Vitamin K1 Administration Increases the Level of Circulating Carboxylated Osteocalcin in Critically Ill Patients
Highlights
- Vitamin K metabolism can be affected in critically ill patients in the intensive care unit (ICU), which could impair the function of vitamin K-dependent proteins (VKDPs).
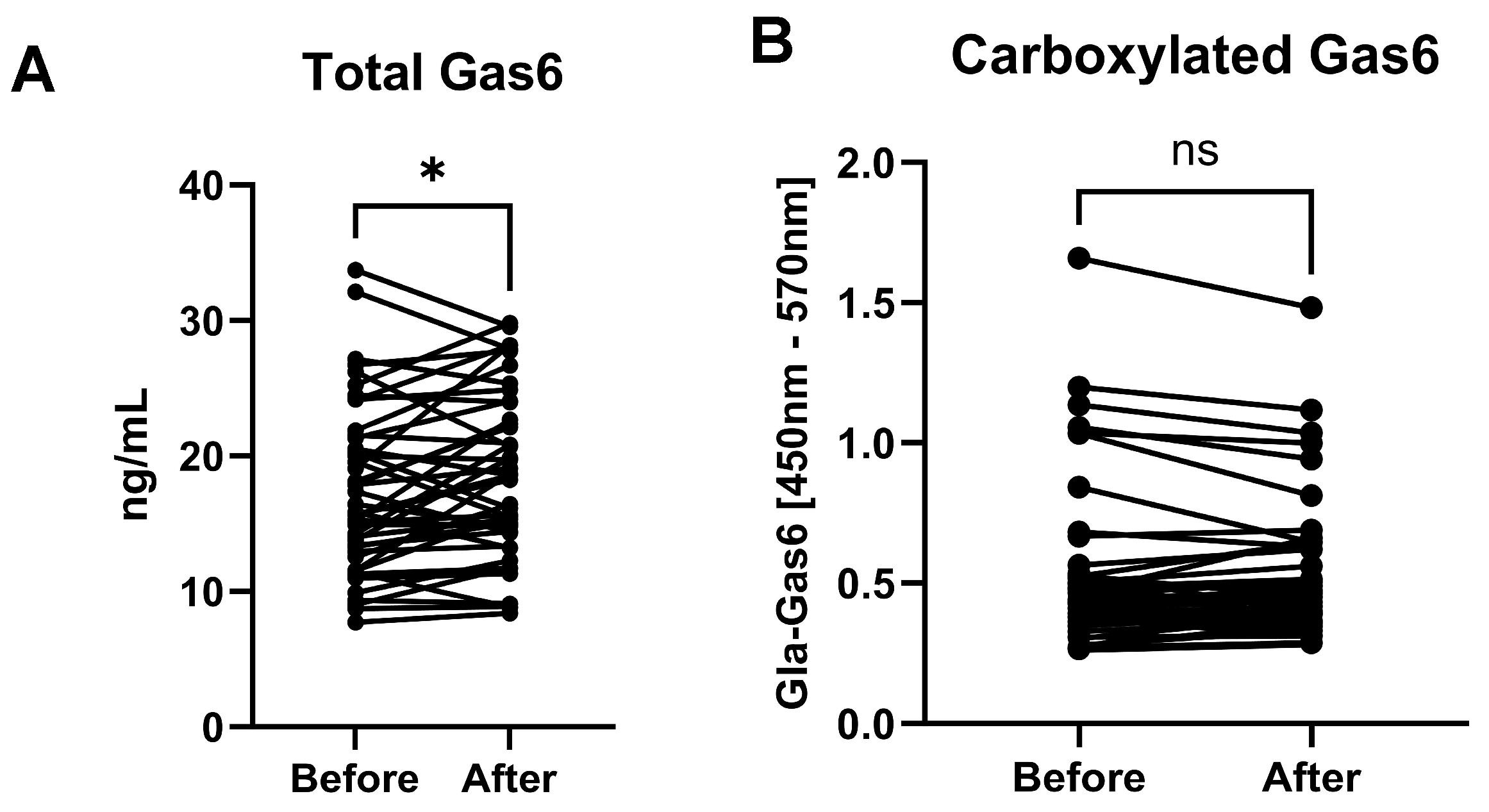
- Intravenous vitamin K1 administration to ICU patients resulted in raised plasma levels of two VKDPs, carboxylated osteocalcin and Gas6, after 24 hours.
- This study demonstrates the direct post-translational modifying effect of vitamin K on a VKDP in humans.
- The study also demonstrates the potential of analysis of VKDPs as surrogate markers of vitamin K metabolism in human subjects.
- As several VKDPs are implicated in inflammation regulation, vitamin K administration may be of benefit to the management of critically ill patients.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Enzyme-Linked Immunosorbent Assays (ELISAs) for the Quantification of VKDPs in Human Plasma
2.3. Statistical Analyses
3. Results
3.1. Patient Population
3.2. Total Gas6 and Gla-Gas6 Plasma Concentrations Before and After Vitamin K
3.3. Undercarboxylated and Carboxylated Osteocalcin Plasma Concentrations Before and After Vitamin K
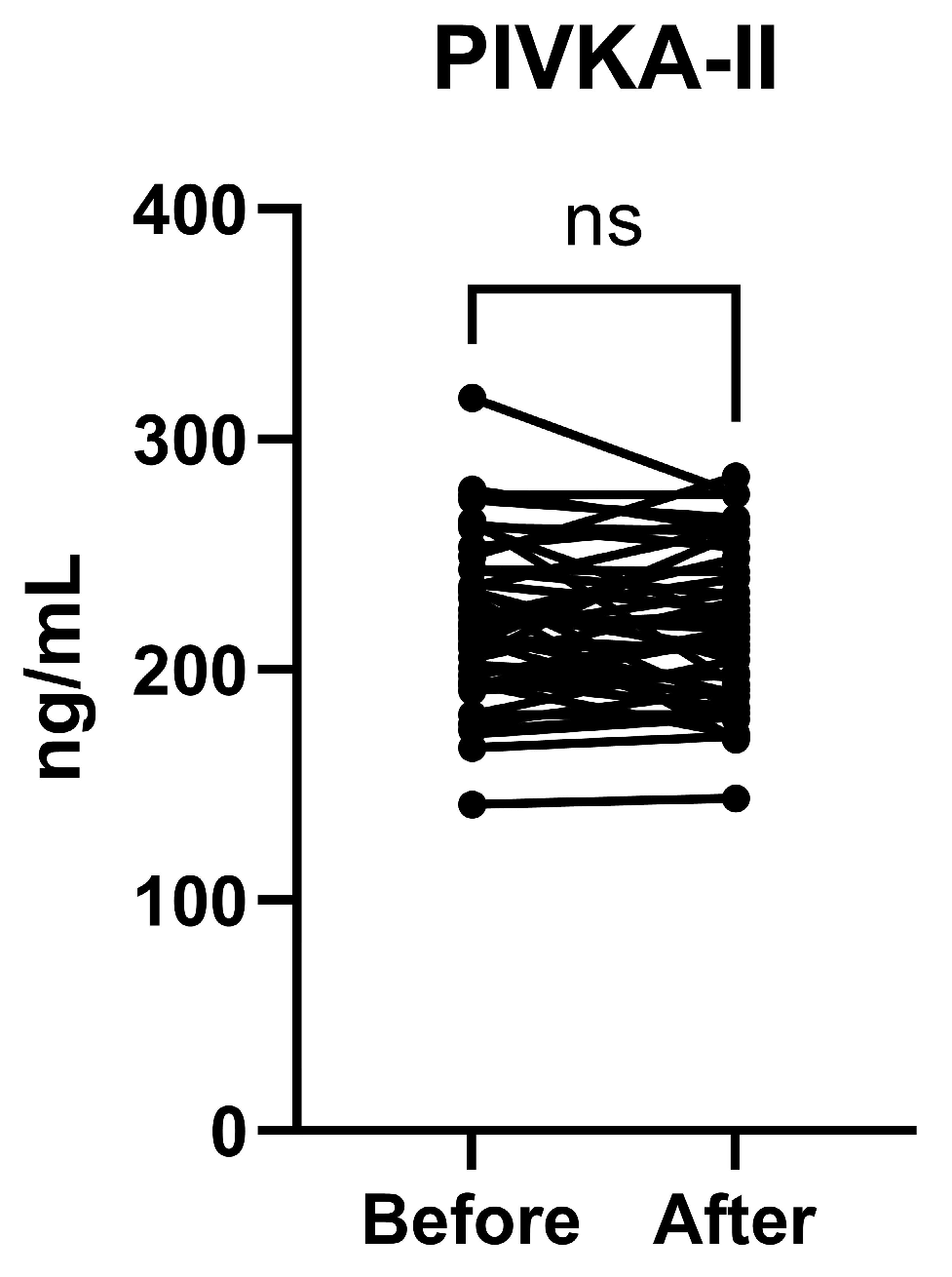
3.4. PIVKA-II Plasma Concentrations Before and After Vitamin K
3.5. Correlations Between Analyte Levels in This Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermeer, C. Vitamin K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56, 5329. [Google Scholar] [CrossRef] [PubMed]
- Berkner, K.L. The vitamin K-dependent carboxylase. Annu. Rev. Nutr. 2005, 25, 127–149. [Google Scholar] [CrossRef]
- Rishavy, M.A.; Hallgren, K.W.; Wilson, L.A.; Usubalieva, A.; Runge, K.W.; Berkner, K.L. The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation. J. Biol. Chem. 2013, 288, 31556–31566. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Marinova, M.; Müller-Reible, C.; Watzka, M. The vitamin K cycle. Vitam. Horm. 2008, 78, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of emerging vitamin K-dependent proteins: Growth arrest-specific protein 6, Gla-rich protein and periostin (Review). Int. J. Mol. Med. 2021, 47, 2. [Google Scholar] [CrossRef]
- Wallin, R.; Cain, D.; Sane, D.C. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells—A cell system which resembles the system in bone cells. Thromb. Haemost. 1999, 82, 1764–1767. [Google Scholar]
- Aydin, N.; Ouliass, B.; Ferland, G.; Hafizi, S. Modification of Gas6 Protein in the Brain by a Functional Endogenous Tissue Vitamin K Cycle. Cells 2024, 13, 873. [Google Scholar] [CrossRef]
- Berkner, K.L.; Runge, K.W. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J. Thromb. Haemost. 2004, 2, 2118–2132. [Google Scholar] [CrossRef]
- Binkley, N.; Harke, J.; Krueger, D.; Engelke, J.; Vallarta-Ast, N.; Gemar, D.; Checovich, M.; Chappell, R.; Suttie, J. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J. Bone Miner. Res. 2009, 24, 983–991. [Google Scholar] [CrossRef]
- Paulus, M.C.; Drent, M.; Kouw, I.W.K.; Balvers, M.G.J.; Bast, A.; van Zanten, A.R.H. Vitamin K: A potential missing link in critical illness-a scoping review. Crit. Care 2024, 28, 212. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, S.; Schurgers, L.; Schött, U.; Kander, T. Vitamin K deficiency in critical ill patients; a prospective observational study. J. Crit. Care 2019, 49, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Dahlbäck, B. Gas6 and protein S: Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Carrera-Silva, E.A.; Bosurgi, L.; Ghosh, S. TAM receptor signaling in immune homeostasis. Annu. Rev. Immunol. 2015, 33, 355–391. [Google Scholar] [CrossRef]
- Ekman, C.; Linder, A.; Akesson, P.; Dahlbäck, B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit. Care 2010, 14, R158. [Google Scholar] [CrossRef] [PubMed]
- Schött, U.; Augustsson, C.; Lilover, L.; Nilsson, C.U.; Walther-Sturesson, L.; Kander, T. Vitamin K Effects on Gas6 and Soluble Axl Receptors in Intensive Care Patients: An Observational Screening Study. Nutrients 2021, 13, 4101. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Gao, Y.; Hu, X.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in Hefei City, China: Diagnostic value in organ injuries. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, S.; Schött, U.; Eriksson, E.; Tahirsylaj, Y.; Schurgers, L.; Kander, T. Intravenous Vitamin K1 for the Correction of Prolonged Prothrombin Times in Non-Bleeding Critically Ill Patients: A Prospective Observational Study. Nutrients 2021, 13, 2580. [Google Scholar] [CrossRef]
- Dahlberg, S.; Schött, U.; Kander, T. The effect of vitamin K on prothrombin time in critically ill patients: An observational registry study. J. Intensive Care 2021, 9, 11. [Google Scholar] [CrossRef]
- Dahlberg, S.; Ede, J.; Schurgers, L.; Vermeer, C.; Kander, T.; Klarin, B.; Schött, U. Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients. Nutrients 2018, 10, 46. [Google Scholar] [CrossRef]
- Braam, L.; McKeown, N.; Jacques, P.; Lichtenstein, A.; Vermeer, C.; Wilson, P.; Booth, S. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J. Am. Diet. Assoc. 2004, 104, 1410–1414. [Google Scholar] [CrossRef]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Van Roosbroeck, D.; Vanhove, P.; Wouters, P.J.; De Pourcq, L.; Bouillon, R. Bone turnover in prolonged critical illness: Effect of vitamin D. J. Clin. Endocrinol. Metab. 2003, 88, 4623–4632. [Google Scholar] [CrossRef]
- Schwetz, V.; Schnedl, C.; Urbanic-Purkart, T.; Trummer, C.; Dimai, H.P.; Fahrleitner-Pammer, A.; Putz-Bankuti, C.; Christopher, K.B.; Obermayer-Pietsch, B.; Pieber, T.R.; et al. Effect of vitamin D3 on bone turnover markers in critical illness: Post hoc analysis from the VITdAL-ICU study. Osteoporos. Int. 2017, 28, 3347–3354. [Google Scholar] [CrossRef]
- Arrieta, F.; Martinez-Vaello, V.; Bengoa, N.; Rosillo, M.; de Pablo, A.; Voguel, C.; Pintor, R.; Belanger-Quintana, A.; Mateo-Lobo, R.; Candela, A.; et al. Stress Hyperglycemia and Osteocalcin in COVID-19 Critically Ill Patients on Artificial Nutrition. Nutrients 2021, 13, 3010. [Google Scholar] [CrossRef] [PubMed]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced Vitamin K Status as a Potentially Modifiable Risk Factor of Severe Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e4039–e4046. [Google Scholar] [CrossRef]
- Visser, M.P.J.; Dofferhoff, A.S.M.; van den Ouweland, J.M.W.; de Jong, P.A.; Zanen, P.; van Daal, H.; Theeuwen, E.B.; Kramers, C.; Janssen, R.; Walk, J. Vitamin K2 Supplementation in Hospitalised COVID-19 Patients: A Randomised Controlled Trial. J. Clin. Med. 2024, 13, 3476. [Google Scholar] [CrossRef] [PubMed]
- Razny, U.; Fedak, D.; Kiec-Wilk, B.; Goralska, J.; Gruca, A.; Zdzienicka, A.; Kiec-Klimczak, M.; Solnica, B.; Hubalewska-Dydejczyk, A.; Malczewska-Malec, M. Carboxylated and undercarboxylated osteocalcin in metabolic complications of human obesity and prediabetes. Diabetes Metab. Res. Rev. 2017, 33, e2862. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Sokoll, L.J.; Booth, S.L.; O’Brien, M.E.; Davidson, K.W.; Tsaioun, K.I.; Sadowski, J.A. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma-carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am. J. Clin. Nutr. 1997, 65, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Vermeer, C.; Koos, R.; Boumans, M.L.; Hackeng, T.M.; Bouwman, F.G.; Kwaijtaal, M.; Brandenburg, V.M.; Ketteler, M.; Schurgers, L.J. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J. Vasc. Res. 2008, 45, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Prynne, C.J.; Thane, C.W.; Prentice, A.; Wadsworth, M.E. Intake and sources of phylloquinone (vitamin K1) in 4-year-old British children: Comparison between 1950 and the 1990s. Public Health Nutr. 2005, 8, 171–180. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary reference values for vitamin K. EFSA J. 2017, 15, e04780. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.M.; Stein, K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog. Food Nutr. Sci. 1992, 16, 307–343. [Google Scholar] [PubMed]
- Karl, J.P.; Fu, X.; Wang, X.; Zhao, Y.; Shen, J.; Zhang, C.; Wolfe, B.E.; Saltzman, E.; Zhao, L.; Booth, S.L. Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am. J. Clin. Nutr. 2015, 102, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Choonara, I.A.; Scott, A.K.; Haynes, B.P.; Cholerton, S.; Breckenridge, A.M.; Park, B.K. Vitamin K1 metabolism in relation to pharmacodynamic response in anticoagulated patients. Br. J. Clin. Pharmacol. 1985, 20, 643–648. [Google Scholar] [CrossRef]
- Sokoll, L.J.; Sadowski, J.A. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am. J. Clin. Nutr. 1996, 63, 566–573. [Google Scholar] [CrossRef]
- Strople, J.; Lovell, G.; Heubi, J. Prevalence of subclinical vitamin K deficiency in cholestatic liver disease. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dauti, F.; Jonsson, M.H.; Hillarp, A.; Bentzer, P.; Schött, U. Perioperative changes in PIVKA-II. Scand. J. Clin. Lab. Invest. 2015, 75, 562–567. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, N.; Kander, T.; Schött, U.; Hafizi, S. Vitamin K1 Administration Increases the Level of Circulating Carboxylated Osteocalcin in Critically Ill Patients. Nutrients 2025, 17, 348. https://doi.org/10.3390/nu17020348
Aydin N, Kander T, Schött U, Hafizi S. Vitamin K1 Administration Increases the Level of Circulating Carboxylated Osteocalcin in Critically Ill Patients. Nutrients. 2025; 17(2):348. https://doi.org/10.3390/nu17020348
Chicago/Turabian StyleAydin, Nadide, Thomas Kander, Ulf Schött, and Sassan Hafizi. 2025. "Vitamin K1 Administration Increases the Level of Circulating Carboxylated Osteocalcin in Critically Ill Patients" Nutrients 17, no. 2: 348. https://doi.org/10.3390/nu17020348
APA StyleAydin, N., Kander, T., Schött, U., & Hafizi, S. (2025). Vitamin K1 Administration Increases the Level of Circulating Carboxylated Osteocalcin in Critically Ill Patients. Nutrients, 17(2), 348. https://doi.org/10.3390/nu17020348








