Traditional Uses, Phytochemistry, and Pharmacological Activities of Vernonia cinerea (L.) Less.: An Updated Review
Abstract
1. Introduction
2. Taxonomic Position
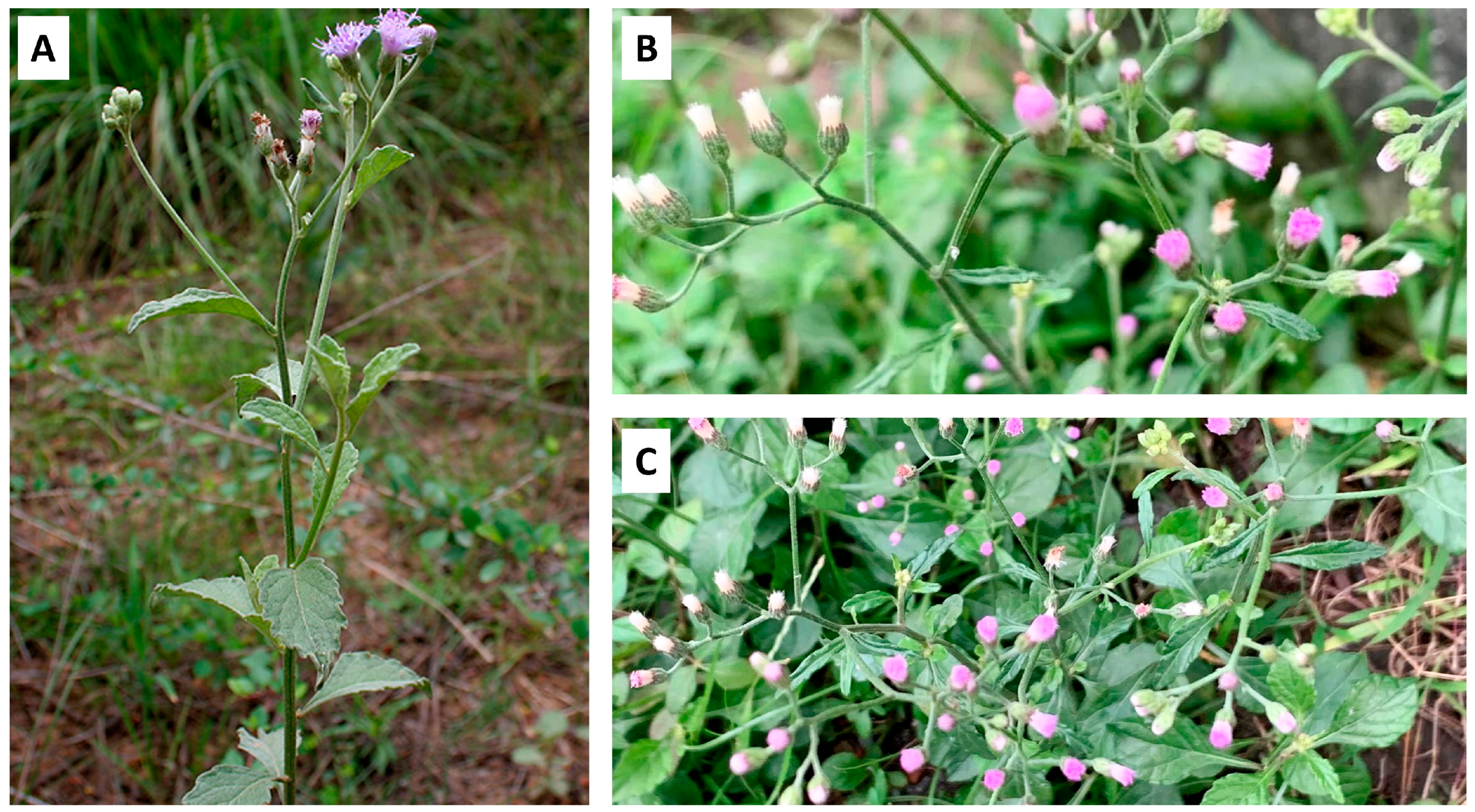
3. Botanical Characterization and Distribution
4. Ethnopharmacological and Traditional Uses
4.1. India
4.2. Other Asian Countries
4.3. African Countries
4.4. West Indies Countries
5. Phytochemistry
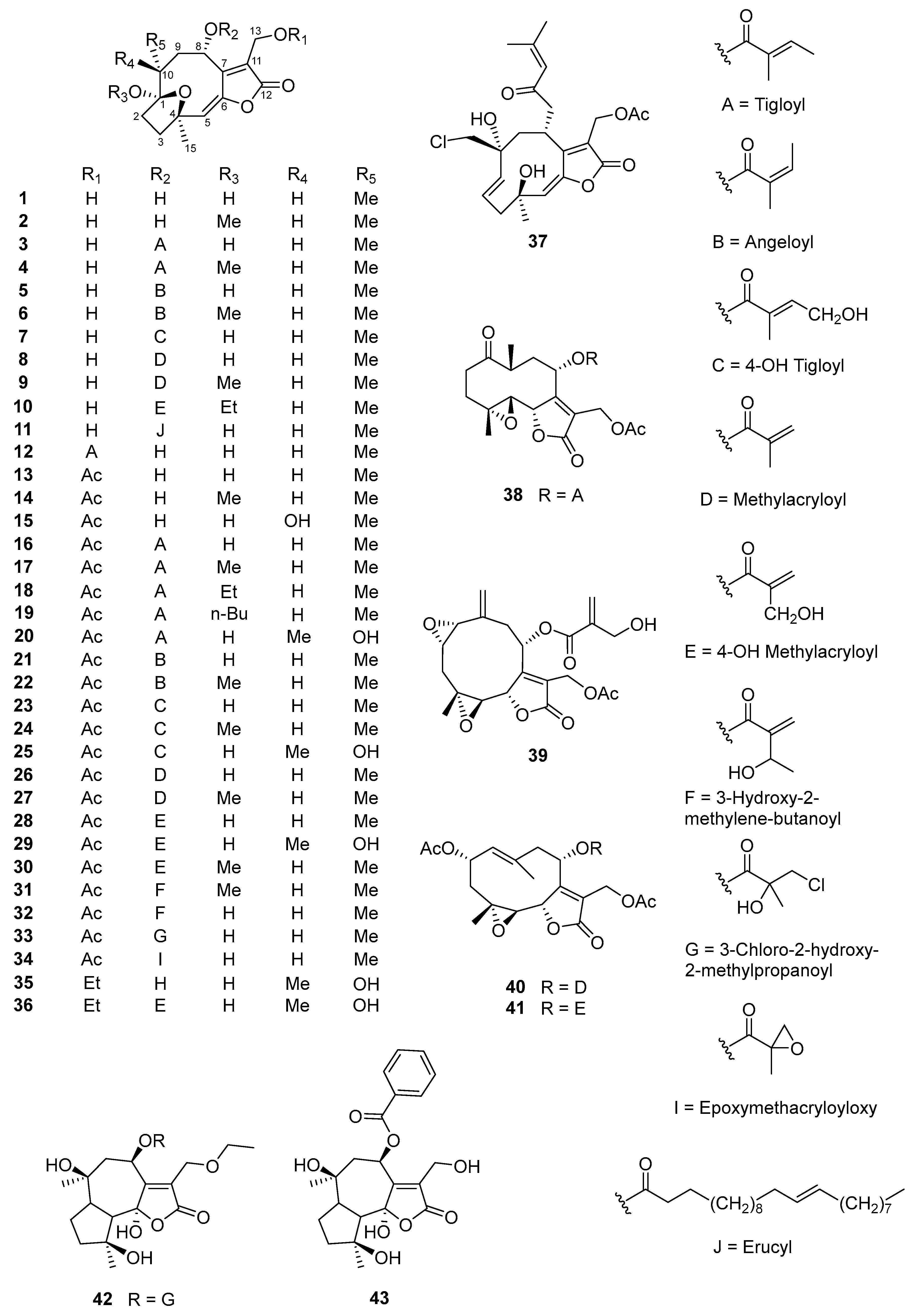
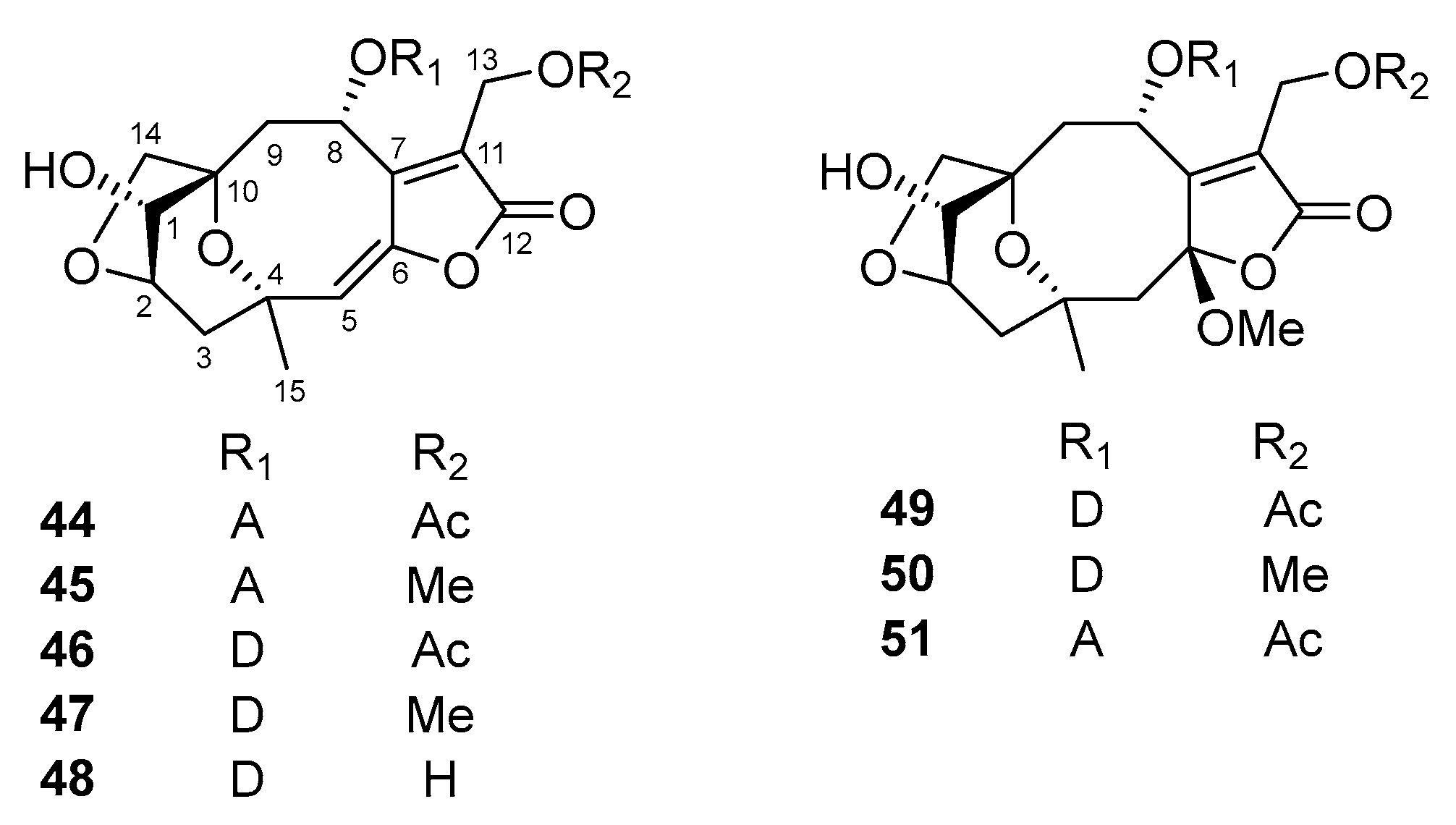
5.1. Sesquiterpene Lactones
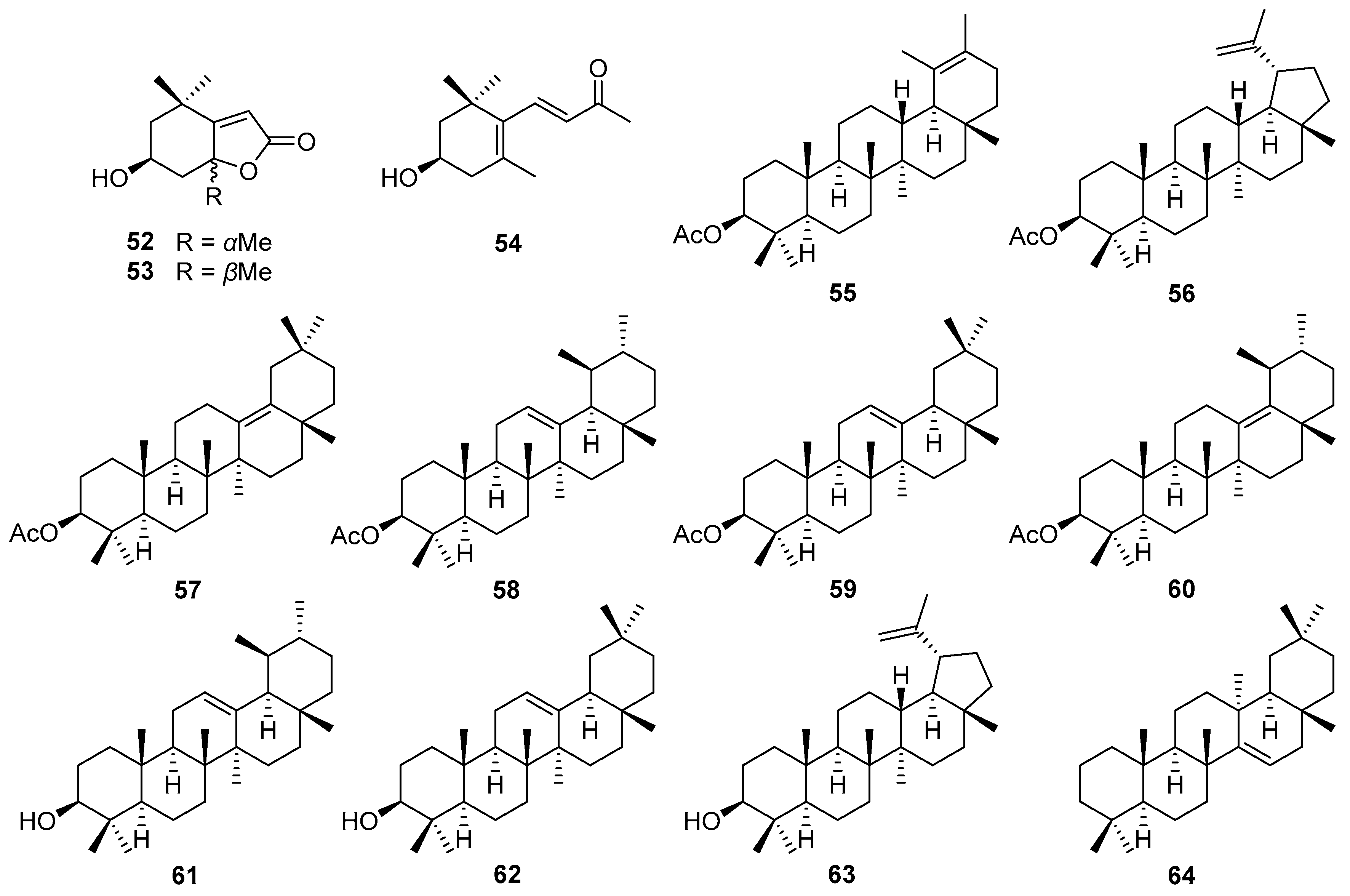
5.2. Norisoprenoids and Triterpenes
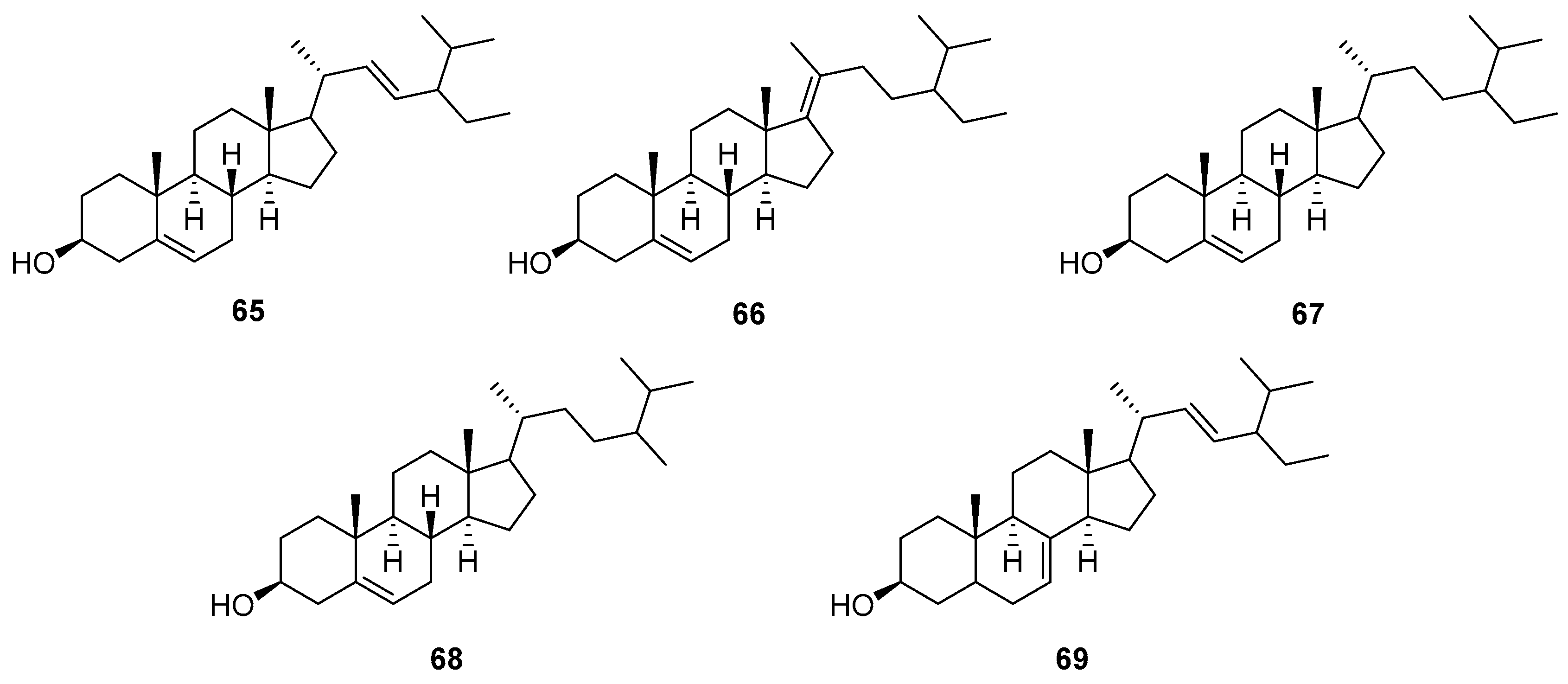
5.3. Steroids
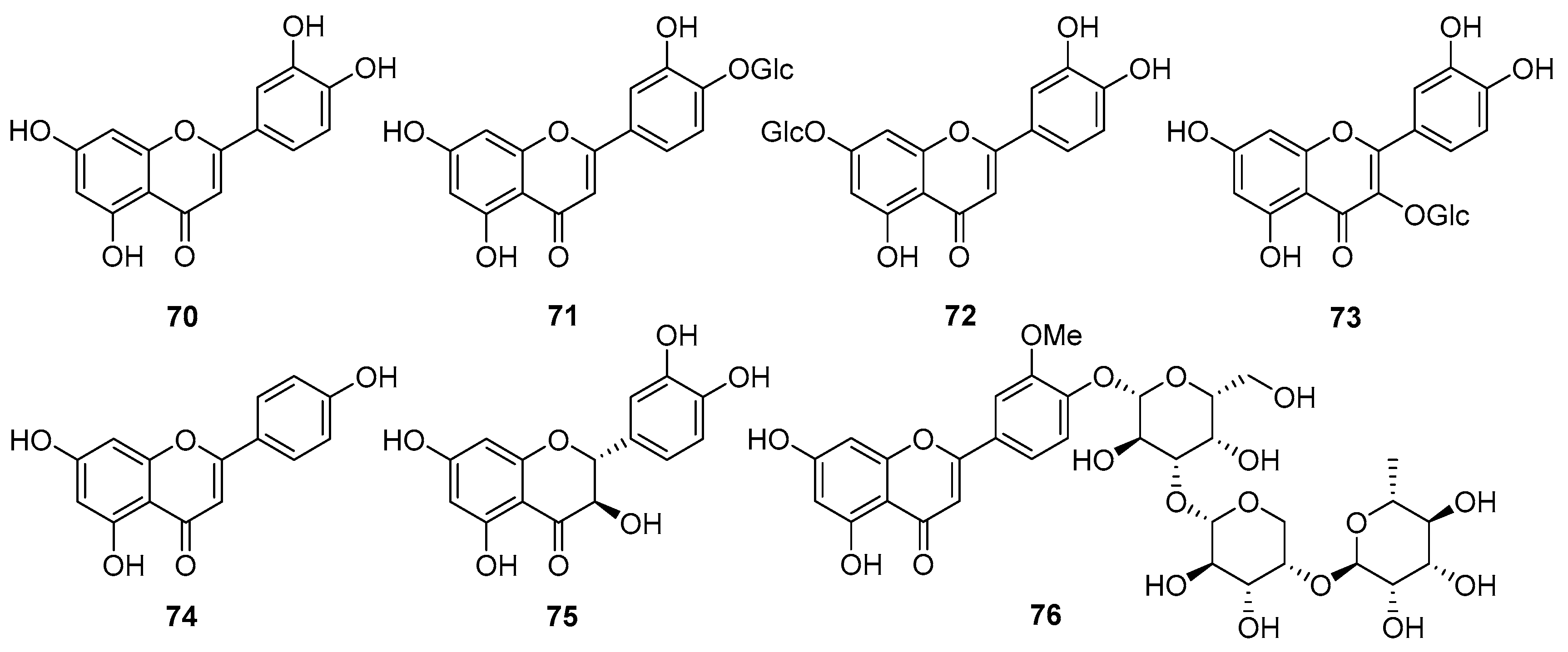
5.4. Flavonoids
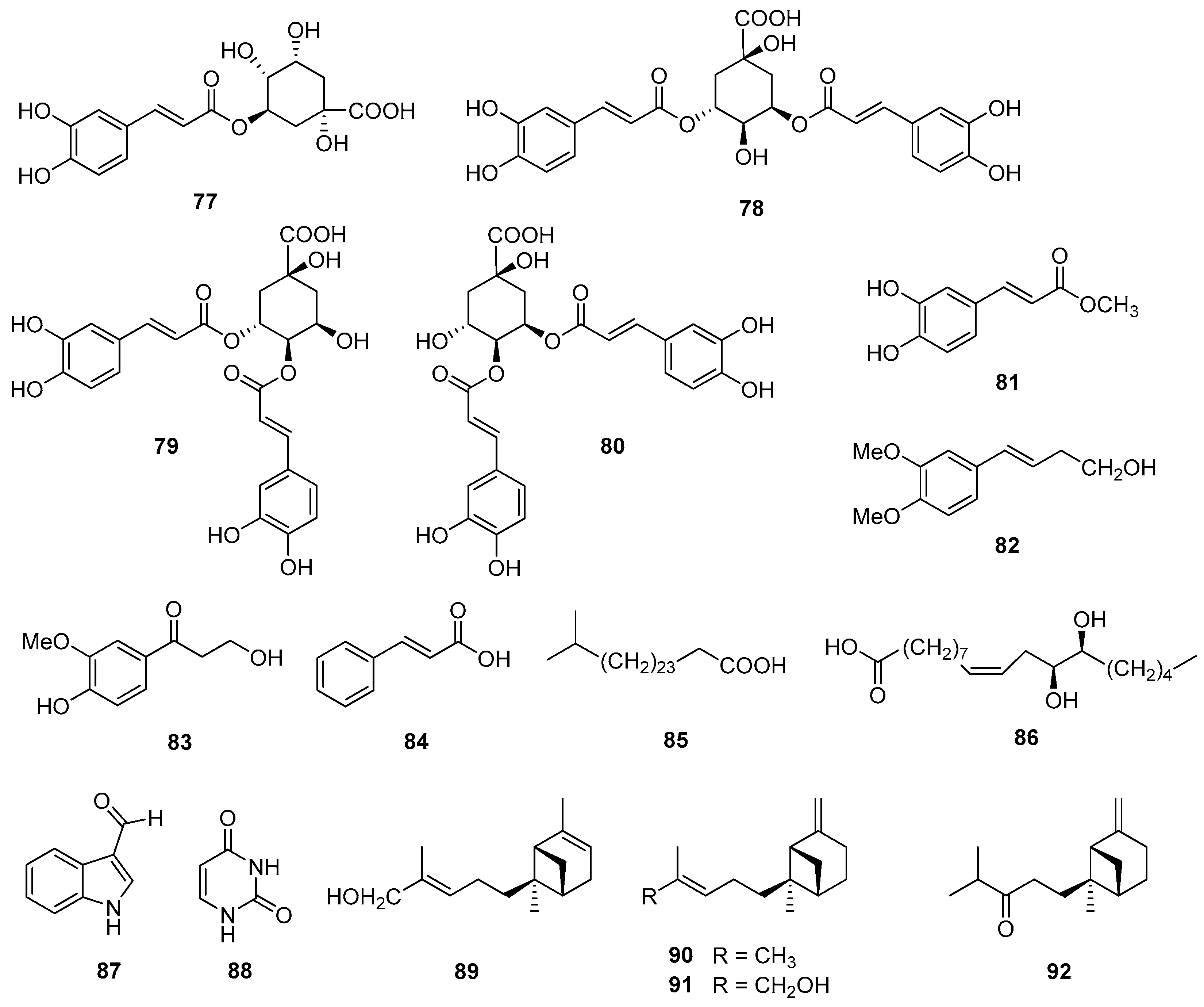
5.5. Phenolics and Other Compounds
5.6. HPLC and GC-MS Identifications
| No. | Compound Name | Plant Part | References |
|---|---|---|---|
| Sesquiterpene lactones | |||
| 1 | 8α-Hydroxyhirsutinolide | Flowers, leaves, stems | [15,45] |
| 2 | 8α-Hydroxyl-1-O-methylhirsutinolide | Flowers | [15] |
| 3 | 8α-Tigloyloxyhirsutinolide | Flowers, leaves, stems | [15,16,44,45,71,72] |
| 4 | Vernolide-A | Flowers, leaves, stems | [15,42,44,45,71,72] |
| 5 | 8α-(2′Z-tigloyloxy)-hirsutinolide | Leaves, stems | [45] |
| 6 | 8α-(2′Z-tigloyloxy)-1α-methoxyhirsutinolide | Aerial parts | [73] |
| 7 | 8α-(4-Hydroxytigloyloxy)-hirsutinolide | Leaves, stems | [45,72] |
| 8 | 8α-(2-Methylacryloyloxy)-hirsutinolide | Aerial parts, leaves | [44,45,71,72] |
| 9 | 8α-(2-Methylacryloyloxy)-1α-methoxyhirsutinolide | Aerial parts, leaves | [44,71] |
| 10 | Cyanolide D | Aerial parts | [44] |
| 11 | 8α-Erucyl-1α-hydroxyl-hirustinolide | Leaves | [74] |
| 12 | 8α-Hydroxy-13-O-tigloyl-hirsutinolide | Leaves, stems | [45] |
| 13 | Hirsutinolide-13-O-acetate | Leaves, flowers | [15,45,72,74] |
| 14 | Vernolide E | Whole plant | [72] |
| 15 | Piptocarphin D | Aerial parts | [16] |
| 16 | 8α-Tigloyloxyhirsutinolide-13-O-acetate | Flowers, leaves, stems | [15,16,40,43,45,71,72] |
| 17 | Vernolide-B | Flowers, leaves, stems | [15,42,43,45,71,72] |
| 18 | Cyanolide B | Aerial parts | [44] |
| 19 | Cyanolide C | Aerial parts | [44] |
| 20 | Piptocarphin B | Aerial parts | [44] |
| 21 | 8α-(2′Z-tigloyloxy)-hirsutinolide-13-O-acetate | Leaves, stems | [45] |
| 22 | Vernolide F | Whole plant | [72] |
| 23 | 8α-(4-Hydroxytigloyloxy)-hirsutinolide-13-O-acetate | Aerial parts | [16,40,71,72] |
| 24 | Vernolide G | Whole plant | [72] |
| 25 | 8α-(4-Hydroxytigloxyloxy)-10α-hydroxyhirsutinolide-13-O-acetate | Aerial parts | [40,71] |
| 26 | 8α-(2-Methylacryloyloxy)-hirsutinolide-13-O-acetate | Flowers, leaves, stems | [15,43,45,71,72] |
| 27 | 8α-(2-Methylacryloyloxy)-1α-methoxyhirsutinolide-13-O-acetate | Flowers | [15,43,72] |
| 28 | 8α-(4-Hydroxymethacryloyloxy)-hirsutinolide-13-O-acetate | Aerial parts | [16,40,72] |
| 29 | 8α-(4-Hydroxymethacryloyloxy)-10α-hydroxyhirsutinolide-13-O-acetate | Aerial parts | [40] |
| 30 | 8α-(2′-Hydroxymethylacryloyloxy)-1α-methoxyhirsutinolide-13-O-acetate | Aerial parts | [72,73] |
| 31 | Vernolide I | Whole plant | [72] |
| 32 | Vernolide J | Whole plant | [72] |
| 33 | Vernolide C | Aerial parts, leaves | [16,44] |
| 34 | 8α-Epoxymethacryloyloxy-hirsutinolide-13-O-acetate | Aerial parts | [16] |
| 35 | (1S*,4R*,8S*,10R*)-1,4-epoxy-13-ethoxy-1,8,10-trihydroxygermacra-5E,7(11)-dien-6,12-olide | Aerial parts | [44] |
| 36 | Vernobockolide B | Whole plant, leaves | [44,72] |
| 37 | Cyanolide A | Aerial parts | [44] |
| 38 | Stilpnotomentolide-8-O-tiglate | Aerial parts | [40] |
| 39 | Vernocinerolide-8-O-(4-hydroxymethacrylate) | Aerial parts | [40] |
| 40 | Glaucolide E | Aerial parts | [40] |
| 41 | 19-Hydroxyglaucolide E | Aerial parts | [40] |
| 42 | Vernocinolide A | Aerial parts | [71] |
| 43 | 8β-[benzoic acid]-4β,6α,10β,13-tetrahydroxyl-7(11)-guaiaen-12,6-olide | Leaves | [74] |
| 44 | Vercinolide A | Whole plant | [43] |
| 45 | Vercinolide B | Whole plant | [43] |
| 46 | Vercinolide C | Whole plant | [43] |
| 47 | Vercinolide D | Whole plant | [43] |
| 48 | Vercinolide E | Whole plant | [43] |
| 49 | Vercinolide F | Whole plant | [43] |
| 50 | Vercinolide G | Whole plant | [43] |
| 51 | Vercinolide H | Whole plant | [43] |
| Norisoprenoids | |||
| 52 | Loliolide | Leaves, stems | [45] |
| 53 | Isololiolide | Leaves, stems | [45] |
| 54 | (3R)-3-Hydroxyionone | Leaves, stems | [45] |
| Triterpenes | |||
| 55 | 3β-Acetoxyurs-19-ene | Roots | [55] |
| 56 | Lupeol acetate | Roots | [55] |
| 57 | δ-Amyrin acetate | Roots | [54] |
| 58 | α-Amyrin acetate | Roots | [54] |
| 59 | β-Amyrin acetate | Roots | [54,75] |
| 60 | 3β-Acetoxyurs-13(18)-ene | Roots | [54] |
| 61 | α-Amyrin | Roots | [54] |
| 62 | β-Amyrin | Roots | [54] |
| 63 | Lupeol | Leaves | [75,76] |
| 64 | 24-Hydroxytaraxer-14-ene | Roots | [53] |
| Steroids | |||
| 65 | Stigmast-5,17(20)-dien-3β-ol | Roots | [58] |
| 66 | Stigmasterol | Roots | [58,75] |
| 67 | Sitosterol | Roots | [58,75] |
| 68 | Campesterol | Roots | [53] |
| 69 | α-Spinasterol | Roots | [53] |
| Flavonoids | |||
| 70 | Luteolin | Aerial parts, roots | [61,62] |
| 71 | Luteolin 4′-O-glucoside | Aerial parts | [27] |
| 72 | Luteolin 7-O-glucoside | Aerial parts | [62] |
| 73 | Quercetin 3-O-β-D-glucopyranoside | Aerial parts | [63] |
| 74 | Apigenin | Leaves, stems | [45] |
| 75 | Taxifolin | Roots | [61] |
| 76 | 5,7,4′-Trihydroxy-3′-methoxyflavone-4′-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-arabinopyranosyl-(1→3)-O-β-D-galactopyranoside | Roots | [61] |
| Phenolic compounds | |||
| 77 | Chlorogenic acid | Aerial parts | [27] |
| 78 | 3,5-Dicaffeoylquinic acid | Aerial parts | [27] |
| 79 | 3,4-Dicaffeoylquinic acid | Aerial parts | [27] |
| 80 | 4,5-Dicaffeoylquinic acid | Aerial parts | [27] |
| 81 | Methyl caffeate | Aerial parts | [27] |
| 82 | (E)-4-(3,4-Dimethoxyphenyl)but-3-en-1-ol | Aerial parts | [63] |
| 83 | 3-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-propan-1-one | Aerial parts | [63] |
| 84 | trans-Cinnamic acid | Aerial parts | [63] |
| Other compounds | |||
| 85 | 26-Methylheptacosanoic acid | Roots | [58] |
| 86 | (9Z,12S,13S)-Dihydroxy-9-octadecanoic acid | Leaves, stems | [45] |
| 87 | 1H-Indole-3-carbaldehyde | Aerial parts | [63] |
| 88 | Uracil | Aerial parts | [63] |
| 89 | (E)-trans-α-Bergamotenol | Flowers, roots | [64] |
| 90 | trans-β-Bergamotene | Flowers, roots | [64] |
| 91 | (E)-trans-β-Bergamotenol | Flowers, roots | [64] |
| 92 | trans–β–Bergamotenone | Flowers, roots | [64] |
6. Pharmacological Activities
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Antipyretic Activity
6.4. Cholinesterase Inhibition
6.5. Antitumor Activities
6.6. Hepatoprotective Activity
6.7. Diuretic Effect and Antiurolithiasis Activity
6.8. Antidiarrheal Effects
6.9. Antifeedant Effects
6.10. Antidiabetic Activity
6.11. Antiprotozoal and Larvicidal Activity
6.12. Antimalarial Activity
6.13. Antifungal and Antimicrobial Activity
6.14. Antiviral Activity
6.15. Antiarthritic Activity
6.16. Analgesic Effect
6.17. Effective in Smoking Cessation
6.18. Antianxiety Effect
6.19. Antivenom Activity
6.20. Toxicological Effect
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Government of India. Department of Indian Systems of Medicine & Homoeopathy. In The Ayurvedic Pharmacopoeia of India; Government of India, Ministry of Health and Family Welfare: New Delhi, India, 2001. [Google Scholar]
- Chi, V.V. Dictionary of medicinal plants in Vietnam. Vietnam. Publ. Med. 2012, 1, 99–100. [Google Scholar]
- Toyang, N.J.; Verpoorte, R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Plant Names: Common Names, Scientific Names, Eponyms. Synonyms, and Etymology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar] [CrossRef]
- Dogra, N.K.; Kumar, S. A review on ethno-medicinal uses and pharmacology of Vernonia cinerea Less. Nat. Prod. Res. 2015, 29, 1102–1117. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.K.; Manikandan, L.; Haldar, P.K.; Bhattacharya, S.; Kandar, C.C. Antibacterial activity of Vernonia cinerea. Fitoterapia 2003, 74, 148–150. [Google Scholar] [CrossRef]
- Allabi, A.C.; Busia, K.; Ekanmian, V.; Bakiono, F. The use of medicinal plants in self-care in the Agonlin region of Benin. J. Ethnopharmacol. 2011, 133, 234–243. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Kuttan, G. Protective role of Vernonia cinerea L. against gamma radiation—Induced immunosupression and oxidative stress in mice. Hum. Exp. Toxicol. 2010, 30, 1022–1038. [Google Scholar] [CrossRef]
- Mazumder, U.K.; Gupta, M.; Manikandan, L.; Bhattacharya, S.; Haldar, P.K.; Roy, S. Evaluation of anti-inflammatory activity of Vernonia cinerea Less. extract in rats. Phytomedicine 2003, 10, 185–188. [Google Scholar] [CrossRef]
- Johnson, J.; Varghese, L. Tumor reduction potentials of Vernonia cinerea sesquiterpenes by induction of ferroptosis. J. Herbs Spices Med. Plants 2023, 29, 438–451. [Google Scholar] [CrossRef]
- Haque, M.A.; Abdullah, C.S.; Romana, B.; Rafique, M.B.; Zia-ulHuda, G.M.; Hossain, S.F.; Begum, B. Evaluation of anti-diarrheal and anti-diabetic activities of the stem, barks and leaves of the plant Vernonia cinerea (Family: Asteraceae). J. Appl. Pharm. Sci. 2013, 3, 69–72. [Google Scholar] [CrossRef][Green Version]
- Lertsinudom, S.; Sawanyawisuth, K.; Srisoi, S.; Areemit, J.; Hansuri, N.; Tawinkan, N.; Theeranut, A.; Sripanidkulchai, B.; Pranboon, S. Vernonia cinerea pastilles is effective for smoking cessation. J. Tradit. Complement. Med. 2021, 11, 90–94. [Google Scholar] [CrossRef]
- Shelar, D.; Tikole, S.; Kakade, T. Vernonia cinerea: A review. J. Curr. Pharma Res. 2014, 4, 1194–1200. [Google Scholar]
- Theja, D.D.; Nirmala, S. A review of Vernonia cinerea L. ethno-medicinal uses and pharmacology shows that it could be a useful plant for medical purposes. Intell. Pharm. 2023, in press. [Google Scholar] [CrossRef]
- Youn, U.J.; Park, E.-J.; Kondratyuk, T.P.; Simmons, C.J.; Borris, R.P.; Tanamatayarat, P.; Wongwiwatthananukit, S.; Toyama, O.; Songsak, T.; Pezzuto, J.M.; et al. Anti-inflammatory sesquiterpene lactones from the flower of Vernonia cinerea. Bioorg. Med. Chem. Lett. 2012, 22, 5559–5562. [Google Scholar] [CrossRef] [PubMed]
- Chea, A.; Hout, S.; Long, C.; Marcourt, L.; Faure, R.; Azas, N.; Elias, R. Antimalarial activity of sesquiterpene lactones from Vernonia cinerea. Chem. Pharm. Bull. 2006, 54, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, Y. First report of powdery mildew caused by Podosphaera xanthii on Vernonia cinerea in China. Plant Dis. 2023, 107, 4024. [Google Scholar] [CrossRef]
- Dahanukar, S.A.; Thatte, U.M. Current status of ayurveda in phytomedicine. Phytomedicine 1997, 4, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Varghese, K.J.; Anila, J.; Nagalekshmi, R.; Resiya, S.; Sonu, J. Dasapushpam: The traditional uses and the therapeutic potential of ten sacred plants of Kerala state in India. Int. J. Pharm. Sci. Res. 2010, 1, 50. [Google Scholar]
- Ida, B.; Seema, D.; Shital, D.; Riva, S.; Astrida, R. Antimicrobial activity of ten common herbs, commonly known as Dashapushpam from Kerala, India. Afr. J. Microbiol. Res. 2010, 4, 2357–2362. [Google Scholar]
- Alagesaboopathi, C. Ethnomedicinal plants and their utilization by villagers in Kumaragiri hills of Salem district of Tamilnadu, India. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 222–227. [Google Scholar] [CrossRef][Green Version]
- Padal, S.; Murty, P.P.; Rao, D.S.; Venkaiah, M. Ethnomedicinal plants from Paderu division of Visakhapatnam District, AP, India. J. Phytol. 2010, 2, 70–91. [Google Scholar]
- Anitha, B.; Mohan, V.R.; Athiperumalsami, T.; Sutha, S. Ethnomedicinal plants used by the Kanikkars of Tirunelveli District, Tamil Nadu, India to treat skin diseases. Ethnobot. Leafl. 2008, 2008, 171–180. [Google Scholar]
- Singh, A.K.; Raghubanshi, A.S.; Singh, J.S. Medical ethnobotany of the tribals of Sonaghati of Sonbhadra district, Uttar Pradesh, India. J. Ethnopharmacol. 2002, 81, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, M.; Ignacimuthu, S. Traditional knowledge of Kani tribals in Kouthalai of Tirunelveli hills, Tamil Nadu, India. J. Ethnopharmacol. 2005, 102, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, P.K. An ethnobotanical study of medicinal plants in Chandauli District of Uttar Pradesh, India. J. Ethnopharmacol. 2009, 121, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, A.M.; de Silva, K.T.D.; de Silva, S.R.P.; Sirimanne, V.D.P.; Labadie, R.P.; van den Berg, A.J.J.; Vander Sluis, W. Inhibition of chemiluminescence generated by zymosan-activated polymorphonuclear leucocytes by phenolic constituents of Vernonia cinerea. Fitoterapia 1999, 70, 317–319. [Google Scholar] [CrossRef]
- Joshi, A.R.; Joshi, K. Indigenous knowledge and uses of medicinal plants by local communities of the Kali Gandaki Watershed Area, Nepal. J. Ethnopharmacol. 2000, 73, 175–183. [Google Scholar] [CrossRef]
- Lin, K.W. Ethnobotanical study of medicinal plants used by the Jah Hut peoples in Malaysia. Indian J. Med. Sci. 2005, 59, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Nordiana, M. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia 1999, 70, 502–513. [Google Scholar] [CrossRef]
- Inta, A.; Trisonthi, P.; Trisonthi, C. Analysis of traditional knowledge in medicinal plants used by Yuan in Thailand. J. Ethnopharmacol. 2013, 149, 344–351. [Google Scholar] [CrossRef]
- Neamsuvan, O.; Tuwaemaengae, T.; Bensulong, F.; Asae, A.; Mosamae, K. A survey of folk remedies for gastrointestinal tract diseases from Thailand’s three southern border provinces. J. Ethnopharmacol. 2012, 144, 11–21. [Google Scholar] [CrossRef]
- Igoli, J.; Ogaji, O.; Tor-Anyiin, T.; Igoli, N. Traditional medicine practice amongst the Igede people of Nigeria. Part II. Afr. J. Tradit. Complement. Altern. Med. 2005, 2, 134–152. [Google Scholar] [CrossRef]
- Hamill, F.A.; Apio, S.; Mubiru, N.K.; Bukenya-Ziraba, R.; Mosango, M.; Maganyi, O.W.; Soejarto, D.D. Traditional herbal drugs of Southern Uganda, II: Literature analysis and antimicrobial assays. J. Ethnopharmacol. 2003, 84, 57–78. [Google Scholar] [CrossRef]
- Akendengué, B. Medicinal plants used by the Fang traditional healers in Equatorial Guinea. J. Ethnopharmacol. 1992, 37, 165–173. [Google Scholar] [CrossRef]
- Ssegawa, P.; Kasenene, J.M. Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. J. Ethnopharmacol. 2007, 113, 521–540. [Google Scholar] [CrossRef]
- Brussell, D.E. A medicinal plant collection from Montserrat, West Indies. Econ. Bot. 2004, 58, S203–S220. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Ayad, R.; Akkal, S. Chapter 12—Phytochemistry and biological activities of algerian Centaurea and related genera. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 357–414. [Google Scholar]
- Jakupovic, J.; Banerjee, S.; Castro, V.; Bohlmann, F.; Schuster, A.; Msonthi, J.D.; Keeley, S. Poskeanolide, a seco-germacranolide and other sesquiterpene lactones from Vernonia species. Phytochemistry 1986, 25, 1359–1364. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Kuo, Y.-J.; Yu, A.-S.; Wu, M.-D.; Ong, C.-W.; Yang Kuo, L.-M.; Huang, J.-T.; Chen, C.-F.; Li, S.-Y. Two novel sesquiterpene lactones, cytotoxic vernolide-A and -B, from Vernonia cinerea. Chem. Pharm. Bull. 2003, 51, 425–426. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.; Wei, Y.; Wall, M.; Songsak, T.; Wongwiwatthananukit, S.; Chang, L.C. Bioactive sesquiterpene lactones isolated from the whole plants of Vernonia cinerea. J. Nat. Prod. 2019, 82, 2124–2131. [Google Scholar] [CrossRef]
- Ang, S.; Liu, C.; Hong, P.; Yang, L.; Hu, G.e.; Zheng, X.; Jin, J.; Wu, R.; Wong, W.-L.; Zhang, K.; et al. Hirsutinolide-type sesquiterpenoids with anti-prostate cancer activity from Cyanthillium cinereum. Phytochemistry 2023, 216, 113887. [Google Scholar] [CrossRef]
- Youn, U.J.; Miklossy, G.; Chai, X.; Wongwiwatthananukit, S.; Toyama, O.; Songsak, T.; Turkson, J.; Chang, L.C. Bioactive sesquiterpene lactones and other compounds isolated from Vernonia cinerea. Fitoterapia 2014, 93, 194–200. [Google Scholar] [CrossRef]
- Blundell, R.; Azzopardi, J.; Briffa, J.; Rasul, A.; Vargas-de la Cruz, C.; Shah, M.A. Chapter 13—Analysis of pentaterpenoids. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 457–475. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.-Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. Engl. 2012, 51, 1124–1137. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [CrossRef]
- Kushiro, T.; Ebizuka, Y. 1.18—Triterpenes. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 673–708. [Google Scholar] [CrossRef]
- Ba Vinh, L.; Jang, H.-J.; Viet Phong, N.; Dan, G.; Won Cho, K.; Ho Kim, Y.; Young Yang, S. Bioactive triterpene glycosides from the fruit of Stauntonia hexaphylla and insights into the molecular mechanism of its inflammatory effects. Bioorg. Med. Chem. Lett. 2019, 29, 2085–2089. [Google Scholar] [CrossRef]
- Silva, M.D.L.e.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Vinh, L.B.; Phong, N.V.; Ali, I.; Dan, G.; Koh, Y.S.; Anh, H.L.T.; Van Anh, D.T.; Yang, S.Y.; Kim, Y.H. Identification of potential anti-inflammatory and melanoma cytotoxic compounds from Aegiceras corniculatum. Med. Chem. Res. 2020, 29, 2020–2027. [Google Scholar] [CrossRef]
- Misra, T.N.; Singh, R.S.; Upadhyay, J.; Srivastava, R. Chemical constituents of Vernonia cinerea. Isolation and structure elucidation of a new pentacyclic triterpenoid. J. Nat. Prod. 1984, 47, 865–867. [Google Scholar] [CrossRef]
- Misra, T.N.; Singh, R.S.; Upadhyay, J.; Srivastava, R. Chemical constituents of Vernonia cinerea, Part I. Isolation and spectral studies of triterpenes. J. Nat. Prod. 1984, 47, 368–372. [Google Scholar] [CrossRef]
- Misra, T.N.; Singh, R.S.; Srivastava, R.; Pandey, H.S.; Prasad, C.; Singh, S. A new triterpenoidal from Vernonia cinerea. Planta Med. 1993, 59, 458–460. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frígola, A. Chapter 11—Bioactive components from leaf vegetable products. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 321–346. [Google Scholar]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef] [PubMed]
- Misra, T.N.; Singh, R.S.; Upadhyay, J.; Srivastava, R. Isolation of a natural sterol and an aliphatic acid from Vernonia cinerea. Phytochemistry 1984, 23, 415–417. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Murkovic, M. Phenolic Compounds. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4507–4514. [Google Scholar] [CrossRef]
- Yadava, R.N.; Raj, M. A new antiviral flavone glycoside from Vernonia cinerea Less. Asian J. Chem. 2013, 25, 3542–3544. [Google Scholar] [CrossRef]
- Gunasingh, C.; Barnabas, G.; Nagarajan, S. Flavonoids of the flowers of Vernonia cinerea. Indian J. Pharm. Sci. 1981, 43, 114. [Google Scholar]
- Youn, U.J.; Chang, L.C. Chemical constituents from the aerial parts of Vernonia cinerea L. and their anti-inflammatory activity. Korean J. Crop Sci. 2016, 24, 437–443. [Google Scholar] [CrossRef]
- Boué, G.B.; Boti, J.B.; Tonzibo, Z.F.; Paoli, M.; Bighelli, A. New trans–β–bergamotene derivatives in the root and the flower essential oils of Cyanthillium cinereum (L.) H. Rob. from Côte d’Ivoire. Nat. Prod. Res. 2019, 33, 2795–2800. [Google Scholar] [CrossRef] [PubMed]
- Yunjiao, X.; Panpan, W.; Yijun, R.; Peiying, S.; Hong, Y. Qualitative and quantitative analysis of the major ingredients of a herbal preparation, Ciwujia injection by combination of HPLC-Q-TOF-MS, HPLC-TQ-MS/MS and UPLC-PDA. Curr. Pharm. Anal. 2019, 15, 388–398. [Google Scholar] [CrossRef]
- Vinh, L.B.; Nguyet, N.T.M.; Ye, L.; Dan, G.; Phong, N.V.; Anh, H.L.T.; Kim, Y.H.; Kang, J.S.; Yang, S.Y.; Hwang, I. Enhancement of an in vivo anti-inflammatory activity of oleanolic acid through glycosylation occurring naturally in Stauntonia hexaphylla. Molecules 2020, 25, 3699. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Ramesh, P.R.; Mahesh, K.; Anandan, E.M.; Praveen, M.; Balachandran, I. Metabolite profiling of Cyanthillium cinereum (L.) H. Rob. and its herbal formulation by tandem mass spectroscopic analysis. Nat. Prod. Res. 2022, 36, 3726–3730. [Google Scholar] [CrossRef]
- Rajamurugan, R.; Selvaganabathy, N.; Kumaravel, S.; Ramamurthy, C.H.; Sujatha, V.; Suresh Kumar, M.; Thirunavukkarasu, C. Identification, quantification of bioactive constituents, evaluation of antioxidant and in vivo acute toxicity property from the methanol extract of Vernonia cinerea leaf extract. Pharm. Biol. 2011, 49, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H. Kinetics studies on effects of extraction techniques on bioactive compounds from Vernonia cinerea leaf. J. Food Sci. Technol. 2019, 56, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. GC/MS analysis of the essential oil of Vernonia cinerea. Nat. Prod. Commun. 2015, 10, 1934578X1501000746. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, Z.-J.; Yue, J.-M. Sesquiterpenoids from Vernonia cinerea. Nat. Prod. Res. 2006, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.-M.Y.; Tseng, P.-Y.; Lin, Y.-C.; Liaw, C.-C.; Zhang, L.-J.; Tsai, K.-C.; Lin, Z.-H.; Ho, H.-O.; Kuo, Y.-H. New hirsutinolide-type sesquiterpenoids from Vernonia cinerea inhibit nitric oxide production in LPS-stimulated RAW264.7 cells. Planta Med. 2018, 84, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Youn, U.J.; Wongwiwatthananukit, S.; Songsak, T.; Chang, L.C. Sesquiterpene lactones from Vernonia cinerea. Chem. Nat. Compd. 2018, 54, 235–237. [Google Scholar] [CrossRef]
- Dharani, J.; Ravi, S. Isolation of sesquiterpene lactones and the antioxidant and anticancer activities of crude extracts from Cyanthillium cinereum. Chem. Nat. Compd. 2022, 58, 40–46. [Google Scholar] [CrossRef]
- Haque, M.A.; Hassan, M.M.; Das, A.; Begum, B.; Ali, M.Y.; Morshed, H. Phytochemical investigation of Vernonia cinerea (Family: Asteraceae). J. Appl. Pharm. Sci. 2012, 2, 79. [Google Scholar] [CrossRef]
- Asha, R.; Gayathri Devi, V.; Abraham, A. Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea attenuate selenite induced cataract formation in Sprague Dawley rat pups. Chem. Biol. Interact. 2016, 245, 20–29. [Google Scholar] [CrossRef]
- Pukumpuang, W.; Thongwai, N.; Tragoolpua, Y. Total phenolic contents, antibacterial and antioxidant activities of some Thai medicinal plant extracts. J. Med. Plant Res. 2012, 6, 4953–4960. [Google Scholar] [CrossRef]
- Danish Rizvi, S.M.; Biswas, D.; Arif, J.M.; Zeeshan, M. In-vitro antibacterial and antioxidant potential of leaf and flower extracts of Vernonia cinerea and their phytochemical constituents. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 164–169. [Google Scholar]
- Haque, M.A.; Abdullah, C.S.; Hassan, M.M.; Parvin, M.N.; Rafique, M.B.; Mostofa, A.G.M. Evaluation of the antioxidant and anti-cholineesterase activities of the stem, barks and leaves of the plant Vernonia cinerea (Family: Asteraceae). J. Appl. Pharm. Sci. 2012, 2, 174–176. [Google Scholar] [CrossRef][Green Version]
- Sonibare, M.A.; Aremu, O.T.; Okorie, P.N. Antioxidant and antimicrobial activities of solvent fractions of Vernonia cinerea (L.) Less leaf extract. Afr. Health Sci. 2016, 16, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Luetragoon, T.; Sranujit, R.P.; Noysang, C.; Thongsri, Y.; Potup, P.; Somboonjun, J.; Maichandi, N.; Suphrom, N.; Sangouam, S.; Usuwanthim, K. Evaluation of anti-inflammatory effect of Moringa oleifera Lam. and Cyanthillium cinereum (Less) H. Rob. Lozenges in volunteer smokers. Plants 2021, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Monton, C.; Kittiratpattana, P.; Nakyai, S.; Sutapakul, T.; Navabhatra, A.; Wunnakup, T.; Chankana, N.; Suksaeree, J. Microwave-assisted extraction of Clausena anisata leaves and Vernonia cinerea whole plants to maximize nitrate content: Optimization approach, antioxidant activity, and cytotoxicity. Adv. Tradit. Med. 2022, 22, 697–711. [Google Scholar] [CrossRef]
- Iwalewa, E.O.; Iwalewa, O.J.; Adeboye, J.O. Analgesic, antipyretic, anti-inflammatory effects of methanol, chloroform and ether extracts of Vernonia cinerea Less leaf. J. Ethnopharmacol. 2003, 86, 229–234. [Google Scholar] [CrossRef]
- Pratheesh Kumar, P.; Kuttan, G. Vernonia cinerea L. scavenges free radicals and regulates nitric oxide and proinflammatory cytokines profile in carrageenan induced paw edema model. Immunopharmacol. Immunotoxicol. 2009, 31, 94–102. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Kuttan, G. Modulation of immune response by Vernonia cinerea L. inhibits the proinflammatory cytokine profile, iNOS, and COX-2 expression in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Saraphanchotiwitthaya, A.; Sripalakit, P. Anti-inflammatory activity of a Vernonia cinerea methanolic extract in vitro. ScienceAsia 2015, 41, 392–399. [Google Scholar] [CrossRef]
- Laosim, T.; Chuchawankul, S.; Tencomnao, T. Immunomodulatory effect of hexane extract of Vernonia cinerea Less. trunk on human peripheral blood mononuclear cells. J. Chem. Pharm. Res. 2011, 3, 188–195. [Google Scholar]
- Gupta, M.; Mazumder, U.K.; Manikandan, L.; Bhattacharya, S.; Haldar, P.K.; Roy, S. Evaluation of antipyretic potential of Vernonia cinerea extract in rats. Phytother. Res. 2003, 17, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.V.; Trang, V.T.; Tuan Anh, H.L.; Vinh, L.B.; Phong, N.V.; Thuan, T.Q.; Hieu, N.V.; Dat, N.T.; Nhan, L.V.; Tuan, D.T.; et al. Acetylcholinesterase inhibition studies of alkaloid components from Crinum asiaticum var. sinicum: In vitro assessments by molecular docking and molecular dynamics simulations. J. Asian Nat. Prod. Res. 2023, 26, 652–662. [Google Scholar] [CrossRef]
- Vinh, L.B.; Dan, G.; Phong, N.V.; Cho, K.; Kim, Y.H.; Yang, S.Y. In vitro investigation of acetylcholinesterase inhibitors isolated from the fruit of Stauntonia hexaphylla. Chem. Nat. Compd. 2021, 57, 784–787. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Amuthan, A.; Devi, V.; Shreedhara, C.S.; Rao, V.; Jasphin, S.; Kumar, N. Vernonia cinerea regenerates tubular epithelial cells in cisplatin induced nephrotoxicity in cancer bearing mice without affecting antitumor activity. J. Tradit. Complement. Med. 2021, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative imbalance and kidney damage: New study perspectives from animal models to hospitalized patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Rankin, G.O.; Tyree, C.; Pope, D.; Tate, J.; Racine, C.; Anestis, D.K.; Brown, K.C.; Dial, M.; Valentovic, M.A. Role of free radicals and biotransformation in trichloronitrobenzene-induced nephrotoxicity in vitro. Int. J. Mol. Sci. 2017, 18, 1165. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, A.; Bharathi, K.; Prasad, K.V.S.R.G. Effect of Vernonia cinerea aerial parts against cisplatin-induced nephrotoxicity in rats. Pharmacologyonline 2011, 2, 548–555. [Google Scholar]
- Pratheeshkumar, P.; Kuttan, G. Ameliorative action of Vernonia cinerea L. on cyclophosphamide-induced immunosuppression and oxidative stress in mice. Inflammopharmacology 2010, 18, 197–207. [Google Scholar] [CrossRef]
- Ueda, J.-y.; Tezuka, Y.; Banskota, A.H.; Tran, Q.L.; Tran, Q.K.; Harimaya, Y.; Saiki, I.; Kadota, S. Antiproliferative activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2002, 25, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Kuttan, G. Vernonia cinerea Less. inhibits tumor cell invasion and pulmonary metastasis in C57BL/6 mice. Integr. Cancer Ther. 2011, 10, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Latha, L.Y.; Darah, I.; Jain, K.; Sasidharan, S. Toxicity study of Vernonia cinerea. Pharm. Biol. 2010, 48, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Kuttan, G. Effect of vernolide-A, a sesquiterpene lactone from Vernonia cinerea L., on cell-mediated immune response in B16F-10 metastatic melanoma-bearing mice. Immunopharmacol. Immunotoxicol. 2011, 33, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Kuttan, G. Modulation of cytotoxic T lymphocyte, natural killer cell, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by Vernonia cinerea L. and vernolide-A in BALB/c mice via enhanced production of cytokines IL-2 and IFN-γ. Immunopharmacol. Immunotoxicol. 2012, 34, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pouyfung, P.; Choonate, S.; Wongnoppavich, A.; Rongnoparut, P.; Chairatvit, K. Anti-proliferative effect of 8α-tigloyloxyhirsutinolide-13-O-acetate (8αTGH) isolated from Vernonia cinerea on oral squamous cell carcinoma through inhibition of STAT3 and STAT2 phosphorylation. Phytomedicine 2019, 52, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.M.; Vinh, L.B.; Thanh, N.V.; Phong, N.V. Inhibition of PTP1B by isosinensetin, a polymethoxylated flavone isolated from trifoliate orange peel: Kinetic studies, molecular docking, and molecular dynamics simulation. Chem. Pap. 2023, 77, 1751–1757. [Google Scholar] [CrossRef]
- Maiti, P.; Nand, M.; Joshi, T.; Ramakrishnan, M.A.; Chandra, S. Identification of luteolin-7-glucoside and epicatechin gallate from Vernonia cinerea, as novel EGFR L858R kinase inhibitors against lung cancer: Docking and simulation-based study. J. Biomol. Struct. Dyn. 2021, 39, 5048–5057. [Google Scholar] [CrossRef] [PubMed]
- Beeran, A.A.; Udupa, N.; Maliyakkal, N. The dichloromethane fraction of Vernonia cinerea impart pro-apoptotic, genotoxic, cell cycle arrest, and drug efflux inhibitory effects on human adenocarcinoma cells. Recent Pat. Anti-Cancer Drug Discov. 2020, 15, 239–256. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Toriumi, K.; Horikoshi, Y.; Yoshiyuki Osamura, R.; Yamamoto, Y.; Nakamura, N.; Takekoshi, S. Carbon tetrachloride-induced hepatic injury through formation of oxidized diacylglycerol and activation of the PKC/NF-κB pathway. Lab. Investig. 2013, 93, 218–229. [Google Scholar] [CrossRef]
- Leelaprakash, G.; Mohan Dass, S.; Sivajothi, V. Antioxidant and hepatoprotective activities of Vernonia cinerea extract against CCl4 induced hepatotoxicity in albino rats. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 30–34. [Google Scholar]
- Adeboye, J.O.; Asije, W.; Awe, S.O. Diuretic and antidiuretic diuretic of the leaf extracts of Vernonia cinerea (Less) (Fam. Compositae). Phytother. Res. 1997, 11, 454–456. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Hiremath, R.D.; Jalalpure, S.S. Effect of hydro-alcoholic extract of Vernonia cinerea Less. against ethylene glycol-induced urolithiasis in rats. Indian J. Pharmacol. 2016, 48, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Shukla, Y.N.; Tripathi, A.K.; Singh, S.C. Insect antifeedant principles from Vernonia cinerea. Phytother. Res. 1998, 12, 195–199. [Google Scholar] [CrossRef]
- Naowaboot, J.; Wannasiri, S.; Pannangpetch, P. Vernonia cinerea water extract improves insulin resistance in high-fat diet–induced obese mice. Nutr. Res. 2018, 56, 51–60. [Google Scholar] [CrossRef]
- Pomjunya, A.; Ratthanophart, J.; Fungfuang, W. Effects of Vernonia cinerea on reproductive performance in streptozotocin-induced diabetic rats. J. Vet. Med. Sci. 2017, 79, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.S.B.; Mostofa, A.G.M.; Ferdous, F.M.T.I.; Islam, M.S. A randomized, placebo-controlled, crossover study of an herbal preparation containing Vernonia cinerea in the treatment of type 2 diabetes. J. Altern. Complement. Med. 2013, 19, 767–771. [Google Scholar] [CrossRef]
- Camacho, M.d.R.; Phillipson, J.D.; Croft, S.L.; Solis, P.N.; Marshall, S.J.; Ghazanfar, S.A. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol. 2003, 89, 185–191. [Google Scholar] [CrossRef]
- Arivoli, S.; Tennyson, S.; Martin, J.J. Larvicidal efficacy of Vernonia cinerea (L.)(Asteraceae) leaf extracts against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). J. Biopest. 2011, 4, 37. [Google Scholar]
- Yadav, R.; Tikar, S.; Sharma, A.; Tyagi, V.; Sukumaran, D.; Jain, A.; Veer, V. Screening of some weeds for larvicidal activity against Aedes albopictus, a vector of dengue and chikungunya. J. Vector Borne Dis. 2015, 52, 88–94. [Google Scholar] [PubMed]
- Shanker, K.; Khare, P.; Tiwari, N.; Mohanty, S.; Bawankule, D.U.; Pal, A. Synthesis of gold mediated biocompatible nanocomposite of lactone enriched fraction from sahadevi (Vernonia cinerea Lees): An assessment of antimalarial potential. Curr. Top. Med. Chem. 2016, 16, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Hout, S.; Chea, A.; Bun, S.-S.; Elias, R.; Gasquet, M.; Timon-David, P.; Balansard, G.; Azas, N. Screening of selected indigenous plants of Cambodia for antiplasmodial activity. J. Ethnopharmacol. 2006, 107, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H.T.; Nordskjold, J.B.; Smitt, U.W.; Nyman, U.; Palpu, P.; Joshi, P.; Varughese, G. In vitro screening of Indian medicinal plants for antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Ilondu, E. Phytochemical composition and efficacy of ethanolic leaf extracts of some Vernonia species against two phytopathogenic fungi. J. Biopest. 2013, 6, 165. [Google Scholar] [CrossRef]
- Latha, L.Y.; Darah, I.; Jain, K.; Sasidharan, S. Effects of Vernonia cinerea Less methanol extract on growth and morphogenesis of Candida albicans. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 543–549. [Google Scholar] [PubMed]
- Latha, L.Y.; Darah, I.; Kassim, M.J.N.M.; Sasidharan, S. Antibacterial activity and morphological changes of Pseudomonas aeruginosa cells after exposure to Vernonia cinerea extract. Ultrastruct. Pathol. 2010, 34, 219–225. [Google Scholar] [CrossRef]
- Joshi, T.; Pandey, S.C.; Maiti, P.; Tripathi, M.; Paliwal, A.; Nand, M.; Sharma, P.; Samant, M.; Pande, V.; Chandra, S. Antimicrobial activity of methanolic extracts of Vernonia cinerea against Xanthomonas oryzae and identification of their compounds using in silico techniques. PLoS ONE 2021, 16, e0252759. [Google Scholar] [CrossRef]
- Dhanalakshmi, P.; Jaya, A.; Priya, P.; Elumalai, S.; Lakshmi, Y.; Manimaran, A.; Sivalingam, S.; Arumugam, A. Evaluation of inhibitory effect of Vernonia cinerea L. leaf extracts on different fungal species. Int. J. Pharm. Pharm. Sci. 2013, 5, 414–416. [Google Scholar]
- Chen, C.-P.; Lin, C.-C.; Tsuneo, N. Screening of Taiwanese crude drugs for antibacterial activity against Streptococcus mutans. J. Ethnopharmacol. 1989, 27, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bagchi, G.D.; Darokar, M.P. Antibacterial activity observed in the seeds of some Coprophilous plants. Int. J. Pharmacogn. 1997, 35, 179–184. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Huang, T.-L. Screening of anti-Helicobacter pylori herbs deriving from Taiwanese folk medicinal plants. FEMS Immunol. Med. Microbiol. 2005, 43, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Latha, R.M.; Geetha, T.; Varalakshmi, P. Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen. Pharmacol.-The Vasc. Syst. 1998, 31, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M. Tobacco smoking: The leading cause of preventable disease worldwide. Thorac. Surg. Clin. 2013, 23, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Leelarungrayub, D.; Pratanaphon, S.; Pothongsunun, P.; Sriboonreung, T.; Yankai, A.; Bloomer, R.J. Vernonia cinerea Less. supplementation and strenuous exercise reduce smoking rate: Relation to oxidative stress status and beta-endorphin release in active smokers. J. Int. Soc. Sports Nutr. 2010, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Puttarak, P.; Pornpanyanukul, P.; Meetam, T.; Bunditanukul, K.; Chaiyakunapruk, N. Efficacy and safety of Vernonia cinerea (L.) Less. for smoking cessation: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2018, 37, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.-H.; Han, S.; Lim, Y.-R.; Park, H.-G.; Chun, Y.-J.; Park, S.-W.; Kim, D. Directed-evolution analysis of human cytochrome P450 2A6 for enhanced enzymatic catalysis. J. Toxicol. Environ. Health Part A 2014, 77, 1409–1418. [Google Scholar] [CrossRef]
- Park, S.L.; Murphy, S.E.; Wilkens, L.R.; Stram, D.O.; Hecht, S.S.; Le Marchand, L. Association of CYP2A6 activity with lung cancer incidence in smokers: The multiethnic cohort study. PLoS ONE 2017, 12, e0178435. [Google Scholar] [CrossRef]
- Pouyfung, P.; Sarapusit, S.; Rongnoparut, P. Effects of Vernonia cinerea compounds on drug-metabolizing cytochrome P450s in human liver microsomes. Phytother. Res. 2017, 31, 1916–1925. [Google Scholar] [CrossRef]
- Boonruang, S.; Prakobsri, K.; Pouyfung, P.; Srisook, E.; Prasopthum, A.; Rongnoparut, P.; Sarapusit, S. Inhibition of human cytochromes P450 2A6 and 2A13 by flavonoids, acetylenic thiophenes and sesquiterpene lactones from Pluchea indica and Vernonia cinerea. J. Enzyme Inhib. Med. Chem. 2017, 32, 1136–1142. [Google Scholar] [CrossRef]
- Prasopthum, A.; Pouyfung, P.; Sarapusit, S.; Srisook, E.; Rongnoparut, P. Inhibition effects of Vernonia cinerea active compounds against cytochrome P450 2A6 and human monoamine oxidases, possible targets for reduction of tobacco dependence. Drug Metab. Pharmacokinet. 2015, 30, 174–181. [Google Scholar] [CrossRef]
- Li, T.; Ma, X.; Xue, G.; Ju, X.; Liu, J.; Wang, L. Determination of sunset yellow in beverage based on solution-gated graphene transistors with multi-walled carbon nanotube functionalized gate electrodes. J. Electroanal. Chem. 2022, 922, 116758. [Google Scholar] [CrossRef]
- Krishnan, N.S.; Talari, D.; Rajangam, J.; Pujari, L.; Palei, N.N.; Balaji, A.; Surendran, V. Protective effect of Vernonia cinerea against sunset yellow induced anxiogenic behaviour in mice model: Counteract oxidative damage based approach. Nat. Prod. J. 2021, 11, 537–545. [Google Scholar] [CrossRef]
- Suji, S.; Dinesh, M.D.; Keerthi, K.U.; Anagha, K.P.; Arya, J.; Anju, K.V. Evaluation of neutralization potential of Naja naja and Daboia russelii snake venom by root extract of Cyanthillium cinereum. Indian J. Crit. Care Med. 2023, 27, 821–829. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Yogeeta, S.K.; Subashini, R.; Devaki, T. Attenuation of cyclophosphamide induced toxicity by squalene in experimental rats. Chem. Biol. Interact. 2006, 160, 252–260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trang, N.M.; Vinh, L.B.; Phong, N.V.; Yang, S.Y. Traditional Uses, Phytochemistry, and Pharmacological Activities of Vernonia cinerea (L.) Less.: An Updated Review. Nutrients 2024, 16, 1396. https://doi.org/10.3390/nu16091396
Trang NM, Vinh LB, Phong NV, Yang SY. Traditional Uses, Phytochemistry, and Pharmacological Activities of Vernonia cinerea (L.) Less.: An Updated Review. Nutrients. 2024; 16(9):1396. https://doi.org/10.3390/nu16091396
Chicago/Turabian StyleTrang, Nguyen Minh, Le Ba Vinh, Nguyen Viet Phong, and Seo Young Yang. 2024. "Traditional Uses, Phytochemistry, and Pharmacological Activities of Vernonia cinerea (L.) Less.: An Updated Review" Nutrients 16, no. 9: 1396. https://doi.org/10.3390/nu16091396
APA StyleTrang, N. M., Vinh, L. B., Phong, N. V., & Yang, S. Y. (2024). Traditional Uses, Phytochemistry, and Pharmacological Activities of Vernonia cinerea (L.) Less.: An Updated Review. Nutrients, 16(9), 1396. https://doi.org/10.3390/nu16091396





