Isoeugenol Inhibits Adipogenesis in 3T3-L1 Preadipocytes with Impaired Mitotic Clonal Expansion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Adipocyte Differentiation
2.3. Oil Red O (ORO) Staining
2.4. Western Blotting Analysis
2.5. Quantitative Polymerase Chain Reaction (qPCR)
2.6. Cell Proliferation
2.7. Flow Cytometry
2.8. Measurement of Intracellular ROS
2.9. Statistical Analysis
3. Results
3.1. IEG Inhibits MDI-Induced Adipogenesis in 3T3-L1 Cells
3.2. IEG Suppresses the Early Stage of Adipocyte Differentiation
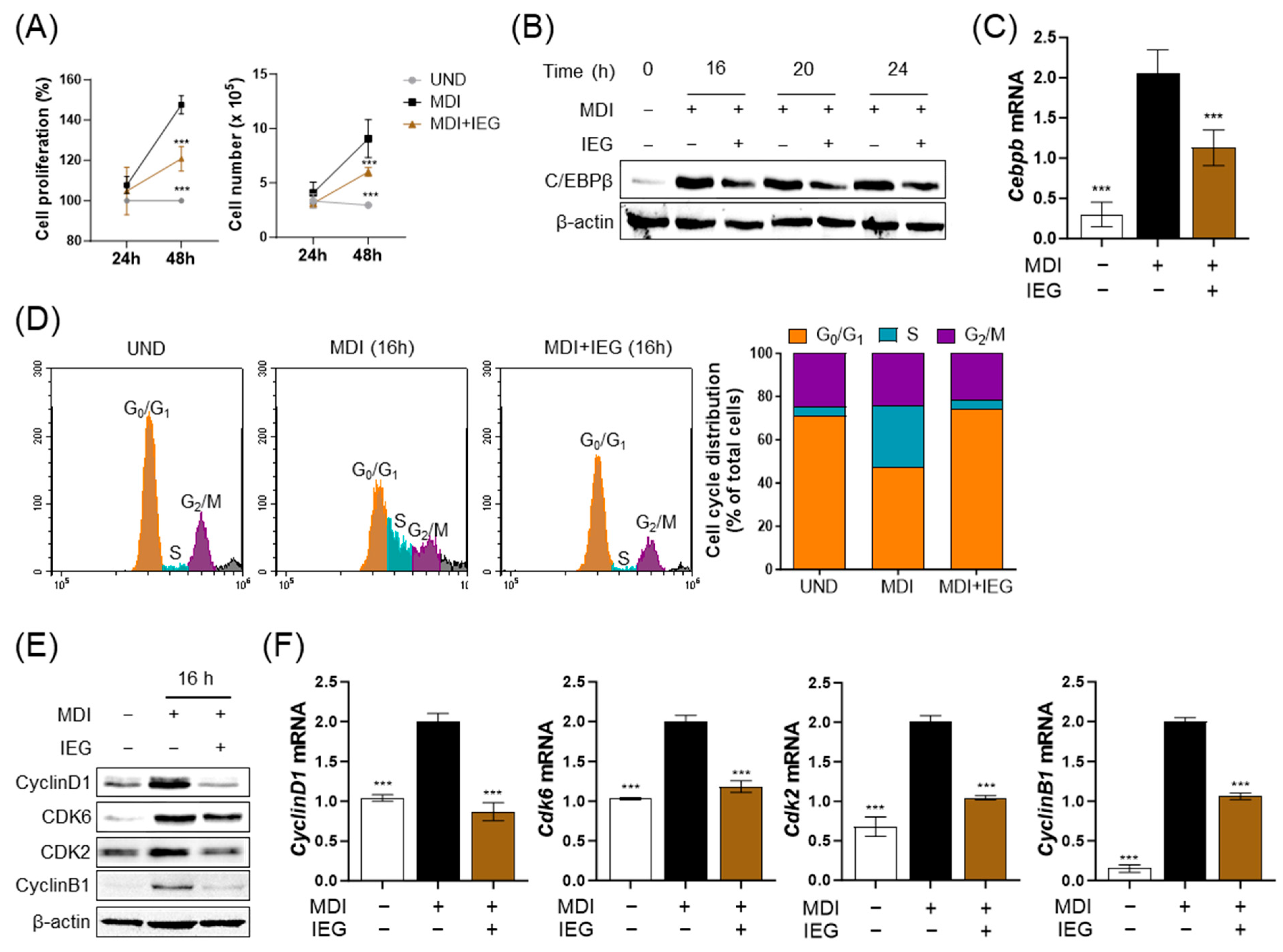
3.3. IEG Suppresses MDI-Induced Cell Cycle Progression in the Early Stage of Differentiation
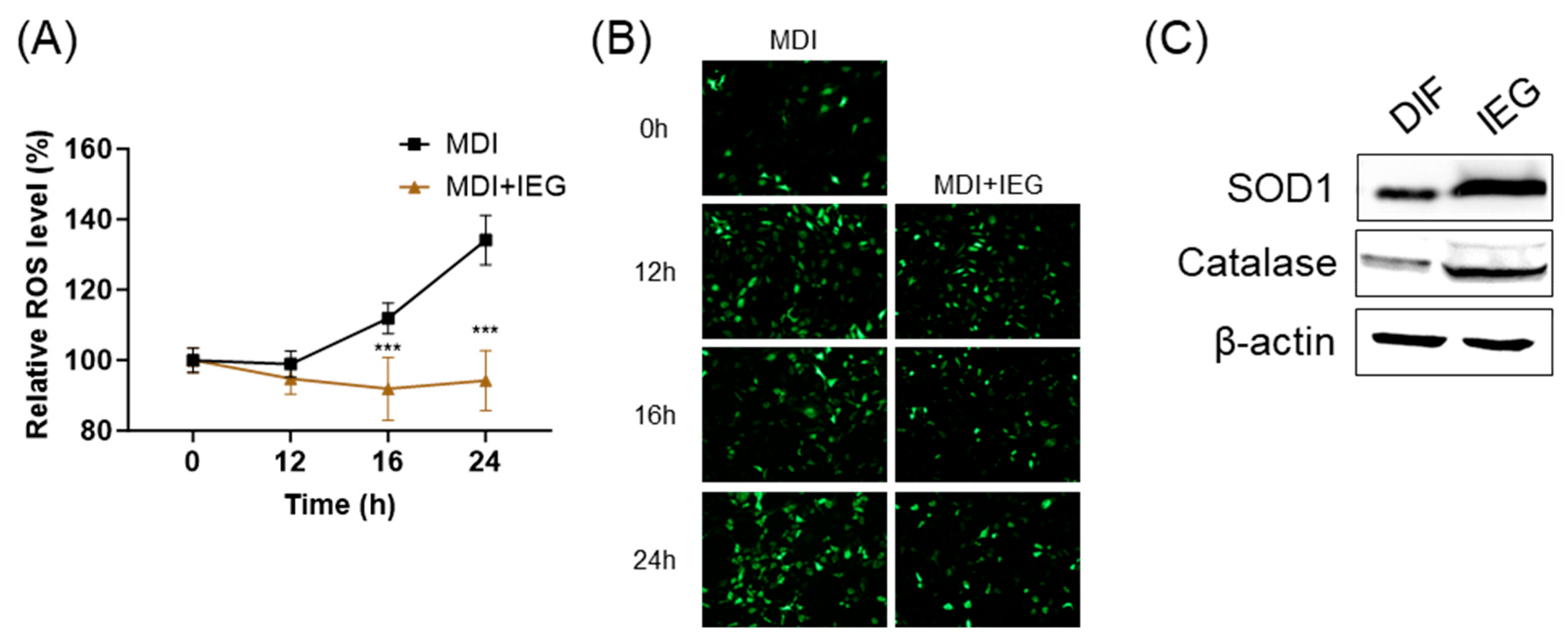
3.4. IEG Reduces Intracellular ROS Generation in the Early Stage of Differentiation
3.5. IEG Attenuated the Expression of AKT and ERK in the Early Stage of Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; DeMarsilis, A.; Mantzoros, C.S. Obesity and diabetes. Diabetes Res. Clin. Pract. 2023, 202, 110773. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini-Silva, C.; Zingg, J.-M.; Fassini, P.G.; Suen, V.M.M. Bioactive dietary components—Anti-obesity effects related to energy metabolism and inflammation. BioFactors 2023, 49, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Tang, Q.-Q.; Vinson, C.; Lane, M.D. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 2004, 101, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Yamamoto, A.; Yoneda, T.; Hori, S.; Okochi, N.; Kagotani, K.; Okumura, K.; Takebayashi, S.-I. Reorganization of the DNA replication landscape during adipogenesis is closely linked with adipogenic gene expression. J. Cell Sci. 2023, 136, jcs260778. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Klemm, D.J.; Vinson, C.; Lane, M.D. Role of CREB in Transcriptional Regulation of CCAAT/Enhancer-binding Protein β Gene during Adipogenesis. J. Biol. Chem. 2004, 279, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.G.; Giordano, A.P.; Gambaro, S.E.; Alzamendi, A.; Frontini-López, Y.R.; Moreno, G.; Spinedi, E.; Giovambattista, A. Dexamethasone primes adipocyte precursor cells for differentiation by enhancing adipogenic competency. Life Sci. 2020, 261, 118363. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Li, Y.; Matye, D.J.; Wang, Y.; Ding, W.-X.; Li, T. Inhibition of insulin/PI3K/AKT signaling decreases adipose Sortilin 1 in mice and 3T3-L1 adipocytes. Biochim. Biophys. Acta (BBA)–Mol. Basis Dis. 2017, 1863, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Park, J.; Rahman, S.; Jin, J.; Choi, I.; Kim, J. Adipogenic function of tetranectin mediated by enhancing mitotic clonal expansion via ERK signaling. BMB Rep. 2021, 54, 374. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.-W. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Shibamoto, T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]. Food Chem. 2001, 74, 443–448. [Google Scholar] [CrossRef]
- Fabry, P.; Weber, S.; Teipel, J.; Richling, E.; Walch, S.G.; Lachenmeier, D.W. Quantitative NMR Spectrometry of Phenylpropanoids, including Isoeugenol in Herbs, Spices, and Essential Oils. Foods 2024, 13, 720. [Google Scholar] [CrossRef]
- Filiciotto, L.; Márquez-Medina, M.D.; Pineda, A.; Balu, A.M.; Romero, A.A.; Angelici, C.; de Jong, E.; van der Waal, J.C.; Luque, R. Continuous flow study of isoeugenol to vanillin: A bio-based iron oxide catalyst. Catal. Today 2021, 368, 281–290. [Google Scholar] [CrossRef]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicol. Vitr. 2005, 19, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhang, L.F.; Xu, J.G.; Hu, Q.P. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017, 61, 1353356. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gu, Z.; Wang, Y.; Wang, S.; Chen, H.; Zhang, H.; Chen, W.; Chen, Y.Q. Clove extract functions as a natural fatty acid synthesis inhibitor and prevents obesity in a mouse model. Food Funct. 2017, 8, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Y.; Wang, Y.; Geng, R.; Fang, J.; Kang, S.-G.; Huang, K.; Tong, T. Eugenol, A Major Component of Clove Oil, Attenuates Adiposity, and Modulates Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2022, 66, 2200387. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.M.; Lane, M.D. Mitotic Clonal Expansion during Preadipocyte Differentiation: Calpain-mediated Turnover of p27. J. Biol. Chem. 2000, 275, 17653–17660. [Google Scholar] [CrossRef] [PubMed]
- Marcon, B.H.; Shigunov, P.; Spangenberg, L.; Pereira, I.T.; de Aguiar, A.M.; Amorín, R.; Rebelatto, C.K.; Correa, A.; Dallagiovanna, B. Cell cycle genes are downregulated after adipogenic triggering in human adipose tissue-derived stem cells by regulation of mRNA abundance. Sci. Rep. 2019, 9, 5611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.W.; Grønborg, M.; Urlaub, H.; Lane, M.D.; Tang, Q.-Q. Role of cdk2 in the sequential phosphorylation/activation of C/EBPβ during adipocyte differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 11597–11602. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lee, M.-H.; Hsu, C.-C.; Wei, C.-L.; Tsai, Y.-C. Methyl Cinnamate Inhibits Adipocyte Differentiation via Activation of the CaMKK2–AMPK Pathway in 3T3-L1 Preadipocytes. J. Agric. Food Chem. 2012, 60, 955–963. [Google Scholar] [CrossRef]
- Choi, Y.R.; Kim, Y.-S.; Kim, M.J. Cinnamyl Alcohol Attenuates Adipogenesis in 3T3-L1 Cells by Arresting the Cell Cycle. Int. J. Mol. Sci. 2024, 25, 693. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; Patten, D.A.; Harper, M.-E. Reactive Oxygen Species and Oxidative Stress in Obesity—Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, C.U.; Casalini, J.R.; Pradeep, S.; Scherer, S.D.; Greiner, D.; Bayik, D.; Watson, D.C.; Olson, G.S.; Lathia, J.D.; Johnson, J.S. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. Elife 2023, 12, e85494. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Jimoh, A.; Tanko, Y.; Ahmed, A.; Mohammed, A.; Ayo, J.O. Resveratrol prevents high-fat diet-induced obesity and oxidative stress in rabbits. Pathophysiology 2018, 25, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Jang, H.-D. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. Int. J. Mol. Sci. 2021, 22, 6096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Huang, J.; Düvel, K.; Boback, B.; Wu, S.; Squillace, R.M.; Wu, C.L.; Manning, B.D. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS ONE 2009, 4, e6189. [Google Scholar] [CrossRef] [PubMed]
- Sri Devi, S.; Ashokkumar, N. Citral, a Monoterpene Inhibits Adipogenesis Through Modulation of Adipogenic Transcription Factors in 3T3-L1 Cells. Indian J. Clin. Biochem. 2018, 33, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Seo, S.G.; Yue, S.; Cheng, J.-X.; Lee, K.W.; Kim, K.-H. An inhibitory effect of resveratrol in the mitotic clonal expansion and insulin signaling pathway in the early phase of adipogenesis. Nutr. Res. 2012, 32, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Wei, L.; Castro-Muñozledo, F.; Koo, W.L. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011, 89, 779–785. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Zhang, Y.; Deng, R.; Shao, L.; Liu, Y.; Li, F.; Wang, X.; Zhou, L. Identification of suitable reference genes for quantitative RT-PCR during 3T3-L1 adipocyte differentiation. Int. J. Mol. Med. 2014, 33, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Cahyadi, D.D.; Warita, T.; Irie, N.; Mizoguchi, K.; Tashiro, J.; Hosaka, Y.Z.; Warita, K. Housekeeping gene expression variability in differentiating and non-differentiating 3T3-L1 cells. Adipocyte 2023, 12, 2235081. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| Pparg | Forward | AAGGATTCATGACCAGGGAGTTCC |
| Reverse | GCGGTCTCCACTGAGAATAATG | |
| Cebpα | Forward | GTGGACAAGAACAGCAACGAGT |
| Reverse | AGGCGGTCATTGTCACTGGTCAA | |
| Fapb4 | Forward | GTGGGCTTTGCCACAAGGAAAGT |
| Reverse | GGTGATTTCATCGAATTCCACGCC | |
| Adipoq | Forward | AGCCGCTTATGTGTATCGCTCAG |
| Reverse | CCCGGAATGTTGCAGTAGAACT | |
| Fasn | Forward | TGGGTTTGGTGAATTGTCTCCG |
| Reverse | ACACGTTCATCACGAGGTCATG | |
| Cebpb | Forward | AACAACATCGCGGTGCGCAA |
| Reverse | AACAAGTTCCGCAGGGTGCTGA | |
| CyclinD1 | Forward | AAGCAGACCATCCGCAAGCA |
| Reverse | GGTAGCAGGAGAGGAAGTTGTT | |
| Cdk6 | Forward | TTTTCAGATGGCCCTTACCTCG |
| Reverse | CCACGAAAAAGAGGCTTTCTGC | |
| Cdk2 | Forward | CGAGCACCTGAAATTCTTCTGG |
| Reverse | AGAGTCCGAAAGATCCGGAA | |
| CyclinB1 | Forward | GCATCTAAAGTCGGAGAGGT |
| Reverse | GGTGTCCATTCACCGTTGTC | |
| 18S | Forward | GTAACCCGTTGAACCCCATT |
| Reverse | CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.R.; Na, H.-J.; Lee, J.; Kim, Y.-S.; Kim, M.J. Isoeugenol Inhibits Adipogenesis in 3T3-L1 Preadipocytes with Impaired Mitotic Clonal Expansion. Nutrients 2024, 16, 1262. https://doi.org/10.3390/nu16091262
Choi YR, Na H-J, Lee J, Kim Y-S, Kim MJ. Isoeugenol Inhibits Adipogenesis in 3T3-L1 Preadipocytes with Impaired Mitotic Clonal Expansion. Nutrients. 2024; 16(9):1262. https://doi.org/10.3390/nu16091262
Chicago/Turabian StyleChoi, Yae Rim, Hyun-Jin Na, Jaekwang Lee, Young-Suk Kim, and Min Jung Kim. 2024. "Isoeugenol Inhibits Adipogenesis in 3T3-L1 Preadipocytes with Impaired Mitotic Clonal Expansion" Nutrients 16, no. 9: 1262. https://doi.org/10.3390/nu16091262
APA StyleChoi, Y. R., Na, H.-J., Lee, J., Kim, Y.-S., & Kim, M. J. (2024). Isoeugenol Inhibits Adipogenesis in 3T3-L1 Preadipocytes with Impaired Mitotic Clonal Expansion. Nutrients, 16(9), 1262. https://doi.org/10.3390/nu16091262







