Vitamin D Binding Protein Gene Polymorphisms (rs4588 and rs7041) and VDBP Levels in Total Hip Replacement Outcomes
Abstract
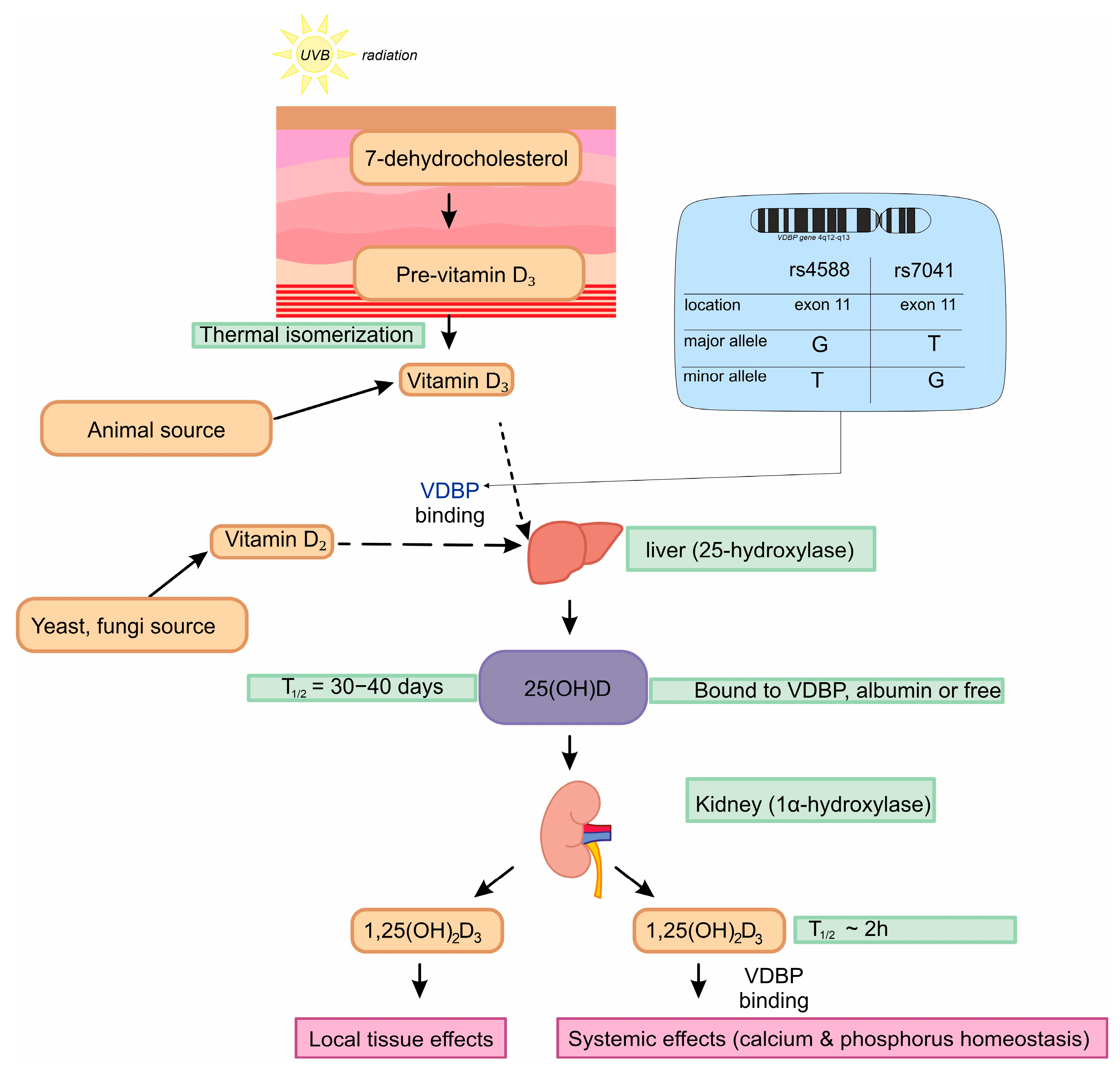
:1. Introduction
2. Materials and Methods
2.1. Characteristic of Patients and Control Groups
2.1.1. Recruitment and Sample Collection
2.1.2. Exclusion and Inclusion Criteria
2.2. Biological Material Collection
2.3. Concentration Assessment and Calculation
2.3.1. 25(OH)D Serum Concentration
2.3.2. Vitamin D Binding Protein Concentration
2.4. Genotyping
2.4.1. rs7041
2.4.2. rs4588
2.5. Statistical Analysis
3. Results
3.1. Frequencies
3.2. Logistic Regression Analysis of rs4588 and rs7041
3.3. VDBP Concentration
3.3.1. VDBP Among the Studied Groups
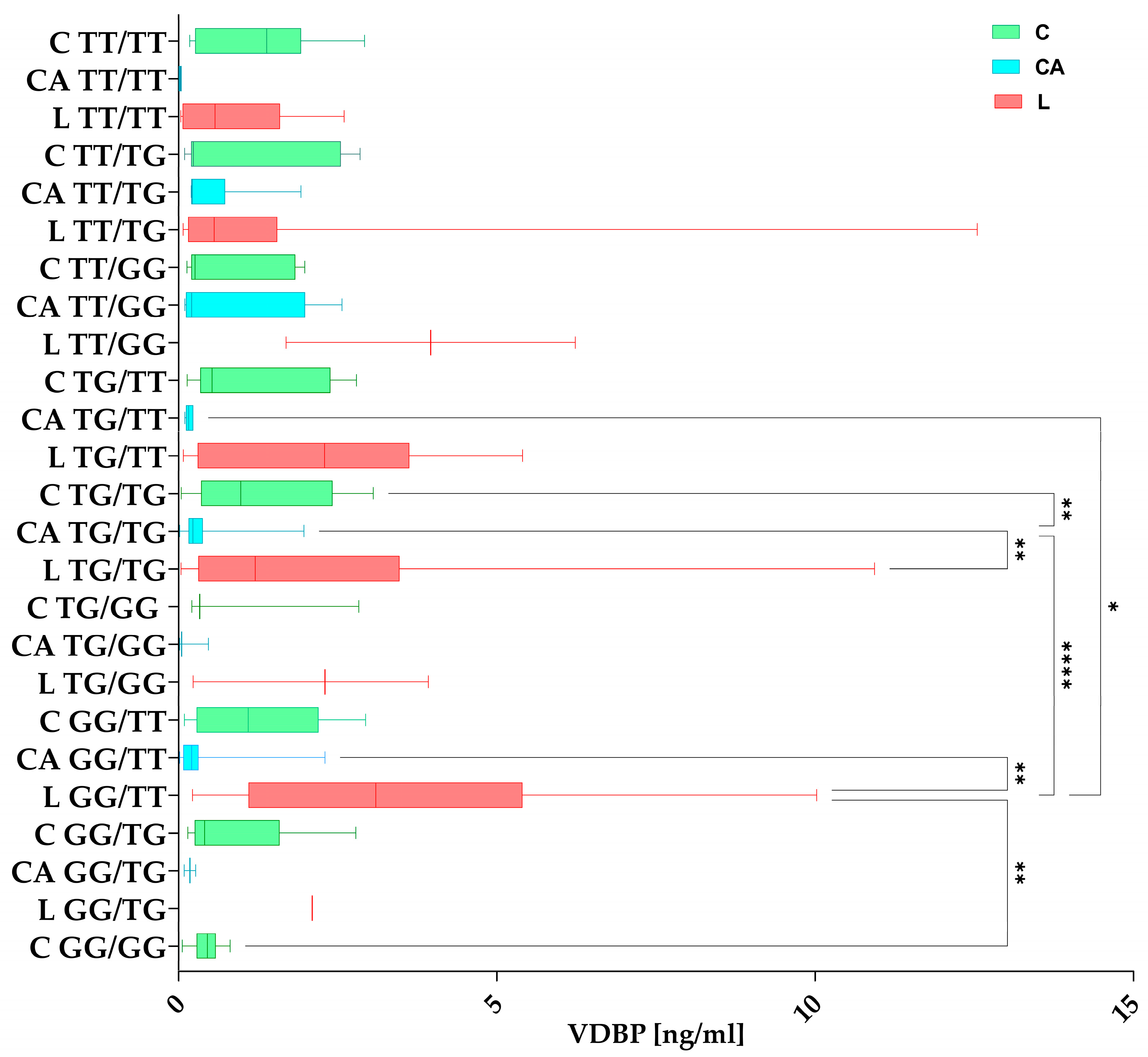
3.3.2. VDBP Concentrations in Haplotypes Among the Studied Groups
3.4. 25(OH)D Concentration
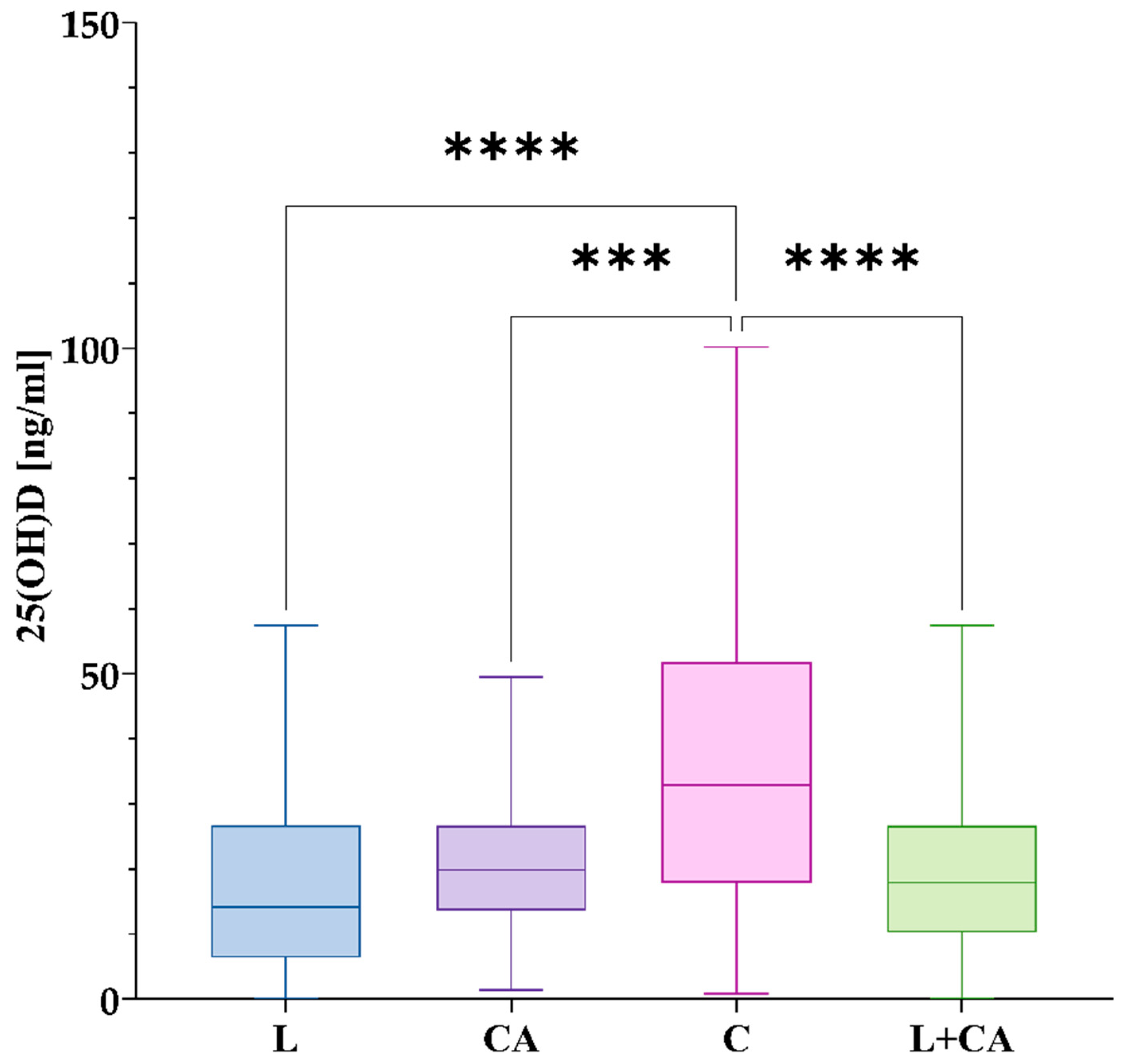
3.4.1. 25(OH)D Among the Studied Groups
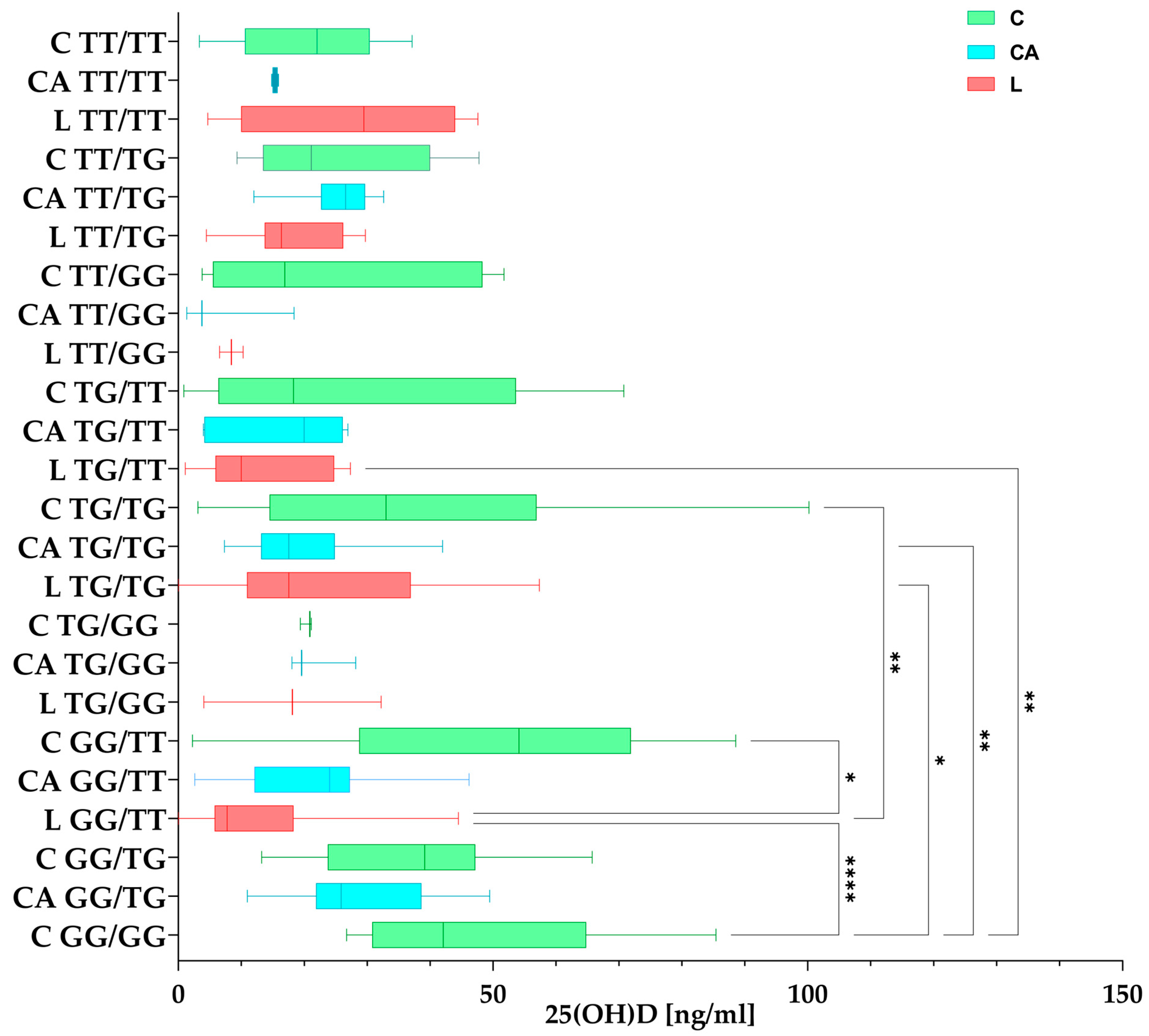
3.4.2. 25(OH)D Concentrations in Haplotypes Among the Studied Groups
3.5. Correlation Between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieczka, Ł.; Bohatyrewicz, A.; Pituch, S. Endoprotezy stawów biodrowych wczoraj i dziś. Rheumatol. Forum 2017, 3, 216–221. [Google Scholar]
- Yang, Q.; Wang, J.; Xu, Y.; Chen, Y.; Lian, Q.; Zhang, Y. Incidence and Risk Factors of In-Hospital Prosthesis-Related Complications Following Total Hip Arthroplasty: A Retrospective Nationwide Inpatient Sample Database Study. Int. Orthop. 2020, 44, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.; Nham, F.; Zalikha, A.K.; El-Othmani, M.M. Epidemiology of Total Hip Arthroplasty: Demographics, Comorbidities and Outcomes. Arthroplasty 2023, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Number of Hip Replacements in OECD-Countries 2021. Available online: https://www.statista.com/statistics/283234/number-of-knee-replacements-in-selected-countries/ (accessed on 3 January 2025).
- Total Hip Replacement. Available online: https://hipkneeinfo.org/hip-care/total-hip-replacement/ (accessed on 17 January 2025).
- Rejestr Endoprotez-Default. Available online: https://shiny.nfz.gov.pl/app/re_endoprotezy (accessed on 17 January 2025).
- W-Dahl, A.; Kärrholm, J.; Rogmark, C.; Nåtman, J.; Bülow, E.; Arani, P.; Mohaddes, M.; Rolfson, O. The Swedish Arthroplasty Register Annual Report 2023. Available online: https://www.researchgate.net/publication/378941757_The_Swedish_Arthroplasty_Register_Annual_Report_2023 (accessed on 17 January 2025).
- Furnes, O.; Gjertsen, J.-E.; Hallan, G.; Visnes, H.; Gundersen, T.; Fenstad, A.; Dybvik, E.; Stenvik, S.; Kvinnesland, I.; Mulpuri, K.; et al. Yearly Report Norwegian National Advisory Unit on Arthroplasty and Hip Fractures Norwegian Arthroplasty Register Norwegian Cruciate Ligament Register Norwegian Hip Fracture Register Norwegian Paediatric Hip Register Report 2022. Available online: https://www.researchgate.net/publication/366006951_Yearly_Report_Norwegian_National_Advisory_Unit_on_Arthroplasty_and_Hip_Fractures_Norwegian_Arthroplasty_Register_Norwegian_Cruciate_Ligament_Register_Norwegian_Hip_Fracture_Register_Norwegian_Paediatr (accessed on 17 January 2025).
- Available online: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2021st%20Annual%20Report%202024_Hips.pdf (accessed on 17 January 2025).
- Kenanidis, E.; Kakoulidis, P.; Karponis, D.; Tsiridis, E. The Effect of Perioperative Vitamin D Levels on the Functional, Patient-Related Outcome Measures and the Risk of Infection Following Hip and Knee Arthroplasty: A Systematic Review. Patient Relat. Outcome Meas. 2020, 11, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Mjöberg, B. Hip Prosthetic Loosening: A Very Personal Review. World J. Orthop. 2021, 12, 629–639. [Google Scholar] [CrossRef]
- Basit, S. Vitamin D in Health and Disease: A Literature Review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for Skeletal and Non-Skeletal Health: What We Should Know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Kim, T.-K.; Slominski, R.M.; Pyza, E.; Kleszczynski, K.; Tuckey, R.C.; Reiter, R.J.; Holick, M.F.; Slominski, A.T. Evolutionary Formation of Melatonin and Vitamin D in Early Life Forms: Insects Take Centre Stage. Biol. Rev. Camb. Philos. Soc. 2024, 99, 1772–1790. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Speeckaert, R.; van Geel, N.; Delanghe, J.R. Chapter One—Vitamin D Binding Protein: A Multifunctional Protein of Clinical Importance. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 63, pp. 1–57. [Google Scholar]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the Scenes of Vitamin D Binding Protein: More than Vitamin D Binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef]
- Hirschfeld, J. Immune-Electrophoretic Demonstration of Qualitative Differences in Human Sera and Their Relation to the Haptoglobins. Acta Pathol. Microbiol. Scand. 2009, 47, 160–168. [Google Scholar] [CrossRef]
- Pop, T.L.; Sîrbe, C.; Benţa, G.; Mititelu, A.; Grama, A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10705. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Safadi, F.F.; Thornton, P.; Magiera, H.; Hollis, B.W.; Gentile, M.; Haddad, J.G.; Liebhaber, S.A.; Cooke, N.E. Osteopathy and Resistance to Vitamin D Toxicity in Mice Null for Vitamin D Binding Protein. J. Clin. Investig. 1999, 103, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M. Contributions of Sunlight and Diet to Vitamin D Status. Calcif. Tissue Int. 2013, 92, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L. Role of Vitamin D in Health and Disease: How Diet May Improve Vitamin D Absorption. Int. J. Food Sci. Nutr. 2023, 74, 121–123. [Google Scholar] [CrossRef]
- Holick, M. Environmental Factors That Influence the Cutaneous Production of Vitamin D. Am. J. Clin. Nutr. 1995, 61, 638S–645S. [Google Scholar] [CrossRef]
- Lehmann, B.; Meurer, M. Vitamin D Metabolism. Dermatol. Ther. 2010, 23, 2–12. [Google Scholar] [CrossRef]
- Slominski, A.T.; Tuckey, R.C.; Jetten, A.M.; Holick, M.F. Recent Advances in Vitamin D Biology: Something New under the Sun. J. Investig. Dermatol. 2023, 143, 2340–2342. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of Novel CYP11A1-Derived Secosteroids in the Human Epidermis and Serum and Pig Adrenal Gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef]
- Slominski, A.T.; Tuckey, R.C.; Jenkinson, C.; Li, W.; Jetten, A.M. Chapter 6—Alternative Pathways for Vitamin D Metabolism. In Feldman and Pike’ s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovannucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 85–109. ISBN 978-0-323-91386-7. [Google Scholar]
- Rozmus, D.; Fiedorowicz, E.; Grzybowski, R.; Płomiński, J.; Cieślińska, A. VDR Gene Polymorphisms (BsmI, FokI, TaqI, ApaI) in Total Hip Arthroplasty Outcome Patients. Int. J. Mol. Sci. 2024, 25, 8225. [Google Scholar] [CrossRef]
- Song, Y.-H.; Naumova, A.K.; Liebhaber, S.A.; Cooke, N.E. Physical and Meiotic Mapping of the Region of Human Chromosome 4q11–Q13 Encompassing the Vitamin D Binding Protein DBP/Gc-Globulin and Albumin Multigene Cluster. Genome Res. 1999, 9, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Piquette, C.A.; Robinson-Hill, R.; Webster, R.O. Human Monocyte Chemotaxis to Complement-Derived Chemotaxins Is Enhanced by Gc-Globulin. J. Leukoc. Biol. 1994, 55, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Papiha, S.S.; Allcroft, L.C.; Kanan, R.M.; Francis, R.M.; Datta, H.K. Vitamin D Binding Protein Gene in Male Osteoporosis: Association of Plasma DBP and Bone Mineral Density with (TAAA)n-Alu Polymorphism in DBP. Calcif. Tissue Int. 1999, 65, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms—The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Usama; Khan, Z.; Ali, A.; Shah, M.; Imran, M. Differential Glycosylation in Mutant Vitamin D-Binding Protein Decimates the Binding Stability of Vitamin D. J. Biomol. Struct. Dyn. 2024, 42, 5365–5375. [Google Scholar] [CrossRef]
- Quesada-Colloto, P.; Avello-Llano, N.; García-Romero, R.; Garriga-García, M.; Álvarez-Beltrán, M.; Reyes-Domínguez, A.I.; Fernández-Lorenzo, A.E.; Gil-Peña, H.; Gómez-Alonso, C.; García-Gil-Albert, C.; et al. Polymorphisms of the Vitamin D Binding Protein (VDBP) and Free Vitamin D in Patients with Cystic Fibrosis. Nutrients 2024, 16, 3850. [Google Scholar] [CrossRef]
- Rozmus, D.; Plomiński, J.; Augustyn, K.; Cieślińska, A. Rs7041 and Rs4588 Polymorphisms in Vitamin D Binding Protein Gene (VDBP) and the Risk of Diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef]
- Rs4588 RefSNP Report-dbSNP-NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs4588 (accessed on 7 January 2022).
- Rs7041 RefSNP Report-dbSNP-NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs7041 (accessed on 7 January 2022).
- Murthykumar, K.; Arjunkumar, R.; Jayaseelan, V.P. Association of Vitamin D Receptor Gene Polymorphism (Rs10735810) and Chronic Periodontitis. J. Investig. Clin. Dent. 2019, 10, e12440. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Han, W.; Geng, C.; Chen, D.; Wu, B.; Zhang, J.; Wang, C.; Jiang, P. Association of Leptin and Leptin Receptor Polymorphisms with Coronary Artery Disease in a North Chinese Han Population. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190388. [Google Scholar] [CrossRef]
- Brambilla, L.; Peretti, G.M.; Sirtori, P.; Maffulli, N.; Mangiavini, L. Outcome of Total Hip and Total Knee Arthroplasty and Vitamin D Homeostasis. Br. Med. Bull. 2020, 135, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Maniar, R.N.; Patil, A.M.; Maniar, A.R.; Gangaraju, B.; Singh, J. Effect of Preoperative Vitamin D Levels on Functional Performance after Total Knee Arthroplasty. Clin. Orthop. Surg. 2016, 8, 153–156. [Google Scholar] [CrossRef]
- Mouli, V.H.; Schudrowitz, N.; Carrera, C.X.; Uzosike, A.C.; Fitz, W.; Rajaee, S.S. High-Dose Vitamin D Supplementation Can Correct Hypovitaminosis D Prior to Total Knee Arthroplasty. J. Arthroplast. 2022, 37, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Arshi, A.; Wang, C.; Buser, Z.; Wang, J.C.; Jensen, A.R.; Adams, J.S.; Zeegen, E.N.; Bernthal, N.M. Preoperative Vitamin D Deficiency Is Associated With Higher Postoperative Complication Rates in Total Knee Arthroplasty. Orthopedics 2018, 41, e489–e495. [Google Scholar] [CrossRef] [PubMed]
- Fadason, T.; Farrow, S.; Gokuladhas, S.; Golovina, E.; Nyaga, D.; O’Sullivan, J.M.; Schierding, W. Assigning Function to SNPs: Considerations When Interpreting Genetic Variation. Semin. Cell Dev. Biol. 2022, 121, 135–142. [Google Scholar] [CrossRef]
- Ramensky, V.; Bork, P.; Sunyaev, S. Human Non-synonymous SNPs: Server and Survey. Nucleic Acids Res. 2002, 30, 3894–3900. [Google Scholar] [CrossRef]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (Synonymous) SNPs: Should We Care about Them? Methods Mol. Biol. 2009, 578, 23–39. [Google Scholar] [CrossRef]
- Shastry, B.S. SNPs: Impact on Gene Function and Phenotype. In Single Nucleotide Polymorphisms: Methods and Protocols; Komar, A.A., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 3–22. ISBN 978-1-60327-411-1. [Google Scholar]
- Newton, D.A.; Baatz, J.E.; Kindy, M.S.; Gattoni-Celli, S.; Shary, J.R.; Hollis, B.W.; Wagner, C.L. Vitamin D Binding Protein Polymorphisms Significantly Impact Vitamin D Status in Children. Pediatr. Res. 2019, 86, 662–669. [Google Scholar] [CrossRef]
- Shnar, V.A.; Алексеевна, В.Ш.; Paskidov, D.V.; Владимирoвич, П.Д.; Bograya, M.M.; Михайлoвна, Б.М.; Gazatova, N.D.; Динисламoвна, Г.Н.; Voronova, S.S.; Сергеевна, В.С.; et al. The association of the rs4588 and rs7041 polymorphisms of the VDBP gene and vitamin D status in obese individuals. Cytokines Inflamm. 2024, 21, 46–53. [Google Scholar] [CrossRef]
- Zhou, C.; Gan, X.; Ye, Z.; Zhang, Y.; Yang, S.; He, P.; Zhang, Y.; Liu, M.; Wu, Q.; Qin, X. Serum 25-Hydroxyvitamin D, Vitamin D Receptor, and Vitamin-D-Binding Protein Gene Polymorphisms and Risk of Dementia Among Older Adults With Prediabetes. J. Gerontol. Ser. A 2024, 79, glae015. [Google Scholar] [CrossRef]
- Karcıoğlu Batur, L.; Dokur, M.; Koç, S.; Karabay, M.; Akcay, Z.N.; Gunger, E.; Hekim, N. Investigation of the Relationship between Vitamin D Deficiency and Vitamin D-Binding Protein Polymorphisms in Severe COVID-19 Patients. Diagnostics 2024, 14, 1941. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Dursun, E.; Alaylıoglu, M.; Gezen-Ak, D.; Al Anouti, F.; Pilz, S.; Pludowski, P.; Jude, E.; Kotsa, K. Upregulation of Irisin and Vitamin D-Binding Protein Concentrations by Increasing Maternal 25-Hydrovitamin D Concentrations in Combination with Specific Genotypes of Vitamin D-Binding Protein Polymorphisms. Nutrients 2022, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Omidifar, A.; Varzandi, T.; Salehnezhad, T.; Sahraian, M.A. Reduction in Circulating Vitamin D Binding Protein in Patients with Multiple Sclerosis. BMC Neurol. 2021, 21, 168. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Jung, Y.-K.; Ju, S.; Lee, S.J.; Cho, Y.J.; Jeong, Y.Y.; Lee, J.D.; Cho, M.-C. Serum Vitamin D Binding Protein Level, but Not Serum Total, Bioavailable, Free Vitamin D, Is Higher in 30-Days Survivors than in Nonsurvivors with Sepsis. Medicine 2020, 99, e20756. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bains, K. Protein, Lysine and Vitamin D: Critical Role in Muscle and Bone Health. Crit. Rev. Food Sci. Nutr. 2022, 62, 2548–2559. [Google Scholar] [CrossRef]
- Taylor, S.N. Vitamin D in Toddlers, Preschool Children, and Adolescents. Ann. Nutr. Metab. 2020, 76, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.Y.; Nguyen, M.N.; Benson, H.A.E.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In Vivo Evidence for a Novel Pathway of Vitamin D3 Metabolism Initiated by P450scc and Modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef]
- Slominski, R.M.; Tuckey, R.C.; Manna, P.R.; Jetten, A.M.; Postlethwaite, A.; Raman, C.; Slominski, A.T. Extra-Adrenal Glucocorticoid Biosynthesis: Implications for Autoimmune and Inflammatory Disorders. Genes Immun. 2020, 21, 150–168. [Google Scholar] [CrossRef]
- Da Cunha, B.M.; Gava, A.D.; de Oliveira, S.B.; de David, A.C.; dos Santos-Neto, L.L. Vitamin d Is Related to Gait Recovery after Total Hip Arthroplasty: A Prospective Analysis. Gait Posture 2016, 50, 96–101. [Google Scholar] [CrossRef]
- Unnanuntana, A.; Rebolledo, B.J.; Gladnick, B.P.; Nguyen, J.T.; Sculco, T.P.; Cornell, C.N.; Lane, J.M. Does Vitamin D Status Affect the Attainment of In-Hospital Functional Milestones After Total Hip Arthroplasty? J. Arthroplast. 2012, 27, 482–489. [Google Scholar] [CrossRef]
- Hussain, S.M.; Daly, R.M.; Wang, Y.; Shaw, J.E.; Magliano, D.J.; Graves, S.; Ebeling, P.R.; Wluka, A.E.; Cicuttini, F.M. Association between Serum Concentration of 25-Hydroxyvitamin D and the Risk of Hip Arthroplasty for Osteoarthritis: Result from a Prospective Cohort Study. Osteoarthr. Cartil. 2015, 23, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Zebaze, R.M.; Ghasem-Zadeh, A.; Bohte, A.; Iuliano-Burns, S.; Mirams, M.; Price, R.I.; Mackie, E.J.; Seeman, E. Intracortical Remodelling and Porosity in the Distal Radius and Post-Mortem Femurs of Women: A Cross-Sectional Study. Lancet 2010, 375, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Mellish, R.W.E.; Garrahan, N.J. Age-Related Changes in Iliac Crest Trabecular Microanatomic Bone Structure in Man. Bone 1987, 8, 289–292. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Mathews, C.H.; Villanueva, A.R.; Kleerekoper, M.; Frame, B.; Rao, D.S. Relationships between Surface, Volume, and Thickness of Iliac Trabecular Bone in Aging and in Osteoporosis. Implications for the Microanatomic and Cellular Mechanisms of Bone Loss. J. Clin. Investig. 1983, 72, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.S.; Maus, U.; Lazovic, D.; Horas, K.; Roth, K.E.; Kurth, A.A. Is There an Association between Low Serum 25-OH-D Levels and the Length of Hospital Stay in Orthopaedic Patients after Arthroplasty? J. Orthop. Traumatol. 2016, 17, 297–302. [Google Scholar] [CrossRef]
- Traven, S.A.; Chiaramonti, A.M.; Barfield, W.R.; Kirkland, P.A.; Demos, H.A.; Schutte, H.D.; Drew, J.M. Fewer Complications Following Revision Hip and Knee Arthroplasty in Patients With Normal Vitamin D Levels. J. Arthroplast. 2017, 32, S193–S196. [Google Scholar] [CrossRef]
- Zajonz, D.; Prager, F.; Edel, M.; Möbius, R.; Daikos, A.; Fakler, J.K.; Josten, C.; Kratzsch, J.; Roth, A. The Significance of the Vitamin D Metabolism in the Development of Periprosthetic Infections after THA and TKA: A Prospective Matched-Pair Analysis of 240 Patients. Clin. Interv. Aging 2018, 13, 1429–1435. [Google Scholar] [CrossRef]
- Visser, E.; de Roos, N.M.; Oosting, E.; Endenburg, S.C.; Dronkers, J.J. Association Between Preoperative Vitamin D Status and Short-Term Physical Performance after Total Hip Arthroplasty: A Prospective Study. Ann. Nutr. Metab. 2018, 73, 252–260. [Google Scholar] [CrossRef]
- Martínez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramírez-Salazar, E.G.; Reyes-Grajeda, J.P.; De la Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramírez-Palacios, P.; Quiterio, M.; et al. Serum Proteomic Analysis Reveals Vitamin D-Binding Protein (VDBP) as a Potential Biomarker for Low Bone Mineral Density in Mexican Postmenopausal Women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef]
- Bhan, I. Vitamin D Binding Protein and Bone Health. Int. J. Endocrinol. 2014, 2014, e561214. [Google Scholar] [CrossRef]
- Takiar, R.; Lutsey, P.L.; Zhao, D.; Guallar, E.; Schneider, A.L.C.; Grams, M.E.; Appel, L.J.; Selvin, E.; Michos, E.D. The Associations of 25-Hydroxyvitamin D Levels, Vitamin D Binding Protein Gene Polymorphisms, and Race with Risk of Incident Fracture-Related Hospitalization: Twenty-Year Follow-up in a Bi-Ethnic Cohort (the ARIC Study). Bone 2015, 78, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Duan, X.; Wang, J.; Shu, B.; Li, X.; Ba, Q.; Li, J.; Wang, Y.; Wang, H. Bioavailable 25(OH)D but Not Total 25(OH)D Is an Independent Determinant for Bone Mineral Density in Chinese Postmenopausal Women. eBioMedicine 2017, 15, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Unnanuntana, A.; Saleh, A.; Nguyen, J.T.; Sculco, T.P.; Cornell, C.N.; Mancuso, C.A.; Lane, J.M. Low Vitamin D Status Does Not Adversely Affect Short-Term Functional Outcome After Total Hip Arthroplasty. J. Arthroplast. 2013, 28, 315–322.e2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jang, G.; Park, J.W.; Lee, Y.-K.; Koo, K.-H. Vitamin D Deficiency and Sarcopenia in Hip Fracture Patients. J. Bone Metab. 2021, 28, 79–83. [Google Scholar] [CrossRef]



| Characteristics | L (n = 112) | CA (n = 87) | C (n = 249) | p-Value (L vs. CA; L vs. C; CA vs. C) |
|---|---|---|---|---|
| Age: years mean (SD) | 53.57 (12.37) | 48.77 (14.52) | 44.53 (13.23) | 0.0156/<0.0001/0.344 * |
| Gender: n (%) | ||||
| Female: | 50 (45) | 25 (29) | 122 (49) | |
| Male: | 62 (55) | 62 (71) | 127 (51) | |
| χ2 (2, 448) = 10.7693 | 0.0046 | |||
| rs7041 | rs4588 | |||||
|---|---|---|---|---|---|---|
| Genotype | Control [n (%)] | Control Arthroplasty [n (%)] | Loosening [n (%)] | Control [n (%)] | Control Arthroplasty [n (%)] | Loosening [n (%)] |
| C | CA | L | C | CA | L | |
| TT | 50 (20) | 15 (17.2) | 23 (20.5) | 119 (47.7) | 60 (68.9) | 51 (45.5) |
| TG | 144 (58) | 52 (59.7) | 56 (50.1) | 39 (15.6) | 8 (9.1) | 12 (10.7) |
| GG | 55 (22) | 20 (22.9) | 33 (29.4) | 91 (36.5) | 19 (21.8) | 49 (43.7) |
| Genotype χ2 (4, 448) = 0.3205; p = 0.5242 | Genotype χ2 (4, 448) = 16.14; p = 0.0028 | |||||
| L vs. CA + C | L vs. C | L vs. CA | L vs. CA + C | L vs. C | L vs. CA | |
| Dominant OR | 0.68 | 0.68 | 0.72 | 1.36 | 1.55 | 2.49 |
| (95% CI; p) | (0.43–1.11; 0.12) | (0.41–1.12; 0.13) | (0.38–1.36; 0.31) | (0.69–2.67; 0.38) | (0.78–3.08; 0.21) | (1.14–5.42; 0.02) |
| Recessive OR | 2.8 | 2.8 | 2.8 | 1.02 | 0.97 | 0.93 |
| (95% CI; p) | (1.71–4.59; <0.0001) | (1.69–4.65; 0.0001) | (1.56–5.00; 0.0005) | (0.67–1.55; 0.93) | (0.62–1.51; 0.87) | (0.55–1.56; 0.78) |
| TT OR | 1 (reference) | |||||
| vs. TG OR | 1.23 | 1.18 | 1.42 | 1.57 | 1.26 | 3.33 |
| (95% CI; p) | (0.71–2.17; 0.46) | (0.66–2.12; 0.57) | (0.67–3.02; 0.36) | (0.99–2.47; 0.06) | (0.78–2.03; 0.34) | (1.66–6.68; 0.0007) |
| vs. GG OR | 0.80 | 0.77 | 0.93 | 1.75 | 1.75 | 5.44 |
| (95% CI; p) | (0.43–1.51; 0.50) | (0.40–1.48; 0.43) | (0.40–2.19; 0.87) | (0.85–3.58; 0.13) | (0.83–3.65; 0.14) | (2.17–13.67; 0.0003) |
| L vs. CA + C Haplotype | Loosening (Freq) | Control Arthroplasty + Control (Freq) | χ2 | Fisher’s p | Pearson’s p | OR [95% CI] |
|---|---|---|---|---|---|---|
| rs7041/4588 | ||||||
| TT | 34 (0.151) | 117 (0.174) | 0.597 | 0.472 | 0.439 | 0.848 [0.56–1.286] |
| TG | 68 (0.303) | 209 (0.311) | 0.043 | 0.867 | 0.834 | 0.965 [0.695–1.34] |
| GT | 115 (0.513) | 282 (0.419) | 5.983 | 0.016 | 0.014 | 1.459 [1.077–1.976] |
| GG | 7 (0.031) | 64 (0.095) | 9.427 | 0.001 | 0.002 | 0.306 [0.138–0.678] |
| L vs. CA haplotype | Loosening (freq) | Control Arthroplasty (freq) | χ2 | Fisher’s p | Pearson’s p | OR [95% CI] |
| rs7041/4588 | ||||||
| TT | 34 (0.151) | 17 (0.097) | 2.564 | 0.13 | 0.109 | 1.652 [0.889–3.07] |
| TG | 68 (0.303) | 65 (0.373) | 2.156 | 0.163 | 0.142 | 0.73 [0.48–1.111] |
| GT | 115 (0.513) | 81 (0.465) | 0.898 | 0.364 | 0.343 | 1.211 [0.814–1.801] |
| GG | 7 (0.031) | 11 (0.063) | 2.317 | 0.148 | 0.127 | 0.478 [0.181–1.259] |
| L vs. C haplotype | Loosening (freq) | Control (freq) | χ2 | Fisher’s p | Pearson’s p | OR [95% CI] |
| rs7041/4588 | ||||||
| TT | 34 (0.151) | 100 (0.2) | 2.456 | 0.121 | 0.117 | 0.712 [0.465–1.09] |
| TG | 68 (0.303) | 144 (0.289) | 0.154 | 0.724 | 0.694 | 1.071 [0.759–1.512] |
| GT | 115 (0.513) | 201 (0.403) | 7.565 | 0.007 | 0.005 | 1.558 [1.135–2.141] |
| GG | 7 (0.031) | 53 (0.106) | 11.459 | 0.0004 | 0.0007 | 0.27 [0.121–0.605] |
| Results | Study Cohort | Case | References |
|---|---|---|---|
| 25(OH)D Conc. | |||
| Weak correlation between 25(OH)D levels and peak stretch or peak power | 110 subjects | THR | [60] |
| 23 vitamin D-deficient, 32 vitamin D-insufficient, and 32 vitamin D-sufficient patients; 25(OH)D status not related to performance after surgery | 87 subjects | THR | [69] |
| 40% of patients were noted to have low 25(OH)D levels | 200 subjects; 187—osteoarthritis (OA), 6—osteonecrosis, 3—hip dysplasia, and post-traumatic OA—4 | THR | [74] |
| Correlation of increased 25(OH)D serum concentrations and increased risk of total hip replacements in OA among males, but not among females | 9135 subjects | THR, BMD | [62] |
| Insufficient serum levels of 25-OH-D were observed in 86% of patients, with over 60% classified as vitamin D deficient | 1083 subjects | THR/TKA | [66] |
| 25(OH)D levels lower among patients for THR n for HFS | 70 subjects for HFS; 100 subjects for THR | HFS, primary THR | [75] |
| No association of 25(OH)D with a re-admission risk, but elevated risk of 90-day complications and periprosthetic joint infections | 126 subjects | revision THR | [67] |
| No significant differences in 25(OH)D and studied groups | 240 subjects | THR/TKA, osteoporosis | [68] |
| Deficient levels of 25(OH)D associated with increased fracture risk. Racial differences in fracture risk may be related to differences in bioavailable vitamin D due to the single nucleotide polymorphism | 12,781 middle-aged subjects | fracture risk | [72] |
| VDBP conc. | |||
| Low VDBP serum levels are associated with a low BMD | 425 postmenopausal women | BMD | [70] |
| VDBP SNP | |||
| TT variants of rs7041 were more frequent (0.543) than TG (0.370) and GG (0.74) and rs4588 GT was as frequent (0.436) as GG (0.446), but TT had the lowest frequency (0.118) rs4588 has no association with blood VDBP | 967 postmenopausal women | BMD | [73] |
| No independent association of rs4588 and rs7041 with fractures | 12,781 middle-aged subjects | fracture risk | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozmus, D.; Fiedorowicz, E.; Płomiński, J.; Cieślińska, A. Vitamin D Binding Protein Gene Polymorphisms (rs4588 and rs7041) and VDBP Levels in Total Hip Replacement Outcomes. Nutrients 2025, 17, 378. https://doi.org/10.3390/nu17030378
Rozmus D, Fiedorowicz E, Płomiński J, Cieślińska A. Vitamin D Binding Protein Gene Polymorphisms (rs4588 and rs7041) and VDBP Levels in Total Hip Replacement Outcomes. Nutrients. 2025; 17(3):378. https://doi.org/10.3390/nu17030378
Chicago/Turabian StyleRozmus, Dominika, Ewa Fiedorowicz, Janusz Płomiński, and Anna Cieślińska. 2025. "Vitamin D Binding Protein Gene Polymorphisms (rs4588 and rs7041) and VDBP Levels in Total Hip Replacement Outcomes" Nutrients 17, no. 3: 378. https://doi.org/10.3390/nu17030378
APA StyleRozmus, D., Fiedorowicz, E., Płomiński, J., & Cieślińska, A. (2025). Vitamin D Binding Protein Gene Polymorphisms (rs4588 and rs7041) and VDBP Levels in Total Hip Replacement Outcomes. Nutrients, 17(3), 378. https://doi.org/10.3390/nu17030378







