Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models
Abstract
1. Introduction and Background
1.1. Obesity-Associated Low-Grade Inflammation
1.2. The Role of Neutrophils in Obesity-Associated Meta-Inflammation
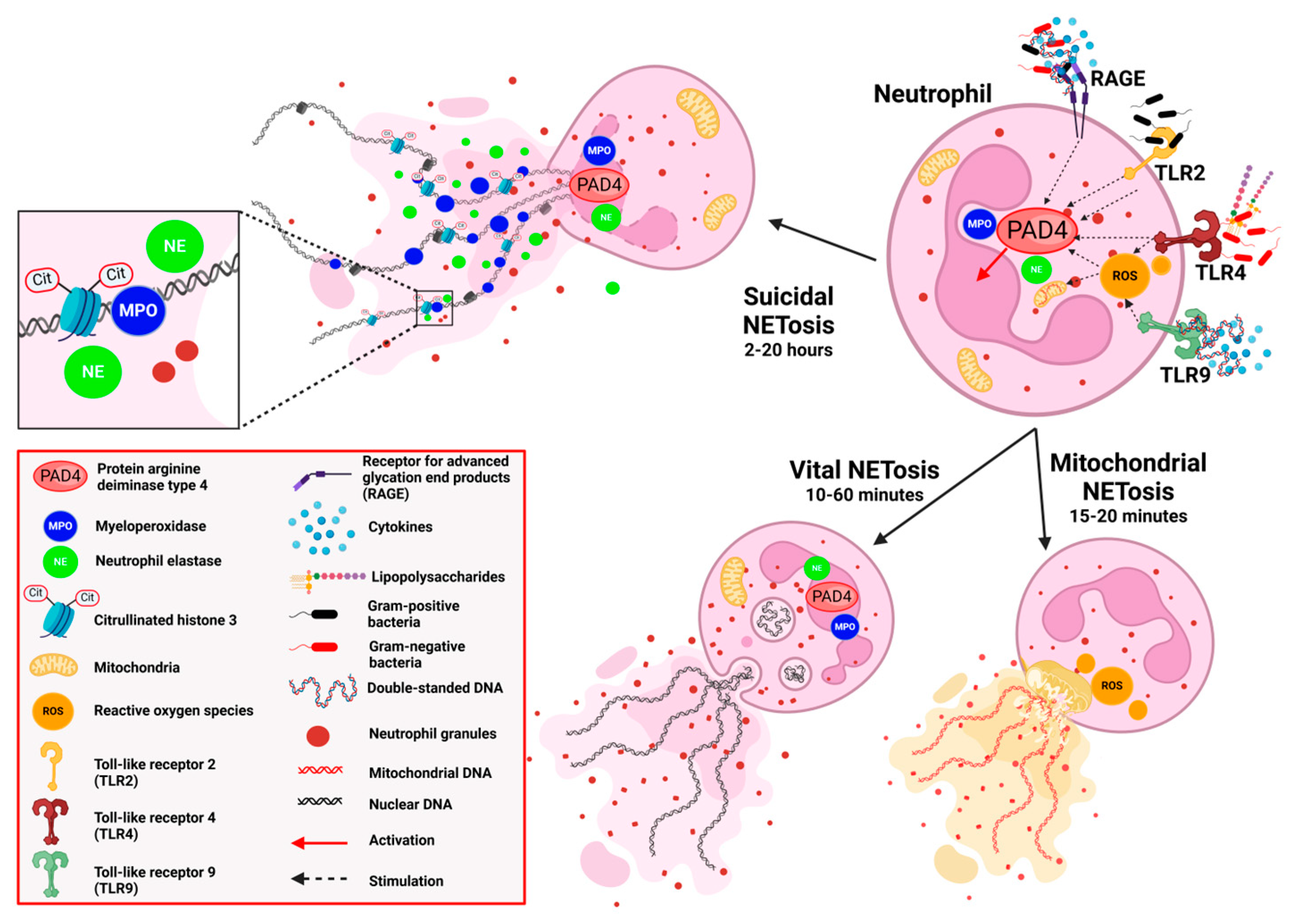
2. Formation of Neutrophil Extracellular Traps
2.1. Pathogen- and Danger-Associated Molecular Patterns
2.2. Forms of NETosis
2.2.1. Suicidal NETosis
2.2.2. Vital NETosis
2.2.3. Mitochondrial NETosis
2.3. Metabolic Pathways in NETs Formation
2.4. Interplay of Neutrophils and NETs with Other Immune Cells
2.5. Sex Differences
2.6. Age Differences
2.7. Priming of NETosis by Sterile Stimuli
2.8. Association of NETosis with Inflammatory Status
2.9. Effects of Temperature on NETs Formation
3. Obesity-Associated Neutrophilia
3.1. Human Studies
Summary
3.2. Experimental Studies
4. Neutrophil Extracellular Traps in Obesity
4.1. Human Studies
Summary
4.2. Animal Models
4.2.1. Rodent Models of Obesity
4.2.2. NETosis in Dietary-Induced Obesity Models
4.2.3. Summary
5. Neutrophil Extracellular Traps in Hypertension
5.1. Human Studies on Hypertension and NET Formation
5.2. NETosis in Rodent Models of Hypertension
5.3. Summary and Perspectives
6. Dyslipidemia and NET Formation
6.1. Human Studies on NETs in Steatohepatitis
6.2. Rodent Studies on NETs in Steatohepatitis
6.3. Summary and Perspectives
7. Knockout Animal Models in the Obesity and Metabolic Syndrome Research
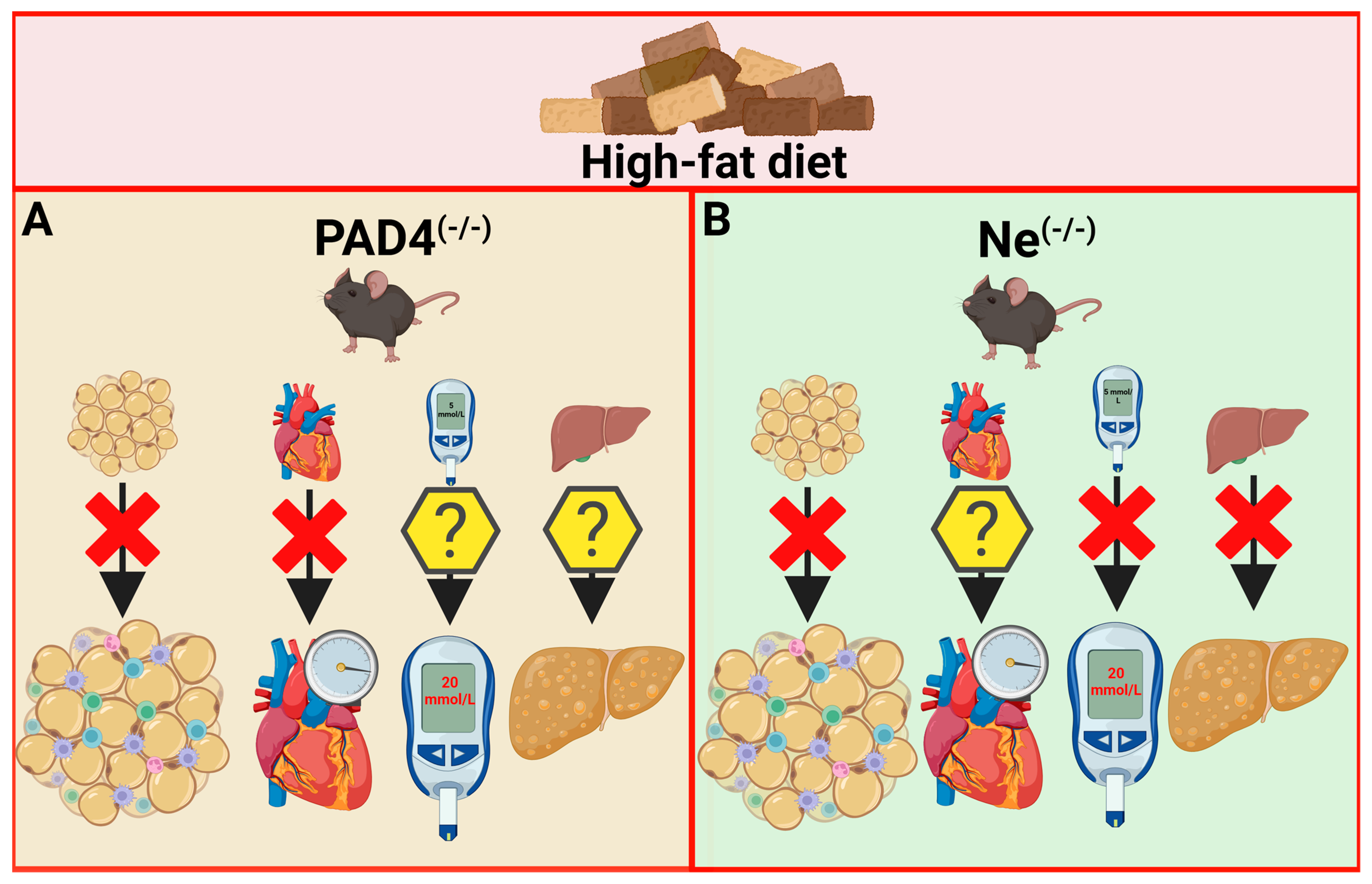
7.1. PAD4 Deficiency
7.2. MPO Deficiency and Inhibition
7.3. Neutrophil Elastase Deficiency
8. Discussion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WOF. World Obesity Atlas 2024; World Obesity Federation: London, UK, 2024; Available online: https://data.worldobesity.org/publications/?cat=22 (accessed on 4 March 2024).
- NCD-RisC. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Yanovski, J.A. Obesity: Trends in underweight and obesity—Scale of the problem. Nat. Rev. Endocrinol. 2018, 14, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Neeland, I.J.; Sanders, P.; St-Onge, M.P. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q. Definitions, Classification, and Epidemiology of Obesity. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Bluher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur. Heart J. 2024, 45, 4063–4098. [Google Scholar] [CrossRef]
- Gugliucci, A. Biomarkers of dysfunctional visceral fat. Adv. Clin. Chem. 2022, 109, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Neutrophils and Bacterial Immune Evasion. J. Innate Immun. 2018, 10, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. How human neutrophils kill and degrade microbes: An integrated view. Immunol. Rev. 2007, 219, 88–102. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Moutsopoulos, N.M. Neutrophils and neutrophil extracellular traps in oral health and disease. Exp. Mol. Med. 2024, 56, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Artemniak-Wojtowicz, D.; Kucharska, A.; Pyrżak, B. Obesity and chronic inflammation crosslinking. Cent. Eur. J. Immunol. 2020, 45, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Braster, Q.; Silvestre Roig, C.; Hartwig, H.; Beckers, L.; den Toom, M.; Döring, Y.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Inhibition of NET Release Fails to Reduce Adipose Tissue Inflammation in Mice. PLoS ONE 2016, 11, e0163922. [Google Scholar] [CrossRef]
- Fadini, G.P.; Menegazzo, L.; Scattolini, V.; Gintoli, M.; Albiero, M.; Avogaro, A. A perspective on NETosis in diabetes and cardiometabolic disorders. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Asiamah, E.K.; Ekwemalor, K.; Adjei-Fremah, S.; Osei, B.; Newman, R.; Worku, M. Natural and synthetic pathogen associated molecular patterns modulate galectin expression in cow blood. J. Anim. Sci. Technol. 2019, 61, 245–253. [Google Scholar] [CrossRef]
- Garg, A.D.; Vandenberk, L.; Fang, S.; Fasche, T.; Van Eygen, S.; Maes, J.; Van Woensel, M.; Koks, C.; Vanthillo, N.; Graf, N.; et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017, 24, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Cicchinelli, S.; Pignataro, G.; Gemma, S.; Piccioni, A.; Picozzi, D.; Ojetti, V.; Franceschi, F.; Candelli, M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024, 25, 962. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Block, H.; Rossaint, J.; Zarbock, A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction. Cells 2022, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Boettcher, M.; Dölling, M.; Heuer, A.; Hohberger, B.; Leppkes, M.; Naschberger, E.; Schapher, M.; Schauer, C.; Schoen, J.; et al. Moonlighting chromatin: When DNA escapes nuclear control. Cell Death Differ. 2023, 30, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, S.; Pan, J.; Lin, Q.; Yang, S.; Ambe, P.C.; Basharat, Z.; Zimmer, V.; Wang, W.; Hong, W. Damage associated molecular patterns and neutrophil extracellular traps in acute pancreatitis. Front. Cell. Infect. Microbiol. 2022, 12, 927193. [Google Scholar] [CrossRef]
- Cicco, S.; Cicco, G.; Racanelli, V.; Vacca, A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediat. Inflamm. 2020, 2020, 7527953. [Google Scholar] [CrossRef]
- Halverson, T.W.; Wilton, M.; Poon, K.K.; Petri, B.; Lewenza, S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015, 11, e1004593. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, C.; Zhang, Y.; Zhu, L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules 2024, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Yin, M.; Xie, S.; Shi, L.; Nie, W.; Shi, B.; Yu, G. The molecular mechanism of neutrophil extracellular traps and its role in bone and joint disease. Heliyon 2023, 9, e22920. [Google Scholar] [CrossRef]
- Burgener, S.S.; Schroder, K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020, 12, a037028. [Google Scholar] [CrossRef]
- Guillotin, F.; Fortier, M.; Portes, M.; Demattei, C.; Mousty, E.; Nouvellon, E.; Mercier, E.; Chea, M.; Letouzey, V.; Gris, J.C.; et al. Vital NETosis vs. suicidal NETosis during normal pregnancy and preeclampsia. Front. Cell Dev. Biol. 2022, 10, 1099038. [Google Scholar] [CrossRef]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm 2022, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Su, C.g.; Liao, Z.; Pei, Y.; Wang, J.; Li, Z.; Fu, S.; Liu, J. The effect of neutrophil extracellular traps in venous thrombosis. Thromb. J. 2023, 21, 67. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Aziz, M.; Wang, P. The vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef]
- Takishita, Y.; Yasuda, H.; Shimizu, M.; Matsuo, A.; Morita, A.; Tsutsumi, T.; Tsuchiya, M.; Sato, E.F. Formation of neutrophil extracellular traps in mitochondrial DNA-deficient cells. J. Clin. Biochem. Nutr. 2020, 66, 15–23. [Google Scholar] [CrossRef]
- Boilard, E.; Fortin, P.R. Mitochondria drive NETosis and inflammation in SLE. Nat. Rev. Rheumatol. 2016, 12, 195–196. [Google Scholar] [CrossRef]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Lotze, M.T.; Zeh, H.J.; Rubartelli, A.; Sparvero, L.J.; Amoscato, A.A.; Washburn, N.R.; Devera, M.E.; Liang, X.; Tör, M.; Billiar, T. The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007, 220, 60–81. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Andersson, S.G.; Karlberg, O.; Canbäck, B.; Kurland, C.G. On the origin of mitochondria: A genomics perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 165–177; discussion 177–179. [Google Scholar] [CrossRef]
- van Raam, B.J.; Sluiter, W.; de Wit, E.; Roos, D.; Verhoeven, A.J.; Kuijpers, T.W. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS ONE 2008, 3, e2013. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Rochael, N.C.; Guimarães-Costa, A.B.; de Souza-Vieira, T.S.; Ganilho, J.; Saraiva, E.M.; Palhano, F.L.; Foguel, D. A Metabolic Shift toward Pentose Phosphate Pathway Is Necessary for Amyloid Fibril- and Phorbol 12-Myristate 13-Acetate-induced Neutrophil Extracellular Trap (NET) Formation. J. Biol. Chem. 2015, 290, 22174–22183. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dikshit, M. Metabolic Insight of Neutrophils in Health and Disease. Front. Immunol. 2019, 10, 2099. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef]
- Kirchner, T.; Möller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat. Inflamm. 2012, 2012, 849136. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, D.; Amini, P.; Oberson, K.; Sokollik, C.; Duppenthaler, A.; Simon, H.U.; Yousefi, S. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 2017, 216, 4073–4090. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Sadaf, S.; Chandra, T.; Kumar, S.; Dikshit, M. Glycolysis dependent lactate formation in neutrophils: A metabolic link between NOX-dependent and independent NETosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165542. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front. Immunol. 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Injarabian, L.; Devin, A.; Ransac, S.; Marteyn, B.S. Neutrophil Metabolic Shift during their Lifecycle: Impact on their Survival and Activation. Int. J. Mol. Sci. 2020, 21, 287. [Google Scholar] [CrossRef]
- Joshi, M.B.; Lad, A.; Bharath Prasad, A.S.; Balakrishnan, A.; Ramachandra, L.; Satyamoorthy, K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013, 587, 2241–2246. [Google Scholar] [CrossRef]
- Ohinata, H.; Obama, T.; Makiyama, T.; Watanabe, Y.; Itabe, H. High-Density Lipoprotein Suppresses Neutrophil Extracellular Traps Enhanced by Oxidized Low-Density Lipoprotein or Oxidized Phospholipids. Int. J. Mol. Sci. 2022, 23, 13992. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Palladino, E.N.D.; Katunga, L.A.; Kolar, G.R.; Ford, D.A. 2-Chlorofatty acids: Lipid mediators of neutrophil extracellular trap formation. J. Lipid Res. 2018, 59, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Lazzaretto, B.; Fadeel, B. Intra- and Extracellular Degradation of Neutrophil Extracellular Traps by Macrophages and Dendritic Cells. J. Immunol. 2019, 203, 2276–2290. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Tillack, K.; Breiden, P.; Martin, R.; Sospedra, M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J. Immunol. 2012, 188, 3150–3159. [Google Scholar] [CrossRef]
- Wilson, A.S.; Randall, K.L.; Pettitt, J.A.; Ellyard, J.I.; Blumenthal, A.; Enders, A.; Quah, B.J.; Bopp, T.; Parish, C.R.; Brüstle, A. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat. Commun. 2022, 13, 528. [Google Scholar] [CrossRef]
- Gestermann, N.; Di Domizio, J.; Lande, R.; Demaria, O.; Frasca, L.; Feldmeyer, L.; Di Lucca, J.; Gilliet, M. Netting Neutrophils Activate Autoreactive B Cells in Lupus. J. Immunol. 2018, 200, 3364–3371. [Google Scholar] [CrossRef]
- Karmakar, U.; Vermeren, S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology 2021, 164, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Stark, K. Platelet-neutrophil crosstalk and netosis. HemaSphere 2019, 3, 89–91. [Google Scholar] [CrossRef]
- Andrews, R.K.; Arthur, J.F.; Gardiner, E.E. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb. Haemost. 2014, 112, 659–665. [Google Scholar] [CrossRef]
- Donkel, S.J.; Wolters, F.J.; Ikram, M.A.; de Maat, M.P.M. Circulating Myeloperoxidase (MPO)-DNA complexes as marker for Neutrophil Extracellular Traps (NETs) levels and the association with cardiovascular risk factors in the general population. PLoS ONE 2021, 16, e0253698. [Google Scholar] [CrossRef]
- Pastorek, M.; Konečná, B.; Janko, J.; Janovičová, Ľ.; Podracká, Ľ.; Záhumenský, J.; Šteňová, E.; Dúbrava, M.; Hodosy, J.; Vlková, B.; et al. Mitochondria-induced formation of neutrophil extracellular traps is enhanced in the elderly via Toll-like receptor 9. J. Leukoc. Biol. 2023, 114, 651–665. [Google Scholar] [CrossRef]
- D’Abbondanza, M.; Martorelli, E.E.; Ricci, M.A.; De Vuono, S.; Migliola, E.N.; Godino, C.; Corradetti, S.; Siepi, D.; Paganelli, M.T.; Maugeri, N.; et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci. Rep. 2019, 9, 14678. [Google Scholar] [CrossRef] [PubMed]
- Celec, P.; Janovičová, Ĺ.; Gurecká, R.; Koborová, I.; Gardlík, R.; Šebeková, K. Circulating extracellular DNA is in association with continuous metabolic syndrome score in healthy adolescents. Physiol. Genom. 2021, 53, 309–318. [Google Scholar] [CrossRef]
- Itagaki, K.; Kaczmarek, E.; Lee, Y.T.; Tang, I.T.; Isal, B.; Adibnia, Y.; Sandler, N.; Grimm, M.J.; Segal, B.H.; Otterbein, L.E.; et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS ONE 2015, 10, e0120549. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.J.; Sursal, T.; Rodriguez, E.K.; Appleton, P.T.; Zhang, Q.; Itagaki, K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J. Orthop. Trauma. 2010, 24, 534–538. [Google Scholar] [CrossRef]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Ondracek, A.S.; Mangold, A.; Scherz, T.; Nechvile, J.; Seidl, V.; Brostjan, C.; Lang, I.M. Neutrophil Extracellular Traps Induce MCP-1 at the Culprit Site in ST-Segment Elevation Myocardial Infarction. Front. Cell Dev. Biol. 2020, 8, 564169. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sinha, S.; Mohapatra, S.K. Analysis of transcriptomic data sets supports the role of IL-6 in NETosis and immunothrombosis in severe COVID-19. BMC Genom. Data 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Janko, J.; Bečka, E.; Kmeťová, K.; Hudecová, L.; Konečná, B.; Celec, P.; Bajaj-Elliott, M.; Pastorek, M. Neutrophil extracellular traps formation and clearance is enhanced in fever and attenuated in hypothermia. Front. Immunol. 2023, 14, 1257422. [Google Scholar] [CrossRef]
- Herishanu, Y.; Rogowski, O.; Polliack, A.; Marilus, R. Leukocytosis in obese individuals: Possible link in patients with unexplained persistent neutrophilia. Eur. J. Haematol. 2006, 76, 516–520. [Google Scholar] [CrossRef]
- Sanchez-Pino, M.D.; Richardson, W.S.; Zabaleta, J.; Puttalingaiah, R.T.; Chapple, A.G.; Liu, J.; Kim, Y.; Ponder, M.; DeArmitt, R.; Baiamonte, L.B.; et al. Increased inflammatory low-density neutrophils in severe obesity and effect of bariatric surgery: Results from case-control and prospective cohort studies. eBioMedicine 2022, 77, 103910. [Google Scholar] [CrossRef]
- Shantaram, D.; Hoyd, R.; Blaszczak, A.M.; Antwi, L.; Jalilvand, A.; Wright, V.P.; Liu, J.; Smith, A.J.; Bradley, D.; Lafuse, W.; et al. Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils. Nat. Commun. 2024, 15, 5434. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Freitas, D.F.; Colón, D.F.; Silva, R.L.; Santos, E.M.; Guimarães, V.H.D.; Ribeiro, G.H.M.; de Paula, A.M.B.; Guimarães, A.L.S.; Dos Reis, S.T.; Cunha, F.Q.; et al. Neutrophil extracellular traps (NETs) modulate inflammatory profile in obese humans and mice: Adipose tissue role on NETs levels. Mol. Biol. Rep. 2022, 49, 3225–3236. [Google Scholar] [CrossRef]
- Roberts, H.M.; Grant, M.M.; Hubber, N.; Super, P.; Singhal, R.; Chapple, I.L.C. Impact of Bariatric Surgical Intervention on Peripheral Blood Neutrophil (PBN) Function in Obesity. Obes. Surg. 2018, 28, 1611–1621. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Wood, L.G.; Dowling, L.R.; Stanton, S.; Baines, K.J. Neutrophil extracellular traps in obese asthma. Proc. Nutr. Soc. 2023, 82, E161. [Google Scholar] [CrossRef]
- Lewis, S.R.; Ahmed, S.; Dym, C.; Khaimova, E.; Kest, B.; Bodnar, R.J. Inbred mouse strain survey of sucrose intake. Physiol. Behav. 2005, 85, 546–556. [Google Scholar] [CrossRef]
- Graneri, L.T.; Mamo, J.C.L.; D’Alonzo, Z.; Lam, V.; Takechi, R. Chronic Intake of Energy Drinks and Their Sugar Free Substitution Similarly Promotes Metabolic Syndrome. Nutrients 2021, 13, 1202. [Google Scholar] [CrossRef] [PubMed]
- Lieder, B.; Čonka, J.; Reiner, A.T.; Zabel, V.; Ameur, D.; Somoza, M.M.; Šebeková, K.; Celec, P.; Somoza, V. Long-Term Consumption of a Sugar-Sweetened Soft Drink in Combination with a Western-Type Diet Is Associated with Morphological and Molecular Changes of Taste Markers Independent of Body Weight Development in Mice. Nutrients 2022, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. Combined effects of cafeteria and tube-feeding on energy balance in the rat. Proc. Nutr. Soc. 1979, 38, 5a. [Google Scholar] [CrossRef] [PubMed]
- Higa, T.S.; Spinola, A.V.; Fonseca-Alaniz, M.H.; Evangelista, F.S. Comparison between cafeteria and high-fat diets in the induction of metabolic dysfunction in mice. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 47–54. [Google Scholar] [PubMed]
- Shafat, A.; Murray, B.; Rumsey, D. Energy density in cafeteria diet induced hyperphagia in the rat. Appetite 2009, 52, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.R.; Singh, S.; Youssef, F.F. Cafeteria-diet induced obesity results in impaired cognitive functioning in a rodent model. Heliyon 2019, 5, e01412. [Google Scholar] [CrossRef]
- Lalanza, J.F.; Snoeren, E.M.S. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci. Biobehav. Rev. 2021, 122, 92–119. [Google Scholar] [CrossRef] [PubMed]
- Buyukdere, Y.; Gulec, A.; Mutlu, A.A. Effect of cafeteria diet and high fat diet on body composition and biochemical parameters in rats. Clin. Nutr. 2018, 37, S269–S270. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Fu, L.-C.; Chong, H.-C.; Tang, S.-T.; Yang, S.-C.; Huang, W.-C.; Yang, Y.-C.S.; Chen, Y.-L. Consumption of a Taiwanese cafeteria diet induces metabolic disorders and fecal flora changes in obese rats. Nutrition 2024, 117, 112230. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Hasselwander, S.; Li, H.; Xia, N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci. Rep. 2019, 9, 19556. [Google Scholar] [CrossRef]
- Gasparin, F.R.S.; Carreño, F.O.; Mewes, J.M.; Gilglioni, E.H.; Pagadigorria, C.L.S.; Natali, M.R.M.; Utsunomiya, K.S.; Constantin, R.P.; Ouchida, A.T.; Curti, C.; et al. Sex differences in the development of hepatic steatosis in cafeteria diet-induced obesity in young mice. Biochim. Biophys. Acta 2018, 1864, 2495–2509. [Google Scholar] [CrossRef]
- Coatmellec-Taglioni, G.; Dausse, J.-P.; Giudicelli, Y.; Ribière, C. Gender difference in diet-induced obesity hypertension: Implication of renal α2-adrenergic receptors. Am. J. Hypertens. 2002, 15, 143–149. [Google Scholar] [CrossRef]
- Maric, I.; Krieger, J.P.; van der Velden, P.; Börchers, S.; Asker, M.; Vujicic, M.; Wernstedt Asterholm, I.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 828522. [Google Scholar] [CrossRef]
- Šebeková, K.; Brouder Šebeková, K. Glycated proteins in nutrition: Friend or foe? Exp. Gerontol. 2019, 117, 76–90. [Google Scholar] [CrossRef]
- Collison, K.S.; Parhar, R.S.; Saleh, S.S.; Meyer, B.F.; Kwaasi, A.A.; Hammami, M.M.; Schmidt, A.M.; Stern, D.M.; Al-Mohanna, F.A. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J. Leukoc. Biol. 2002, 71, 433–444. [Google Scholar] [CrossRef]
- Tadié, J.M.; Bae, H.B.; Banerjee, S.; Zmijewski, J.W.; Abraham, E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am. J. Physiol. Cell Physiol. 2012, 302, C249–C256. [Google Scholar] [CrossRef]
- Cichon, I.; Ortmann, W.; Santocki, M.; Opydo-Chanek, M.; Kolaczkowska, E. Scrutinizing Mechanisms of the ‘Obesity Paradox in Sepsis’: Obesity Is Accompanied by Diminished Formation of Neutrophil Extracellular Traps (NETs) Due to Restricted Neutrophil-Platelet Interactions. Cells 2021, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Cichon, I.; Ortmann, W.; Kolaczkowska, E. Metabolic Pathways Involved in Formation of Spontaneous and Lipopolysaccharide-Induced Neutrophil Extracellular Traps (NETs) Differ in Obesity and Systemic Inflammation. Int. J. Mol. Sci. 2021, 22, 7718. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Venugopal, J.; Wang, J.; Kleiman, K.; Guo, C.; Eitzman, D.T. Obesity-induced Endothelial Dysfunction is Prevented by Neutrophil Extracellular Trap Inhibition. Sci. Rep. 2018, 8, 4881. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Yang, S.; Zhang, L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front. Immunol. 2017, 8, 928. [Google Scholar] [CrossRef]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T.; the California Pandemic (H1N1) Working Group. A Novel Risk Factor for a Novel Virus: Obesity and 2009 Pandemic Influenza A (H1N1). Clin. Infect. Dis. 2011, 52, 301–312. [Google Scholar] [CrossRef]
- Moorthy, A.N.; Tan, K.B.; Wang, S.; Narasaraju, T.; Chow, V.T. Effect of High-Fat Diet on the Formation of Pulmonary Neutrophil Extracellular Traps during Influenza Pneumonia in BALB/c Mice. Front. Immunol. 2016, 7, 289. [Google Scholar] [CrossRef] [PubMed]
- Kian, N.; Bagheri, A.; Salmanpour, F.; Soltani, A.; Mohajer, Z.; Samieefar, N.; Barekatain, B.; Kelishadi, R. Breast feeding, obesity, and asthma association: Clinical and molecular views. Clin. Mol. Allergy 2023, 21, 8. [Google Scholar] [CrossRef]
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. npj Prim. Care Respir. Med. 2023, 33, 7. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Wei, L.; Bao, L.; Hu, H.; Liu, L.; Tan, W.; Tong, X.; Huang, F. Involucrasin B suppresses airway inflammation in obese asthma by inhibiting the TLR4-NF-κB-NLRP3 pathway. Phytomedicine 2024, 132, 155850. [Google Scholar] [CrossRef]
- Koyanagi, Y.N.; Matsuo, K.; Ito, H.; Tamakoshi, A.; Sugawara, Y.; Hidaka, A.; Wada, K.; Oze, I.; Kitamura, Y.; Liu, R.; et al. Body-Mass Index and Pancreatic Cancer Incidence: A Pooled Analysis of Nine Population-Based Cohort Studies With More Than 340,000 Japanese Subjects. J. Epidemiol. 2018, 28, 245–252. [Google Scholar] [CrossRef]
- Zohar, L.; Rottenberg, Y.; Twig, G.; Katz, L.; Leiba, A.; Derazne, E.; Tzur, D.; Eizenstein, S.; Keinan-Boker, L.; Afek, A.; et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: A nationwide study of 1.79 million Israeli adolescents. Cancer 2019, 125, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, C.C.; Chang, C.Y.; Li, J.R.; Ou, Y.C.; Chen, W.Y.; Liao, S.L.; Wang, J.D. Metformin Mitigated Obesity-Driven Cancer Aggressiveness in Tumor-Bearing Mice. Int. J. Mol. Sci. 2022, 23, 9134. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gao, H.; Dai, S.; Li, M.; Gao, Y.; Yin, L.; Zhang, K.; Zhang, J.; Jiang, K.; Miao, Y.; et al. Metformin inhibits neutrophil extracellular traps-promoted pancreatic carcinogenesis in obese mice. Cancer Lett. 2023, 562, 216155. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Okura, A.; Hoshide, S.; Mogi, M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens. Res. 2024, 47, 1099–1102. [Google Scholar] [CrossRef]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients With Psoriasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Nosalski, R.; Maffia, P.; Drummond, G.R. Immune and inflammatory mechanisms in hypertension. Nat. Rev. Cardiol. 2024, 21, 396–416. [Google Scholar] [CrossRef] [PubMed]
- Siedlinski, M.; Jozefczuk, E.; Xu, X.; Teumer, A.; Evangelou, E.; Schnabel, R.B.; Welsh, P.; Maffia, P.; Erdmann, J.; Tomaszewski, M.; et al. White Blood Cells and Blood Pressure: A Mendelian Randomization Study. Circulation 2020, 141, 1307–1317. [Google Scholar] [CrossRef]
- Tatsukawa, Y.; Hsu, W.L.; Yamada, M.; Cologne, J.B.; Suzuki, G.; Yamamoto, H.; Yamane, K.; Akahoshi, M.; Fujiwara, S.; Kohno, N. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens. Res. 2008, 31, 1391–1397. [Google Scholar] [CrossRef]
- Sarejloo, S.; Dehesh, M.; Fathi, M.; Khanzadeh, M.; Lucke-Wold, B.; Ghaedi, A.; Khanzadeh, S. Meta-analysis of differences in neutrophil to lymphocyte ratio between hypertensive and non-hypertensive individuals. BMC Cardiovasc. Disord. 2023, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.M.; Sobey, C.G.; Latz, E.; Mansell, A.; Drummond, G.R. IL-1β and IL-18: Inflammatory markers or mediators of hypertension? Br. J. Pharmacol. 2014, 171, 5589–5602. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.E.; Vera, L.M.; Arenas, I.A.; Gamarra, G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J. Hum. Hypertens. 2005, 19, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, K.; Chen, F.; Liu, Q.; Ni, J.; Cao, W.; Hua, Y.; He, F.; Liu, Z.; Li, L.; et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front. Immunol. 2022, 13, 975367. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.L.; Lewis, C.; Murrah, N.V.; Anderson, G.T.; Vaccarino, V. Relation of C-reactive protein and tumor necrosis factor-alpha to ambulatory blood pressure variability in healthy adults. Am. J. Cardiol. 2006, 98, 649–652. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Jackson, M.H.; Collier, A.; Nicoll, J.J.; Muir, A.L.; Dawes, J.; Clarke, B.F.; Bell, D. Neutrophil count and activation in vascular disease. Scott. Med. J. 1992, 37, 41–43. [Google Scholar] [CrossRef]
- El-Eshmawy, M.M.; El-Adawy, E.H.; Mousa, A.A.; Zeidan, A.E.; El-Baiomy, A.A.; Abdel-Samie, E.R.; Saleh, O.M. Elevated serum neutrophil elastase is related to prehypertension and airflow limitation in obese women. BMC Womens Health 2011, 11, 1. [Google Scholar] [CrossRef][Green Version]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Downey, G.P. Leukocyte elastase: Physiological functions and role in acute lung injury. Am. J. Respir. Crit. Care Med. 2001, 164, 896–904. [Google Scholar] [CrossRef]
- Bank, U.; Ansorge, S. More than destructive: Neutrophil-derived serine proteases in cytokine bioactivity control. J. Leukoc. Biol. 2001, 69, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, A.J.; Miyagawa, K.; Rhodes, C.J.; Taylor, S.; Del Rosario, P.A.; Hsi, A.; Haddad, F.; Spiekerkoetter, E.; Bental-Roof, M.; Bland, R.D.; et al. Severe Pulmonary Arterial Hypertension Is Characterized by Increased Neutrophil Elastase and Relative Elafin Deficiency. Chest 2021, 160, 1442–1458. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Gkaliagkousi, E.; Lazaridis, A.; Arelaki, S.; Pateinakis, P.; Ntinopoulou, M.; Mitsios, A.; Antoniadou, C.; Argyriou, C.; Georgiadis, G.S.; et al. Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension. JCI Insight 2021, 6, e148668. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, T.; Scherz, T.; Müller, J.; Heidari, H.; Staier, N.; Panzenböck, A.; Mangold, A.; Lang, I.M. Arterial hypertension enhances neutrophil extracellular trap formation via an angiotensin-II-dependent pathway. Atherosclerosis 2017, 263, e67–e68. [Google Scholar] [CrossRef]
- Li, J.; Tong, D.; Song, B.; Xie, F.; Zhang, G.; Hao, X.; Li, W.; Chi, H.; Wang, W.; Shao, Y. Inflammatory cytokines induce neutrophil extracellular traps interaction with activated platelets and endothelial cells exacerbate coagulation in moderate and severe essential hypertension. J. Hypertens. 2022, 40, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Aldabbous, L.; Abdul-Salam, V.; McKinnon, T.; Duluc, L.; Pepke-Zaba, J.; Southwood, M.; Ainscough, A.J.; Hadinnapola, C.; Wilkins, M.R.; Toshner, M.; et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arter. Thromb. Vasc. Biol. 2016, 36, 2078–2087. [Google Scholar] [CrossRef]
- Fang, X.; Ma, L.; Wang, Y.; Ren, F.; Yu, Y.; Yuan, Z.; Wei, H.; Zhang, H.; Sun, Y. Neutrophil extracellular traps accelerate vascular smooth muscle cell proliferation via Akt/CDKN1b/TK1 accompanying with the occurrence of hypertension. J. Hypertens. 2022, 40, 2045–2057. [Google Scholar] [CrossRef]
- Hilscher, M.B.; Sehrawat, T.; Arab, J.P.; Zeng, Z.; Gao, J.; Liu, M.; Kostallari, E.; Gao, Y.; Simonetto, D.A.; Yaqoob, U.; et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology 2019, 157, 193–209.e9. [Google Scholar] [CrossRef]
- Stadler, J.T.; Marsche, G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020, 21, 8985. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Janovičová, Ľ.; Čonka, J.; Lauková, L.; Celec, P. Variability of endogenous deoxyribonuclease activity and its pathophysiological consequences. Mol. Cell. Probes 2022, 65, 101844. [Google Scholar] [CrossRef]
- Arelaki, S.; Koletsa, T.; Sinakos, E.; Papadopoulos, V.; Arvanitakis, K.; Skendros, P.; Akriviadis, E.; Ritis, K.; Germanidis, G.; Hytiroglou, P. Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 2022, 481, 455–465. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; He, T.; Zhang, S.; Wang, Y.; Xie, Z.; Xu, W.; Ding, C.; Shuai, Y.; Hao, H.; et al. Polyunsaturated fatty acids drive neutrophil extracellular trap formation in nonalcoholic steatohepatitis. Eur. J. Pharmacol. 2023, 945, 175618. [Google Scholar] [CrossRef]
- Sun, R.; Xu, D.; Wei, Q.; Zhang, B.; Aa, J.; Wang, G.; Xie, Y. Silybin ameliorates hepatic lipid accumulation and modulates global metabolism in an NAFLD mouse model. Biomed. Pharmacother. 2020, 123, 109721. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, X.; Jia, T.; Sun, Y.; Du, Y.; Wei, S.; Wang, W.; Zhang, Y.; Chen, W.; Zhang, S. Tanshinone IIA Ameliorates Nonalcoholic Steatohepatitis in Mice by Modulating Neutrophil Extracellular Traps and Hepatocyte Apoptosis. Evid. Based Complement. Altern. Med. 2022, 2022, 5769350. [Google Scholar] [CrossRef]
- Xu, Z.J.; Fan, J.G.; Ding, X.D.; Qiao, L.; Wang, G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010, 55, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Terauchi, Y. Lessons from Mouse Models of High-Fat Diet-Induced NAFLD. Int. J. Mol. Sci. 2013, 14, 21240–21257. [Google Scholar] [CrossRef]
- Kucera, O.; Cervinkova, Z. Experimental models of non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2014, 20, 8364–8376. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, H.B.; Andringa, K.K.; Betancourt, A.M.; King, A.L.; Mantena, S.K.; Swain, T.M.; Tinsley, H.N.; Nolte, R.N.; Nagy, T.R.; Abrams, G.A.; et al. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid. Redox Signal. 2011, 15, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, H.; Xu, H.; Li, J.; Golovko, M.; Cheng, H.; Lynch, E.C.; Liu, L.; McCauley, N.; Kennedy, L.; et al. Maternal diet intervention before pregnancy primes offspring lipid metabolism in liver. Lab. Investig. 2020, 100, 553–569. [Google Scholar] [CrossRef]
- Moeckli, B.; Delaune, V.; Prados, J.; Tihy, M.; Peloso, A.; Oldani, G.; Delmi, T.; Slits, F.; Gex, Q.; Rubbia-Brandt, L.; et al. Impact of Maternal Obesity on Liver Disease in the Offspring: A Comprehensive Transcriptomic Analysis and Confirmation of Results in a Murine Model. Biomedicines 2022, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Mihalovičová, L.; Kunšteková, V.; Miláček, D.; Janko, J.; Pastorek, M.; Konečná, B.; Gurecká, R.; Rausová, Z.; Uličná, O.; Celec, P.; et al. Severe gestational diabetes mellitus in lean dams is associated with low IL-1α levels and affects the growth of the juvenile mouse offspring. Sci. Rep. 2023, 13, 1700. [Google Scholar] [CrossRef]
- Van Bruggen, S.; Sheehy, C.E.; Kraisin, S.; Frederix, L.; Wagner, D.D.; Martinod, K. Neutrophil peptidylarginine deiminase 4 plays a systemic role in obesity-induced chronic inflammation in mice. J. Thromb. Haemost. 2024, 22, 1496–1509. [Google Scholar] [CrossRef]
- Krishnan, J.; Hennen, E.M.; Ao, M.; Kirabo, A.; Ahmad, T.; de la Visitación, N.; Patrick, D.M. NETosis Drives Blood Pressure Elevation and Vascular Dysfunction in Hypertension. Circ. Res. 2024, 134, 1483–1494. [Google Scholar] [CrossRef]
- Liu, W.Q.; Zhang, Y.Z.; Wu, Y.; Zhang, J.J.; Li, T.B.; Jiang, T.; Xiong, X.M.; Luo, X.J.; Ma, Q.L.; Peng, J. Myeloperoxidase-derived hypochlorous acid promotes ox-LDL-induced senescence of endothelial cells through a mechanism involving β-catenin signaling in hyperlipidemia. Biochem. Biophys. Res. Commun. 2015, 467, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Piek, A.; Koonen, D.P.Y.; Schouten, E.-M.; Lindtstedt, E.L.; Michaëlsson, E.; de Boer, R.A.; Silljé, H.H.W. Pharmacological myeloperoxidase (MPO) inhibition in an obese/hypertensive mouse model attenuates obesity and liver damage, but not cardiac remodeling. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Lehners, A.; Lange, S.; Niemann, G.; Rosendahl, A.; Meyer-Schwesinger, C.; Oh, J.; Stahl, R.; Ehmke, H.; Benndorf, R.; Klinke, A.; et al. Myeloperoxidase deficiency ameliorates progression of chronic kidney disease in mice. Am. J. Physiol. Physiol. 2014, 307, F407–F417. [Google Scholar] [CrossRef] [PubMed]
- Tokuhiro, T.; Ishikawa, A.; Sato, H.; Takita, S.; Yoshikawa, A.; Anzai, R.; Sato, S.; Aoyagi, R.; Arita, M.; Shibuya, T.; et al. Oxidized Phospholipids and Neutrophil Elastase Coordinately Play Critical Roles in NET Formation. Front. Cell Dev. Biol. 2021, 9, 718586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sano, S.; Oshima, K.; Sano, M.; Watanabe, Y.; Katanasaka, Y.; Yura, Y.; Jung, C.; Anzai, A.; Swirski, F.K.; et al. Wnt5a-Mediated Neutrophil Recruitment Has an Obligatory Rol in Pressure Overload-Induced Cardiac Dysfunction. Circulation 2019, 140, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Zhou, Q.L.; Xie, X.; Gong, Z.; Huang, J.-Y.; Khan, A.R.; Aubert, G.; Candelaria, K.; Thomas, S.; Shin, D.-J.; et al. Imbalance between Neutrophil Elastase and its Inhibitor α1-Antitrypsin in Obesity Alters Insulin Sensitivity, Inflammation, and Energy Expenditure. Cell Metab. 2013, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Ushakumari, C.J.; Zhou, Q.L.; Wang, Y.H.; Na, S.; Rigor, M.C.; Zhou, C.Y.; Kroll, M.K.; Lin, B.D.; Jiang, Z.Y. Neutrophil Elastase Increases Vascular Permeability and Leukocyte Transmigration in Cultured Endothelial Cells and Obese Mice. Cells 2022, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feješ, A.; Šebeková, K.; Borbélyová, V. Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models. Nutrients 2025, 17, 241. https://doi.org/10.3390/nu17020241
Feješ A, Šebeková K, Borbélyová V. Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models. Nutrients. 2025; 17(2):241. https://doi.org/10.3390/nu17020241
Chicago/Turabian StyleFeješ, Andrej, Katarína Šebeková, and Veronika Borbélyová. 2025. "Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models" Nutrients 17, no. 2: 241. https://doi.org/10.3390/nu17020241
APA StyleFeješ, A., Šebeková, K., & Borbélyová, V. (2025). Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models. Nutrients, 17(2), 241. https://doi.org/10.3390/nu17020241





