Creatine Supplementation Beyond Athletics: Benefits of Different Types of Creatine for Women, Vegans, and Clinical Populations—A Narrative Review
Abstract
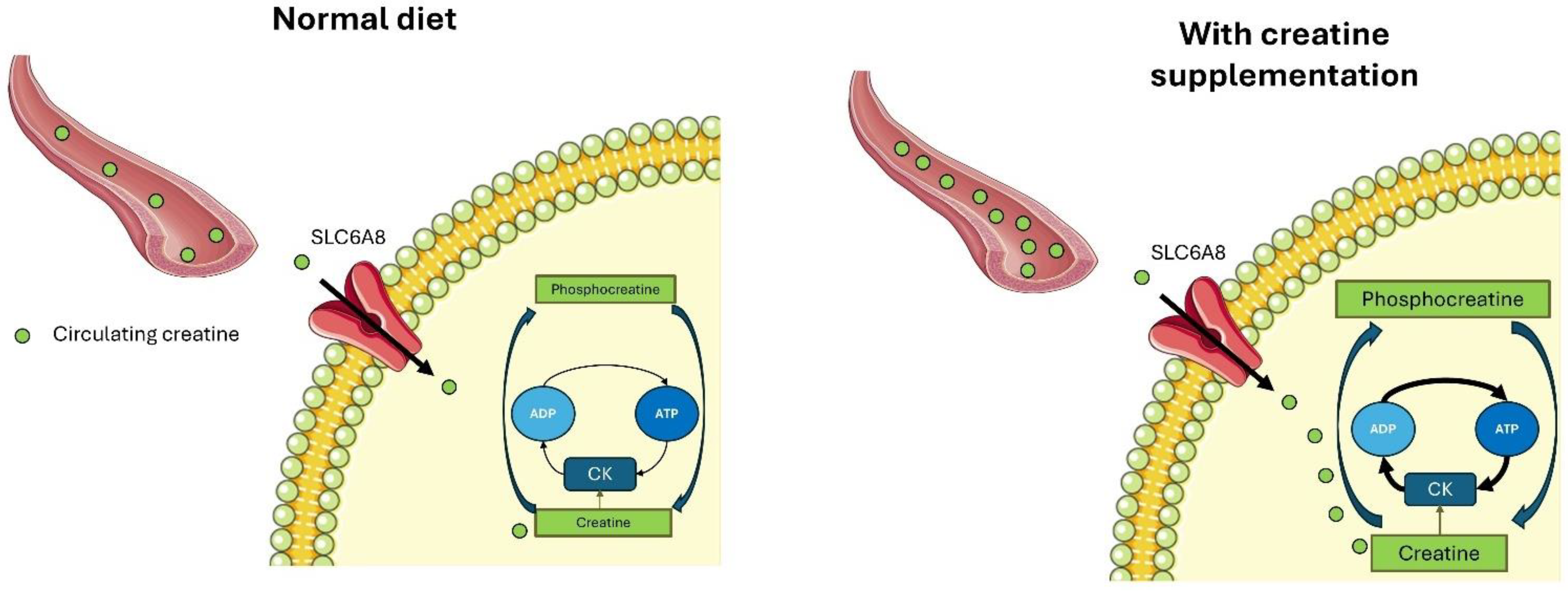
:1. Creatine
2. Types of Creatine
2.1. Creatine Monohydrate
2.2. Creatine Ethyl Ester
2.3. Creatine Gluconate
2.4. Creatine Citrate
2.5. Creatine Magnesium Chelate
3. Physiological Effect of Creatine
4. Creatine and Physical Exercise
Sports Physiology and Creatine
5. Potential Adverse Effects of Creatine Supplementation
5.1. Fluid Retention During Supplementation with Creatine Monohydrate
5.2. Renal Function and Creatine Monohydrate Supplementation
6. Creatine Supplementation Beyond Athletics
7. Creatine Supplementation in Women
8. Creatine Supplementation for Vegetarians/Vegans
9. Therapeutic Potential of Creatine Supplementation in Diseases
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barcelos, R.P.; Stefanello, S.T.; Mauriz, J.L.; Gonzalez-Gallego, J.; Soares, F.A. Creatine and the Liver: Metabolism and Possible Interactions. Mini Rev. Med. Chem. 2016, 16, 12–18. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Brault, J.J.; Abraham, K.A.; Terjung, R.L. Muscle creatine uptake and creatine transporter expression in response to creatine supplementation and depletion. J. Appl. Physiol. 2003, 94, 2173–2180. [Google Scholar] [CrossRef]
- Balsom, P.D.; Söderlund, K.; Ekblom, B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994, 18, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Fazio, C.; Elder, C.L.; Harris, M.M. Efficacy of Alternative Forms of Creatine Supplementation on Improving Performance and Body Composition in Healthy Subjects: A Systematic Review. J. Strength. Cond. Res. 2022, 36, 2663–2670. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Edison, E.E.; da Silva, R.; Brosnan, J.T. New insights into creatine function and synthesis. Adv. Enzym. Regul. 2007, 47, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, T. Separation methods applicable to urinary creatine and creatinine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 781, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Sallsten, G.; Barregard, L. Variability of Urinary Creatinine in Healthy Individuals. Int. J. Environ. Res. Public Health 2021, 18, 3166. [Google Scholar] [CrossRef]
- da Silva, R.P.; Nissim, I.; Brosnan, M.E.; Brosnan, J.T. Creatine synthesis: Hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E256–E261. [Google Scholar] [CrossRef] [PubMed]
- Bemben, M.G.; Lamont, H.S. Creatine supplementation and exercise performance: Recent findings. Sports Med. 2005, 35, 107–125. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Rawson, E.S.; Volek, J.S. Effects of creatine supplementation and resistance training on muscle strength and weightlifting performance. J. Strength Cond. Res. 2003, 17, 822–831. [Google Scholar] [CrossRef]
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health 2018, 10, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Colombini, A.; Machado, M. Creatine supplementation improves performance, but is it safe? Double-blind placebo-controlled study. J. Sports Med. Phys. Fit. 2020, 60, 1034–1039. [Google Scholar] [CrossRef]
- Nunes, J.P.; Ribeiro, A.S.; Schoenfeld, B.J.; Tomeleri, C.M.; Avelar, A.; Trindade, M.C.; Nabuco, H.C.; Cavalcante, E.F.; Junior, P.S.; Fernandes, R.R.; et al. Creatine supplementation elicits greater muscle hypertrophy in upper than lower limbs and trunk in resistance-trained men. Nutr. Health 2017, 23, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Zabriskie, H.A.; Erickson, J.L.; Molling, P.E.; Kerksick, C.M.; Jagim, A.R. Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: A brief review. J. Int. Soc. Sports Nutr. 2018, 15, 41. [Google Scholar] [CrossRef]
- Kreider, R.B.; Jäger, R.; Purpura, M. Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review. Nutrients 2022, 14, 1035. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, C.K.; Carpentier, A.; Poortmans, J.R. Studies on the safety of creatine supplementation. Amino Acids 2011, 40, 1409–1418. [Google Scholar] [CrossRef]
- Pashayee-Khamene, F.; Heidari, Z.; Asbaghi, O.; Ashtary-Larky, D.; Goudarzi, K.; Forbes, S.C.; Candow, D.G.; Bagheri, R.; Ghanavati, M.; Dutheil, F. Creatine supplementation protocols with or without training interventions on body composition: A GRADE-assessed systematic review and dose-response meta-analysis. J. Int. Soc. Sports Nutr. 2024, 21, 2380058. [Google Scholar] [CrossRef]
- Burke, R.; Piñero, A.; Coleman, M.; Mohan, A.; Sapuppo, M.; Augustin, F.; Aragon, A.A.; Candow, D.G.; Forbes, S.C.; Swinton, P.; et al. The Effects of Creatine Supplementation Combined with Resistance Training on Regional Measures of Muscle Hypertrophy: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B. Effects of creatine supplementation on performance and training adaptations. Mol. Cell. Biochem. 2003, 244, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Arnold, B.L.; Weltman, A.L.; Perrin, D.H.; Mistry, D.; Kahler, D.M.; Kraemer, W.; Volek, J. Creatine Supplementation Increases Total Body Water Without Altering Fluid Distribution. J. Athl. Train. 2003, 38, 44–50. [Google Scholar]
- Tarnopolsky, M.A. Clinical use of creatine in neuromuscular and neurometabolic disorders. Sub-Cell. Biochem. 2007, 46, 183–204. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sá Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Januário, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996. [Google Scholar] [CrossRef]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Candow, D.G.; Mahoney, D.; Tarnopolsky, M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med. Sci. Sports Exerc. 2003, 35, 1946–1955. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Beal, M.F. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann. Neurol. 2001, 49, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Trojian, T.H. Creatine supplementation. Curr. Sports Med. Rep. 2013, 12, 240–244. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Francaux, M. Adverse effects of creatine supplementation: Fact or fiction? Sports Med. 2000, 30, 155–170. [Google Scholar] [CrossRef]
- Francaux, M.; Poortmans, J.R. Side effects of creatine supplementation in athletes. Int. J. Sports Physiol. Perform. 2006, 1, 311–323. [Google Scholar] [CrossRef]
- Adriano, E.; Gulino, M.; Arkel, M.; Salis, A.; Damonte, G.; Liessi, N.; Millo, E.; Garbati, P.; Balestrino, M. Di-acetyl creatine ethyl ester, a new creatine derivative for the possible treatment of creatine transporter deficiency. Neurosci. Lett. 2018, 665, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Adriano, E.; Balestrino, M.; Panfoli, I. Creatine ethyl ester: A new substrate for creatine kinase. Mol. Biol. 2012, 46, 162–165. [Google Scholar] [CrossRef]
- Andres, S.; Ziegenhagen, R.; Trefflich, I.; Pevny, S.; Schultrich, K.; Braun, H.; Schänzer, W.; Hirsch-Ernst, K.I.; Schäfer, B.; Lampen, A. Creatine and creatine forms intended for sports nutrition. Mol. Nutr. Food Res. 2017, 61, 1600772. [Google Scholar] [CrossRef]
- Green, A.L.; Hultman, E.; Macdonald, I.A.; Sewell, D.A.; Greenhaff, P.L. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am. J. Physiol.-Endocrinol. Metab. 1996, 271, E821–E826. [Google Scholar] [CrossRef]
- Steenge, G.R.; Lambourne, J.; Casey, A.; Macdonald, I.A.; Greenhaff, P.L. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 1998, 275, E974–E979. [Google Scholar] [CrossRef]
- Theodorou, A.S.; Havenetidis, K.; Zanker, C.L.; O’Hara, J.P.; King, R.F.; Hood, C.; Paradisis, G.; Cooke, C.B. Effects of acute creatine loading with or without carbohydrate on repeated bouts of maximal swimming in high-performance swimmers. J. Strength Cond. Res. 2005, 19, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Jayappa, S.; Dash, A.K. Evaluation of the stability of creatine in solution prepared from effervescent creatine formulations. AAPS PharmSciTech 2003, 4, E25. [Google Scholar] [CrossRef]
- Brilla, L.R.; Giroux, M.S.; Taylor, A.; Knutzen, K.M. Magnesium-creatine supplementation effects on body water. Metab. Clin. Exp. 2003, 52, 1136–1140. [Google Scholar] [CrossRef]
- Zajac, A.; Golas, A.; Chycki, J.; Halz, M.; Michalczyk, M.M. The Effects of Long-Term Magnesium Creatine Chelate Supplementation on Repeated Sprint Ability (RAST) in Elite Soccer Players. Nutrients 2020, 12, 2961. [Google Scholar] [CrossRef]
- Hall, M.; Manetta, E.; Tupper, K. Creatine Supplementation: An Update. Curr. Sports Med. Rep. 2021, 20, 338–344. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Caffeine and creatine use in sport. Ann. Nutr. Metab. 2010, 57 (Suppl. S2), 1–8. [Google Scholar] [CrossRef]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281 Pt 1, 21–40. [Google Scholar] [CrossRef]
- Borchel, A.; Verleih, M.; Kühn, C.; Rebl, A.; Goldammer, T. Evolutionary expression differences of creatine synthesis-related genes: Implications for skeletal muscle metabolism in fish. Sci. Rep. 2019, 9, 5429. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.D.; Parameswaran, B.; Molloy, G.R. Expression of the rat brain creatine kinase gene in C6 glioma cells. J. Neurosci. Res. 1993, 35, 92–102. [Google Scholar] [CrossRef]
- Wilson, C.D.; Shen, W.; Molloy, G.R. Expression of the brain creatine kinase gene is low in neuroblastoma cell lines. Dev. Neurosci. 1997, 19, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, R.J.; Friedman, D.L.; Olson, E.N.; Ma, T.S.; Cortez, M.D.; Goodman, C.; Puleo, P.R.; Perryman, M.B. Muscle creatine kinase isoenzyme expression in adult human brain. J. Biol. Chem. 1990, 265, 6403–6409. [Google Scholar] [CrossRef]
- Harris, R.C.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef]
- Cribb, P.J.; Williams, A.D.; Stathis, C.G.; Carey, M.F.; Hayes, A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med. Sci. Sports Exerc. 2007, 39, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Ibañez, J.; González-Badillo, J.J.; Gorostiaga, E.M. Effects of creatine supplementation on muscle power, endurance, and sprint performance. Med. Sci. Sports Exerc. 2002, 34, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Hultman, E.; Söderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. (1985) 1996, 81, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef]
- Preen, D.; Dawson, B.; Goodman, C.; Beilby, J.; Ching, S. Creatine supplementation: A comparison of loading and maintenance protocols on creatine uptake by human skeletal muscle. Int. J. Sport. Nutr. Exerc. Metab. 2003, 13, 97–111. [Google Scholar] [CrossRef]
- van Loon, L.J.; Murphy, R.; Oosterlaar, A.M.; Cameron-Smith, D.; Hargreaves, M.; Wagenmakers, A.J.; Snow, R. Creatine supplementation increases glycogen storage but not GLUT-4 expression in human skeletal muscle. Clin. Sci. 2004, 106, 99–106. [Google Scholar] [CrossRef]
- Yáñez-Silva, A.; Buzzachera, C.F.; Piçarro, I.D.C.; Januario, R.S.B.; Ferreira, L.H.B.; McAnulty, S.R.; Utter, A.C.; Souza-Junior, T.P. Effect of low dose, short-term creatine supplementation on muscle power output in elite youth soccer players. J. Int. Soc. Sports Nutr. 2017, 14, 5. [Google Scholar] [CrossRef]
- Mujika, I.; Burke, L.M. Nutrition in team sports. Ann. Nutr. Metab. 2010, 57 (Suppl. S2), 26–35. [Google Scholar] [CrossRef]
- Fernández-Landa, J.; Santibañez-Gutierrez, A.; Todorovic, N.; Stajer, V.; Ostojic, S.M. Effects of Creatine Monohydrate on Endurance Performance in a Trained Population: A Systematic Review and Meta-analysis. Sports Med. 2023, 53, 1017–1027. [Google Scholar] [CrossRef]
- Fiorenza, M.; Hostrup, M.; Gunnarsson, T.P.; Shirai, Y.; Schena, F.; Iaia, F.M.; Bangsbo, J. Neuromuscular Fatigue and Metabolism during High-Intensity Intermittent Exercise. Med. Sci. Sports Exerc. 2019, 51, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.R.; Gastin, P.B. Energy system contribution during 200- to 1500-m running in highly trained athletes. Med. Sci. Sports Exerc. 2001, 33, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Rawson, E.S. Scientific basis and practical aspects of creatine supplementation for athletes. Nutrition 2004, 20, 609–614. [Google Scholar] [CrossRef]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef] [PubMed]
- Terjung, R.L.; Clarkson, P.; Eichner, E.R.; Greenhaff, P.L.; Hespel, P.J.; Israel, R.G.; Kraemer, W.J.; Meyer, R.A.; Spriet, L.L.; Tarnopolsky, M.A.; et al. American College of Sports Medicine roundtable. Physiological and health effects of oral creatine supplementation. Med. Sci. Sports Exerc. 2000, 32, 706–717. [Google Scholar] [CrossRef]
- Ribeiro, F.; Longobardi, I.; Perim, P.; Duarte, B.; Ferreira, P.; Gualano, B.; Roschel, H.; Saunders, B. Timing of Creatine Supplementation around Exercise: A Real Concern? Nutrients 2021, 13, 2844. [Google Scholar] [CrossRef]
- Kutz, M.R.; Gunter, M.J. Creatine monohydrate supplementation on body weight and percent body fat. J. Strength Cond. Res. 2003, 17, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Becque, M.D.; Lochmann, J.D.; Melrose, D.R. Effects of oral creatine supplementation on muscular strength and body composition. Med. Sci. Sports Exerc. 2000, 32, 654–658. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Volek, J.S. Creatine supplementation: Its role in human performance. Clin. Sports Med. 1999, 18, 651–666. [Google Scholar] [CrossRef]
- Moore, S.R.; Gordon, A.N.; Cabre, H.E.; Hackney, A.C.; Smith-Ryan, A.E. A Randomized Controlled Trial of Changes in Fluid Distribution across Menstrual Phases with Creatine Supplementation. Nutrients 2023, 15, 429. [Google Scholar] [CrossRef]
- Andre, T.L.; Gann, J.J.; McKinley-Barnard, S.K.; Willoughby, D.S. Effects of Five Weeks of Resistance Training and Relatively-Dosed Creatine Monohydrate Supplementation on Body Composition and Muscle Strength, and Whole-Body Creatine Metabolism in Resistance-Trained Males. Int. J. Kinesiol. Sports Sci. 2016, 4, 27–35. [Google Scholar]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R.; Francaux, M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med. Sci. Sports Exerc. 1999, 31, 1108–1110. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Mayhew, J.L.; Ware, J.S. Effects of long-term creatine supplementation on liver and kidney functions in American college football players. Int. J. Sport. Nutr. Exerc. Metab. 2002, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, I.; Gualano, B.; Seguro, A.C.; Roschel, H. Is It Time for a Requiem for Creatine Supplementation-Induced Kidney Failure? A Narrative Review. Nutrients 2023, 15, 1466. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.C.B.; Schincaglia, R.M.; Candow, D.G.; Pimentel, G.D. Effect of Creatine Supplementation on Body Composition and Malnutrition-Inflammation Score in Hemodialysis Patients: An Exploratory 1-Year, Balanced, Double-Blind Design. Nutrients 2024, 16, 615. [Google Scholar] [CrossRef]
- Post, A.; Tsikas, D.; Bakker, S.J.L. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef]
- Forsberg, A.M.; Nilsson, E.; Werneman, J.; Bergström, J.; Hultman, E. Muscle composition in relation to age and sex. Clin. Sci. 1991, 81, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef]
- Gordon, A.N.; Moore, S.R.; Patterson, N.D.; Hostetter, M.E.; Cabre, H.E.; Hirsch, K.R.; Hackney, A.C.; Smith-Ryan, A.E. The Effects of Creatine Monohydrate Loading on Exercise Recovery in Active Women throughout the Menstrual Cycle. Nutrients 2023, 15, 3567. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595. [Google Scholar] [CrossRef]
- Sales, L.P.; Pinto, A.J.; Rodrigues, S.F.; Alvarenga, J.C.; Gonçalves, N.; Sampaio-Barros, M.M.; Benatti, F.B.; Gualano, B.; Rodrigues Pereira, R.M. Creatine Supplementation (3 g/d) and Bone Health in Older Women: A 2-Year, Randomized, Placebo-Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 931–938. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Cabre, H.E.; Eckerson, J.M.; Candow, D.G. Creatine Supplementation in Women’s Health: A Lifespan Perspective. Nutrients 2021, 13, 877. [Google Scholar] [CrossRef]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef]
- Delanghe, J.; De Slypere, J.P.; De Buyzere, M.; Robbrecht, J.; Wieme, R.; Vermeulen, A. Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Clin. Chem. 1989, 35, 1802–1803. [Google Scholar] [CrossRef]
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29, S20–S34. discussion S35-27. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Benzi, G.; Ceci, A. Creatine as nutritional supplementation and medicinal product. J. Sports Med. Phys. Fit. 2001, 41, 1–10. [Google Scholar]
- Venderley, A.M.; Campbell, W.W. Vegetarian diets: Nutritional considerations for athletes. Sports Med. 2006, 36, 293–305. [Google Scholar] [CrossRef]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine for treating muscle disorders. Cochrane Database Syst. Rev. 2013, 2013, Cd004760. [Google Scholar] [CrossRef]
- Walter, M.C.; Lochmüller, H.; Reilich, P.; Klopstock, T.; Huber, R.; Hartard, M.; Hennig, M.; Pongratz, D.; Müller-Felber, W. Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study. Neurology 2000, 54, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Triantafyllidis, K.K.; Kechagias, K.S.; Forbes, S.C.; Candow, D.G. Effects of creatine supplementation on memory in healthy individuals: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2023, 81, 416–427. [Google Scholar] [CrossRef]
- Xu, C.; Bi, S.; Zhang, W.; Luo, L. The effects of creatine supplementation on cognitive function in adults: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1424972. [Google Scholar] [CrossRef]
- Rae, C.; Digney, A.L.; McEwan, S.R.; Bates, T.C. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proc. Biol. Sci. 2003, 270, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Elechi, J.O.G.; Guandique, D.M.A.; Cannataro, R. Creatine in Cognitive Performance: A Commentary. Curr. Mol. Pharmacol. 2024, 17, e18761429272915. [Google Scholar] [CrossRef]
- Benton, D.; Donohoe, R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011, 105, 1100–1105. [Google Scholar] [CrossRef]
- Moriarty, T.; Bourbeau, K.; Dorman, K.; Runyon, L.; Glaser, N.; Brandt, J.; Hoodjer, M.; Forbes, S.C.; Candow, D.G. Dose-Response of Creatine Supplementation on Cognitive Function in Healthy Young Adults. Brain Sci. 2023, 13, 1276. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Schifitto, G.; Oakes, D.; Bredlau, A.L.; Meyers, C.M.; Nahin, R.; Rosas, H.D. The CREST-E study of creatine for Huntington disease: A randomized controlled trial. Neurology 2017, 89, 594–601. [Google Scholar] [CrossRef]
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Müller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef]
- Clarke, H.; Hickner, R.C.; Ormsbee, M.J. The Potential Role of Creatine in Vascular Health. Nutrients 2021, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Kim, D.H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef]
- Kuethe, F.; Krack, A.; Richartz, B.M.; Figulla, H.R. Creatine supplementation improves muscle strength in patients with congestive heart failure. Pharmazie. 2006, 61, 218–222. [Google Scholar] [PubMed]
- Aksentijević, D.; Zervou, S.; Faller, K.M.; McAndrew, D.J.; Schneider, J.E.; Neubauer, S.; Lygate, C.A. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS ONE 2014, 9, e109021. [Google Scholar] [CrossRef]
- Gordon, A.; Hultman, E.; Kaijser, L.; Kristjansson, S.; Rolf, C.J.; Nyquist, O.; Sylvén, C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc. Res. 1995, 30, 413–418. [Google Scholar] [CrossRef]
- Harmon, K.K.; Stout, J.R.; Fukuda, D.H.; Pabian, P.S.; Rawson, E.S.; Stock, M.S. The Application of Creatine Supplementation in Medical Rehabilitation. Nutrients 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Hellín, J.; Del Coso, J.; Franco-Andrés, A.; Gamonales, J.M.; Espada, M.C.; González-García, J.; López-Moreno, M.; Varillas-Delgado, D. Creatine Supplementation Beyond Athletics: Benefits of Different Types of Creatine for Women, Vegans, and Clinical Populations—A Narrative Review. Nutrients 2025, 17, 95. https://doi.org/10.3390/nu17010095
Gutiérrez-Hellín J, Del Coso J, Franco-Andrés A, Gamonales JM, Espada MC, González-García J, López-Moreno M, Varillas-Delgado D. Creatine Supplementation Beyond Athletics: Benefits of Different Types of Creatine for Women, Vegans, and Clinical Populations—A Narrative Review. Nutrients. 2025; 17(1):95. https://doi.org/10.3390/nu17010095
Chicago/Turabian StyleGutiérrez-Hellín, Jorge, Juan Del Coso, Arturo Franco-Andrés, José M. Gamonales, Mário C. Espada, Jaime González-García, Miguel López-Moreno, and David Varillas-Delgado. 2025. "Creatine Supplementation Beyond Athletics: Benefits of Different Types of Creatine for Women, Vegans, and Clinical Populations—A Narrative Review" Nutrients 17, no. 1: 95. https://doi.org/10.3390/nu17010095
APA StyleGutiérrez-Hellín, J., Del Coso, J., Franco-Andrés, A., Gamonales, J. M., Espada, M. C., González-García, J., López-Moreno, M., & Varillas-Delgado, D. (2025). Creatine Supplementation Beyond Athletics: Benefits of Different Types of Creatine for Women, Vegans, and Clinical Populations—A Narrative Review. Nutrients, 17(1), 95. https://doi.org/10.3390/nu17010095









