Prevention of Male Late-Onset Hypogonadism by Natural Polyphenolic Antioxidants
Abstract
:1. Introduction
2. Testosterone Synthesis by Leydig Cells
3. Development of Late-Onset Male Hypogonadism
4. Natural Antioxidants Contributing to the Optimal Production of Androgen
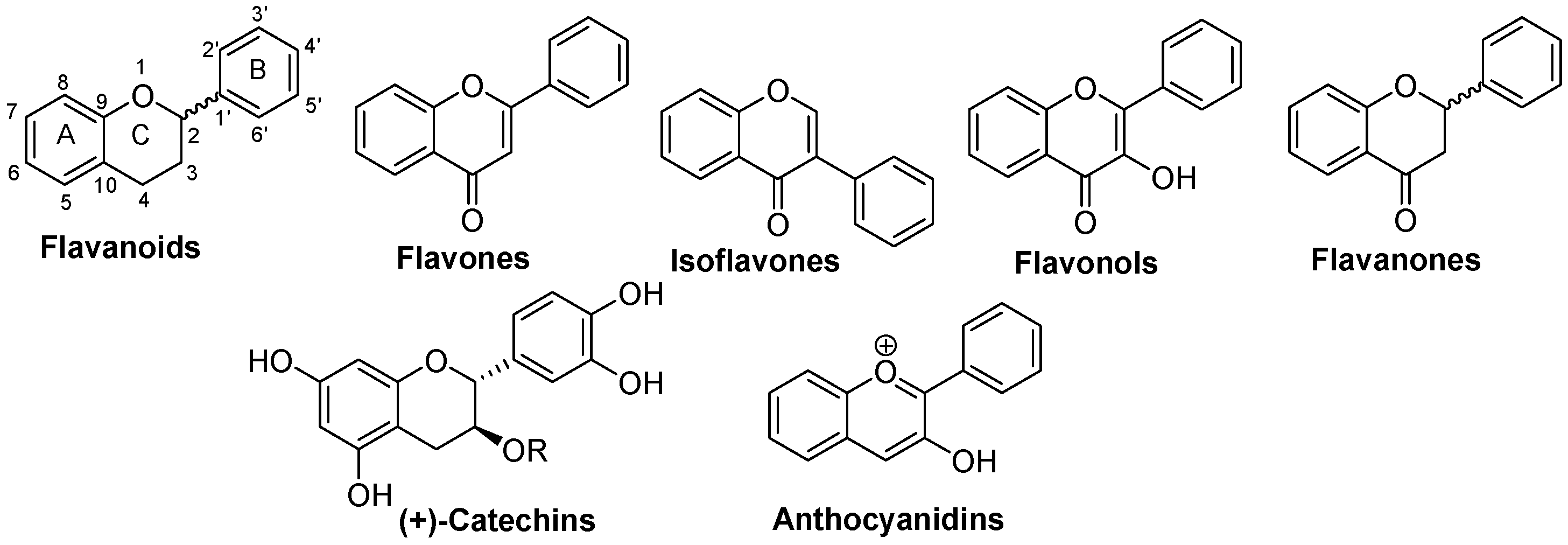
4.1. Flavonoids
4.1.1. Flavones
4.1.2. Isoflavones
4.1.3. Flavonols
4.1.4. Flavanones
4.1.5. Catechins
4.1.6. Anthocyanidins
4.2. Hydroxycinnamic Acid Phenethyl Ester Derivatives
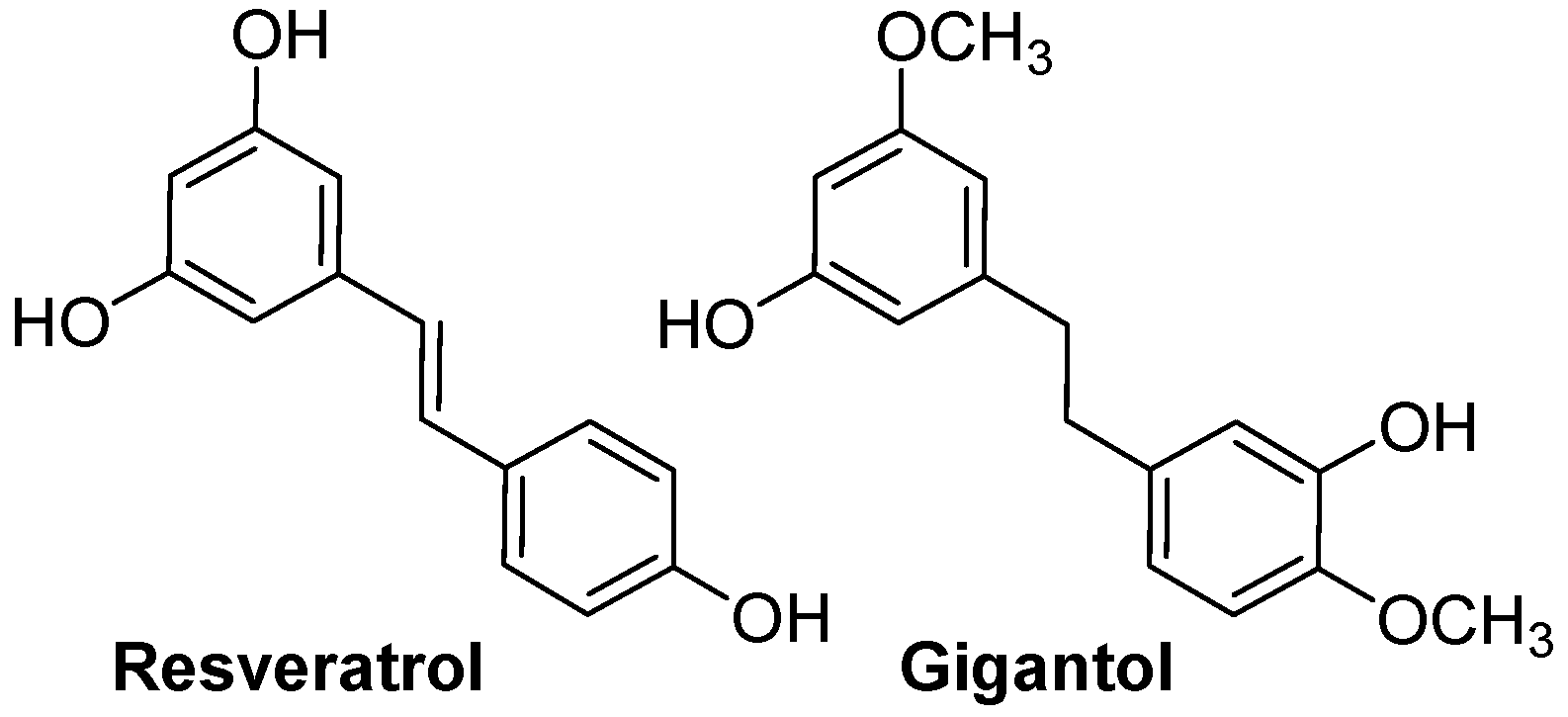
4.3. Resveratrol and Gigantol
5. Other Actions of Natural Antioxidants
5.1. Effects of Natural Antioxidants on the Hypothalamus
5.2. Effects of Natural Antioxidants on the Pituitary Gland
5.3. Influences of Natural Antioxidants on Liver Function and Testosterone Bioavailability
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gray, A.; Feldman, H.A.; McKinlay, J.B.; Longcope, C. Age, Disease, and Changing Sex Hormone Levels in Middle-Aged Men: Results of the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 1991, 73, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Rosenberg, M.T. A Practical Guide to Male Hypogonadism in the Primary Care Setting. Int. J. Clin. Pract. 2010, 64, 682–696. [Google Scholar] [CrossRef]
- Midzak, A.S.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Leydig Cell Aging and the Mechanisms of Reduced Testosterone Synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.M. Andropause: Clinical Implications of the Decline in Serum Testosterone Levels with Aging in Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M76–M99. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, N.; Thakur, D.S.; Patidar, A. Male Hypogonadism: Symptoms and Treatment. J. Adv. Pharm. Technol. Res. 2010, 1, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Ghouili, F.; Roumaud, P.; Martin, L.J.; Touaibia, M. Influence of Flavonols and Quercetin Derivative Compounds on MA-10 Leydig Cells Steroidogenic Genes Expressions. Toxicol. In Vitro 2017, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Ghouili, F.; Roumaud, P.; Bauer, W.; Touaibia, M.; Martin, L.J. Influences of Flavones on Cell Viability and cAMP-Dependent Steroidogenic Gene Regulation in MA-10 Leydig Cells. Cell Biol. Toxicol. 2018, 34, 23–38. [Google Scholar] [CrossRef]
- Basque, A.; Nguyen, H.T.; Touaibia, M.; Martin, L.J. Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells. Curr. Issues Mol. Biol. 2022, 44, 73–93. [Google Scholar] [CrossRef]
- Basque, A.; Touaibia, M.; Martin, L.J. Sinapic and Ferulic Acid Phenethyl Esters Increase the Expression of Steroidogenic Genes in MA-10 Tumor Leydig Cells. Toxicol. In Vitro 2023, 86, 105505. [Google Scholar] [CrossRef]
- Couture, R.; Mora, N.; Al Bittar, S.; Najih, M.; Touaibia, M.; Martin, L.J. Luteolin Modulates Gene Expression Related to Steroidogenesis, Apoptosis, and Stress Response in Rat LC540 Tumor Leydig Cells. Cell Biol. Toxicol. 2020, 36, 31–49. [Google Scholar] [CrossRef]
- Niu, Y.; Liao, J.; Zhou, H.; Wang, C.-C.; Wang, L.; Fan, Y. Flavonoids from Lycium Barbarum Leaves Exhibit Anti-Aging Effects through the Redox-Modulation. Molecules 2022, 27, 4952. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Russo, M.; Cafeo, G.; Caruso, D.; Falliti, G.; Dugo, P.; Dossena, S.; et al. Mechanisms Underlying the Anti-Aging Activity of Bergamot (Citrus bergamia) Extract in Human Red Blood Cells. Front. Physiol. 2023, 14, 1225552. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic Application of Quercetin in Aging-Related Diseases: SIRT1 as a Potential Mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Genissel, C.; Levallet, J.; Carreau, S. Regulation of Cytochrome P450 Aromatase Gene Expression in Adult Rat Leydig Cells: Comparison with Estradiol Production. J. Endocrinol. 2001, 168, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Abney, T.O. The Potential Roles of Estrogens in Regulating Leydig Cell Development and Function: A Review. Steroids 1999, 64, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Eacker, S.M.; Agrawal, N.; Qian, K.; Dichek, H.L.; Gong, E.-Y.; Lee, K.; Braun, R.E. Hormonal Regulation of Testicular Steroid and Cholesterol Homeostasis. Mol. Endocrinol. 2008, 22, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. Tracking the Role of a Star in the Sky of the New Millennium. Mol. Endocrinol. 2001, 15, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Gazouli, M.; Yao, Z.-X.; Boujrad, N.; Corton, J.C.; Culty, M.; Papadopoulos, V. Effect of Peroxisome Proliferators on Leydig Cell Peripheral-Type Benzodiazepine Receptor Gene Expression, Hormone-Stimulated Cholesterol Transport, and Steroidogenesis: Role of the Peroxisome Proliferator-Activator Receptor Alpha. Endocrinology 2002, 143, 2571–2583. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Culty, M.; Luo, L.; Yao, Z.-X.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Cholesterol Transport, Peripheral Benzodiazepine Receptor, and Steroidogenesis in Aging Leydig Cells. J. Androl. 2002, 23, 439–447. [Google Scholar] [CrossRef]
- Leers-Sucheta, S.; Stocco, D.M.; Azhar, S. Down-Regulation of Steroidogenic Acute Regulatory (StAR) Protein in Rat Leydig Cells: Implications for Regulation of Testosterone Production during Aging. Mech. Ageing Dev. 1999, 107, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stocco, D.M. The Decline in Testosterone Biosynthesis during Male Aging: A Consequence of Multiple Alterations. Mol. Cell. Endocrinol. 2005, 238, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Evaul, K.; Hammes, S.R. Cross-Talk between G Protein-Coupled and Epidermal Growth Factor Receptors Regulates Gonadotropin-Mediated Steroidogenesis in Leydig Cells. J. Biol. Chem. 2008, 283, 27525–27533. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, M.E.; Yamashita, S.; Ascoli, M. The ERK1/2 Pathway Regulates Testosterone Synthesis by Coordinately Regulating the Expression of Steroidogenic Genes in Leydig Cells. Mol. Cell. Endocrinol. 2013, 370, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Roumaud, P.; Martin, L.J. Roles of Leptin, Adiponectin and Resistin in the Transcriptional Regulation of Steroidogenic Genes Contributing to Decreased Leydig Cells Function in Obesity. Horm. Mol. Biol. Clin. Investig. 2015, 24, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Landry, D.A.; Sormany, F.; Haché, J.; Roumaud, P.; Martin, L.J. Steroidogenic Genes Expressions Are Repressed by High Levels of Leptin and the JAK/STAT Signaling Pathway in MA-10 Leydig Cells. Mol. Cell. Biochem. 2017, 433, 79–95. [Google Scholar] [CrossRef]
- Martin, L.J.; Boucher, N.; Brousseau, C.; Tremblay, J.J. The Orphan Nuclear Receptor NUR77 Regulates Hormone-Induced StAR Transcription in Leydig Cells through Cooperation with Ca2+/Calmodulin-Dependent Protein Kinase I. Mol. Endocrinol. 2008, 22, 2021–2037. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Jo, Y.; Stocco, D.M. Regulation of Leydig Cell Steroidogenesis by Extracellular Signal-Regulated Kinase 1/2: Role of Protein Kinase A and Protein Kinase C Signaling. J. Endocrinol. 2007, 193, 53–63. [Google Scholar] [CrossRef]
- Jo, Y.; King, S.R.; Khan, S.A.; Stocco, D.M. Involvement of Protein Kinase C and Cyclic Adenosine 3′,5′-Monophosphate-Dependent Kinase in Steroidogenic Acute Regulatory Protein Expression and Steroid Biosynthesis in Leydig Cells. Biol. Reprod. 2005, 73, 244–255. [Google Scholar] [CrossRef]
- Garza, S.; Papadopoulos, V. Testosterone Recovery Therapy Targeting Dysfunctional Leydig Cells. Andrology 2023, 11, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Basualto-Alarcón, C.; Varela, D.; Duran, J.; Maass, R.; Estrada, M. Sarcopenia and Androgens: A Link between Pathology and Treatment. Front. Endocrinol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Tuck, S.; Francis, R. Testosterone, Bone and Osteoporosis. Front. Horm. Res. 2009, 37, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Maggi, M. The Role of Testosterone in Erectile Dysfunction. Nat. Rev. Urol. 2010, 7, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Straftis, A.A.; Gray, P.B. Sex, Energy, Well-Being and Low Testosterone: An Exploratory Survey of U.S. Men’s Experiences on Prescription Testosterone. Int. J. Environ. Res. Public Health 2019, 16, 3261. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A Metabolic Hormone in Health and Disease. J. Endocrinol. 2013, 217, R25–R45. [Google Scholar] [CrossRef] [PubMed]
- Blaya, R.; Blaya, P.; Rhoden, L.; Rhoden, E.L. Low Testosterone Levels and Metabolic Syndrome in Aging Male. Curr. Pharm. Des. 2017, 23, 4470–4474. [Google Scholar] [CrossRef] [PubMed]
- Moffat, S.D.; Zonderman, A.B.; Metter, E.J.; Blackman, M.R.; Harman, S.M.; Resnick, S.M. Longitudinal Assessment of Serum Free Testosterone Concentration Predicts Memory Performance and Cognitive Status in Elderly Men. J. Clin. Endocrinol. Metab. 2002, 87, 5001–5007. [Google Scholar] [CrossRef]
- Huang, G.; Wharton, W.; Bhasin, S.; Harman, S.M.; Pencina, K.M.; Tsitouras, P.; Li, Z.; Hally, K.A.; Asthana, S.; Storer, T.W.; et al. Effects of Long-Term Testosterone Administration on Cognition in Older Men with Low or Low-to-Normal Testosterone Concentrations: A Prespecified Secondary Analysis of Data from the Randomised, Double-Blind, Placebo-Controlled TEAAM Trial. Lancet Diabetes Endocrinol. 2016, 4, 657–665. [Google Scholar] [CrossRef]
- Beauchet, O. Testosterone and Cognitive Function: Current Clinical Evidence of a Relationship. Eur. J. Endocrinol. 2006, 155, 773–781. [Google Scholar] [CrossRef]
- Lv, W.; Du, N.; Liu, Y.; Fan, X.; Wang, Y.; Jia, X.; Hou, X.; Wang, B. Low Testosterone Level and Risk of Alzheimer’s Disease in the Elderly Men: A Systematic Review and Meta-Analysis. Mol. Neurobiol. 2016, 53, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; van Schoor, N.M.; de Ronde, W.; Schaap, L.A.; Comijs, H.C.; Beekman, A.T.F.; Lips, P. Low Free Testosterone Levels Are Associated with Prevalence and Incidence of Depressive Symptoms in Older Men. Clin. Endocrinol. 2010, 72, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.-A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef] [PubMed]
- Robaire, B.; Hamzeh, M. Androgen Action in the Epididymis. J. Androl. 2011, 32, 592–599. [Google Scholar] [CrossRef]
- Tsuji, M.; Shima, H.; Cunha, G.R. Morphogenetic and Proliferative Effects of Testosterone and Insulin on the Neonatal Mouse Seminal Vesicle in Vitro. Endocrinology 1991, 129, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D. The Critical Role of Androgens in Prostate Development. Endocrinol. Metab. Clin. N. Am. 2011, 40, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Baskin, L.S.; Sutherland, R.S.; DiSandro, M.J.; Hayward, S.W.; Lipschutz, J.; Cunha, G.R. The Effect of Testosterone on Androgen Receptors and Human Penile Growth. J. Urol. 1997, 158, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of Testosterone on Muscle Mass and Muscle Protein Synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L.; Finkelstein, J.S.; Schoenfeld, D.A.; Rosenthal, D.I.; Anderson, E.J.; Klibanski, A. Increase in Bone Density and Lean Body Mass during Testosterone Administration in Men with Acquired Hypogonadism. J. Clin. Endocrinol. Metab. 1996, 81, 4358–4365. [Google Scholar] [CrossRef]
- Brodnitz, F.S. Hormones and the Human Voice. Bull. N. Y. Acad. Med. 1971, 47, 183–191. [Google Scholar]
- Randall, V.A. Androgens and Hair Growth. Dermatol. Ther. 2008, 21, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Testosterone Signaling and the Regulation of Spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, A.J.; Clarke, I.J. Negative Feedback Regulation of the Secretion and Actions of Gonadotropin-Releasing Hormone in Males. Biol. Reprod. 2001, 64, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Lee, G.; Yun, J.M.; Cho, B. Testosterone Replacement, Muscle Strength, and Physical Function. World J. Mens Health 2018, 36, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Soelaiman, I.-N.; Chin, K.-Y. A Concise Review of Testosterone and Bone Health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Saggese, G.; Baroncelli, G.I.; Bertelloni, S. Puberty and Bone Development. Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Richmond, E.J.; Rogol, A.D. Male Pubertal Development and the Role of Androgen Therapy. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Beggs, L.A.; Yarrow, J.F.; Conover, C.F.; Meuleman, J.R.; Beck, D.T.; Morrow, M.; Zou, B.; Shuster, J.J.; Borst, S.E. Testosterone Alters Iron Metabolism and Stimulates Red Blood Cell Production Independently of Dihydrotestosterone. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E456–E461. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Alexander, G.; Berman, N.; Salehian, B.; Davidson, T.; McDonald, V.; Steiner, B.; Hull, L.; Callegari, C.; Swerdloff, R.S. Testosterone Replacement Therapy Improves Mood in Hypogonadal Men--a Clinical Research Center Study. J. Clin. Endocrinol. Metab. 1996, 81, 3578–3583. [Google Scholar] [CrossRef]
- Westley, C.J.; Amdur, R.L.; Irwig, M.S. High Rates of Depression and Depressive Symptoms among Men Referred for Borderline Testosterone Levels. J. Sex. Med. 2015, 12, 1753–1760. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone, Mood, Behaviour and Quality of Life. Andrology 2020, 8, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Nachtigall, L.B.; Stern, T.A. The Effect of Testosterone Levels on Mood in Men: A Review. Psychosomatics 2013, 54, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Maggi, M. The Role of Testosterone in Male Sexual Function. Rev. Endocr. Metab. Disord. 2022, 23, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Corona, G.; Maggi, M. Testosterone and Sexual Function in Men. Maturitas 2018, 112, 46–52. [Google Scholar] [CrossRef] [PubMed]
- De Maddalena, C.; Vodo, S.; Petroni, A.; Aloisi, A.M. Impact of Testosterone on Body Fat Composition. J. Cell. Physiol. 2012, 227, 3744–3748. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Rauter, A.P.; Ennis, M.; Hellwich, K.-H.; Herold, B.J.; Horton, D.; Moss, G.P.; Schomburg, I. Nomenclature of Flavonoids (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 1429–1486. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.-S.; Zhang, Y. A Systematic Review on Biological Activities of Prenylated Flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids--Food Sources and Health Benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Jana, K.; Yin, X.; Schiffer, R.B.; Chen, J.-J.; Pandey, A.K.; Stocco, D.M.; Grammas, P.; Wang, X. Chrysin, a Natural Flavonoid Enhances Steroidogenesis and Steroidogenic Acute Regulatory Protein Gene Expression in Mouse Leydig Cells. J. Endocrinol. 2008, 197, 315–323. [Google Scholar] [CrossRef]
- Li, W.; Pandey, A.K.; Yin, X.; Chen, J.-J.; Stocco, D.M.; Grammas, P.; Wang, X. Effects of Apigenin on Steroidogenesis and Steroidogenic Acute Regulatory Gene Expression in Mouse Leydig Cells. J. Nutr. Biochem. 2011, 22, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, C.-L.; Dyson, M.T.; Eimerl, S.; Orly, J.; Hutson, J.C.; Stocco, D.M. Cyclooxygenase-2 Regulation of the Age-Related Decline in Testosterone Biosynthesis. Endocrinology 2005, 146, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Li, X.; Liu, J.; Hong, T.; Zhu, Q.; Huang, P.; Ge, R.-S. Suppression of Rat and Human Androgen Biosynthetic Enzymes by Apigenin: Possible Use for the Treatment of Prostate Cancer. Fitoterapia 2016, 111, 66–72. [Google Scholar] [CrossRef]
- Ohlsson, A.; Ullerås, E.; Cedergreen, N.; Oskarsson, A. Mixture Effects of Dietary Flavonoids on Steroid Hormone Synthesis in the Human Adrenocortical H295R Cell Line. Food Chem. Toxicol. 2010, 48, 3194–3200. [Google Scholar] [CrossRef]
- Hasegawa, E.; Nakagawa, S.; Sato, M.; Tachikawa, E.; Yamato, S. Effect of Polyphenols on Production of Steroid Hormones from Human Adrenocortical NCI-H295R Cells. Biol. Pharm. Bull. 2013, 36, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J. Natural Flavonoids in StAR Gene Expression and Testosterone Biosynthesis in Leydig Cell AgingBasic and Clinical Endocrinology Up-to-Date; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Chen, C.-Y.; Peng, W.-H.; Tsai, K.-D.; Hsu, S.-L. Luteolin Suppresses Inflammation-Associated Gene Expression by Blocking NF-kappaB and AP-1 Activation Pathway in Mouse Alveolar Macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Ha, S.K.; Moon, E.; Kim, S.Y. Chrysin Suppresses LPS-Stimulated Proinflammatory Responses by Blocking NF-κB and JNK Activations in Microglia Cells. Neurosci. Lett. 2010, 485, 143–147. [Google Scholar] [CrossRef]
- Jeong, H.J.; Shin, Y.G.; Kim, I.H.; Pezzuto, J.M. Inhibition of Aromatase Activity by Flavonoids. Arch. Pharm. Res. 1999, 22, 309–312. [Google Scholar] [CrossRef]
- Kellis, J.T.; Vickery, L.E. Inhibition of Human Estrogen Synthetase (Aromatase) by Flavones. Science 1984, 225, 1032–1034. [Google Scholar] [CrossRef]
- Ciftci, O.; Ozdemir, I.; Aydin, M.; Beytur, A. Beneficial Effects of Chrysin on the Reproductive System of Adult Male Rats. Andrologia 2012, 44, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, C.; Rossi, R.; Sommavilla, M.; Ferranti, C.; Rossi, R.; Ciculi, C.; Gizzi, S.; Micheletti, A.; Rufini, S. Effects of Chrysin on Urinary Testosterone Levels in Human Males. J. Med. Food 2003, 6, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, L.; Jesse, C.R.; de Gomes, M.G.; Borges Filho, C.; Donato, F.; Souza, L.C.; Goes, A.R.; Furian, A.F.; Boeira, S.P. The Flavonoid Chrysin Protects against Zearalenone Induced Reproductive Toxicity in Male Mice. Toxicon Off. J. Int. Soc. Toxinol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, K.; Kumar, S.; Sharma, A. Beneficial Effects of Chrysin and Benzoflavone on Virility in 2-Year-Old Male Rats. J. Med. Food 2002, 5, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Rahmati, M.; Nikravesh, M.R.; Saeedi Nejat, S.; Jalali, M. The Effect of Chrysin on the Distribution of Extracellular Matrix Proteins around Leydig Cells under Heat Stress Injury. J. Food Biochem. 2024, 2024, e9060893. [Google Scholar] [CrossRef]
- Abadi, A.R.R.; Boukani, L.M.; Shokoohi, M.; Vaezi, N.; Mahmoodi, M.; Gharekhani, M.; Kouchesfahani, H.M.; Khaki, A.A. The Flavonoid Chrysin Protects against Testicular Apoptosis Induced by Torsion/Detorsion in Adult Rats. Andrologia 2023, 2023, e6500587. [Google Scholar] [CrossRef]
- Chen, L.-J.; Games, D.E.; Jones, J. Isolation and Identification of Four Flavonoid Constituents from the Seeds of Oroxylum Indicum by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2003, 988, 95–105. [Google Scholar] [CrossRef]
- Nishioka, T.; Kawabata, J.; Aoyama, Y. Baicalein, an Alpha-Glucosidase Inhibitor from Scutellaria Baicalensis. J. Nat. Prod. 1998, 61, 1413–1415. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, J.; Cui, N.; Jiang, L.; Zhou, H.; Zhang, D.; Hao, G. Baicalin Ameliorates Polycystic Ovary Syndrome through AMP-Activated Protein Kinase. J. Ovarian Res. 2019, 12, 109. [Google Scholar] [CrossRef]
- Carreau, S.; Hess, R.A. Oestrogens and Spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef]
- Dorrington, J.H.; Armstrong, D.T. Follicle-Stimulating Hormone Stimulates Estradiol-17beta Synthesis in Cultured Sertoli Cells. Proc. Natl. Acad. Sci. USA 1975, 72, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.; Dufau, M.L.; Catt, K.J. Direct Inhibitory Effect of Estrogen on Leydig Cell Function of Hypophysectomized Rats. Endocrinology 1978, 103, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Valladares, L.E.; Payne, A.H. Induction of Testicular Aromatization by Luteinizing Hormone in Mature Rats. Endocrinology 1979, 105, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, H.; Li, M.; Gao, Z.; Huang, J.; Liu, L.; Huang, X.; Li, Y. Daidzein Impairs Leydig Cell Testosterone Production and Sertoli Cell Function in Neonatal Mouse Testes: An in Vitro Study. Mol. Med. Rep. 2016, 14, 5325–5333. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Nagpal, M.L.; Stocco, D.M.; Lin, T. Effects of Genistein, Resveratrol, and Quercetin on Steroidogenesis and Proliferation of MA-10 Mouse Leydig Tumor Cells. J. Endocrinol. 2007, 192, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Lehraiki, A.; Chamaillard, C.; Krust, A.; Habert, R.; Levacher, C. Genistein Impairs Early Testosterone Production in Fetal Mouse Testis via Estrogen Receptor Alpha. Toxicol. In Vitro 2011, 25, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-X.; Zhao, B.-H.; Chu, Y.-H.; Zhou, H.-Y.; Akingbemi, B.T.; Zheng, Z.-Q.; Ge, R.-S. Effects of Genistein and Equol on Human and Rat Testicular 3beta-Hydroxysteroid Dehydrogenase and 17beta-Hydroxysteroid Dehydrogenase 3 Activities. Asian J. Androl. 2010, 12, 519–526. [Google Scholar] [CrossRef]
- Le Bail, J.C.; Champavier, Y.; Chulia, A.J.; Habrioux, G. Effects of Phytoestrogens on Aromatase, 3beta and 17beta-Hydroxysteroid Dehydrogenase Activities and Human Breast Cancer Cells. Life Sci. 2000, 66, 1281–1291. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Vazquez, G.; Duval, S.J.; Phipps, W.R.; Kurzer, M.S.; Messina, M.J. Clinical Studies Show No Effects of Soy Protein or Isoflavones on Reproductive Hormones in Men: Results of a Meta-Analysis. Fertil. Steril. 2010, 94, 997–1007. [Google Scholar] [CrossRef]
- Jones, S.; Boisvert, A.; Naghi, A.; Hullin-Matsuda, F.; Greimel, P.; Kobayashi, T.; Papadopoulos, V.; Culty, M. Stimulatory Effects of Combined Endocrine Disruptors on MA-10 Leydig Cell Steroid Production and Lipid Homeostasis. Toxicology 2016, 355–356, 21–30. [Google Scholar] [CrossRef]
- Sherrill, J.D.; Sparks, M.; Dennis, J.; Mansour, M.; Kemppainen, B.W.; Bartol, F.F.; Morrison, E.E.; Akingbemi, B.T. Developmental Exposures of Male Rats to Soy Isoflavones Impact Leydig Cell Differentiation. Biol. Reprod. 2010, 83, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Mäkelä, S.; Joshi, S.; Huhtaniemi, I.; Santti, R. Genistein Exerts Estrogen-like Effects in Male Mouse Reproductive Tract. Mol. Cell. Endocrinol. 1998, 144, 83–93. [Google Scholar] [CrossRef]
- Levy, J.R.; Faber, K.A.; Ayyash, L.; Hughes, C.L. The Effect of Prenatal Exposure to the Phytoestrogen Genistein on Sexual Differentiation in Rats. Proc. Soc. Exp. Biol. Med. 1995, 208, 60–66. [Google Scholar] [CrossRef]
- Roberts, D.; Veeramachaneni, D.N.; Schlaff, W.D.; Awoniyi, C.A. Effects of Chronic Dietary Exposure to Genistein, a Phytoestrogen, during Various Stages of Development on Reproductive Hormones and Spermatogenesis in Rats. Endocrine 2000, 13, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.A.; Griffiths, K.; Morton, M.S. Inhibition of 5 Alpha-Reductase in Genital Skin Fibroblasts and Prostate Tissue by Dietary Lignans and Isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.-A.; Son, H.M.; Lee, J.-S.; Kwon, C.-S.; Lim, J.K.; Yeo, Y.K.; Park, Y.S.; Kim, J.-S. Regulation of Male Sex Hormone Levels by Soy Isoflavones in Rats. Nutr. Cancer 2002, 42, 206–210. [Google Scholar] [CrossRef]
- Moyad, M.A. Soy, Disease Prevention, and Prostate Cancer. Semin. Urol. Oncol. 1999, 17, 97–102. [Google Scholar]
- Ferigolo, M.; Nardi, J.; Freddo, N.; Ferramosca, A.; Zara, V.; Dallegrave, E.; Macedo, M.B.; Eller, S.; de Oliveira, A.P.; Biazus, I.C.; et al. Evaluation of Genistein as a Mitochondrial Modulator and Its Effects on Sperm Quality. Int. J. Mol. Sci. 2023, 24, 14260. [Google Scholar] [CrossRef]
- McVey, M.J.; Cooke, G.M.; Curran, I.H.A. Increased Serum and Testicular Androgen Levels in F1 Rats with Lifetime Exposure to Soy Isoflavones. Reprod. Toxicol. 2004, 18, 677–685. [Google Scholar] [CrossRef]
- Al-Shaikh, T.M. Role of Soy Isoflavone in Preventing Aging Changes in Rat Testis: Biochemical and Histological Studies. Saudi J. Biol. Sci. 2022, 29, 103423. [Google Scholar] [CrossRef]
- Caceres, S.; Crespo, B.; Alonso-Diez, A.; de Andrés, P.J.; Millan, P.; Silván, G.; Illera, M.J.; Illera, J.C. Long-Term Exposure to Isoflavones Alters the Hormonal Steroid Homeostasis-Impairing Reproductive Function in Adult Male Wistar Rats. Nutrients 2023, 15, 1261. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Lv, Z.; Hu, C.; Zhang, Q.; Wang, Z.; Hamdard, E.; Dai, H.; Mustafa, S.; Shi, F. Oral Exposure to Genistein during Conception and Lactation Period Affects the Testicular Development of Male Offspring Mice. Animals 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Jeminiwa, B.O.; Knight, R.M.; Braden, T.D.; Cruz-Espindola, C.; Boothe, D.M.; Akingbemi, B.T. Regulation of the Neuroendocrine Axis in Male Rats by Soy-Based Diets Is Independent of Age and Due Specifically to Isoflavone Action†. Biol. Reprod. 2020, 103, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.E.; Camargo, J.; Hamilton-Reeves, J.; Kurzer, M.; Messina, M. Neither Soy nor Isoflavone Intake Affects Male Reproductive Hormones: An Expanded and Updated Meta-Analysis of Clinical Studies. Reprod. Toxicol. 2021, 100, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Scholten, S.D.; Sergeev, I.N.; Song, Q.; Birger, C.B. Effects of Vitamin D and Quercetin, Alone and in Combination, on Cardiorespiratory Fitness and Muscle Function in Physically Active Male Adults. Open Access J. Sports Med. 2015, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Samova, S.; Patel, C.N.; Doctor, H.; Pandya, H.A.; Verma, R.J. The Effect of Bisphenol A on Testicular Steroidogenesis and Its Amelioration by Quercetin: An in Vivo and in Silico Approach. Toxicol. Res. 2018, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- King, S.R.; LaVoie, H.A. Gonadal Transactivation of STARD1, CYP11A1 and HSD3B. Front. Biosci. Landmark Ed. 2012, 17, 824–846. [Google Scholar] [CrossRef]
- Manna, P.R.; Dyson, M.T.; Eubank, D.W.; Clark, B.J.; Lalli, E.; Sassone-Corsi, P.; Zeleznik, A.J.; Stocco, D.M. Regulation of Steroidogenesis and the Steroidogenic Acute Regulatory Protein by a Member of the cAMP Response-Element Binding Protein Family. Mol. Endocrinol. 2002, 16, 184–199. [Google Scholar] [CrossRef]
- Manna, P.R.; Eubank, D.W.; Lalli, E.; Sassone-Corsi, P.; Stocco, D.M. Transcriptional Regulation of the Mouse Steroidogenic Acute Regulatory Protein Gene by the cAMP Response-Element Binding Protein and Steroidogenic Factor 1. J. Mol. Endocrinol. 2003, 30, 381–397. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhai, Q.-Q.; Zhu, Y.-F.; Liu, B.-Y.; Xu, Y. Quercetin Ameliorates Testosterone Secretion Disorder by Inhibiting Endoplasmic Reticulum Stress through the miR-1306-5p/HSD17B7 Axis in Diabetic Rats. Bosn. J. Basic Med. Sci. 2022, 22, 191–204. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Farombi, E.O. Quercetin Ameliorates Atrazine-Induced Changes in the Testicular Function of Rats. Toxicol. Ind. Health 2016, 32, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Ujah, G.A.; Nna, V.U.; Agah, M.I.; Omue, L.O.; Leku, C.B.; Osim, E.E. Effect of Quercetin on Cadmium Chloride-Induced Impairments in Sexual Behaviour and Steroidogenesis in Male Wistar Rats. Andrologia 2018, 50, e12866. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, D.; Lin, J.; Liu, Y.; Xu, L.; Lv, R.; Mo, K.; Lian, X.; Xie, M.; Xu, S.; et al. Icariin Protects Mouse Leydig Cell Testosterone Synthesis from the Adverse Effects of Di(2-Ethylhexyl) Phthalate. Toxicol. Appl. Pharmacol. 2019, 378, 114612. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.M.; Crawford, P.A.; Woodson, K.G.; Polish, J.A.; Olson, L.M.; Sadovsky, Y. Role of Steroidogenic-Factor 1 in Basal and 3′,5′-Cyclic Adenosine Monophosphate-Mediated Regulation of Cytochrome P450 Side-Chain Cleavage Enzyme in the Mouse. Biol. Reprod. 1997, 57, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.E.; Morin, F.; Roussel, V.; Bertucci, M.C.; Boyer, A.; Murphy, B.D. The Role of Steroidogenic Factor 1 (SF-1) in Steroidogenic Cell Function of the Testes and Ovaries of Mature Mice. Reproduction 2023, 165, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of Icariin on Reproductive Functions in Male Rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Zhang, X.; Afedo, S.Y.; Rui, R. Evaluation of the Protective Effects of Icariin on Nicotine-Induced Reproductive Toxicity in Male Mouse—A Pilot Study. Reprod. Biol. Endocrinol. RBE 2020, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hao, J.; Pu, J.; Zhao, L.; Lü, Z.; Hu, J.; Yu, Q.; Wang, Y.; Xie, Y.; Li, G. Icariin Induces Apoptosis in Mouse MLTC-10 Leydig Tumor Cells through Activation of the Mitochondrial Pathway and down-Regulation of the Expression of Piwil4. Int. J. Oncol. 2011, 39, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Badr, G.M.; Sedky, A.; Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M. Rutin Ameliorates Carbon Tetrachloride (CCl4)-Induced Hepatorenal Toxicity and Hypogonadism in Male Rats. PeerJ 2019, 7, e7011. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Iserhienrhien, B.O.; Badejo, T.A. Rutin- and Selenium-Attenuated Cadmium-Induced Testicular Pathophysiology in Rats. Hum. Exp. Toxicol. 2013, 32, 395–406. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Olufemi, P.D.; Lawrence, C.J.; Wekere, F.C.; Ochulor, A.C.; Barikuma, A.M. Rutin, an Antioxidant Flavonoid, Induces Glutathione and Glutathione Peroxidase Activities to Protect against Ethanol Effects in Cadmium-Induced Oxidative Stress in the Testis of Adult Rats. Andrologia 2017, 49, e12696. [Google Scholar] [CrossRef] [PubMed]
- AbdElrazek, D.A.; Hassan, N.H.; Ibrahim, M.A.; Hassanen, E.I.; Farroh, K.Y.; Abass, H.I. Ameliorative Effects of Rutin and Rutin-Loaded Chitosan Nanoparticles on Testicular Oxidative Stress and Histological Damage Induced by Cyclophosphamide in Male Rats. Food Chem. Toxicol. 2024, 184, 114436. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Li, X.; Zhu, Q.; Wang, Y.; Ge, R.; Jin, X. Rutin Inhibits Androgen Synthesis and Metabolism in Rat Immature Leydig Cells in Vitro. Andrologia 2021, 53, e14221. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Tian, E.; Wang, L.; Li, X.; Zhu, Q.; Wang, Y.; Zhong, Y.; Ge, R.-S. Taxifolin Suppresses Rat and Human Testicular Androgen Biosynthetic Enzymes. Fitoterapia 2018, 125, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Papiez, M.A. Influence of Naringenin on the Activity of Enzymes Participating in Steroidogenesis in Male Rats. Rocz. Akad. Med. W Bialymstoku 1995 2004, 49 (Suppl. S1), 120–122. [Google Scholar]
- Adana, M.Y.; Akang, E.N.; Naidu, E.C.S.; Aniekan, P.I.; Kouame, K.; Offor, U.; Ogedengbe, O.O.; Azu, O.O. Testicular Microanatomical and Hormonal Alterations Following Use of Antiretroviral Therapy in Sprague Dawley Rats: Role of Naringenin. Andrologia 2018, 50, e13137. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Refaie, M.M.M.; Abdelghany, M.I. Naringenin Palliates Cisplatin and Doxorubicin Gonadal Toxicity in Male Rats. Toxicol. Mech. Methods 2019, 29, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Lin, S.; Wang, K.; Wang, H.; Liu, Z. Protective Effect of Naringenin against Cadmium-Induced Testicular Toxicity in Male SD Rats. J. Inorg. Biochem. 2021, 214, 111310. [Google Scholar] [CrossRef] [PubMed]
- Alboghobeish, S.; Mahdavinia, M.; Zeidooni, L.; Samimi, A.; Oroojan, A.A.; Alizadeh, S.; Dehghani, M.A.; Ahangarpour, A.; Khorsandi, L. Efficiency of Naringin against Reproductive Toxicity and Testicular Damages Induced by Bisphenol A in Rats. Iran. J. Basic Med. Sci. 2019, 22, 315–523. [Google Scholar] [CrossRef]
- Okesina, K.B.; Odetayo, A.F.; Adeyemi, W.J.; Ajibare, A.J.; Okesina, A.A.; Olayaki, L.A. Naringin from Sweet Orange Peel Improves Testicular Function in High Fat Diet-Induced Diabetic Rats by Modulating Xanthine Oxidase/Uric Acid Signaling and Maintaining Redox Balance. Lab. Anim. Res. 2024, 40, 5. [Google Scholar] [CrossRef]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a Citrus Flavonoid, Attenuates Testicular Damage in Diabetic Rats via Inhibition of Oxidative Stress, Inflammation, and Apoptosis. Life Sci. 2018, 210, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Helmy, H.S.; Senousy, M.A.; El-Sahar, A.E.; Sayed, R.H.; Saad, M.A.; Elbaz, E.M. Aberrations of miR-126-3p, miR-181a and Sirtuin1 Network Mediate Di-(2-Ethylhexyl) Phthalate-Induced Testicular Damage in Rats: The Protective Role of Hesperidin. Toxicology 2020, 433–434, 152406. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Bharathi, B.; Jaya Prakash, G.; Krishna, K.M.; Ravi Krishna, C.H.; Sivanarayana, T.; Madan, K.; Rama Raju, G.A.; Annapurna, A. Protective Effect of Alpha Glucosyl Hesperidin (G-Hesperidin) on Chronic Vanadium Induced Testicular Toxicity and Sperm Nuclear DNA Damage in Male Sprague Dawley Rats. Andrologia 2015, 47, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.-L.; Pu, H.-F.; Chen, S.-Y.; Wang, S.-W.; Wang, P.S. Effects of Catechin, Epicatechin and Epigallocatechin Gallate on Testosterone Production in Rat Leydig Cells. J. Cell. Biochem. 2010, 110, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Figueiroa, M.S.; César Vieira, J.S.B.; Leite, D.S.; Filho, R.C.O.A.; Ferreira, F.; Gouveia, P.S.; Udrisar, D.P.; Wanderley, M.I. Green Tea Polyphenols Inhibit Testosterone Production in Rat Leydig Cells. Asian J. Androl. 2009, 11, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Assunção, M.; Andrade, J.P.; Neves, D.; Calhau, C.; Azevedo, I. Chronic Green Tea Consumption Decreases Body Mass, Induces Aromatase Expression, and Changes Proliferation and Apoptosis in Adult Male Rat Adipose Tissue. J. Nutr. 2008, 138, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Sakamoto, Y.; Ogata, A.; Nagai, F.; Mikuriya, H.; Numazawa, M.; Yamada, K.; Aoki, N. Inhibition of Aromatase Activity by Green Tea Extract Catechins and Their Endocrinological Effects of Oral Administration in Rats. Food Chem. Toxicol. 2002, 40, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bianco, F.; Grasselli, F. Epigallocatechin-3-Gallate from Green Tea Negatively Affects Swine Granulosa Cell Function. Domest. Anim. Endocrinol. 2005, 28, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- He, Y.; Hu, Y.; Jiang, X.; Chen, T.; Ma, Y.; Wu, S.; Sun, J.; Jiao, R.; Li, X.; Deng, L.; et al. Cyanidin-3-O-Glucoside Inhibits the UVB-Induced ROS/COX-2 Pathway in HaCaT Cells. J. Photochem. Photobiol. B 2017, 177, 24–31. [Google Scholar] [CrossRef]
- Ma, M.-M.; Li, Y.; Liu, X.-Y.; Zhu, W.-W.; Ren, X.; Kong, G.-Q.; Huang, X.; Wang, L.-P.; Luo, L.-Q.; Wang, X.-Z. Cyanidin-3-O-Glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-κB and MAPK Pathways. Inflammation 2015, 38, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Tian, L.; Li, Y.; Bai, W. Cyanidin-3-O-Glucoside Promotes the Biosynthesis of Progesterone through the Protection of Mitochondrial Function in Pb-Exposed Rat Leydig Cells. Food Chem. Toxicol. 2018, 112, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Jiang, X.; Sun, J.; Tian, L.; Jiao, R.; Tang, Y.; Bai, W. Cyanidin-3-O-Glucoside Protects against Cadmium-Induced Dysfunction of Sex Hormone Secretion via the Regulation of Hypothalamus-Pituitary-Gonadal Axis in Male Pubertal Mice. Food Chem. Toxicol. 2019, 129, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Erboga, M.; Kanter, M.; Aktas, C.; Bozdemir Donmez, Y.; Fidanol Erboga, Z.; Aktas, E.; Gurel, A. Anti-Apoptotic and Anti-Oxidant Effects of Caffeic Acid Phenethyl Ester on Cadmium-Induced Testicular Toxicity in Rats. Biol. Trace Elem. Res. 2016, 171, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-X.; Liang, G.; Chu, Y.; Li, X.; Lian, Q.-Q.; Lin, H.; He, Y.; Huang, Y.; Hardy, D.O.; Ge, R.-S. Curcumin Derivatives Inhibit Testicular 17beta-Hydroxysteroid Dehydrogenase 3. Bioorg. Med. Chem. Lett. 2010, 20, 2549–2551. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chiu, C.-H.; Liu, H.-C.; Wang, J.-Y. Curcumin Downregulates 8-Br-cAMP-Induced Steroidogenesis in Mouse Leydig Cells by Suppressing the Expression of Cyp11a1 and StAR Independently of the PKA-CREB Pathway. Endocr. J. 2018, 65, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R.J. The Production of Resveratrol by Vitis Vinifera and Other Members of the Vitaceae as a Response to Infection or Injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Mongioì, L.M.; Perelli, S.; Condorelli, R.A.; Barbagallo, F.; Crafa, A.; Cannarella, R.; La Vignera, S.; Calogero, A.E. The Role of Resveratrol in Human Male Fertility. Molecules 2021, 26, 2495. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Zhu, Q.; Chen, D.; Guo, J.; Yao, W.; Dong, Y.; Wei, J.; Lian, Q.; Ge, R.-S.; et al. Disrupting Androgen Production of Leydig Cells by Resveratrol via Direct Inhibition of Human and Rat 3β-Hydroxysteroid Dehydrogenase. Toxicol. Lett. 2014, 226, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Svechnikov, K.; Spatafora, C.; Svechnikova, I.; Tringali, C.; Söder, O. Effects of Resveratrol Analogs on Steroidogenesis and Mitochondrial Function in Rat Leydig Cells in Vitro. J. Appl. Toxicol. JAT 2009, 29, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Abdou, H.S.; Bergeron, F.; Tremblay, J.J. A Cell-Autonomous Molecular Cascade Initiated by AMP-Activated Protein Kinase Represses Steroidogenesis. Mol. Cell. Biol. 2014, 34, 4257–4271. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Wang, Q.; Lv, Z.-M.; Wang, C.-L.; Li, C.-P.; Rong, Y.-L. Resveratrol Appears to Protect against Oxidative Stress and Steroidogenesis Collapse in Mice Fed High-Calorie and High-Cholesterol Diet. Andrologia 2015, 47, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, S.; Zhu, X.; Lv, Z. Autophagy-Related 7 Proteindependent Autophagy Mediates Resveratrol-Caused Upregulation of Mitochondrial Biogenesis and Steroidogenesis in Aged Leydig Cell. Mol. Biol. Rep. 2023, 51, 28. [Google Scholar] [CrossRef] [PubMed]
- Greifová, H.; Jambor, T.; Tokárová, K.; Speváková, I.; Knížatová, N.; Lukáč, N. Resveratrol Attenuates Hydrogen Peroxide-Induced Oxidative Stress in TM3 Leydig Cells in Vitro. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 585–595. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Li, Z. Resveratrol Protects Leydig Cells from Nicotine-Induced Oxidative Damage through Enhanced Autophagy. Clin. Exp. Pharmacol. Physiol. 2018, 45, 573–580. [Google Scholar] [CrossRef]
- Jambor, T.; Zajickova, T.; Arvay, J.; Ivanisova, E.; Tirdilova, I.; Knizatova, N.; Greifova, H.; Kovacik, A.; Galova, E.; Lukac, N. Exceptional Properties of Lepidium sativum L. Extract and Its Impact on Cell Viability, Ros Production, Steroidogenesis, and Intracellular Communication in Mice Leydig Cells In Vitro. Molecules 2022, 27, 5127. [Google Scholar] [CrossRef]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm Animals: Source of Reproductive Gain or Waste? Antioxidants 2020, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Samodien, E.; Johnson, R.; Pheiffer, C.; Mabasa, L.; Erasmus, M.; Louw, J.; Chellan, N. Diet-Induced Hypothalamic Dysfunction and Metabolic Disease, and the Therapeutic Potential of Polyphenols. Mol. Metab. 2019, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Tian, Y.; Ling, A.; Liu, Z.; Zhao, L.; Cheng, G. Genistein Affects Gonadotrophin-Releasing Hormone Secretion in GT1-7 Cells via Modulating Kisspeptin Receptor and Key Regulators. Syst. Biol. Reprod. Med. 2022, 68, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Shimoi, K. Anti-Stress Effects of Polyphenols: Animal Models and Human Trials. Food Funct. 2020, 11, 5702–5717. [Google Scholar] [CrossRef]
- Pizarro Meléndez, G.P.; Valero-Jara, V.; Acevedo-Hernández, P.; Thomas-Valdés, S. Impact of Polyphenols on Stress and Anxiety: A Systematic Review of Molecular Mechanisms and Clinical Evidence. Crit. Rev. Food Sci. Nutr. 2024, 64, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Hodgson, L.; Bussu, A.; Farhat, G.; Al-Dujaili, E. Effect of Polyphenol-Rich Dark Chocolate on Salivary Cortisol and Mood in Adults. Antioxidants 2019, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Tremblay, J.J. Glucocorticoids Antagonize cAMP-Induced Star Transcription in Leydig Cells through the Orphan Nuclear Receptor NR4A1. J. Mol. Endocrinol. 2008, 41, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of Endocrine Systems and Food Intake by Green Tea Epigallocatechin Gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef]
- McGarvey, C.; Cates, P.A.; Brooks, A.; Swanson, I.A.; Milligan, S.R.; Coen, C.W.; O’Byrne, K.T. Phytoestrogens and Gonadotropin-Releasing Hormone Pulse Generator Activity and Pituitary Luteinizing Hormone Release in the Rat. Endocrinology 2001, 142, 1202–1208. [Google Scholar] [CrossRef]
- Chupradit, S.; Bokov, D.; Zamanian, M.Y.; Heidari, M.; Hakimizadeh, E. Hepatoprotective and Therapeutic Effects of Resveratrol: A Focus on Anti-Inflammatory and Antioxidative Activities. Fundam. Clin. Pharmacol. 2022, 36, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Hekmatdoost, A.; Adibi, P. Resveratrol and Liver: A Systematic Review. J. Res. Med. Sci. 2015, 20, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; El May, M.; Gharbi, N.; Kamoun, A.; El-Fazaâ, S. Resveratrol, a Red Wine Polyphenol, Attenuates Ethanol-Induced Oxidative Stress in Rat Liver. Life Sci. 2007, 80, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-C.; Lee, K.-C.; Huang, Y.-H.; Chou, C.-K.; Lin, H.-C.; Lee, F.-Y. Regulation by Resveratrol of the Cellular Factors Mediating Liver Damage and Regeneration after Acute Toxic Liver Injury. J. Gastroenterol. Hepatol. 2014, 29, 603–613. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, A.C.N.; de Andrade, C.B.V.; Ramos, I.P.R.; Dias, M.L.; Batista, C.M.P.; Pimentel, C.F.; de Carvalho, J.J.; Goldenberg, R.C.D.S. Resveratrol Promotes Liver Regeneration in Drug-Induced Liver Disease in Mice. Food Res. Int. 2021, 142, 110185. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Flores, L.F.; Casas-Grajales, S.; Hernández-Aquino, E.; Vargas-Pozada, E.E.; Muriel, P. Antioxidant, Antiinflammatory, and Antifibrotic Properties of Quercetin in the Liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 653–674. [Google Scholar]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective Effect of Quercetin: From Chemistry to Medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Protective Effects of Quercetin on Liver Injury Induced by Ethanol. Pharmacogn. Mag. 2010, 6, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A Systematic Review on Hepatoprotective Activity of Quercetin against Various Drugs and Toxic Agents: Evidence from Preclinical Studies. Phytother. Res. PTR 2020, 34, 5–32. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-Gallate (EGCG) Reduces Liver Inflammation, Oxidative Stress and Fibrosis in Carbon Tetrachloride (CCl4)-Induced Liver Injury in Mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Tang, G.; Xu, Y.; Zhang, C.; Wang, N.; Li, H.; Feng, Y. Green Tea and Epigallocatechin Gallate (EGCG) for the Management of Nonalcoholic Fatty Liver Diseases (NAFLD): Insights into the Role of Oxidative Stress and Antioxidant Mechanism. Antioxidants 2021, 10, 1076. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Muriel, P. Pharmacological Actions of Curcumin in Liver Diseases or Damage. Liver Int. Off. J. Int. Assoc. Study Liver 2009, 29, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Xue, N.; Li, H.; Huang, B.; Chen, Z.; Wang, X. Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-Canonical NF-κB Pathway In Vivo and In Vitro. Inflammation 2020, 43, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Al-Amarat, W.; Abukhalil, M.H.; Alruhaimi, R.S.; Alqhtani, H.A.; Aldawood, N.; Alfwuaires, M.A.; Althunibat, O.Y.; Aladaileh, S.H.; Algefare, A.I.; Alanezi, A.A.; et al. Upregulation of Nrf2/HO-1 Signaling and Attenuation of Oxidative Stress, Inflammation, and Cell Death Mediate the Protective Effect of Apigenin against Cyclophosphamide Hepatotoxicity. Metabolites 2022, 12, 648. [Google Scholar] [CrossRef]
- Longcope, C.; Feldman, H.A.; McKinlay, J.B.; Araujo, A.B. Diet and Sex Hormone-Binding Globulin. J. Clin. Endocrinol. Metab. 2000, 85, 293–296. [Google Scholar] [CrossRef]



| Function | Description | References |
|---|---|---|
| Development of male reproductive tissues | Promotes the development of the testes, of epididymides and seminal vesicles, of the prostate, as well as of the penis and scrotum | [43,44,45,46,47] |
| Secondary sexual characteristics | Responsible for features such as increased muscle and bone mass, deepening of the voice, and growth of body hair | [48,49,50,51] |
| Adult male fertility | Critical for the initiation and maintenance of spermatogenesis | [52] |
| Involved in the feedback regulation of pituitary gonadotropin production and secretion | [53] | |
| Muscle mass and strength | Enhances muscle growth, increases protein synthesis, and improves physical strength | [48,54] |
| Bone growth and density | Increases bone density and helps maintaining bone health | [49,55] |
| Enhances bone growth during puberty and cessation of growth of long bones at the end of puberty | [56,57] | |
| Red blood cell production | Stimulates the production of red blood cells in the bone marrow | [58] |
| Mood and mental health | Affects mood, energy levels, and overall sense of well-being; low levels can lead to depression and fatigue | [59,60,61,62] |
| Libido and sexual function | Plays a critical role in sex drive and erectile function | [63,64] |
| Fat distribution | Influences the distribution of body fat, often leading to a reduction in fat mass | [65] |
| Cognitive function | Contributes to cognitive functions such as memory and spatial abilities | [38,39] |
| Hair growth | Promotes hair growth on the face and body, while potentially contributing to scalp hair loss | [51] |
| Basic Skeleton | Lipophilicity (Log p) * |
|---|---|
| Flavanoids | 3.47 |
| Flavones/Isoflavones | 3.18 |
| Flavonols | 2.84 |
| Flavanones | 3.18 |
| (+)-Catechin (R = H) | 0.58 |
| Anthocyanidins | 2.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, L.J.; Touaibia, M. Prevention of Male Late-Onset Hypogonadism by Natural Polyphenolic Antioxidants. Nutrients 2024, 16, 1815. https://doi.org/10.3390/nu16121815
Martin LJ, Touaibia M. Prevention of Male Late-Onset Hypogonadism by Natural Polyphenolic Antioxidants. Nutrients. 2024; 16(12):1815. https://doi.org/10.3390/nu16121815
Chicago/Turabian StyleMartin, Luc J., and Mohamed Touaibia. 2024. "Prevention of Male Late-Onset Hypogonadism by Natural Polyphenolic Antioxidants" Nutrients 16, no. 12: 1815. https://doi.org/10.3390/nu16121815
APA StyleMartin, L. J., & Touaibia, M. (2024). Prevention of Male Late-Onset Hypogonadism by Natural Polyphenolic Antioxidants. Nutrients, 16(12), 1815. https://doi.org/10.3390/nu16121815






