Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism
Abstract
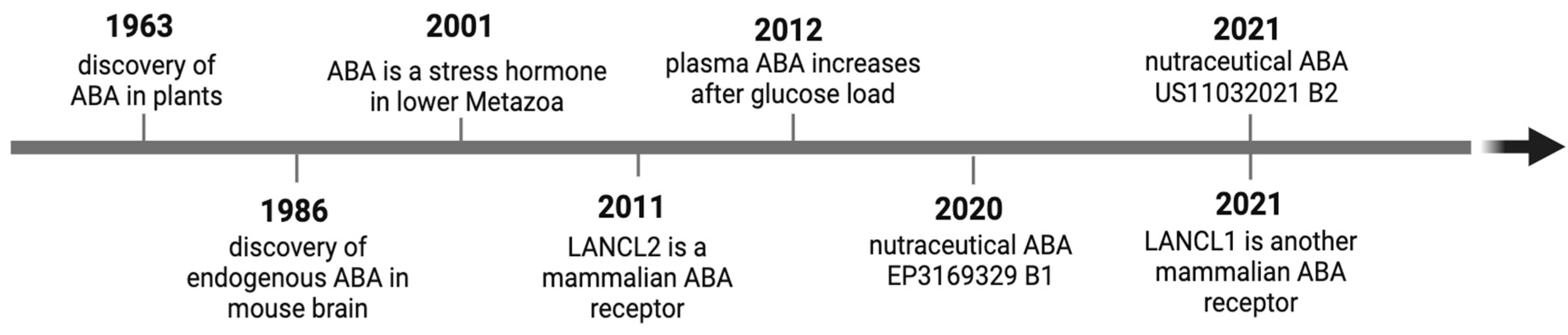
1. Introduction
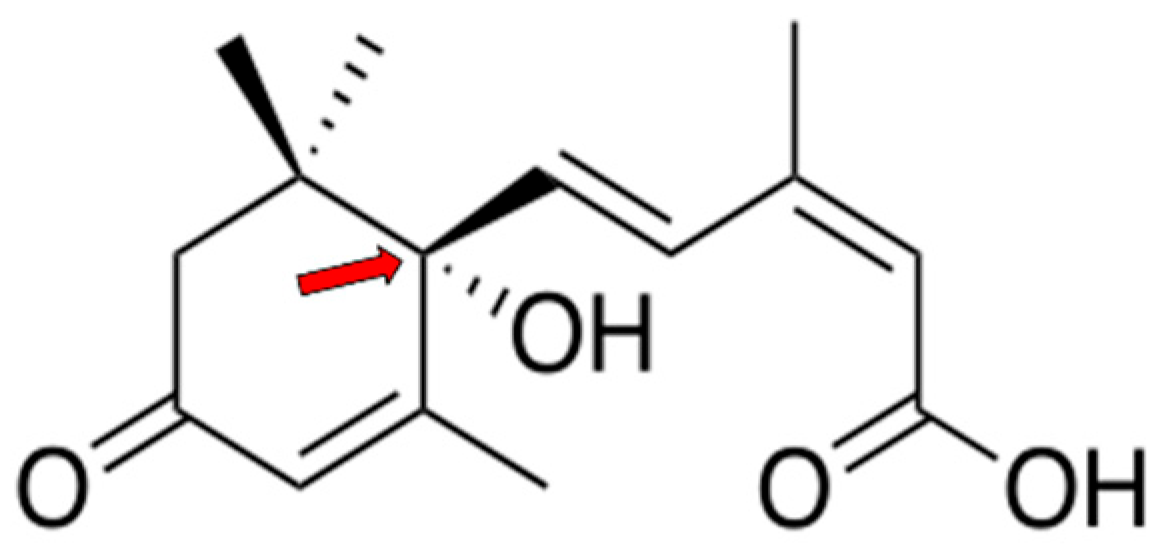
1.1. Role of ABA in Plants
1.2. ABA Content in Fruits and Vegetables
1.3. Manifold Roles of ABA as an Endogenous Animal Hormone
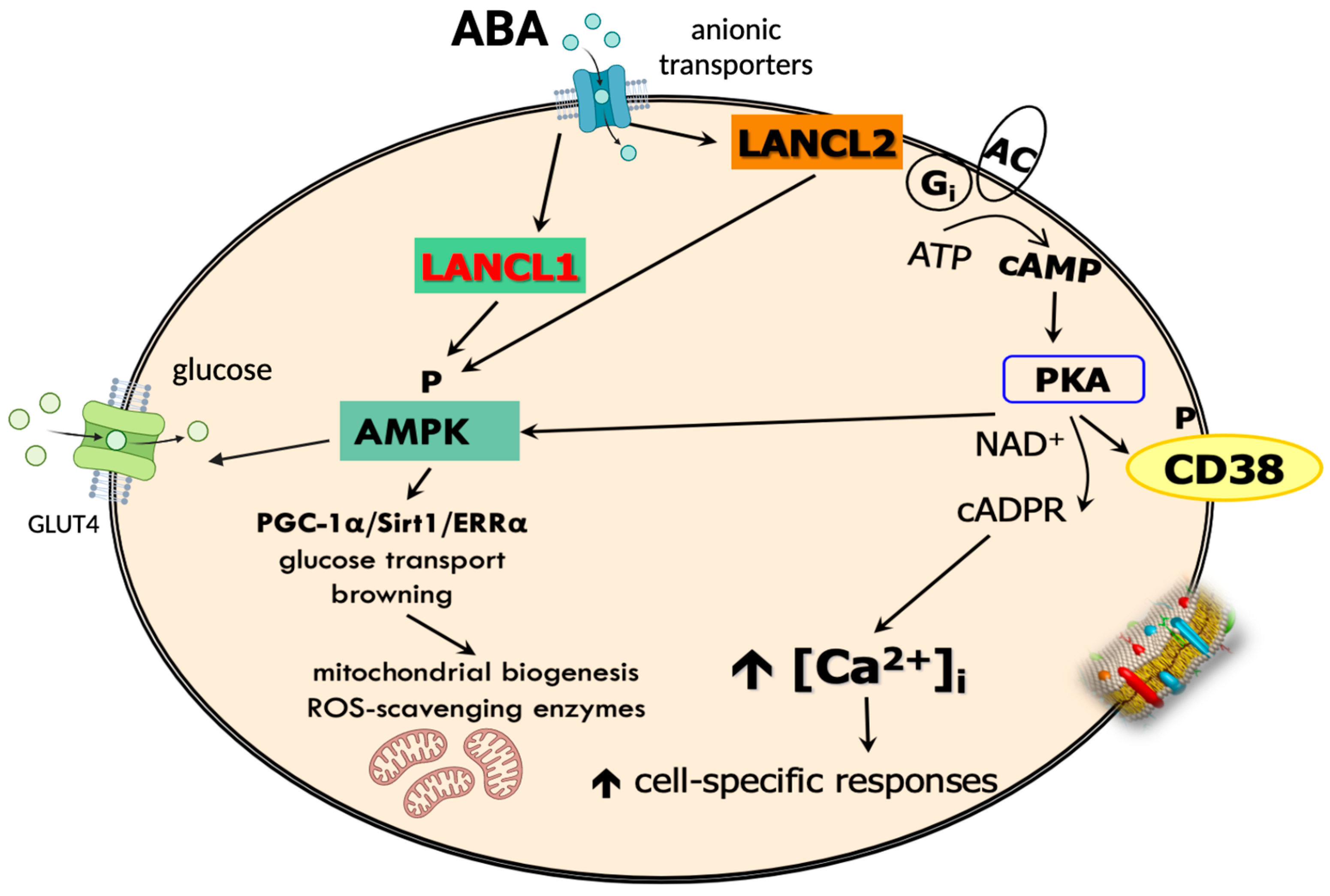
1.4. ABA Receptors and Signaling Pathways
2. Role of ABA in the Control of Glycemia
2.1. Plasma ABA in Healthy and Diabetic Subjects
2.2. ABA Ameliorates Glucose Tolerance in Mice and Healthy Humans
2.3. ABA Stimulates Myocyte, Cardiomyocyte, and Adipocyte Glucose Uptake and Metabolism In Vitro, Ex Vivo, and In Vivo
2.3.1. Skeletal Muscle
2.3.2. Cardiomyocytes
2.3.3. Adipose Tissue
3. Non-Overlapping Roles of ABA and Insulin
4. Open Questions
4.1. Possible ABA Cell Sources
4.2. Role of the ABA-LANCL System in Thermogenesis
4.3. Is the ABA/LANCL System Linked to Genetic Abnormalities?
5. Future Perspectives
5.1. Nutraceutical ABA to Control Glycemia in Prediabetes and Diabetes: Preclinical and Clinical Studies
5.2. Does ABA Increase Body Temperature?
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- In Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 228798. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic acid as an emerging modulator of the responses of plants to low oxygen conditions. Front. Plant Sci. 2021, 12, 661789. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cadenas, A.; Vives, V.; Zandalinas, S.; Manzi, M.; Sanchez-Perez, A.; Perez-Clemente, R.M.; Arbona, V. Abscisic Acid: A versatile phytohormone in plant signaling and beyond. Curr. Protein Pept. Sci. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Sharma, N.; Klahn, P.; Gereke, M.; Bruder, D. Abscisic Acid: A Phytohormone and Mammalian Cytokine as Novel Pharmacon with Potential for Future Development into Clinical Applications. Curr. Med. Chem. 2016, 23, 1549–1570. [Google Scholar] [CrossRef]
- Subodh; Ravina; Priyanka; Narang, J.; Mohan, H. Biosensors for phytohormone Abscisic acid and its role in humans: A review. Sens. Int. 2023, 4, 100234. [Google Scholar] [CrossRef]
- Gharib, A.; Marquez, C.; Meseguer-Beltran, M.; Sanchez-Sarasua, S.; Sanchez-Perez, A.M. Abscisic acid, an evolutionary conserved hormone: Biosynthesis, therapeutic and diagnostic applications in mammals. Biochem. Pharmacol. 2024, 229, 116521. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.; Gastaldelli, A.; De Fronzo, A.R. Insulin: The master regulator of glucose metabolism. J. Metabol. 2022, 129, 155142. [Google Scholar] [CrossRef]
- Le, T.K.C.; Dao, X.D.; Nguyen, D.V.; Luu, D.H.; Bui, T.M.H.; Le, T.H.; Nguyen, H.T.; Le, T.N.; Hosaka, T.; Nguyen, T.T.T. Insulin signaling and its application. Front. Endocrinol. 2023, 14, 1226655. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic Acid: A Conserved Hormone in Plants and Humans and a Promising Aid to Combat Prediabetes and the Metabolic Syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Moreschi, I.; Usai, C.; Guida, L.; Damonte, G.; Salis, A.; Scarfì, S.; Millo, E.; De Flora, A.; Zocchi, E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc. Natl. Acad. Sci. USA 2007, 104, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2021, 35, 199–214. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Magnone, M.; Ameri, P.; Salis, A.; Andraghetti, G.; Emionite, L.; Murialdo, G.; De Flora, A.; Zocchi, E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015, 29, 4783–4793. [Google Scholar] [CrossRef]
- Ohkuma, K.; Lyon, J.L.; Addicott, F.T.; Smith, O.E. Abscisin II, an abscission-accelerating substance from young cotton fruit. Science 1963, 142, 1592–1593. [Google Scholar] [CrossRef]
- Addicott, F.T.; Lyon, J.L.; Ohkuma, K.; Thiessen, W.E.; Carns, H.R.; Smith, O.E.; Cornforth, J.W.; Milborrow, B.V.; Ryback, G.; Wareing, P.F. Abscisic Acid: A New Name for Abscisin II (Dormin). Science 1968, 159, 1493. [Google Scholar] [CrossRef]
- Davis, L.A.; Addicott, F.T. Abscisic Acid: Correlations with abscission and with development in the cotton fruit. Plant Physiol. 1972, 49, 644–648. [Google Scholar] [CrossRef]
- Le Page-Degivry, M.T.; Bidard, J.N.; Rouvier, E.; Bulard, C.; Lazdunski, M. Presence of abscisic acid, a phytohormone, in the mammalian brain. Proc. Natl. Acad. Sci. USA 1986, 83, 1155–1158. [Google Scholar] [CrossRef]
- Zocchi, E.; Carpaneto, A.; CERRαno, C.; Bavestrello, G.; Giovine, M.; Bruzzone, S.; Guida, L.; Franco, L.; Usai, C. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 2001, 98, 14859–14864. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Basile, G.; Cerrano, C.; Bavestrello, G.; Giovine, M.; Bruzzone, S.; Guida, L.; Carpaneto, A.; Magrassi, R.; Usai, C. ABA and cADPR-mediated effects on respiration and filtration downstream of the temperature-signaling cascade in sponges. J. Cell Sci. 2003, 116, 629–636. [Google Scholar] [CrossRef][Green Version]
- Puce, S.; Basile, G.; Bavestrello, G.; Bruzzone, S.; CERRαno, C.; Giovine, M.; Arillo, A.; Zocchi, E. Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J. Biol. Chem. 2004, 279, 39783–39788. [Google Scholar] [CrossRef]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfì, S.; et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. FASEB J. 2012, 26, 1251–1260. [Google Scholar] [CrossRef]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfì, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef]
- Sturla, L.; Fresia, C.; Guida, L.; Grozio, A.; Vigliarolo, T.; Mannino, E.; Millo, E.; Bagnasco, L.; Bruzzone, S.; De Flora, A.; et al. Binding of abscisic acid to human LANCL2. Biochem. Biophys. Res. Commun. 2011, 415, 390–395. [Google Scholar] [CrossRef]
- Lu, P.; Bevan, D.R.; Lewis, S.N.; Hontecillas, R.; Bassaganya-Riera, J. Molecular modeling of lanthionine synthetase component C-like protein 2: A potential target for the discovery of novel type 2 diabetes prophylactics and therapeutics. J. Mol. Model. 2011, 17, 543–553. [Google Scholar] [CrossRef]
- Lu, P.; Hontecillas, R.; Philipson, C.W.; Bassaganya-Riera, J. Lanthionine synthetase component C-like protein 2: A new drug target for inflammatory diseases and diabetes. Curr. Drug Targets 2014, 15, 565–572. [Google Scholar] [CrossRef]
- Spinelli, S.; Begani, G.; Guida, L.; Magnone, M.; Galante, D.; D’Arrigo, C.; Scotti, C.; Iamele, L.; de Jonge, H.; Zocchi, E.; et al. LANCL1 binds abscisic acid and stimulates glucose transport and mitochondrial respiration in muscle cells via the AMPK/PGC-1α/Sirt1 pathway. Mol. Metab. 2021, 53, 101263. [Google Scholar] [CrossRef]
- Guri, A.J.; Hontecillas, R.; Ferrer, G.; Casagran, O.; Wankhade, U.; Noble, A.M.; Eizirik, D.L.; Ortis, F.; Cnop, M.; Liu, D.; et al. Loss of PPAR gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J. Nutr. Biochem. 2008, 19, 216–228. [Google Scholar] [CrossRef]
- Guri, A.J.; Misyak, S.A.; Hontecillas, R.; Hasty, A.; Liu, D.; Si, H.; Bassaganya-Riera, J. Abscisic acid ameliorates atherosclerosis by suppressing macrophage and CD4+ T cell recruitment into the aortic wall. J. Nutr. Biochem. 2010, 21, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Hao, R.L.; Wu, S.S.; Guo, P.C.; Chen, C.J.; Pan, L.P.; Ni, H. Occurrence, function and potential medicinal applications of the phytohormone abscisic acid in animals and humans. Biochem. Pharmacol. 2011, 82, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Guri, A.J.; Lu, P.; Climent, M.; Carbo, A.; Sobral, B.W.; Horne, W.T.; Lewis, S.N.; Bevan, D.R.; Hontecillas, R. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2011, 286, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.J.; Hontecillas, R.; Bassaganya-Riera, J. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin. Nutr. 2010, 29, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas, R.; Roberts, P.C.; Carbo, A.; Vives, C.; Horne, W.T.; Genis, S.; Velayudhan, B.; Bassaganya-Riera, J. Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARgamma-dependent mechanism. J. Nutr. Biochem. 2013, 24, 1019–1027. [Google Scholar] [CrossRef]
- Sanchez-Sarasúa, S.; Moustafa, S.; García-Aviles, A.; Lopez-Climent, M.F.; Gomez-Cadenas, A.; Olucha-Bordonau, F.E.; Sanchez-Perez, A.M. The effect of abscisic acid chronic treatment on neuroinflammatory markers and memory in a rat model of high-fat diet induced neuroinflammation. Nutr. Metab. 2016, 13, 73. [Google Scholar] [CrossRef]
- Ribes-Navarro, A.; Atef, M.; Sanchez-Sarasúa, S.; Beltran-Bretones, M.T.; Olucha-Bordonau, F.; Sanchez-Perez, A.M. Abscisic Acid Supplementation Rescues High Fat Diet-Induced Alterations in Hippocampal Inflammation and IRSs Expression. Mol.Neurobiol. 2018, 56, 454–464. [Google Scholar] [CrossRef]
- Bodrato, N.; Franco, L.; Fresia, C.; Guida, L.; Usai, C.; Salis, A.; Moreschi, I.; FERRαris, C.; Verderio, C.; Basile, G.; et al. Abscisic acid activates the murine microglial cell line N9 through the second messenger cyclic ADP-ribose. J. Biol. Chem. 2009, 284, 14777–14787. [Google Scholar] [CrossRef]
- Magnone, M.; Bruzzone, S.; Guida, L.; Damonte, G.; Millo, E.; Scarfì, S.; Usai, C.; Palombo, D.; Sturla, L.; De Flora, A.; et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cells responses involved in atherogenesis. J. Biol. Chem. 2009, 284, 17808–17818. [Google Scholar] [CrossRef]
- Magnone, M.; Sturla, L.; Jacchetti, E.; Scarfì, S.; Bruzzone, S.; Usai, C.; Guida, L.; Salis, A.; Damonte, G.; De Flora, A.; et al. Autocrine abscisic acid plays a key role in quartz-induced macrophage activation. FASEB J. 2012, 26, 1261–1271. [Google Scholar] [CrossRef]
- Spinelli, S.; Guida, L.; Vigliarolo, T.; Passalacqua, M.; Begani, G.; Magnone, M.; Sturla, L.; Benzi, A.; Ameri, P.; Lazzarini, E.; et al. The ABA-LANCL1/2 Hormone-Receptors System Protects H9c2 Cardiomyocytes from Hypoxia Induced Mitochondrial Injury via an AMPK- and NO-Mediated Mechanism. Cells 2022, 11, 2888. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Guida, L.; Passalacqua, M.; Magnone, M.; Cossu, V.; Sambuceti, G.; Marini, C.; Sturla, L.; Zocchi, E. Abscisic Acid and Its Receptors LANCL1 and LANCL2 Control Cardiomyocyte Mitochondrial Function, Expression of Contractile, Cytoskeletal and Ion Channel Proteins and Cell Proliferation via ERRα. Antioxidants 2023, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Elmasry, A.; El-Nablaway, M.; Othman, G.; Hamed, S.; Khater, Y.; Ashour, R.H.; Hendawy, M.; Rabei, M.R. Cardioprotective effect of abscisic acid in a rat model of type 3 cardio-renal syndrome: Role of NOX-4, P-53, and HSP-70. Biomed. Pharmacother. 2023, 157, 114038. [Google Scholar] [CrossRef]
- Spinelli, S.; Guida, L.; Passalacqua, M.; Magnone, M.; Caushi, B.; Zocchi, E.; Sturla, L. The ABA/LANCL1-2 Hormone/Receptors System Controls ROS Production in Cardiomyocytes through ERRα. Biomedicines 2024, 12, 2071. [Google Scholar] [CrossRef]
- Rafiepour, K.; Esmaeili-Mahani, S.; Salehzadeh, A.; Sheibani, V. Phytohormone Abscisic Acid Protects Human Neuroblastoma SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity Through Its Antioxidant and Antiapoptotic Properties. Rejuvenation Res. 2019, 22, 99–108. [Google Scholar] [CrossRef]
- Khorasani, A.; Abbasnejad, M.; Esmaeili-Mahani, S. Phytohormone abscisic acid ameliorates cognitive impairments in streptozotocin-induced rat model of Alzheimer’s disease through PPARbeta/delta and PKA signaling. Int. J. Neurosci. 2019, 129, 1053–1065. [Google Scholar] [CrossRef]
- Espinosa-Fernández, V.; Mañas-Ojeda, A.; Pacheco-Herrero, M.; Castro-Salazar, E.; Ros-Bernal, F.; Sánchez-Pérez, A.M. Early intervention with ABA prevents neuroinflammation and memory impairment in a triple transgenic mice model of Alzheimer’s disease. Behav. Brain Res. 2019, 374, 112106. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, N.; Ju, Y.J.; Gee, M.S.; Lee, D.; Lee, J.K. Phytohormone Abscisic Acid Improves Memory Impairment and Reduces Neuroinflammation in 5xFAD Mice by Upregulation of LanC-Like Protein 2. Int. J. Mol. Sci. 2020, 21, 8425. [Google Scholar] [CrossRef]
- Maixner, D.W.; Christy, D.; Kong, L.; Viatchenko-Karpinski, V.; Horner, K.A.; Hooks, S.B.; Weng, H.R. Phytohormone abscisic acid ameliorates neuropathic pain via regulating LANCL2 protein abundance and glial activation at the spinal cord. Mol. Pain 2022, 18, 17448069221107781. [Google Scholar] [CrossRef]
- Shabani, M.; Soti, M.; Ranjbar, H.; Naderi, R. Abscisic acid ameliorates motor disabilities in 6-OHDA-induced mice model of Parkinson’s disease. Heliyon 2023, 9, e18473. [Google Scholar] [CrossRef]
- Spinelli, S.; Magnone, M.; Guida, L.; Sturla, L.; Zocchi, E. The ABA/LANCL Hormone/Receptor System in the Control of Glycemia, of Cardiomyocyte Energy Metabolism, and in Neuroprotection: A New Ally in the Treatment of Diabetes Mellitus? Int. J. Mol. Sci. 2023, 24, 1199. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Beltrán, M.; Sánchez-Sarasúa, S.; Landry, M.; Kerekes, N.; Sánchez-Pérez, A.M. Targeting Neuroinflammation with Abscisic Acid Reduces Pain Sensitivity in Females and Hyperactivity in Males of an ADHD Mice Model. Cells 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Xu, Y.; Liu, H.; Sun, L.; Cheng, X.; Shen, Y.; Wei, J. Function and Mechanism of Abscisic Acid on Microglia-Induced Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 4920. [Google Scholar] [CrossRef]
- Birnbaum, E.M.; Xie, L.; Serrano, P.; Rockwell, P.; Figueiredo-Pereira, M.E. BT-11 repurposing potential for Alzheimer’s disease and insights into its mode of actions. bioRxiv 2024. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Villar, A.; Mulà, A.; Zangara, A.; Risco, E.; Smidt, C.R.; Hontecillas, R.; Leber, A.; Bassaganya-Riera, J. Abscisic Acid Standardized Fig (Ficus carica) Extracts Ameliorate Postprandial Glycemic and Insulinemic Responses in Healthy Adults. Nutrients 2019, 11, 1757. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Preti, P.S.; Tenore, G.; Novellino, E. Abscisic Acid Treatment in Patients with Prediabetes. Nutrients 2020, 12, 2931. [Google Scholar] [CrossRef]
- Spinelli, S.; Cossu, V.; Passalacqua, M.; Hansen, J.B.; Guida, L.; Magnone, M.; Sambuceti, G.; Marini, C.; Sturla, L.; Zocchi, E. The ABA/LANCL1/2 Hormone/Receptor System Controls Adipocyte Browning and Energy Expenditure. Int. J. Mol. Sci. 2023, 24, 3489. [Google Scholar] [CrossRef]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR Receptors Play a Vital Role in the Abscisic-Acid-Dependent Responses of Plants to External or Internal Stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Cao, M.J.; Yan, J.; Dong, J.; Chen, M.X.; Yang, J.F.; Li, J.H.; Ying, R.N.; Gao, Y.Y.; Li, L.; et al. Stabilization of dimeric PYR/PYL/RCAR family members relieves abscisic acid-induced inhibition of seed germination. Nat. Commun. 2024, 15, 8077. [Google Scholar] [CrossRef]
- Klingler, J.P.; Batelli, G.; Zhu, J.K. ABA receptors: The START of a new paradigm in phytohormone signalling. J. Exp. Bot. 2010, 61, 3199–3210. [Google Scholar] [CrossRef]
- Chen, J.G.; Ellis, B.E. GCR2 is a new member of the eukaryotic lanthionine synthetase component C-like protein family. Plant Signal Behav. 2008, 3, 307–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnston, C.A.; Temple, B.R.; Chen, J.G.; Gao, Y.; Moriyama, E.N.; Jones, A.M.; Siderovski, D.P.; Willard, F.S. Comment on “A G protein coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid”. Science 2007, 318, 914. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Mayer, H.; Marchler-Bauer, A.; Salzer, U.; Prohaska, R. Characterization of p40/GPR69A as a peripheral membrane protein related to the lantibiotic synthetase component C. Biochem. Biophys. Res. Commun. 2000, 275, 69–74. [Google Scholar] [CrossRef]
- He, C.; Zeng, M.; Dutta, D.; Koh, T.H.; Chen, J.; van der Donk, W.A. LanCL proteins are not Involved in Lanthionine Synthesis in Mammals. Sci. Rep. 2017, 20, 40980. [Google Scholar] [CrossRef]
- Mayer, H.; Bauer, H.; Prohaska, R. Organization and chromosomal localization of the human and mouse genes coding for LanC-like protein 1 (LANCL1). Cytogenet. Cell Genet. 2001, 93, 100–104. [Google Scholar] [CrossRef]
- Eley, G.D.; Reiter, J.L.; Pandita, A.; Park, S.; Jenkins, R.B.; Maihle, N.J.; James, C.D. A chromosomal region 7p11.2 transcript map: Its development and application to the study of EGFR amplicons inglioblastoma. Neuro Oncol. 2002, 4, 86–94. [Google Scholar] [CrossRef][Green Version]
- Gassner, C.; Brönnimann, C.; Merki, Y.; Mattle-Greminger, M.P.; Sigurdardottir, S.; Meyer, E.; Engström, C.; O’Sullivan, J.D.; Jung, H.H.; Frey, B.M. Stepwise partitioning of Xp21: A profiling method for XK deletions causative of the McLeod syndrome. Transfusion. 2017, 57, 2125–2135. [Google Scholar] [CrossRef][Green Version]
- Mayer, H.; Salzer, U.; Breuss, J.; Ziegler, S.; Marchler-Bauer, A.; Prohaska, R. Isolation, molecular characterization, and tissue-specific expression of a novel putative G protein-coupled receptor. Biochim. Biophys. Acta 1998, 1395, 301–308. [Google Scholar] [CrossRef]
- Park, S.; James, C.D. Lanthioninesynthetase component C-like 2increases cellular sensitivity toadriamycin by decreasing theexpression of P-glycoprotein through a transcription-mediatedmechanism. Cancer Res. 2003, 63, 723–727. [Google Scholar]
- Landlinger, C.; Salzer, U.; Prohaska, R. Myristoylation of human LanC-like protein 2 (LANCL2) is essential for the interaction with the plasma membrane and the increase in cellular sensitivity to adriamycin. Biochim. Biophys. Acta 2006, 1758, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Cichero, E.; Fresia, C.; Guida, L.; Booz, V.; Millo, E.; Scotti, C.; Iamele, L.; de Jonge, H.; Galante, D.; De Flora, A.; et al. Identification of a high affinity binding site for abscisic acid on human lanthionine synthetase component C-like protein 2. Int. J. Biochem. Cell Biol. 2018, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Scarano, N.; Di Palma, F.; Origlia, N.; Musumeci, F.; Schenone, S.; Spinelli, S.; Passalacqua, M.; Zocchi, E.; Sturla, L.; Cichero, E.; et al. New Insights into the LANCL2-ABA Binding Mode towards the Evaluation of New LANCL Agonists. Pharmaceutics 2023, 15, 2754. [Google Scholar] [CrossRef] [PubMed]
- Vigliarolo, T.; Zocchi, E.; Fresia, C.; Booz, V.; Guida, L. Abscisic acid influx into human nucleated cells occurs through the anion exchanger AE2. Int. J. Biochem. Cell Biol. 2016, 75, 99–103. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Bruzzone, S.; Magnone, M.; Mannino, E.; Sociali, G.; Sturla, L.; Fresia, C.; Booz, V.; Emionite, L.; De Flora, A.; Zocchi, E. Abscisic Acid Stimulates Glucagon-Like Peptide-1 Secretion from L-Cells and Its Oral Administration Increases Plasma Glucagon-Like Peptide-1 Levels in Rats. PLoS ONE 2015, 10, e0140588. [Google Scholar] [CrossRef]
- Booz, V.; Bayer Christiansen, C.; Ehrenreich Kuhre, R.; Yosifova Saltiel, M.; Sociali, G.; Schaltenberg, N.; Fischer, A.W.; Heeren, J.; Zocchi, E.; Holst, J.J.; et al. Abscisic acid stimulates the release of insulin and of GLP-1 in the rat perfused pancreas and intestine. Diabetes/Metab. Res. Rev. 2018, 35, e3102. [Google Scholar] [CrossRef]
- Ameri, P.; Bruzzone, S.; Mannino, E.; Sociali, G.; Andraghetti, G.; Salis, A.; Ponta, M.L.; Briatore, L.; Adami, G.F.; Ferraiolo, A.; et al. Impaired increase of plasma abscisic acid in response to oral glucose load in type 2 diabetes and in gestational diabetes. PLoS ONE 2015, 10, e0115992. [Google Scholar] [CrossRef]
- Magnone, M.; Spinelli, S.; Begani, G.; Guida, L.; Sturla, L.; Emionite, L.; Zocchi, E. Abscisic acid improves insulin action on glycemia in insulin-deficient mouse models of Type 1 Diabetes. Metabolites 2022, 12, 523. [Google Scholar] [CrossRef]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 29, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Tominaga, M. TRPM2 modulates insulin secretion in pancreatic β-cells. Islets 2011, 3, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Mannino, E.; Scarfì, S.; Bruzzone, S.; Magnone, M.; Sociali, G.; Booz, V.; Guida, L.; Vigliarolo, T.; Fresia, C.; et al. Abscisic acid enhances glucose disposal and induces brown fat activity in adipocytes in vitro and in vivo. Biochim. Biophys. Acta 2017, 1862, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Leoncini, G.; Vigliarolo, T.; Emionite, L.; Sturla, L.; Zocchi, E.; Murialdo, G. Chronic Intake of Micrograms of Abscisic Acid Improves Glycemia and Lipidemia in a Human Study and in High-Glucose Fed Mice. Nutrients 2018, 10, 1454–1458. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Vainshtein, A.; Sandri, M. Signaling Pathways That Control Muscle Mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle—A focus on the molecular mechanisms of insulin resistance. Mol. Cell Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Li, L.; Li, L. Skeletal muscle non-shivering thermogenesis as an attractive strategy to combat obesity. Life Sci. 2021, 269, 119024. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, M.; Khathi, A. Prediabetes-Associated Changes in Skeletal Muscle Function and Their Possible Links with Diabetes: A Literature Review. Int. J. Mol. Sci. 2023, 29, 469. [Google Scholar] [CrossRef]
- Xi, X.; Han, J.; Zhang, J.Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J. Biol. Chem. 2001, 276, 4129–41034. [Google Scholar] [CrossRef]
- Jager, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Leick, L.; Fentz, J.; Biensø, R.S.; Knudsen, J.G.; Jeppesen, J.; Kiens, B.; Wojtaszewski, J.F.; Pilegaard, H. PGC1-1α is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E456–E465. [Google Scholar] [CrossRef]
- Wan, Z.; Root-McCaig, J.; Castellani, L.; Kemp, B.E.; Steinberg, G.R.; Wright, D.C. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity 2014, 22, 730–738. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Peifer-Weiß, L.; Al-Hasani, H.; Chadt, A. AMPK and Beyond: The Signaling Network Controlling RabGAPs and Contraction-Mediated Glucose Uptake in Skeletal Muscle. Int. J. Mol. Sci. 2024, 25, 1910. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Leber, A.; Hontecillas, R.; Tubau-Juni, N.; Zoccoli-Rodriguez, V.; Goodpaster, B.; Bassaganya-Riera, J. Abscisic acid enriched fig extract promotes insulin sensitivity by decreasing systemic inflammation and activating LANCL2 in skeletal muscle. Sci. Rep. 2020, 10, 10463. [Google Scholar] [CrossRef]
- Maurya, S.K.; Periasamy, M. Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacol. Res. 2015, 102, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Pant, M.; Bal, N.C.; Periasamy, M. Sarcolipin: A Key Thermogenic and Metabolic Regulator in Skeletal Muscle. Trends Endocrinol. Metab. 2016, 27, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523.e5. [Google Scholar] [CrossRef] [PubMed]
- Della Guardia, L.; Luzi, L.; Codella, R. Muscle-UCP3 in the regulation of energy metabolism. Mitochondrion 2024, 76, 101872. [Google Scholar] [CrossRef]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Hooks, B.G.; McPherson, R.; Dent, R.R.M.; Harper, M.E. Targeting skeletal muscle mitochondrial health in obesity. Clin. Sci. 2022, 136, 1081–1110. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; She, Y.; Yu, M.; Min, W.; Shang, W.; Zhang, Z. Adipose-Muscle crosstalk in age-related metabolic disorders: The emerging roles of adipo-myokines. Ageing Res. Rev. 2023, 84, 101829. [Google Scholar] [CrossRef]
- Yang, K.C.; Bonini, M.G.; Dudley, S.C., Jr. Mitochondria and arrhythmias. Free Radic. Biol. Med. 2014, 71, 351–361. [Google Scholar] [CrossRef]
- Miragoli, M.; Cabassi, A. Mitochondrial Mechanosensor Microdomains in Cardiovascular Disorders. Adv. Exp. Med. Biol. 2017, 982, 247–264. [Google Scholar] [CrossRef]
- Morciano, G.; Boncompagni, C.; Ramaccini, D.; Pedriali, G.; Bouhamida, E.; Tremoli, E.; Giorgi, C.; Pinton, P. Comprehensive Analysis of Mitochondrial Dynamics Alterations in Heart Diseases. Int. J. Mol. Sci. 2023, 24, 3414. [Google Scholar] [CrossRef]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Vernier, M.; Dufour, C.R.; McGuirk, S.; Scholtes, C.; Li, X.; Bourmeau, G.; Kuasne, H.; Park, M.; St-Pierre, J.; Audet-Walsh, E.; et al. Estrogen-related Receptors are Targetable ROS sensors. Genes Dev. 2020, 34, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, C.; Giguère, V. Transcriptional Regulation of ROS Homeostasis by the ERR Subfamily of Nuclear Receptors. Antioxidants 2021, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, M.; Pang, D.; Bi, D.; Zou, Y.; Xia, X.; Yang, W.; Luo, L.; Deng, R.; Tan, H.; et al. Developmental and activity-dependent expression of LanCL1 confers antioxidant activity required for neuronal survival. Dev. Cell. 2014, 30, 479–487. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, Q.; Chen, X.; Tong, S.; Sun, J.; Lv, R.; Wang, S.; Gou, Y.; Tan, L.; Xu, J.; et al. sLanCL1 protects prostate cancer cells from oxidative stress via suppression of JNK pathway. Cell Death Dis. 2018, 9, 197. [Google Scholar] [CrossRef]
- Downey, A.; Olcott, M.; Spector, D.; Bird, K.; Ter Doest, A.; Pierce, Z.; Quach, E.; Sparks, S.; Super, C.; Naifeh, J.; et al. Stable knockout of lanthionine synthase C-like protein-1 (LanCL1) from HeLa cells indicates a role for LanCL1 in redox regulation of deubiquitinating enzymes. Free Radic. Biol. Med. 2020, 161, 115–124. [Google Scholar] [CrossRef]
- Shi, S.; Wang, J.; Gong, H.; Huang, X.; Mu, B.; Cheng, X.; Feng, B.; Jia, L.; Luo, Q.; Liu, W.; et al. PGC-1alpha-Coordinated Hypothalamic Antioxidant Defense Is Linked to SP1-LanCL1 Axis during High-Fat-Diet-Induced Obesity in Male Mice. Antioxidants 2024, 13, 252. [Google Scholar] [CrossRef]
- Huang, H.; Tsui, Y.M.; Ho, D.W.; Chung, C.Y.; Sze, K.M.; Lee, E.; Cheung, G.C.; Zhang, V.X.; Wang, X.; Lyu, X.; et al. LANCL1, a cell surface protein, promotes liver tumor initiation through FAM49B-Rac1 axis to suppress oxidative stress. Hepatology 2024, 79, 323–340. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Shi, S.; Lan, X.; Cheng, X.; Li, L.; Zou, Y.; Jia, L.; Liu, W.; Luo, Q.; et al. LanCL2 Implicates in Testicular Redox Homeostasis and Acrosomal Maturation. Antioxidants 2024, 13, 534. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Della Guardia, L.; Shin, A.C. Obesity-induced tissue alterations resist weight loss: A mechanistic review. Diabetes Obes. Metab. 2024, 26, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.J.; Hontecillas, R.; Si, H.; Liu, D.; Bassaganya-Riera, J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin. Nutr. 2007, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.J.; Hontecillas, R.; Bassaganya-Riera, J. Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: Possible action of the cAMP/PKA/PPAR γ axis. Clin. Nutr. 2010, 29, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ijichi, N.; Ikeda, K.; Horie-Inoue, K.; Yagi, K.; Okazaki, Y.; Inoue, S. Estrogen-related receptor alpha modulates the expression of adipogenesis-related genes during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2007, 358, 813–818. [Google Scholar] [CrossRef]
- Kubo, M.; Ijichi, N.; Ikeda, K.; Horie-Inoue, K.; Takeda, S.; Inoue, S. Modulation of adipogenesis-related gene expression by estrogen-related receptor gamma during adipocytic differentiation. Biochim. Biophys. Acta 2009, 1789, 71–77. [Google Scholar] [CrossRef]
- Gantner, M.L.; Hazen, B.C.; Eury, E.; Brown, E.L.; Kralli, A. Complementary Roles of Estrogen-Related Receptors in BrownAdipocyte Thermogenic Function. Endocrinology 2016, 157, 4770–4781. [Google Scholar] [CrossRef]
- Brown, E.L.; Hazen, B.C.; Eury, E.; Wattez, J.S.; Gantner, M.L.; Albert, V.; Chau, S.; Sanchez-Alavez, M.; Conti, B.; Kralli, A. Estrogen-Related Receptors Mediate the Adaptive Response of Brown Adipose Tissue to Adrenergic Stimulation. iScience 2018, 2, 221–237. [Google Scholar] [CrossRef]
- Morganstein, D.L.; Wu, P.; Mane, M.R.; Fisk, N.M.; White, R.; Parker, M.G. Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: A role for ERRalpha in human UCP1 expression. Cell Res. 2010, 20, 434–444. [Google Scholar] [CrossRef]
- Yan, M.; Audet-Walsh, E.; Manteghi, S.; Dufour, C.R.; Walker, B.; Baba, M.; St-Pierre, J.; Giguère, V.; Pause, A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016, 30, 1034–1046. [Google Scholar] [CrossRef]
- Emmett, M.J.; Lim, H.W.; Jager, J.; Richter, H.J.; Adlanmerini, M.; Peed, L.C.; Briggs, E.R.; Steger, D.J.; Ma, T.; Sims, C.A.; et al. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature 2017, 546, 544–548. [Google Scholar] [CrossRef]
- Inoue, S.I.; Emmett, M.J.; Lim, H.W.; Midha, M.; Richter, H.J.; Celwyn, I.J.; Mehmood, R.; Chondronikola, M.; Klein, S.; Hauck, A.K.; et al. Short-term cold exposure induces persistent epigenomic memory in brown fat. Cell Metab. 2024, 36, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.; Yen, P.M. Thermogenesis in Adipose Tissue Activated by Thyroid Hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, J.Y.; Ryu, R.; Lee, J.; Cho, S.J.; Kwon, E.Y.; Lee, M.K.; Liu, K.H.; Rina, Y.; Sung, M.K.; et al. Platycodon grandi florus root extract attenuates body fat mass, hepatic steatosis and insulin resistance through the Interplay between the Liver and Adipose Tissue. Nutrients 2016, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocytes via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494. [Google Scholar] [CrossRef]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Takeda, Y.; Harada, Y.; Yoshikawa, T.; Dai, P. Mitochondrial Energy Metabolism in the Regulation of Thermogenic Brown Fats and Human Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 1352. [Google Scholar] [CrossRef]
- Feng, S.; Reuss, L.; Wang, Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef]
- Seo, J.B.; Choe, S.S.; Jeong, H.W.; Park, S.W.; Shin, H.J.; Choi, S.M.; Park, J.Y.; Choi, E.W.; Kim, J.B.; Seen, D.S.; et al. Anti-obesity effects of Lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp. Mol. Med. 2011, 43, 205–215. [Google Scholar] [CrossRef]
- Armoni, M.; Kritz, N.; Harel, C.; Bar-Yoseph, F.; Chen, H.; Quon, M.J.; Karnieli, E. Peroxisome proliferator-activated receptor-γ represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J. Biol. Chem. 2003, 278, 30614–30623. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Alemany, M. The Metabolic Syndrome, a Human Disease. Int. J. Mol. Sci. 2024, 25, 2251. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- Peng, W.; Mu, Y.; Hu, Y.; Li, B.; Raman, J.; Sui, Z. Double Burden of Malnutrition in the Asia-Pacific Region—A Systematic Review and Meta-analysis. J. Epidemiol. Glob. Health 2020, 10, 16–27. [Google Scholar] [CrossRef]
- Ma, P.; He, P.; Xu, C.Y.; Hou, B.Y.; Qiang, G.F.; Du, G.H. Recent developments in natural products for white adipose tissue browning. Chin. J. Nat. Med. 2020, 18, 803–817. [Google Scholar] [CrossRef]
- Choi, Y.; Yu, L. Natural Bioactive Compounds as Potential Browning Agents in White Adipose Tissue. Pharm. Res. 2021, 38, 549–567. [Google Scholar] [CrossRef]
- Zocchi, G.; Fontanelli, F.; Spinelli, S.; Sturla, L.; Passalacqua, M.; Urra, J.C.G.; Delsante, S.; Zocchi, E. Thermal measurements support a role of the ABA/LANCL1-2 hormone/receptors system in thermogenesis. Open Biol. 2024, 14, 240107. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic acid as pathogen effector and immune regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef]
- Karadeniz, A.; Topcuoglu, S.F.; Inan, S. Auxin, gibberellin, cytokinin and abscisic acid production in some bacteria. World J. Microbiol. Biotechnol. 2006, 22, 1061–1064. [Google Scholar] [CrossRef]
- Bruzzone, S.; Bodrato, N.; Usai, C.; Guida, L.; Moreschi, I.; Nano, R.; Antonioli, B.; Fruscione, F.; Magnone, M.; Scarfì, S.; et al. Abscisic Acid Is an Endogenous Stimulator of Insulin Release from Human Pancreatic Islets with Cyclic ADP Ribose as Second Messenger. J. Biol. Chem. 2008, 283, 32188–32197. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chen, M.; Pang, D.; Xia, X.; Du, C.; Yang, W.; Cui, Y.; Huang, C.; Jiang, W.; Bi, D.; et al. LanCL1 promotes motor neuron survival and extends the lifespan of amyotrophic lateral sclerosis mice. Cell Death Differ. 2020, 27, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.; Rainbow, D.; Plagnol, V.; Todd, J.A.; Peterson, L.B.; Wicker, L.S. Interactions between Idd5.1/Ctla4 and other type 1 diabetes genes. J. Immunol. 2007, 179, 8341–8349. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; D’Avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A Pilot screening of agro-food waste products as source nutraceutical formulations to improve simulated postprandial glycaemia and insulinaemia in healthy subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Spinelli, S.; Bruschi, M.; Passalacqua, M.; Guida, L.; Magnone, M.; Sturla, L.; Zocchi, E. Estrogen-Related Receptor alpha: A key transcription factor in the regulation of energy metabolism at an organismic level and a target of the ABA/LANCL hormone receptor system. Int. J. Mol. Sci. 2024, 25, 4796. [Google Scholar] [CrossRef]
- Dotson, C.D.; Zhang, L.; Xu, H.; Shin, Y.K.; Vigues, S.; Ott, S.H.; Elson, A.E.T.; Choi, H.J.; Shaw, H.; Egan, J.M.; et al. Bitter taste receptors influence glucose homeostasis. PLoS ONE 2008, 3, e3974. [Google Scholar] [CrossRef]
- Palau-Rodriguez, M.; Tulipani, S.; Isabel Queipo-Ortuño, M.; Urpi-Sarda, M.; Tinahones, F.J.; Andres-Lacueva, C. Metabolomic insights into the intricate gut microbial-host interaction in the development of obesity and type 2 diabetes. Front. Microbiol. 2015, 6, 1151. [Google Scholar] [CrossRef]
- Pydi, S.P.; Jaggupilli, A.; Nelson, K.M.; Suzanne, R.A.; Bhullar, R.P.; Loewen, M.C.; Chelikani, P. Abscisic acid acts as a blocker of the bitter taste G Protein-Coupled Receptor T2R4. Biochemistry 2015, 54, 2622–2631. [Google Scholar] [CrossRef]
- Rahim, N.E.; Flood, D.; Marcus, M.E.; Theilmann, M.; Aung, T.N.; Agoudavi, K.; Aryal, K.K.; Bahendeka, S.; Bicaba, B.; Bovet, P.; et al. Diabetes risk and provision of diabetes prevention activities in 44 low-income and middle-income countries: A cross-sectional analysis of nationally representative, individual-level survey data. Lancet Glob. Health 2023, 11, e1576–e1586. [Google Scholar] [CrossRef]
- Han, C.; Song, Q.; Ren, Y.; Chen, X.; Jiang, X.; Hu, D.J. Global prevalence of prediabetes in children and adolescents: A systematic review and meta-analysis. Diabetes 2022, 14, 434–441. [Google Scholar] [CrossRef]


| Fruits | mg/Kg | Vegetables | mg/Kg |
|---|---|---|---|
| average content | 0.62 | average content | 0.29 |
| Avocado | 2 | Soybean | 0.79 |
| Citrus | 1.25 | Barley | 0.20 |
| Fig | 0.72 | Tomato | 0.20 |
| Bilberry | 0.4 | Wheat | 0.15 |
| Apricot | 0.32 | Pea | 0.13 |
| Banana | 0.22 | Cucumber | 0.09 |
| In Vitro Studies on Cell Lines | In Vivo and Ex Vivo Studies in Rodents | Clinical Studies in Healthy and in Prediabetic Subjects |
|---|---|---|
| ABA stimulates glucose consumption by adipocytes and myoblasts [24] | ABA improves glucose tolerance and reduces insulinemia in rats [16]; ABA increases blood glucose clearance and skeletal muscle uptake in rats [81] | An apricot extract providing a dose of ABA of 0.5 microg/Kg BW, taken before a carbohydrate-rich meal, reduces glycemia in healthy subjects [16] |
| ABA stimulates glucose uptake in the absence of insulin via an AMPK-dependent mechanism in rat muscle cells [81] and in murine muscle cells [29] | ABA enhances glucose tolerance and elevates muscle glycogen levels in TRPM2-KO mice with low insulin levels [81] | A 75-day administration of a vegetable extract providing a daily dose of ABA of 1.0 microg/Kg BW, reduces glycemia and lipidemia in borderline subjects [84] |
| ABA stimulates glucose uptake and metabolism-inducing brown features in rodent adipocytes [83] | An extract enriched with ABA enhances glucose tolerance and insulin sensitivity and reduces fasting blood glucose levels in mouse models of diet-induced obesity (DIO) and db/db mice [100] | Fig extracts enriched with ABA improve post-meal blood glucose and insulin levels in healthy adults [55] |
| ABA controls human adipocyte browning and energy expenditure via LANCL1/2 [57] | Chronically ABA-treated mice show increased skeletal muscle glycogen content, higher physical performance, and improved glucose tolerance [81] | A 3-month treatment with a dwarf peaches extract titrated in ABA improves glyco-metabolic and inflammatory parameters in prediabetic subjects [154] |
| Rat H9c2 cardiomyocytes overexpressing LANCL1/2 and treated with ABA display increased expression of glucose transporters GLUT4 and GLUT1, glycolytic enzymes, and pyruvate dehydrogenase, increased glucose uptake [42] | ABA improves glucose tolerance in LANCL2 KO mice by stimulating muscle GLUT4 expression via the LANCL1/AMPK/PGC1a axis [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, S.; Humma, Z.; Magnone, M.; Zocchi, E.; Sturla, L. Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism. Nutrients 2025, 17, 13. https://doi.org/10.3390/nu17010013
Spinelli S, Humma Z, Magnone M, Zocchi E, Sturla L. Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism. Nutrients. 2025; 17(1):13. https://doi.org/10.3390/nu17010013
Chicago/Turabian StyleSpinelli, Sonia, Zelle Humma, Mirko Magnone, Elena Zocchi, and Laura Sturla. 2025. "Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism" Nutrients 17, no. 1: 13. https://doi.org/10.3390/nu17010013
APA StyleSpinelli, S., Humma, Z., Magnone, M., Zocchi, E., & Sturla, L. (2025). Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism. Nutrients, 17(1), 13. https://doi.org/10.3390/nu17010013






