Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spore Suspension
2.2. Animals and Experimental Design
2.3. Disease Activity Index (DAI) Assessment
2.4. Histopathological Analyses of the Livers of UC Mice
2.5. Ultra-Structural Analysis of Colonic Tissue
2.6. Measurement of Inflammatory Biomarkers by ELISA
2.7. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analyses
2.8. Gut Microbiota Analysis
2.9. Non-Targeted Fecal Metabonomics
2.10. Statistical Analysis
3. Results
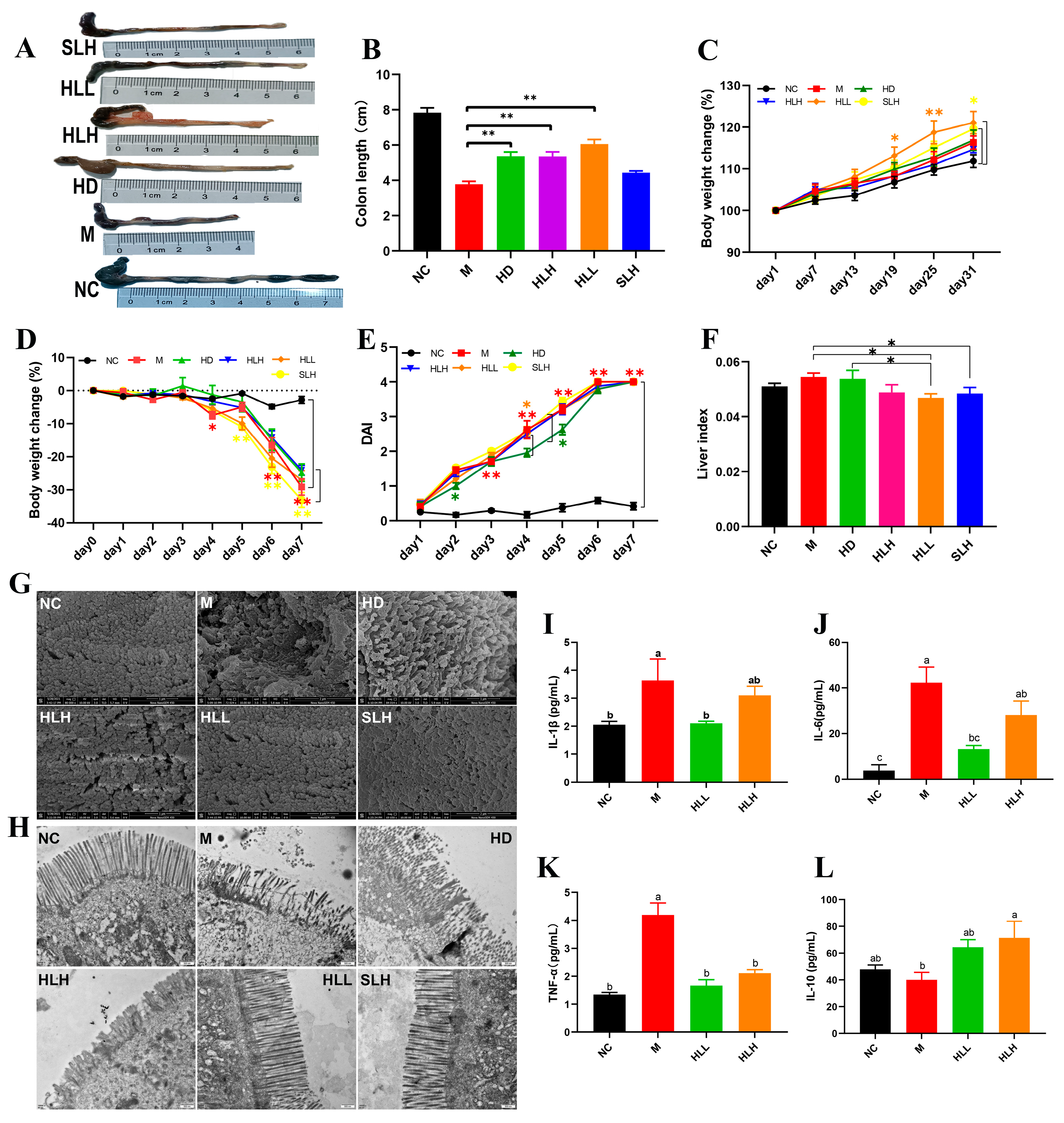
3.1. E. amstelodami Ameliorated DSS-Induced Colitis Mice
3.2. HLL and SLH Administration Improved the Ultrastructure of Colonic Epithelium in Mice
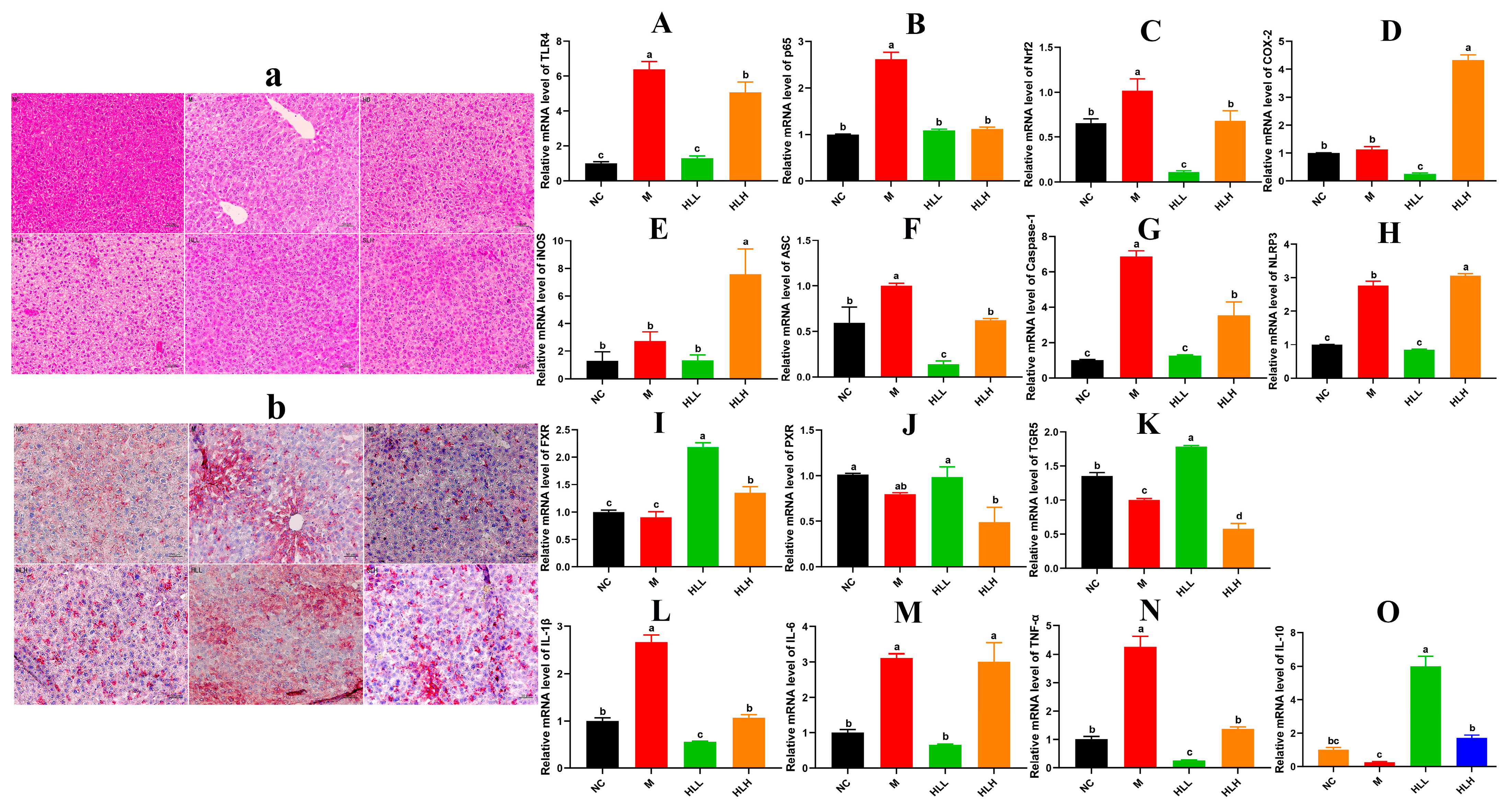
3.3. HLL and SLH Supplementation Alleviates Liver Injury by DSS in Mice
3.4. HLL Treatment Significantly Down-Regulated the Expression of Inflammatory Signal Pathway Related-Gene in Mice Liver
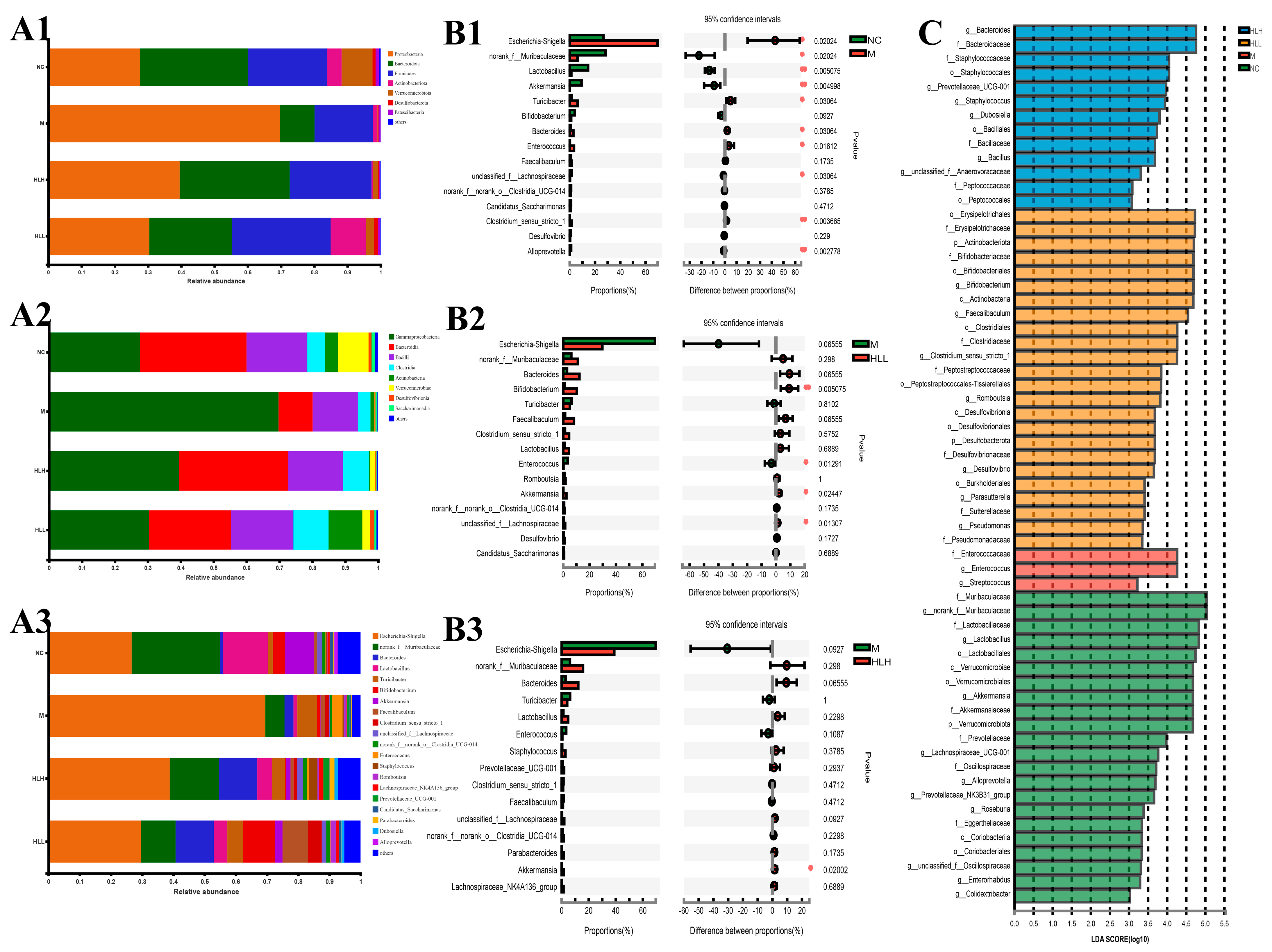
3.5. HLH and HLL Treatment Modulates Bacterial Community Structure in DSS-Induced Mice
3.6. HLL Supplementation Alteres Fungal Community Structure in DSS-Induced Mice
3.7. Correlations between Inflammation-Related Gene Expression and Gut Microorganisms
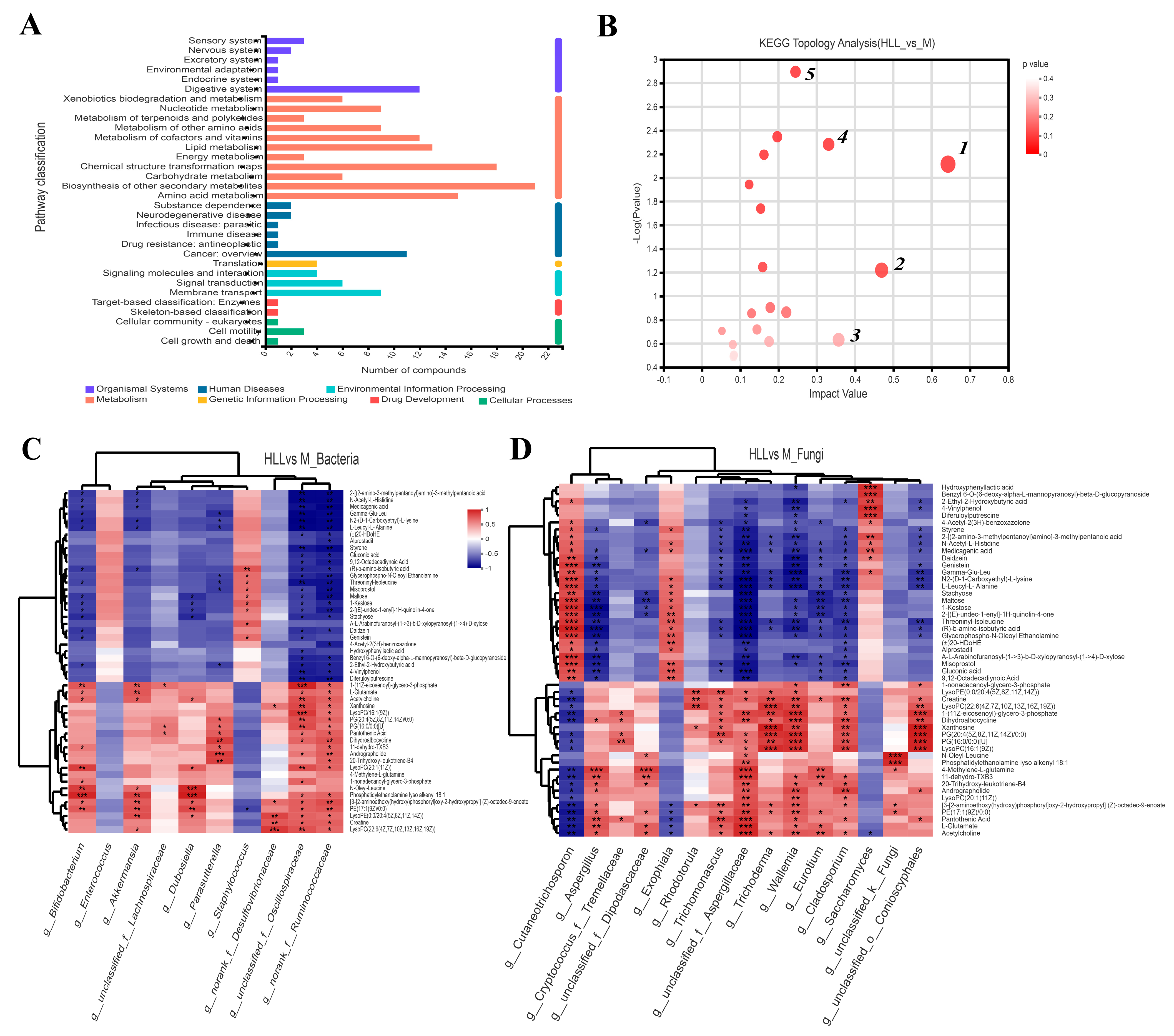
3.8. HLL Intervention Regulates Gut Metabolism in Colitis Mice
3.9. Correlations between Differential Metabolites and Predominant Microorganisms
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiba, M.; Hosoba, M.; Yamada, K. Plant-Based Diet Recommended for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, X.; Chen, Z.; Xiao, H.; Dong, J.; Li, Y.; Zeng, X.; Liu, J.; Wan, G.; Fan, S.; et al. Stable colonization of Akkermansia muciniphila educates host intestinal microecology and immunity to battle against inflammatory intestinal diseases. Exp. Mol. Med. 2023, 55, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Zhang, L.; Ruan, Z. Reparative Effects of Ethanol-Induced Intestinal Barrier Injury by Flavonoid Luteolin via MAPK/NF-kappaB/MLCK and Nrf2 Signaling Pathways. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Cerda, T.; Argüelles-Arias, F.; Macías-García, L.; Vázquez-Román, V.; Tapia, G.; Xie, K.; García-García, M.D.; Merinero, M.; García-Montes, J.-M.; Alcudia, A.; et al. Effects of polyphenolic maqui (Aristotelia chilensis) extract on the inhibition of NLRP3 inflammasome and activation of mast cells in a mouse model of Crohn’s disease-like colitis. Front. Immunol. 2024, 14, 1229767. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Ma, T.; Geng, S.; Ning, D. Walnut oil alleviates DSS–induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb. Pathog. 2021, 154, 104866. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Hu, Z.; Luo, Y.; Zhou, Y.; Yang, F.; Luo, F. Targeting gut-liver axis by dietary lignans ameliorate obesity: Evidences and mechanisms. Crit. Rev. Food Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.J.; Wang, L. Bile acid-activated receptors: A review on FXR and other nuclear receptors. In Bile Acids and Their Receptors; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2019; Volume 256, pp. 51–72. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. Bile acids as hormones: The FXR-FGF15/19 pathway. Dig. Dis. 2015, 33, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, A.; Wang, Y.; Wang, J.; Zhang, B.; Zhang, Y.; Liu, J.; Wang, S. D-Psicose intake exacerbates dextran sulfate sodium-induced colitis in mice through alteration in the gut microbiota and dysfunction of mucosal barrier. Food Sci. Hum. Wellness 2024, 13, 173–182. [Google Scholar] [CrossRef]
- Mao, B.; Guo, W.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H. Blautia producta displays potential probiotic properties against dextran sulfate sodium-induced colitis in mice. Food Sci. Hum. Wellness 2024, 13, 709–720. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, W.; Zu, X.; Xie, H.; Li, H.; Li, Y.; Zhang, W. Integrative metabolic and microbial profiling on patients with spleen-yang-deficiency syndrome. Sci. Rep. 2018, 8, 6619. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, S.; Tang, L.; Chen, Y.; Jiang, S.; Liu, L.; Gao, X. Exploring the effects of Qijiao Shengbai capsule on leukopenic mice from the perspective of intestinbased on metabolomics and 16S rRNA sequencing. Heliyon 2023, 9, e19949. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Ying, J.; Zhang, K.; Hu, Z.; Liu, Z.; Chen, S. Integrated metagenomics and metabolomics analysis reveals changes in the microbiome and metabolites in the rhizosphere soil of Fritillaria unibracteata. Front. Plant Sci. 2023, 14, 1223720. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.T.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H. Malvidin 3-Glucoside Modulated Gut Microbial Dysbiosis and Global Metabolome Disrupted in a Murine Colitis Model Induced by Dextran Sulfate Sodium. Mol. Nutr. Food Res. 2019, 63, e1900455. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 2018, 9, 2872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, K.; Wu, W.; Lv, L.; Bian, X.; Yang, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; et al. Administration of Bifidobacterium bifidum CGMCC 15068 modulates gut microbiota and metabolome in azoxymethane (AOM)/dextran sulphate sodium (DSS)-induced colitis-associated colon cancer (CAC) in mice. Appl. Microbiol. Biotechnol. 2020, 104, 5915–5928. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, C.; Yan, W.; Jiang, H.; Liu, G. Lactobacillus pentosus Increases the Abundance of Akkermansia and Affects the Serum Metabolome to Alleviate DSS-Induced Colitis in a Murine Model. Front. Cell Dev. Biol. 2020, 8, 591408. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, U.; Daniluk, J.; Kucharski, R.; Kowalczyk, T.; Pietrowska, K.; Samczuk, P.; Filimoniuk, A.; Kretowski, A.; Lebensztejn, D.; Ciborowski, M. Untargeted metabolomics and inflammatory markers profiling in children with crohn’s disease and ulcerative colitis—A preliminary study. Inflamm. Bowel Dis. 2019, 25, 1120–1128. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Luo, Y.; Xiao, L.; Wang, K.; Huang, J.; Liu, Z. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. Lebensm.-Wiss. Technol. 2020, 127, 109355. [Google Scholar] [CrossRef]
- Wang, X.; Du, G.; Chen, H.; Zeng, X.; Liu, B.; Guo, C.; Sheng, Q.; Yuan, Y.; Yue, T. Comparative Metagenomics Reveals Microbial Communities and Their Associated Functions in Two Types of Fuzhuan Brick Tea. Front. Microbiol. 2021, 12, 2633–2641. [Google Scholar] [CrossRef]
- Liang, S.; Granato, D.; Zou, C.; Gao, Y.; Zhu, Y.; Zhang, L.; Yin, J.-F.; Zhou, W.; Xu, Y.-Q. Processing technologies for manufacturing tea beverages: From traditional to advanced hybrid processes. Trends Food Sci. Technol. 2021, 118, 431–446. [Google Scholar] [CrossRef]
- Fu, D.; Ryan, E.P.; Huang, J.; Liu, Z.; Weir, T.L.; Snook, R.L.; Ryan, T.P. Fermented Camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Res. Int. 2011, 44, 2999–3005. [Google Scholar] [CrossRef]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 2022, 18, 437. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hernando, A.D.H.; Yassin, M.; Rasmussen, K.; Olsen, J.; Hansen, G.H. Short-term tissue permeability actions of dextran sulfate sodium studied in a colon organ culture system. Tissue Barriers 2020, 8, 1728165. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, H.; Su, Z.; Khoo, C.; Gu, L. Identifying cranberry juice consumers with predictive OPLS-DA models of plasma metabolome and validation of cranberry juice intake biomarkers in a double-blinded, randomized, placebo-controlled, cross-over study. Mol. Nutr. Food Res. 2020, 64, e1901242. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Xie, X.; Chen, T.; Yin, Y.; Zhai, R.; Wang, X.; An, W.; Li, J. Anthocyanins from the fruits of Lycium ruthenicum Murray improve high-fat diet-induced insulin resistance by ameliorating inflammation and oxidative stress in mice. Food Funct. 2021, 12, 3855. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Gut-liver axis modulation of Panax notoginseng saponins in nonalcoholic fatty liver disease. Hepatol. Int. 2021, 15, 350–365. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, L.; Liu, X.; Wang, P.; Wei, S.; Huang, Y.; Gu, W.; Bo, R.; Liu, M.; Yu, J.; et al. Chitosan-coated artesunate protects against ulcerative colitis via STAT6-mediated macrophage M2 polarization and intestinal barrier protection. Int. J. Biol. Macromol. 2024, 254, 127680. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.-W.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Fiaschini, N.; Palone, F.; Vitali, R.; Colantoni, E.; Laudadio, I.; Oliva, S.; Aloi, M.; Cucchiara, S.; Stronati, L. Intestinal inflammation alters the expression of hepatic bile acid receptors causing liver impairment. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The gut microbiota of healthy chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, F.; Li, R.; Liu, Y.; Wang, X.; Zhang, X.; Xu, C.; Li, Y.; Guo, Y.; Yao, Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020, 124, 109829. [Google Scholar] [CrossRef]
- Ihekweazu, F.D.; Fofanova, T.Y.; Queliza, K.; Nagy-Szakal, D.; Stewart, C.J.; Engevik, M.A.; Hulten, K.G.; Tatevian, N.; Graham, D.Y.; Versalovic, J.; et al. Bacteroides ovatus ATCC 8483 monotherapy is superior to traditional fecal transplant and multi-strain bacteriotherapy in a murine colitis model. Gut Microbes 2019, 10, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naïve microbiome in new-onset crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Jin, M.-Y.; Wu, X.-Y.; Li, M.-Y.; Li, X.-T.; Huang, R.-M.; Sun, Y.-M.; Xu, Z.-L. Noni (Morinda citrifolia L.) Fruit Polysaccharides Regulated IBD Mice Via Targeting Gut Microbiota: Association of JNK/ERK/NF-κB Signaling Pathways. J. Agric. Food Chem. 2021, 69, 10151–10162. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; Romão, E.D.L.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Kim, B.-Y.; Bae, J.-M.; Wang, Y.; Jin, Y.-S. Genome-edited Saccharomyces cerevisiae strains for improving quality, safety, and flavor of fermented foods. Food Microbiol. 2022, 104, 103971. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial interactions in alcoholic beverages. Int. Microbiol. 2022, 25, 1–15. [Google Scholar] [CrossRef]
- Rui, Y.; Wan, P.; Chen, G.; Xie, M.; Sun, Y.; Zeng, X.; Liu, Z. Analysis of bacterial and fungal communities by Illumina MiSeq platforms and characterization of Aspergillus cristatus in Fuzhuan brick tea. LWT-Food Sci. Technol. 2019, 110, 168–174. [Google Scholar] [CrossRef]
- Andersen, M.R.; Salazar, M.P.; Schaap, P.J.; van de Vondervoort, P.J.; Culley, D.; Thykaer, J.; Frisvad, J.C.; Nielsen, K.F.; Albang, R.; Albermann, K.; et al. Comparative genomics of citric-acid-producing Aspergillus niger ATCC 1015 versus enzyme-producing CBS 513.88. Genome Res. 2011, 21, 885–897. [Google Scholar] [CrossRef]
- Rui, Y.; Wan, P.; Chen, G.; Xie, M.; Sun, Y.; Zeng, X.; Liu, Z. Simulated digestion and fermentation in vitro by human gut microbiota of intra- and extra-cellular polysaccharides from Aspergillus cristatus. LWT-Food Sci. Technol. 2019, 116, 108508. [Google Scholar] [CrossRef]
- Dai, L.; Tang, Y.; Zhou, W.; Dang, Y.; Sun, Q.; Tang, Z.; Zhu, M.; Ji, G. Gut microbiota and related metabolites were disturbed in ulcerative colitis and partly restored after mesalamine treatment. Front. Pharmacol. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, J.; Zhou, X.; Hu, L.; Sun, Y.; Wang, Z.; Yue, Z.; Shan, A. Dietary supplementation with aromatic amino acids decreased triglycerides and alleviated hepatic steatosis by stimulating bile acid synthesis in mice. Food Funct. 2021, 12, 267–277. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Ma, X.; Xu, B.; Chen, L.; Chen, C.; Liu, W.; Liu, Y.; Xiang, Z. Therapeutic effect of Patrinia villosa on TNBS-induced ulcerative colitis via metabolism, vitamin D receptor and NF-kappaB signaling pathways. J. Ethnopharmacol. 2022, 288, 114989. [Google Scholar] [CrossRef]
- Yu, Z.-W.; Xie, Y.; Huang, Z.-C.; Yang, K.; Wang, Z.-G.; Hu, H.-L. Study of the therapeutic effect of raw and processed Vladimiriae Radix on ulcerative colitis based on intestinal flora, metabolomics and tissue distribution analysis. Phytomedicine 2021, 85, 153538. [Google Scholar] [CrossRef]
- Xie, Z.; Zeng, Z.; Chen, G.; Dong, W.; Peng, Y.; Xu, W.; Sun, Y.; Zeng, X.; Liu, Z. Intracellular Polysaccharides of Aspergillus cristatus from Fuzhuan Brick Tea Leverage the Gut Microbiota and Repair the Intestinal Barrier to Ameliorate DSS-Induced Colitis in Mice. J. Agric. Food Chem. 2023, 71, 8023–8037. [Google Scholar] [CrossRef]
- Lu, X.; Jing, Y.; Zhang, N.; Cao, Y. Eurotium cristatum, a Probiotic Fungus from Fuzhuan Brick Tea, and Its Polysaccharides Ameliorated DSS-Induced Ulcerative Colitis in Mice by Modulating the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 2957–2967. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, J.; Wei, J.; Zhang, Y.; Xu, Y.; Yue, T.; Yuan, Y. Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Nutrients 2024, 16, 1178. https://doi.org/10.3390/nu16081178
Wang X, Liu J, Wei J, Zhang Y, Xu Y, Yue T, Yuan Y. Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Nutrients. 2024; 16(8):1178. https://doi.org/10.3390/nu16081178
Chicago/Turabian StyleWang, Xin, Jinhu Liu, Jianping Wei, Yuxiang Zhang, Yunpeng Xu, Tianli Yue, and Yahong Yuan. 2024. "Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice" Nutrients 16, no. 8: 1178. https://doi.org/10.3390/nu16081178
APA StyleWang, X., Liu, J., Wei, J., Zhang, Y., Xu, Y., Yue, T., & Yuan, Y. (2024). Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Nutrients, 16(8), 1178. https://doi.org/10.3390/nu16081178





