Combating Sarcopenia Through Nutrition: Anti-Inflammatory and Antioxidant Properties of Aronia melanocarpa
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology of Literature Review
2.1.1. Databases and Search Strategy
2.1.2. Inclusion Criteria
2.1.3. Exclusion Criteria
2.1.4. Filters
2.1.5. Strategies for Handling Conflicting Results
2.2. Application of AI in Manuscript Preparation
3. Results
3.1. Overview of Sarcopenia: Definition, Prevalence, and Clinical Relevance
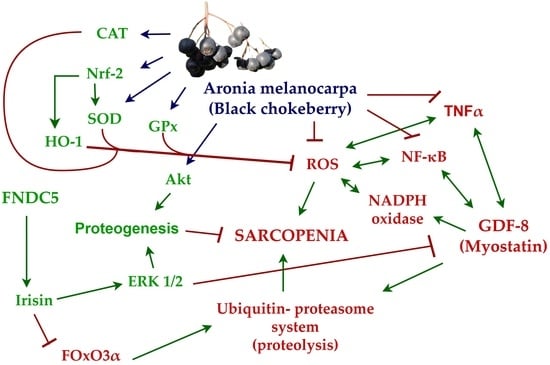
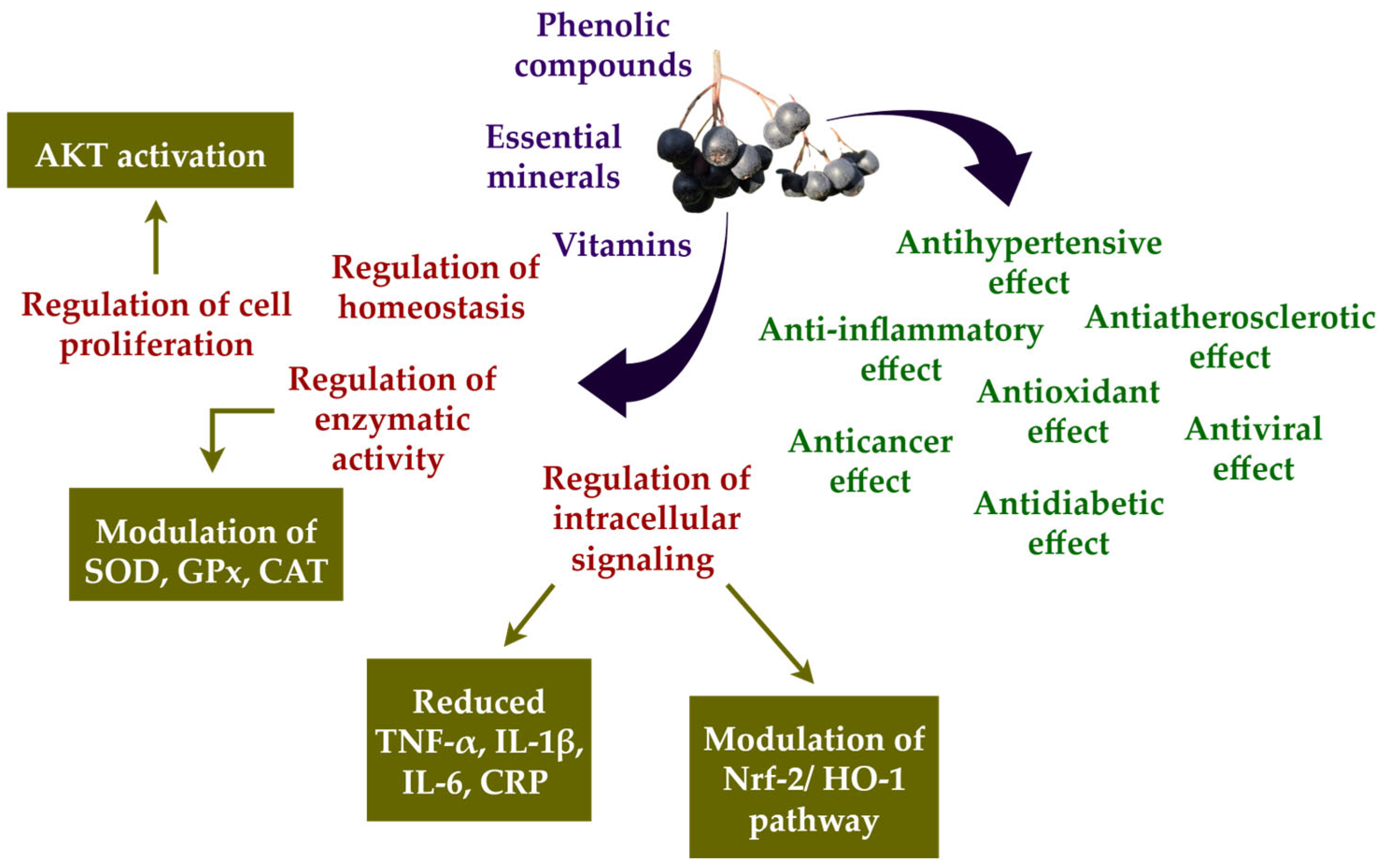
3.2. Aronia melanocarpa: A Nutritional Powerhouse for Reducing Oxidative Stress and Inflammation
3.3. The Role of Irisin and Myostatin in Overall Muscle State
3.4. Role of Oxidative Stress and Inflammation in the Development of Sarcopenia
3.5. Macrophages as Part of the Balance Between Pro- and Anti-Inflammatory Responses
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| CAT | Catalase |
| CD 80 | Cluster of differentiation 80 |
| CD 86 | Cluster of differentiation 86 |
| CD 163 | Cluster of differentiation 163 |
| CD 206 | Cluster of differentiation 206 |
| CRP | C-reactive protein |
| DNA | Deoxyribonucleic acid |
| FNDC-5 | Fibronectin type III domain-containing protein 5 |
| GDF-8 | Growth differentiation factor 8 |
| GPx | Glutathione peroxidase |
| HO-1 | Heme oxygenase 1 |
| IL-1β | Interleukin 1β |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| Keap 1 | Kelch-like ECH-associating protein 1 |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MDA | Malondialdehyde |
| MHC II | Histocompatibility complex molecules class II |
| MSTN | Myostatin |
| mTOR | Mammalian target of rapamycin |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NF-κB | Nuclear factor kappa B |
| NLRP-3 | NOD-, LRP-, and pyrin domain-containing protein 3 |
| NO | Nitric oxide |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| 8-OHdG | 8-hydroxy-2᾿-deoxyguanosine |
| PGE-2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| Raw 264.7 | Cell line derived from a mouse tumor induced by Abelson murine leukemia virus |
| ROS | Reactive oxygen species |
| SIRT 1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
References
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef]
- Henze, H.; Jung, M.; Ahrens, H.E.; Steiner, S.; von Maltzahn, J. Skeletal muscle aging—Stem cells in the spotlight. Mech. Ageing Dev. 2020, 189, 111283. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular senescence: A translational perspective. eBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Gori, C.; Rianda, S. Sarcopenia and nutrition. Adv. Food Nutr. Res. 2014, 71, 101–136. [Google Scholar] [CrossRef]
- Antuña, E.; Cachán-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.E.; So, H.K.; Vuong, T.A.; Na, M.W.; Anh, S.; Lee, H.K.; Kim, K.H.; Kang, J.S.; Bae, G.U.; Lee, S.J. Aronia upregulates myogenic differentiation and augments muscle mass and function through muscle metabolism. Front. Nutr. 2021, 8, 753643. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Grosman, Y.; Kalichman, L. The Intersection of Sarcopenia and Musculoskeletal Pain: Addressing Interconnected Challenges in Aging Care. Int. J. Environ. Res. Public Health 2025, 22, 547. [Google Scholar] [CrossRef]
- Abenavoli, L.; Statsenko, M.; Scarlata, G.G.M.; Morano, D.; Myazin, R.; Emelyanov, D. Sarcopenia and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Narrative Review. Livers 2024, 4, 495–506. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Cho, M.R.; Lee, S.; Song, S.K. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahizadeh, A.; Javankiani, S.; Ebrahimi, P.; Sharifi, F.; Soltani, P.; Amiri, M.; Moodi, M.; Khorashadizadeh, M.; Fakhrzadeh, H.; Ramezani, P.; et al. Sarcopenia association with physical and psychological indices in community-dwelling aged population: Birjand Longitudinal Aging Study (BLAS). Aging Clin. Exp. Res. 2025, 37, 198. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H.; Hwang, Y.C. Sarcopenia in youth. Metabolism 2023, 144, 155557. [Google Scholar] [CrossRef]
- Joseph, A.M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.D.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Szentesi, P.; Csernoch, L.; Dux, L.; Keller-Pintér, A. Changes in redox signaling in the skeletal muscle with aging. Oxid. Med. Cell. Longev. 2019, 2019, 4617801. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Perandini, L.A.; Chimin, P.; Lutkemeyer, D.D.S.; Câmara, N.O.S. Chronic inflammation in skeletal muscle impairs satellite cell function during regeneration: Can physical exercise restore the satellite cell niche? FEBS J. 2018, 285, 1973–1984. [Google Scholar] [CrossRef]
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef]
- Sarıkaya, B.; Kolay, E.; Guney-Coskun, M.; Yiğit-Ziolkowski, A.; Aktaç, Ş. The effect of black chokeberry (Aronia melanocarpa) on human inflammation biomarkers and antioxidant enzymes: A systematic review of randomized controlled trials. Nutr. Rev. 2025, 83, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; Gargan, S.; Swandulla, D.; Ohlendieck, K. Fiber-type shifting in sarcopenia of old age: Proteomic profiling of the contractile apparatus of skeletal muscles. Int. J. Mol. Sci. 2023, 24, 2415. [Google Scholar] [CrossRef]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry (Aronia melanocarpa) in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Platonova, E.Y.; Shaposhnikov, M.V.; Lee, H.Y.; Lee, J.H.; Min, K.J.; Moskalev, A. Black chokeberry (Aronia melanocarpa) extracts in terms of geroprotector criteria. Trends Food Sci. Technol. 2021, 114, 570–584. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential benefits of black chokeberry (Aronia melanocarpa) fruits and their constituents in improving human health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Parzonko, A.; Naruszewicz, M. Cardioprotective effects of Aronia melanocarpa anthocynanins. From laboratory experiments to clinical practice. Curr. Pharm. Des. 2016, 22, 174–179. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.W.; Bae, J.Y.; Heo, J.; Kim, D.; Han, S.Z.; Park, M.S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef]
- Dvorska, D.; Mazurakova, A.; Lackova, L.; Sebova, D.; Kajo, K.; Samec, M.; Brany, D.; Svajdlenka, E.; Treml, J.; Mersakova, S.; et al. Aronia melanocarpa L. fruit peels show anti-cancer effects in preclinical models of breast carcinoma: The perspectives in the chemoprevention and therapy modulation. Front. Oncol. 2024, 14, 1463656. [Google Scholar] [CrossRef]
- Luzak, B.; Golanski, J.; Rozalski, M.; Krajewska, U.; Olas, B.; Watala, C. Extract from Aronia melanocarpa fruits potentiates the inhibition of platelet aggregation in the presence of endothelial cells. Arch. Med. Sci. AMS 2010, 6, 141–144. [Google Scholar] [CrossRef]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V.Y. Antidiabetic Effects of Aronia melanocarpa and Its Other Therapeutic Properties. Front. Nutr. 2017, 4, 53. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Go, M.Y.; Kim, J.; Jeon, C.Y.; Shin, D.W. Functional activities and mechanisms of Aronia melanocarpa in human health. Curr. Issues Mol. Biol. 2024, 46, 8071–8087. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Xie, L.; Lee, S.G.; Vance, T.M.; Wang, Y.; Kim, B.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of aronia berry extract. Food Chem. 2016, 211, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising natural products with diverse pharmacological activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef] [PubMed]
- Case, A.J.; Agraz, D.; Ahmad, I.M.; Zimmerman, M.C. Low-dose Aronia melanocarpa concentrate attenuates paraquat-induced neurotoxicity. Oxid. Med. Cell. Longev. 2016, 2016, 5296271. [Google Scholar] [CrossRef]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-κB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef]
- Sreedharan, S.; Nair, V.; Bhargava, P.; Cisneros-Zevallos, L. Protective role of polyphenols from aronia berry (Aronia melanocarpa) against LPS-induced inflammation in colon cells and macrophages. Nutrients 2025, 17, 1652. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cai, P.J.; Dai, H.C.; Xiao, Y.H.; Jia, C.L.; Sun, A.D. Black chokeberry (Aronia melanocarpa L.) polyphenols attenuate obesity-induced colonic inflammation by regulating gut microbiota and the TLR4/NF-κB signaling pathway in high fat diet-fed rats. Food Funct. 2023, 14, 10014–10030. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Zheng, Y.; Liu, W.; Ding, C. Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota. Sci. Rep. 2021, 11, 20558. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Zheng, M.; Sheng, L.; Shi, D.; Song, K. A comprehensive review of the functional potential and sustainable applications of Aronia melanocarpa in the food industry. Plants 2024, 13, 3557. [Google Scholar] [CrossRef]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological aspects of selected myokines in skeletal muscle: Focus on aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Lee, S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/irisin: Physiology and pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. Res. 2021, 41, 294–303. [Google Scholar] [CrossRef]
- Gerosa, L.; Malvandi, A.M.; Malavolta, M.; Provinciali, M.; Lombardi, G. Exploring cellular senescence in the musculoskeletal system: Any insights for biomarkers discovery? Ageing Res. Rev. 2023, 88, 101943. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, N.; Wiczkowski, A. Analytics of oxidative stress markers in the early diagnosis of oxygen DNA damage. Adv. Clin. Exp. Med. 2017, 26, 155–166. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar] [CrossRef]
- Remacle, J.; Michiels, C.; Raes, M. The importance of antioxidant enzymes in cellular aging and degeneration. EXS 1992, 62, 99–108. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Abdelnaser, M.; Alaaeldin, R.; Attya, M.E.; Fathy, M. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 397, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Mohib, M.; Afnan, K.; Paran, T.Z.; Khan, S.; Sarker, J.; Hasan, N.; Hasan, I.; Sagor, A.T. Beneficial role of citrus fruit polyphenols against hepatic dysfunctions: A review. J. Diet. Suppl. 2018, 15, 223–250. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, X.; Liu, S.; Liu, Y.; Wang, D. Aronia melanocarpa prevents alcohol-induced chronic liver injury via regulation of Nrf2 signaling in C57BL/6 mice. Oxid. Med. Cell. Longev. 2020, 2020, 4054520. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef] [PubMed]
- Namgaladze, D.; Brüne, B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim. Biophys. Acta 2014, 1841, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Shavlakadze, T.; McGeachie, J.; Grounds, M.D. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 2010, 11, 363–376. [Google Scholar] [CrossRef]
- Duong, L.; Radley, H.G.; Lee, B.; Dye, D.E.; Pixley, F.J.; Grounds, M.D.; Nelson, D.J.; Jackaman, C. Macrophage function in the elderly and impact on injury repair and cancer. Immun. Ageing 2021, 18, 4. [Google Scholar] [CrossRef]
- Cui, Y.; Lin, Y.; Meng, X.; Ma, J.; Deng, H.; Liu, X.; He, X.; Zhao, J. Cyanidin-3-galactoside from Aronia melanocarpa ameliorates PM10-induced pulmonary injury by modulating M1/M2 macrophage polarization and NRF2/Sirt1 MAPK signaling. J. Funct. Foods 2021, 78, 104363. [Google Scholar] [CrossRef]

| Features | Type I Fibers | Type IIa Fibers | Type IIb/IIx Fibers |
|---|---|---|---|
| Speed of contraction | Slowest | Intermediate | Fastest |
| Mitochondria | Many | Fewer | Least |
| Glycogen content | Low | High | High |
| Color | Red | Red | White |
| Fatigue resistance | High | Moderate | Low |
| Activity duration | Long | Moderate | Short |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metodieva, K.; Dimitrov, I.; Bivolarska, A. Combating Sarcopenia Through Nutrition: Anti-Inflammatory and Antioxidant Properties of Aronia melanocarpa. Nutrients 2025, 17, 3333. https://doi.org/10.3390/nu17213333
Metodieva K, Dimitrov I, Bivolarska A. Combating Sarcopenia Through Nutrition: Anti-Inflammatory and Antioxidant Properties of Aronia melanocarpa. Nutrients. 2025; 17(21):3333. https://doi.org/10.3390/nu17213333
Chicago/Turabian StyleMetodieva, Kalina, Iliyan Dimitrov, and Anelia Bivolarska. 2025. "Combating Sarcopenia Through Nutrition: Anti-Inflammatory and Antioxidant Properties of Aronia melanocarpa" Nutrients 17, no. 21: 3333. https://doi.org/10.3390/nu17213333
APA StyleMetodieva, K., Dimitrov, I., & Bivolarska, A. (2025). Combating Sarcopenia Through Nutrition: Anti-Inflammatory and Antioxidant Properties of Aronia melanocarpa. Nutrients, 17(21), 3333. https://doi.org/10.3390/nu17213333