Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Sample Preparation for Nitrate and Nitrite Measurements
2.3. Preparation of Samples for Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
2.4. Determination of 15NO3− or 15NO2− Percent Using LC–MS/MS
2.5. Statistical Analysis
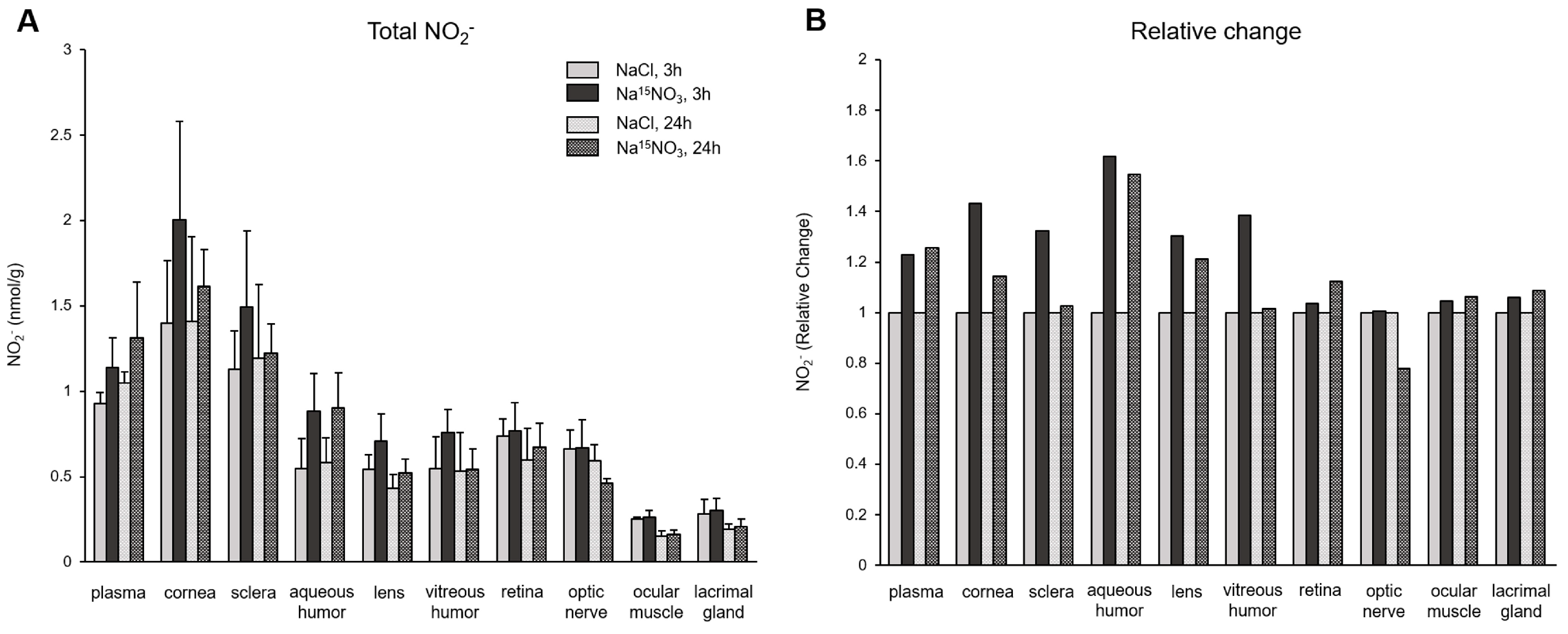
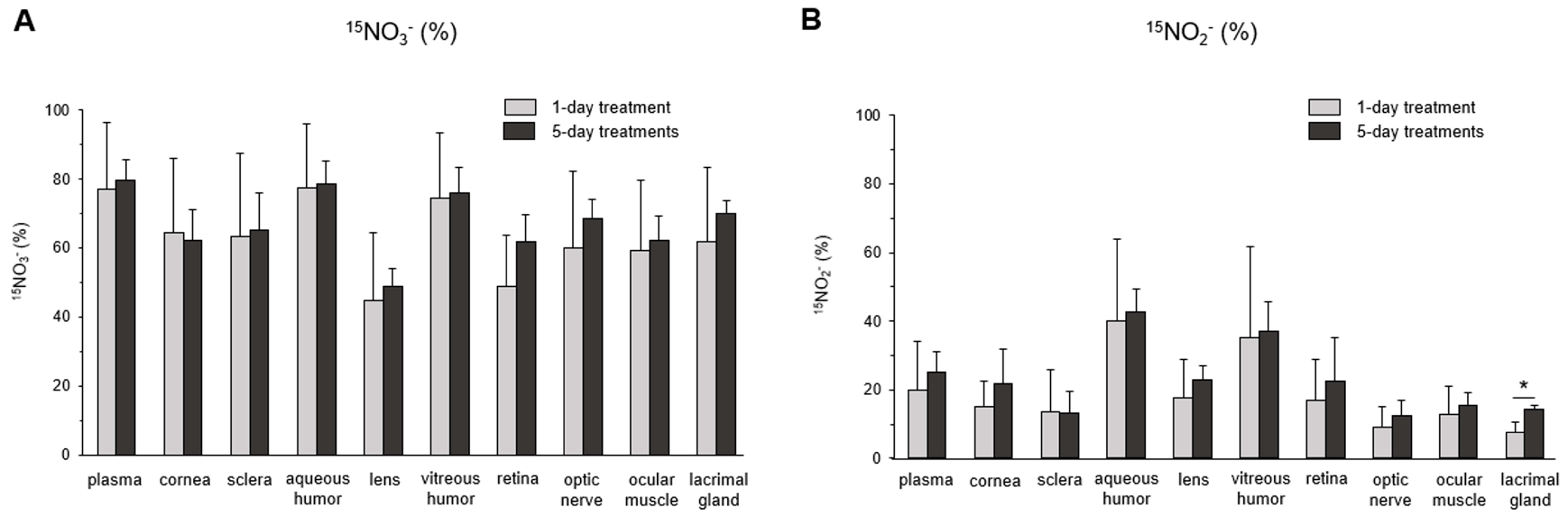
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 2019, 176, 228–245. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Bryan, N.S.; Ahmed, S.; Lefer, D.J.; Hord, N.; von Schwarz, E.R. Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action. Nitric Oxide 2023, 132, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to NO: Potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Samouilov, A.; Liu, X.; Zweier, J.L. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: Evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 2003, 42, 1150–1159. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Kwan Jeff Lam, K.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 2016, 55–56, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Wiklund, N.P.; Engstrand, L.; Weitzberg, E.; Lundberg, J.O. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 2001, 5, 580–586. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Flogel, U.; Drexhage, C.; Hendgen-Cotta, U.; Kelm, M.; Schrader, J. Nitrite reductase function of deoxymyoglobin: Oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007, 100, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Phillipson, M.; Jansson, E.A.; Patzak, A.; Lundberg, J.O.; Holm, L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G718–G724. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary Nitrate and Physical Performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Baranauskas, M.N.; Hinrichs, R.J.; Liu, Z.; Carter, S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Macuh, M.; Knap, B. Effects of Nitrate Supplementation on Exercise Performance in Humans: A Narrative Review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Agron, E.; Peprah, D.; Keenan, T.D.L.; Lawler, T.P.; Mares, J.; Chew, E.Y.; Investigators, A.A. Association of Dietary Nitrate and a Mediterranean Diet with Age-Related Macular Degeneration among US Adults: The Age-Related Eye Disease Study (AREDS) and AREDS2. JAMA Ophthalmol. 2022, 141, 130–139. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Kifley, A.; Lewis, J.R.; Bondonno, C.; Joachim, N.; Hodgson, J.M.; Mitchell, P. Association of Dietary Nitrate Intake with the 15-Year Incidence of Age-Related Macular Degeneration. J. Acad. Nutr. Diet. 2018, 118, 2311–2314. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Lewis, J.R.; Blekkenhorst, L.C.; Bondonno, C.; Burlutsky, G.; Hodgson, J.M.; Mitchell, P. Association of dietary nitrate intake with retinal microvascular structure in older adults. Eur. J. Nutr. 2020, 59, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of Dietary Nitrate Intake with Primary Open-Angle Glaucoma: A Prospective Analysis from the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, J.E.; de Crom, T.O.E.; Blekkenhorst, L.C.; Klaver, C.C.W.; Voortman, T.; Ramdas, W.D. Dietary Nitrate Intake Is Associated with Decreased Incidence of Open-Angle Glaucoma: The Rotterdam Study. Nutrients 2022, 14, 2490. [Google Scholar] [CrossRef] [PubMed]
- Bouchemi, M.; Soualmia, H.; Midani, F.; El Afrit, M.A.; El Asmi, M.; Feki, M. Impaired nitric oxide production in patients with primary open-angle glaucoma. Tunis. Med. 2020, 98, 144–149. [Google Scholar] [PubMed]
- Carr, B.J.; Stell, W.K. Nitric Oxide (NO) Mediates the Inhibition of Form-Deprivation Myopia by Atropine in Chicks. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; DeCory, H.H. The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J. Ocul. Pharmacol. Ther. 2018, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Reina-Torres, E.; De Ieso, M.L.; Pasquale, L.R.; Madekurozwa, M.; van Batenburg-Sherwood, J.; Overby, D.R.; Stamer, W.D. The vital role for nitric oxide in intraocular pressure homeostasis. Prog. Retin. Eye Res. 2021, 83, 100922. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Kuppusamy, R.; Yeo, J.H.; Kumar, N.; New, E.J.; Willcox, M.D.P. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 2021, 21, 37–51. [Google Scholar] [CrossRef]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide 2018, 77, 75–87. [Google Scholar] [CrossRef]
- Kaufman, M.B. Pharmaceutical Approval Update. Pharm. Ther. 2018, 43, 22–60. [Google Scholar]
- Park, J.W.; Piknova, B.; Jenkins, A.; Hellinga, D.; Parver, L.M.; Schechter, A.N. Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye. Sci. Rep. 2020, 10, 13166. [Google Scholar] [CrossRef]
- Hu, C.W.; Chang, Y.J.; Yen, C.C.; Chen, J.L.; Muthukumaran, R.B.; Chao, M.R. 15N-labelled nitrite/nitrate tracer analysis by LC-MS/MS: Urinary and fecal excretion of nitrite/nitrate following oral administration to mice. Free Radic. Biol. Med. 2019, 143, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hughan, K.S.; Wendell, S.G.; Delmastro-Greenwood, M.; Helbling, N.; Corey, C.; Bellavia, L.; Potti, G.; Grimes, G.; Goodpaster, B.; Kim-Shapiro, D.B.; et al. Conjugated Linoleic Acid Modulates Clinical Responses to Oral Nitrite and Nitrate. Hypertension 2017, 70, 634–644. [Google Scholar] [CrossRef]
- Kadach, S.; Park, J.W.; Stoyanov, Z.; Black, M.I.; Vanhatalo, A.; Burnley, M.; Walter, P.J.; Cai, H.; Schechter, A.N.; Piknova, B.; et al. 15N-labelled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiol. 2023, 237, e13924. [Google Scholar] [CrossRef]
- Siervo, M.; Jackson, S.J.; Bluck, L.J. In-vivo nitric oxide synthesis is reduced in obese patients with metabolic syndrome: Application of a novel stable isotopic method. J. Hypertens. 2011, 29, 1515–1527. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Walter, P.J.; Cai, H.; Upanan, S.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Distribution of dietary nitrate and its metabolites in rat tissues after 15N-labeled nitrate administration. Sci. Rep. 2023, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Cassel, K.S.; Gilliard, C.N.; Schechter, A.N. Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method. J. Vis. Exp. 2016, 118, e54879. [Google Scholar] [CrossRef]
- Park, J.W.; Thomas, S.M.; Wylie, L.J.; Jones, A.M.; Vanhatalo, A.; Schechter, A.N.; Piknova, B. Preparation of Rat Skeletal Muscle Homogenates for Nitrate and Nitrite Measurements. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Chao, M.R.; Shih, Y.M.; Hsu, Y.W.; Liu, H.H.; Chang, Y.J.; Lin, B.H.; Hu, C.W. Urinary nitrite/nitrate ratio measured by isotope-dilution LC-MS/MS as a tool to screen for urinary tract infections. Free Radic. Biol. Med. 2016, 93, 77–83. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, J.A.; McKee, M. Alterations of ocular nitric oxide synthase in human glaucoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1774–1784. [Google Scholar]
- Polak, K.; Luksch, A.; Berisha, F.; Fuchsjaeger-Mayrl, G.; Dallinger, S.; Schmetterer, L. Altered nitric oxide system in patients with open-angle glaucoma. Arch. Ophthalmol. 2007, 125, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Fahlke, C.; Beck, C.L.; George, A.L., Jr. A mutation in autosomal dominant myotonia congenita affects pore properties of the muscle chloride channel. Proc. Natl. Acad. Sci. USA 1997, 94, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, X.D.; Liu, X.; Chen, T.Y.; Zhao, M. Chloride channels and transporters in human corneal epithelium. Exp. Eye Res. 2010, 90, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.A.; Schultz, D.S.; Deen, W.M.; Young, V.R.; Tannenbaum, S.R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: Effect of diet supplementation with ascorbic acid. Cancer Res. 1983, 43, 1921–1925. [Google Scholar]
- Kadach, S.; Piknova, B.; Black, M.I.; Park, J.W.; Wylie, L.J.; Stoyanov, Z.; Thomas, S.M.; McMahon, N.F.; Vanhatalo, A.; Schechter, A.N.; et al. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 2022, 121, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Miller, M.J.; Joshi, M.S.; Sadowska-Krowicka, H.; Clark, D.A.; Lancaster, J.R., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 1998, 273, 18709–18713. [Google Scholar] [CrossRef]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R., Jr. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, C.J.; Shui, Y.B.; Holekamp, N.M.; Bai, F.; Beebe, D.C. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5731–5738. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Sairenchi, T.; Machida, T.; Takino, Y.; Kondo, Y.; Mukai, K.; Kobashi, G.; Ishigami, A.; Senoo, T. Reduced aqueous humour ascorbic-acid concentration in women with smaller anterior chamber depth. Sci. Rep. 2019, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Jacques, P.F.; Nowell, T.; Perrone, G.; Blumberg, J.; Handelman, G.; Jozwiak, B.; Nadler, D. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr. Eye Res. 1997, 16, 857–864. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Piknova, B.; Tunau-Spencer, K.J.; Thomas, S.M.; Cai, H.; Walter, P.J.; Jenkins, A.; Hellinga, D.; Parver, L.M.; Schechter, A.N. Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation. Nutrients 2024, 16, 1154. https://doi.org/10.3390/nu16081154
Park JW, Piknova B, Tunau-Spencer KJ, Thomas SM, Cai H, Walter PJ, Jenkins A, Hellinga D, Parver LM, Schechter AN. Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation. Nutrients. 2024; 16(8):1154. https://doi.org/10.3390/nu16081154
Chicago/Turabian StylePark, Ji Won, Barbora Piknova, Khalid J. Tunau-Spencer, Samantha M. Thomas, Hongyi Cai, Peter J. Walter, Audrey Jenkins, David Hellinga, Leonard M. Parver, and Alan N. Schechter. 2024. "Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation" Nutrients 16, no. 8: 1154. https://doi.org/10.3390/nu16081154





