholar] [CrossRef]
- Butler, C.; Lee, Y.-C.A.; Li, S.; Li, Q.; Chen, C.-J.; Hsu, W.-L.; Lou, P.-J.; Zhu, C.; Pan, J.; Shen, H.; et al. Diet and the risk of head and neck cancer among never-smokers and smokers in a Chinese population. Cancer Epidemiol. 2017, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Perloy, A.; Maasland, D.H.E.; Brandt, P.A.; Kremer, B.; Schouten, L.J. Intake of meat and fish and risk of head–neck cancer subtypes in the Netherlands Cohort Study. Cancer Causes Control 2017, 28, 647–656. [Google Scholar] [CrossRef]
- Yan, L.; Qiu, Y.; Chen, F.; Cai, L.; Huang, J.; Liu, F.; Wu, J.; Lin, L.; He, B. Effects of dietary factors on the incidence of tongue cancer in smoking and non-smoking population. Wei Sheng Yan Jiu 2017, 46, 189–195. [Google Scholar] [PubMed]
- Cross, A.; Harnly, J.; Ferrucci, L.; Risch, A.; Mayne, S.; Sinha, R. Developing a heme iron database for meats according to meat type, cooking method and doneness level. Food Nutr. Sci. 2012, 3, 905–913. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Starzyńska, A. Nowotwory Złośliwe Jamy Ustnej—Charakterystyka, Diagnostyka, Postępowanie. Forum Med. Rodz. 2016, 10, 254–262. [Google Scholar]
- Bugshan, A.; Farooq, I. Oral squamous cell carcinoma: Metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Research 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.J. Risk Factors Associated with oral and maxillofacial benign tumors: A case-control ctudy. Cell. Mol. Biol. 2017, 63, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, B.; Chen, F.; Huang, J.; Yan, L.; Hu, Z.; Lin, L.; He, F.; Cai, L. Influencing factors for oral-maxillofacial benign tumors: A case-control study. Zhonghua Yu Fang. Yi Xue Za Zhi 2015, 49, 693–699. [Google Scholar]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.; Sidahmed, E. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Di Maso, M.; Talamini, R.; Bosetti, C.; Montella, M.; Zucchetto, A.; Libra, M.; Negri, E.; Levi, F.; La Vecchia, C.; Franceschi, S.; et al. Red meat and cancer risk in a network of case-control studies focusing on cooking practices. Ann. Oncol. 2013, 24, 3107–3112. [Google Scholar] [CrossRef]
- De Stefani, E.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Case–control study on the role of heterocyclic amines in the etiology of upper aerodigestive cancers in Uruguay. Nutr. Cancer 1998, 32, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Karczmarek-Borowska, B.; Synoś, K.; Zielińska, K. Wstępne doniesienia na temat wpływu żywienia na rodzaj chorób nowotworowych. Probl. Nauk. Stosow. 2018, 8, 163–176. [Google Scholar]
- Franco, E.L.; Kowalski, L.P.; Oliveira, B.V.; Curado, M.P.; Pereira, R.N.; Silva, M.E.; Fava, A.S.; Torloni, H. Risk factors for oral cancer in Brazil: A case–control study. Int. J. Cancer 1989, 43, 992–1000. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chan, W.; Wang, J.; Waymack, B.E.; Wooten, J.B.; Seeman, J.I.; Hajaligol, M.R. On the role of peptides in the pyrolysis of amino acids. J. Anal. Appl. Pyrolysis 2004, 72, 153–163. [Google Scholar] [CrossRef]
- Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control 2017, 80, 45–51. [Google Scholar] [CrossRef]
- Zalega, J.; Szostak-Węgierek, D. Nutrition in cancer prevention. Part. III. Diets of anticancer properties. Prob. Hig. Epidemiol. 2013, 94, 59–70. [Google Scholar]
- Bell, E.B.; Reis, I.M.; Cohen, E.R.; Almuhaimid, T.; Smith, D.H.; Alotaibi, F.; Gordon, C.; Gomez-Fernandez, C.; Goodwin, W.J.; Franzmann, E.J. Green salad intake is associated with improved oral cancer survival and lower soluble CD44 levels. Nutrients 2021, 26, 372. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of Southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef] [PubMed]
- Brewczyński, A.; Jabłońska, B.; Kentnowski, M.; Mrowiec, S.; Składowski, K.; Rutkowski, T. The association between carotenoids and head and neck cancer risk. Nutrients 2022, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, D.T.; Gangarosa, L.M.; Schliebe, B.; Umbach, D.M.; Xu, Z.; MacIntosh, B.; Knize, M.G.; Matthews, P.P.; Swank, A.E.; Sandler, R.S.; et al. Inhibition of Fried Meat-Induced Colorectal DNA Damage and Altered Systemic Genotoxicity in Humans by Crucifera, Chlorophyllin, and Yogurt. PLoS ONE 2011, 6, e18707. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C.C. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int. J. Cancer 2007, 122, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Munter, L.; Maasland, D.H.E.; Brandt, P.V.D.; Kremer, B.; Schouten, L.J. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2015, 102, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Minelli, L.; Rosignoli, P. Apple intake and cancer risk: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2016, 19, 2603–2617. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Maugeri, U.; Popiela, T.; Kulig, J.; Sochacka-Tatara, E.; Pac, E.; Sowa, A.; Musial, A. Case-control study on beneficial effect of regular consumption of apples on colorectal cancer risk in a population with relatively low intake of fruits and vegetables. Eur. J. Cancer Prev. 2010, 19, 42–47. [Google Scholar] [CrossRef]
- Bao, P.-P.; Shu, X.-O.; Zheng, Y.; Cai, H.; Ruan, Z.-X.; Gu, K.; Su, Y.; Gao, Y.-T.; Zheng, W.; Lu, W. Fruit, vegetable and animal food intake and breast cancer risk by hormone receptor status. Nutr. Cancer 2012, 64, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Navarra, M.; Woodside, J.; Cantwell, M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, R.; Deng, T.; Lin, Z.; Li, H.; Yang, Y.; Su, Q.; Wang, J.; Yang, Y.; Wang, J.; et al. Association between dried fruit intake and pan—Cancers incidence risk: A two-sample Mendelian randomization study. Front. Nutr. 2022, 9, 899137. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.; Aune, D.; Noh, H.; Petersen, K.; Freisling, H. Dried fruits, nuts and cancer risk and survival: A review of the evidence and future research directions. Nutrients 2023, 15, 1443. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Report on the Global Tobacco Epidemic, 2008; The MPOWER Package; World Health Organization: Geneva, Switzerland, 2008.
| Food Group | Products |
|---|---|
| Fats | Oil Butter Margarine cubes (for baking or frying) and margarine in cups (for spreading) Sour or sweet cream Other animal fats, e.g., lard, bacon Mayonnaise and dressings, e.g., salad dressings |
| Fruits | Fruits, all types in general Dried fruit, e.g., raisins, apricots, figs, apples, and plums |
| Vegetables and grains | Vegetables, all types in general Potatoes, in various forms, e.g., boiled, roasted, French fries, potato pancakes, and potato dumplings Nuts, e.g., peanuts, hazelnuts, walnuts, almonds, pistachios, cashews, coconut, and chestnuts Seeds, e.g., pumpkin, sesame, sunflower, and wheat |
| Meat and fish | Cold cuts, e.g., ham, tenderloin, poultry, pork, beef, and mixed Sausages, various types, e.g., frankfurters and bacon Meat products and organ meats, e.g., liver, black pudding, brawn, and pâtés Red meat, e.g., pork, beef, and veal Poultry meat, e.g., chicken, duck, and turkey Game, e.g., wild boar, roe deer, and hare Lean fish, e.g., pollock, cod, perch, hake, and carp Oily fish, e.g., salmon, sardines, herring, and mackerel |
| Drinks | Fruit juices and fruit nectars Vegetable and fruit juices Sweetened drinks e.g., Fanta, Coca-cola, and Sprite Beer Wine and drinks Vodka and spirits |
| Method of preparing food | Fried meat Cooked meat Smoked meat Roasted meat Grilled meat |
| Vitamins and dietary supplements | - |
| Study Group | Control Group | |
|---|---|---|
| age [years] | 67.5 ± 12.4 | 53 ± 20.1 |
| weight [kg] | 65.5 ± 16.6 | 80 ± 13 |
| height [cm] | 171 ± 9.9 | 170.5 ± 7.8 |
| BMI [kg/m2] | 22.3 ± 5.2 | 27.5 ± 5.3 |
| Statistical Variable | OR | 95% CI down | 95% CI upper | p |
|---|---|---|---|---|
| Meat products and offal e.g., liver, black pudding, brawn, and pâtés | 2.23 | 1.42 | 3.50 | <0.001 |
| Sausages—various types and bacon | 1.76 | 1.06 | 2.94 | 0.030 |
| Red meat e.g., pork, beef, and veal | 1.42 | 0.82 | 2.48 | 0.213 |
| Poultry meat e.g., poultry meat, chicken, duck, and turkey | 1.16 | 0.60 | 2.21 | 0.663 |
| High-quality meat e.g., ham, tenderloin, poultry, pork, beef, and mixed | 1.09 | 0.70 | 1.70 | 0.696 |
| Game meat e.g., meat from wild boar, roe deer, quail, wild duck, and hare | 0.50 | 0.23 | 1.07 | 0.075 |
| Statistical Variable | OR 1 | 95% CI down 1 | 95% CI upper 1 | p 1 | OR 2 | 95% CI down 2 | 95% CI upper 2 | p 2 |
|---|---|---|---|---|---|---|---|---|
| Red meat e.g., pork, beef, and veal | 2.47 | 1.59 | 3.81 | <0.001 | 1.60 | 0.97 | 2.65 | 0.065 |
| Sausages—various types and bacon | - | - | - | - | 2.06 | 1.36 | 3.13 | <0.001 |
| Statistical Variable | OR | 95% CI down | 95% CI upper | p |
|---|---|---|---|---|
| Sausages—various types and bacon | 1.81 | 1.17 | 2.81 | 0.008 |
| Red meat e.g., pork, beef, and veal | 1.69 | 1.00 | 2.86 | 0.049 |
| Smoking | 4.54 | 1.81 | 11.39 | 0.001 |
| Statistical Variable | OR | 95% CI down | 95% CI upper | p |
|---|---|---|---|---|
| Sausages | 2.12 | 1.32 | 3.36 | 0.002 |
| Red meat | 1.70 | 0.98 | 2.94 | 0.057 |
| Beer | 0.91 | 0.61 | 1.34 | 0.619 |
| Wine/drinks | 0.48 | 0.28 | 0.82 | 0.007 |
| Vodka | 0.99 | 0.97 | 0.58 | 0.965 |
| Statistical Variable | OR | 95% CI down | 95% CI upper | p |
|---|---|---|---|---|
| Smoked meat | 1.69 | 1.13 | 2.51 | 0.010 |
| Fried meeat | 1.49 | 0.99 | 2.23 | 0.055 |
| Boiled meat | 1.39 | 0.95 | 2.03 | 0.088 |
| Roasted meat | 1.24 | 0.80 | 1.92 | 0.335 |
| Grilled meat | 0.65 | 0.41 | 1.01 | 0.055 |
| Statistical Variable | OR | 95% CI down | 95% CI upper | p |
|---|---|---|---|---|
| Fruit | 1.85 | 1.22 | 2.80 | 0.004 |
| Dry fruit | 0.78 | 0.56 | 1.08 | 0.131 |
| Vegetables | 0.62 | 0.42 | 0.92 | 0.018 |
| Fruit juices | 0.94 | 0.71 | 1.24 | 0.645 |
| Vegetable juices | 0.91 | 0.67 | 1.23 | 0.544 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulanda, S.; Lau, K.; Nowak, A.; Łyko-Morawska, D.; Kotylak, A.; Janoszka, B. The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds. Nutrients 2024, 16, 1084. https://doi.org/10.3390/nu16071084
Bulanda S, Lau K, Nowak A, Łyko-Morawska D, Kotylak A, Janoszka B. The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds. Nutrients. 2024; 16(7):1084. https://doi.org/10.3390/nu16071084
Chicago/Turabian StyleBulanda, Sylwia, Karolina Lau, Agnieszka Nowak, Dorota Łyko-Morawska, Anna Kotylak, and Beata Janoszka. 2024. "The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds" Nutrients 16, no. 7: 1084. https://doi.org/10.3390/nu16071084
APA StyleBulanda, S., Lau, K., Nowak, A., Łyko-Morawska, D., Kotylak, A., & Janoszka, B. (2024). The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds. Nutrients, 16(7), 1084. https://doi.org/10.3390/nu16071084