Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Sample Preparation for Nitrate and Nitrite Measurements
2.3. Preparation of Samples for Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
2.4. Determination of 15NO3− or 15NO2− Percent Using LC–MS/MS
2.5. Statistical Analysis
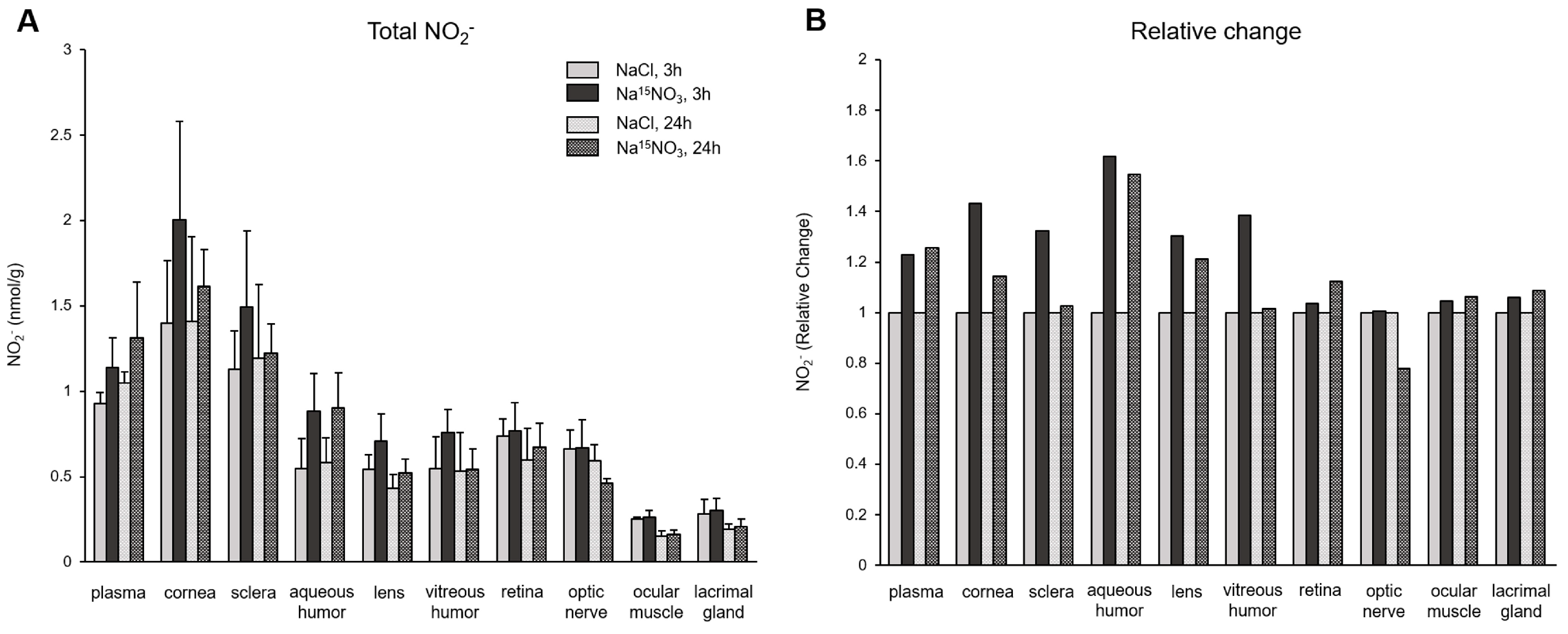
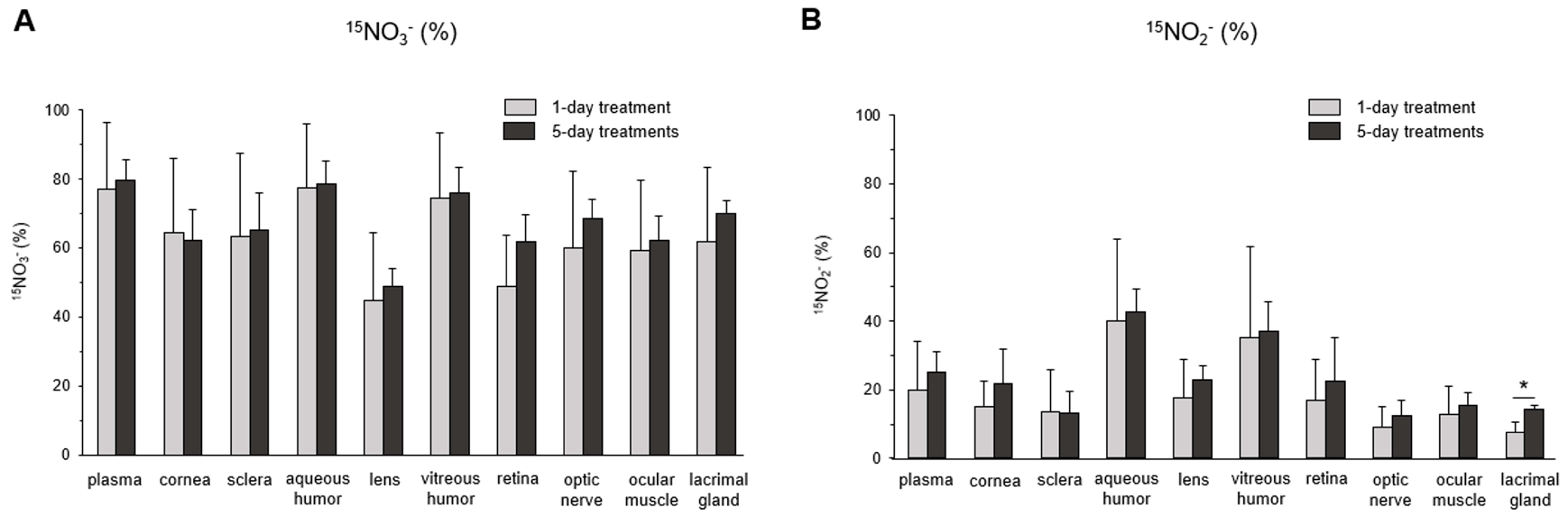
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 2019, 176, 228–245. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Bryan, N.S.; Ahmed, S.; Lefer, D.J.; Hord, N.; von Schwarz, E.R. Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action. Nitric Oxide 2023, 132, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to NO: Potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Samouilov, A.; Liu, X.; Zweier, J.L. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: Evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 2003, 42, 1150–1159. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Kwan Jeff Lam, K.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 2016, 55–56, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Wiklund, N.P.; Engstrand, L.; Weitzberg, E.; Lundberg, J.O. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 2001, 5, 580–586. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Flogel, U.; Drexhage, C.; Hendgen-Cotta, U.; Kelm, M.; Schrader, J. Nitrite reductase function of deoxymyoglobin: Oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007, 100, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Phillipson, M.; Jansson, E.A.; Patzak, A.; Lundberg, J.O.; Holm, L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G718–G724. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary Nitrate and Physical Performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Baranauskas, M.N.; Hinrichs, R.J.; Liu, Z.; Carter, S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Macuh, M.; Knap, B. Effects of Nitrate Supplementation on Exercise Performance in Humans: A Narrative Review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Agron, E.; Peprah, D.; Keenan, T.D.L.; Lawler, T.P.; Mares, J.; Chew, E.Y.; Investigators, A.A. Association of Dietary Nitrate and a Mediterranean Diet with Age-Related Macular Degeneration among US Adults: The Age-Related Eye Disease Study (AREDS) and AREDS2. JAMA Ophthalmol. 2022, 141, 130–139. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Kifley, A.; Lewis, J.R.; Bondonno, C.; Joachim, N.; Hodgson, J.M.; Mitchell, P. Association of Dietary Nitrate Intake with the 15-Year Incidence of Age-Related Macular Degeneration. J. Acad. Nutr. Diet. 2018, 118, 2311–2314. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Lewis, J.R.; Blekkenhorst, L.C.; Bondonno, C.; Burlutsky, G.; Hodgson, J.M.; Mitchell, P. Association of dietary nitrate intake with retinal microvascular structure in older adults. Eur. J. Nutr. 2020, 59, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of Dietary Nitrate Intake with Primary Open-Angle Glaucoma: A Prospective Analysis from the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, J.E.; de Crom, T.O.E.; Blekkenhorst, L.C.; Klaver, C.C.W.; Voortman, T.; Ramdas, W.D. Dietary Nitrate Intake Is Associated with Decreased Incidence of Open-Angle Glaucoma: The Rotterdam Study. Nutrients 2022, 14, 2490. [Google Scholar] [CrossRef] [PubMed]
- Bouchemi, M.; Soualmia, H.; Midani, F.; El Afrit, M.A.; El Asmi, M.; Feki, M. Impaired nitric oxide production in patients with primary open-angle glaucoma. Tunis. Med. 2020, 98, 144–149. [Google Scholar] [PubMed]
- Carr, B.J.; Stell, W.K. Nitric Oxide (NO) Mediates the Inhibition of Form-Deprivation Myopia by Atropine in Chicks. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; DeCory, H.H. The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J. Ocul. Pharmacol. Ther. 2018, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Reina-Torres, E.; De Ieso, M.L.; Pasquale, L.R.; Madekurozwa, M.; van Batenburg-Sherwood, J.; Overby, D.R.; Stamer, W.D. The vital role for nitric oxide in intraocular pressure homeostasis. Prog. Retin. Eye Res. 2021, 83, 100922. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Kuppusamy, R.; Yeo, J.H.; Kumar, N.; New, E.J.; Willcox, M.D.P. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 2021, 21, 37–51. [Google Scholar] [CrossRef]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide 2018, 77, 75–87. [Google Scholar] [CrossRef]
- Kaufman, M.B. Pharmaceutical Approval Update. Pharm. Ther. 2018, 43, 22–60. [Google Scholar]
- Park, J.W.; Piknova, B.; Jenkins, A.; Hellinga, D.; Parver, L.M.; Schechter, A.N. Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye. Sci. Rep. 2020, 10, 13166. [Google Scholar] [CrossRef]
- Hu, C.W.; Chang, Y.J.; Yen, C.C.; Chen, J.L.; Muthukumaran, R.B.; Chao, M.R. 15N-labelled nitrite/nitrate tracer analysis by LC-MS/MS: Urinary and fecal excretion of nitrite/nitrate following oral administration to mice. Free Radic. Biol. Med. 2019, 143, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hughan, K.S.; Wendell, S.G.; Delmastro-Greenwood, M.; Helbling, N.; Corey, C.; Bellavia, L.; Potti, G.; Grimes, G.; Goodpaster, B.; Kim-Shapiro, D.B.; et al. Conjugated Linoleic Acid Modulates Clinical Responses to Oral Nitrite and Nitrate. Hypertension 2017, 70, 634–644. [Google Scholar] [CrossRef]
- Kadach, S.; Park, J.W.; Stoyanov, Z.; Black, M.I.; Vanhatalo, A.; Burnley, M.; Walter, P.J.; Cai, H.; Schechter, A.N.; Piknova, B.; et al. 15N-labelled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiol. 2023, 237, e13924. [Google Scholar] [CrossRef]
- Siervo, M.; Jackson, S.J.; Bluck, L.J. In-vivo nitric oxide synthesis is reduced in obese patients with metabolic syndrome: Application of a novel stable isotopic method. J. Hypertens. 2011, 29, 1515–1527. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Walter, P.J.; Cai, H.; Upanan, S.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Distribution of dietary nitrate and its metabolites in rat tissues after 15N-labeled nitrate administration. Sci. Rep. 2023, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Cassel, K.S.; Gilliard, C.N.; Schechter, A.N. Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method. J. Vis. Exp. 2016, 118, e54879. [Google Scholar] [CrossRef]
- Park, J.W.; Thomas, S.M.; Wylie, L.J.; Jones, A.M.; Vanhatalo, A.; Schechter, A.N.; Piknova, B. Preparation of Rat Skeletal Muscle Homogenates for Nitrate and Nitrite Measurements. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Chao, M.R.; Shih, Y.M.; Hsu, Y.W.; Liu, H.H.; Chang, Y.J.; Lin, B.H.; Hu, C.W. Urinary nitrite/nitrate ratio measured by isotope-dilution LC-MS/MS as a tool to screen for urinary tract infections. Free Radic. Biol. Med. 2016, 93, 77–83. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, J.A.; McKee, M. Alterations of ocular nitric oxide synthase in human glaucoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1774–1784. [Google Scholar]
- Polak, K.; Luksch, A.; Berisha, F.; Fuchsjaeger-Mayrl, G.; Dallinger, S.; Schmetterer, L. Altered nitric oxide system in patients with open-angle glaucoma. Arch. Ophthalmol. 2007, 125, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Fahlke, C.; Beck, C.L.; George, A.L., Jr. A mutation in autosomal dominant myotonia congenita affects pore properties of the muscle chloride channel. Proc. Natl. Acad. Sci. USA 1997, 94, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, X.D.; Liu, X.; Chen, T.Y.; Zhao, M. Chloride channels and transporters in human corneal epithelium. Exp. Eye Res. 2010, 90, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.A.; Schultz, D.S.; Deen, W.M.; Young, V.R.; Tannenbaum, S.R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: Effect of diet supplementation with ascorbic acid. Cancer Res. 1983, 43, 1921–1925. [Google Scholar]
- Kadach, S.; Piknova, B.; Black, M.I.; Park, J.W.; Wylie, L.J.; Stoyanov, Z.; Thomas, S.M.; McMahon, N.F.; Vanhatalo, A.; Schechter, A.N.; et al. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 2022, 121, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Miller, M.J.; Joshi, M.S.; Sadowska-Krowicka, H.; Clark, D.A.; Lancaster, J.R., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 1998, 273, 18709–18713. [Google Scholar] [CrossRef]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R., Jr. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, C.J.; Shui, Y.B.; Holekamp, N.M.; Bai, F.; Beebe, D.C. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5731–5738. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Sairenchi, T.; Machida, T.; Takino, Y.; Kondo, Y.; Mukai, K.; Kobashi, G.; Ishigami, A.; Senoo, T. Reduced aqueous humour ascorbic-acid concentration in women with smaller anterior chamber depth. Sci. Rep. 2019, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Jacques, P.F.; Nowell, T.; Perrone, G.; Blumberg, J.; Handelman, G.; Jozwiak, B.; Nadler, D. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr. Eye Res. 1997, 16, 857–864. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Piknova, B.; Tunau-Spencer, K.J.; Thomas, S.M.; Cai, H.; Walter, P.J.; Jenkins, A.; Hellinga, D.; Parver, L.M.; Schechter, A.N. Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation. Nutrients 2024, 16, 1154. https://doi.org/10.3390/nu16081154
Park JW, Piknova B, Tunau-Spencer KJ, Thomas SM, Cai H, Walter PJ, Jenkins A, Hellinga D, Parver LM, Schechter AN. Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation. Nutrients. 2024; 16(8):1154. https://doi.org/10.3390/nu16081154
Chicago/Turabian StylePark, Ji Won, Barbora Piknova, Khalid J. Tunau-Spencer, Samantha M. Thomas, Hongyi Cai, Peter J. Walter, Audrey Jenkins, David Hellinga, Leonard M. Parver, and Alan N. Schechter. 2024. "Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation" Nutrients 16, no. 8: 1154. https://doi.org/10.3390/nu16081154
APA StylePark, J. W., Piknova, B., Tunau-Spencer, K. J., Thomas, S. M., Cai, H., Walter, P. J., Jenkins, A., Hellinga, D., Parver, L. M., & Schechter, A. N. (2024). Dietary Nitrate Metabolism in Porcine Ocular Tissues Determined Using 15N-Labeled Sodium Nitrate Supplementation. Nutrients, 16(8), 1154. https://doi.org/10.3390/nu16081154





