Lipoprotein(a) Response to Dietary Saturated Fat Reduction: Relationship to Apolipoprotein(a) Size Polymorphism in African Americans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Intervention Diets
2.3. Determinations of Lp(a) and Allele-Specific apo(a) Levels and Apo(a) Characteristics
2.4. Statistics
3. Results
3.1. Participant Characteristics
3.2. Lp(a) Response by Carrier Status of a Small Size Apo(a), Apo(a) Phenotype and Dominance Pattern
3.3. Lp(a) Response by Tertiles of Combined apo(a) Kringle Sizes in Participants with Two Expressed Apo(a) Isoforms
3.4. Determinants of Changes in Lp(a) Levels from the AAD to the DASH-Type Diet in All Participants
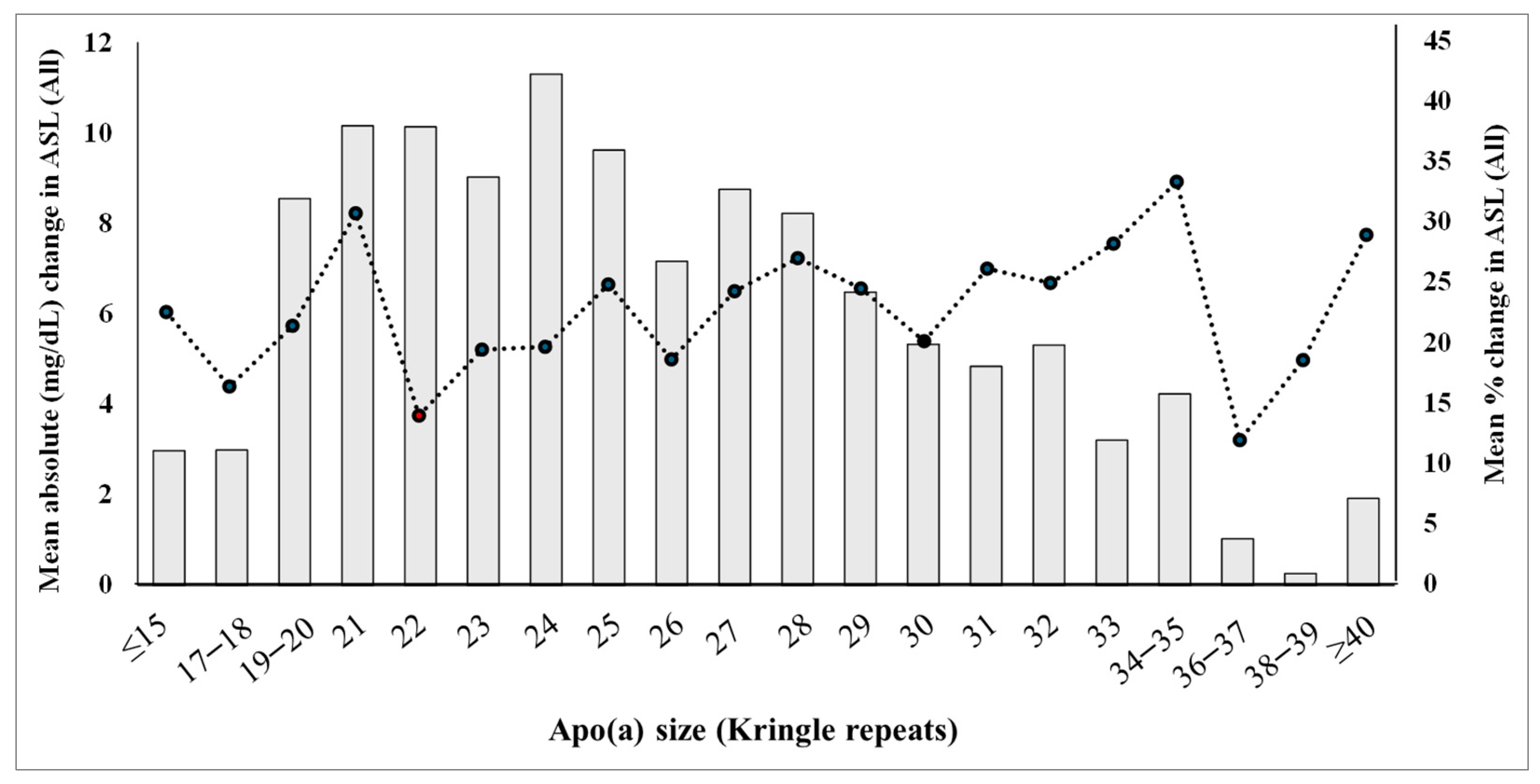
3.5. Changes in Allele-Specific Apo(a) Levels Across the Apo(a) Size Spectrum in All Participants
3.6. LDL-C Response and Apo(a) Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Average American Diet. |
| Apo(a) | Apolipoprotein(a). |
| ASL | Allele-specific apo(a) level. |
| CKD | Chronic kidney disease. |
| CVD | Cardiovascular disease. |
| DASH | Dietary Approaches to Stop Hypertension. |
| K | Kringles. |
| Lp(a) | Lipoprotein(a). |
| SFA | Saturated fatty acid. |
References
- Utermann, G.; Menzel, H.J.; Kraft, H.G.; Duba, H.C.; Kemmler, H.G.; Seitz, C. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J. Clin. Investig. 1987, 80, 458–465. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.W.; Tomlinson, J.E.; Kuang, W.J.; Eaton, D.L.; Chen, E.Y.; Fless, G.M.; Scanu, A.M.; Lawn, R.M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 1987, 330, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein (a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, M.L.; Beisiegel, U.; Henne-Bruns, D.; Eaton, D.L.; Lawn, R.M. Apolipoprotein(a) size heterogeneity is related to variable number of repeat sequences in its mRNA. Biochemistry 1990, 29, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Erqou, S.; Thompson, A.; Di Angelantonio, E.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) isoforms and the risk of vascular disease: Systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010, 55, 2160–2167. [Google Scholar] [CrossRef]
- Kronenberg, F.; Utermann, G. Lipoprotein(a): Resurrected by genetics. J. Intern. Med. 2013, 273, 6–30. [Google Scholar] [CrossRef]
- Kraft, H.G.; Kochl, S.; Menzel, H.J.; Sandholzer, C.; Utermann, G. The apolipoprotein (a) gene: A transcribed hypervariable locus controlling plasma lipoprotein (a) concentration. Hum. Genet. 1992, 90, 220–230. [Google Scholar] [CrossRef]
- Kraft, H.G.; Lingenhel, A.; Kochl, S.; Hoppichler, F.; Kronenberg, F.; Abe, A.; Muhlberger, V.; Schonitzer, D.; Utermann, G. Apolipoprotein(a) kringle IV repeat number predicts risk for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 713–719. [Google Scholar] [CrossRef]
- Helmhold, M.; Bigge, J.; Muche, R.; Mainoo, J.; Thiery, J.; Seidel, D.; Armstrong, V.W. Contribution of the apo[a] phenotype to plasma Lp[a] concentrations shows considerable ethnic variation. J. Lipid Res. 1991, 32, 1919–1928. [Google Scholar] [CrossRef]
- Sandholzer, C.; Hallman, D.M.; Saha, N.; Sigurdsson, G.; Lackner, C.; Csaszar, A.; Boerwinkle, E.; Utermann, G. Effects of the apolipoprotein(a) size polymorphism on the lipoprotein(a) concentration in 7 ethnic groups. Hum. Genet. 1991, 86, 607–614. [Google Scholar] [CrossRef]
- Paultre, F.; Pearson, T.A.; Weil, H.F.; Tuck, C.H.; Myerson, M.; Rubin, J.; Francis, C.K.; Marx, H.F.; Philbin, E.F.; Reed, R.G.; et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Albers, J.J.; Wijsman, E.; Zhang, Z.; Chapman, N.H.; Kennedy, H. Differences in Lp[a] concentrations and apo[a] polymorphs between black and white Americans. J. Lipid Res. 1996, 37, 2569–2585. [Google Scholar] [CrossRef]
- Mehta, A.; Jain, V.; Saeed, A.; Saseen, J.J.; Gulati, M.; Ballantyne, C.M.; Virani, S.S. Lipoprotein(a) and ethnicities. Atherosclerosis 2022, 349, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Kris-Etherton, P.; Dennis, B.; Elmer, P.J.; Ershow, A.; Lefevre, M.; Pearson, T.; Roheim, P.; Ramakrishnan, R.; Reed, R.; et al. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: The DELTA Study, protocol 1. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Lefevre, M.; Ginsberg, H.N.; Kris-Etherton, P.M.; Elmer, P.J.; Stewart, P.W.; Ershow, A.; Pearson, T.A.; Dennis, B.H.; Roheim, P.S.; et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: Studies in the fasting and postprandial states. Am. J. Clin. Nutr. 2007, 86, 1611–1620. [Google Scholar] [CrossRef]
- Law, H.G.; Meyers, F.J.; Berglund, L.; Enkhmaa, B. Lipoprotein(a) and diet-a challenge for a role of saturated fat in cardiovascular disease risk reduction? Am. J. Clin. Nutr. 2023, 118, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Law, H.G.; Khan, M.A.; Zhang, W.; Bang, H.; Rood, J.; Most, M.; Lefevre, M.; Berglund, L.; Enkhmaa, B. Reducing saturated fat intake lowers LDL-C but increases Lp(a) levels in African Americans: The GET-READI feeding trial. J. Lipid Res. 2023, 64, 100420. [Google Scholar] [CrossRef]
- Kamboh, M.I.; Ferrell, R.E.; Kottke, B.A. Expressed hypervariable polymorphism of apolipoprotein (a). Am. J. Hum. Genet. 1991, 49, 1063–1074. [Google Scholar]
- Enkhmaa, B.; Anuurad, E.; Zhang, W.; Yue, K.; Li, C.S.; Berglund, L. The roles of apo(a) size, phenotype, and dominance pattern in PCSK9-inhibition-induced reduction in Lp(a) with alirocumab. J. Lipid Res. 2017, 58, 2008–2016. [Google Scholar] [CrossRef]
- Tsimikas, S.; Fazio, S.; Ferdinand, K.C.; Ginsberg, H.N.; Koschinsky, M.L.; Marcovina, S.M.; Moriarty, P.M.; Rader, D.J.; Remaley, A.T.; Reyes-Soffer, G.; et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 177–192. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.L.; Lambert, G.; Mach, F.; et al. Frequent questions and responses on the 2022 lipoprotein(a) consensus statement of the European Atherosclerosis Society. Atherosclerosis 2023, 374, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Stroes, E.S.G. The dedicated “Lp(a) clinic”: A concept whose time has arrived? Atherosclerosis 2020, 300, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Causes and consequences of lipoprotein(a) abnormalities in kidney disease. Clin. Exp. Nephrol. 2014, 18, 234–237. [Google Scholar] [CrossRef]
- Hopewell, J.C.; Haynes, R.; Baigent, C. The Role of Lipoprotein(a) in Chronic Kidney Disease. J. Lipid Res. 2018, 59, 577–585. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; Lopez, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Balog, C.; Swerdlow, D.I.; Scrimgeour, A.C.; Rambaran, C.; Wilson, R.J.; Boyce, M.; Ray, K.K.; Cho, L.; et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals With Elevated Plasma Lipoprotein(a) Levels. JAMA 2022, 327, 1679–1687. [Google Scholar] [CrossRef]
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA 2023, 330, 2075–2083. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nissen, S.E.; Fleming, C.; Urva, S.; Suico, J.; Berg, P.H.; Linnebjerg, H.; Ruotolo, G.; Turner, P.K.; Michael, L.F. Muvalaplin, an Oral Small Molecule Inhibitor of Lipoprotein(a) Formation: A Randomized Clinical Trial. JAMA 2023, 330, 1042–1053. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein(a) and cardiovascular disease. Lancet 2024, 404, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Kennedy, H.; Bittolo Bon, G.; Cazzolato, G.; Galli, C.; Casiglia, E.; Puato, M.; Pauletto, P. Fish intake, independent of apo(a) size, accounts for lower plasma lipoprotein(a) levels in Bantu fishermen of Tanzania: The Lugalawa Study. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Matveyenko, A.; Seid, H.; Kim, K.; Ramakrishnan, R.; Thomas, T.; Matienzo, N.; Reyes-Soffer, G. Association of free-living diet composition with plasma lipoprotein(a) levels in healthy adults. Lipids Health Dis. 2023, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.M.; Sapp, P.A.; Kris-Etherton, P.M.; Petersen, K.S. Effects of saturated fatty acid consumption on lipoprotein (a): A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2024, 120, 619–629. [Google Scholar] [CrossRef]
- Chemello, K.; Chan, D.C.; Lambert, G.; Watts, G.F. Recent advances in demystifying the metabolism of lipoprotein(a). Atherosclerosis 2022, 349, 82–91. [Google Scholar] [CrossRef]
- Brunner, C.; Lobentanz, E.M.; Petho-Schramm, A.; Ernst, A.; Kang, C.; Dieplinger, H.; Muller, H.J.; Utermann, G. The number of identical kringle IV repeats in apolipoprotein(a) affects its processing and secretion by HepG2 cells. J. Biol. Chem. 1996, 271, 32403–32410. [Google Scholar] [CrossRef]
- Haring, B.; von Ballmoos, M.C.; Appel, L.J.; Sacks, F.M. Healthy dietary interventions and lipoprotein (a) plasma levels: Results from the Omni Heart Trial. PLoS ONE 2014, 9, e114859. [Google Scholar] [CrossRef]

| Variables | All |
|---|---|
| Lp(a) level | |
| Average American Diet (mg/dL) | 44 (22–80) |
| DASH-type diet (mg/dL) | 58 (29–94) |
| Unit (mg/dL) change | 11 + 11 1 |
| Percent (%) change | 24 + 25 1 |
| Apo(a) isoform size (kringle repeats) 2 | |
| Larger isoform | 32 (29–34) |
| Smaller isoform | 27 (24–29) |
| Carrier status of a small (<22 kringles) apo(a) size 2 | |
| Carriers, n (%) | 26 (16%) |
| Non-carriers, n (%) | 139 (84%) |
| Apo(a) phenotype 2 | |
| Single expressed isoform, n (%) | 48 (29%) |
| Two expressed isoforms, n (%) | 117 (71%) |
| Apo(a) isoform dominance pattern 3 | |
| Co-dominating, n (%) | 73 (63%) |
| Smaller-dominating, n (%) | 39 (33%) |
| Larger-dominating, n (%) | 5 (4%) |
| Lp(a) Level | Carriers (n = 26) | Non-Carriers (n = 139) | p-Value * |
|---|---|---|---|
| Average American Diet (mg/dL) | 100 (47–120) | 38 (21–68) | <0.0001 |
| DASH-type diet (mg/dL) | 111 (53–129) | 52 (26–85) | <0.0001 |
| Unit (mg/dL) change | 15 ± 14 | 11 ± 11 | 0.114 |
| Percent (%) change | 21 ± 20 | 25 ± 26 | 0.518 |
| Variables | Tertiles of Combined Apo(a) Kringles * | |||
|---|---|---|---|---|
| Tertile 1 (<54 K) (n = 34) | Tertile 2 (>54 K and <61 K) (n = 43) | Tertile 3 (>61 K) (n = 40) | p-Value # | |
| Lp(a) level | ||||
| Average American Diet (mg/dL) | 98 (80–113) | 46 (33–77) | 29 (18–50) | <0.0001 |
| DASH-type diet (mg/dL) | 111 (88–129) | 64 (41–91) | 39 (20–62) | <0.0001 |
| Unit (mg/dL) change | 15 ± 14 | 14 ± 10 | 8 ± 9 | 0.024/0.006 |
| Percent (%) change | 17 ± 16 | 28 ± 19 | 24 ± 31 | 0.104/0.298 |
| Apo(a) dominance pattern | ||||
| Co-dominating, n (%) | 23 (68) | 31 (72) | 19 (48) | 0.060 |
| Smaller-dominating, n (%) | 8 (23) | 10 (23) | 21 (52) | 0.009 |
| Larger-dominating, n (%) | 3 (9) | 2 (5) | 0 (0) | 0.191 |
| Prevalence of a small (<22 kringles) size apo(a) | ||||
| Carrier, n (%) | 19 (56) | 3 (7) | 0 (0) | <0.001 |
| Non-carrier, n (%) | 15 (44) | 40 (93) | 40 (100) | <0.001 |
| Variables | Level Change (mg/dL) | Relative Change (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before Adjustment | After Adjustment | Before Adjustment | After Adjustment | |||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Age | −0.011 | 0.898 | −0.005 | 0.946 | −0.017 | 0.930 | 0.012 | 0.954 |
| Sex | 0.630 | 0.746 | −0.679 | 0.722 | −3.334 | 0.443 | −1.629 | 0.722 |
| AAD Lp(a) level | 0.113 | <0.001 | 0.098 | <0.001 | −0.126 | 0.014 | −0.144 | 0.032 |
| Apo(a) phenotype (single or two) | 2.698 | 0.171 | −1.510 | 0.871 | −2.592 | 0.560 | 0.412 | 0.986 |
| Size of small apo(a) isoform | −0.832 | <0.001 | −0.354 | 0.404 | 0.472 | 0.355 | −0.344 | 0.743 |
| Combined kringle repeats | −0.010 | 0.872 | 0.040 | 0.886 | −0.017 | 0.901 | −0.013 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, H.G.; Myagmarsuren, M.; Bang, H.; Zhang, W.; Lefevre, M.; Berglund, L.; Enkhmaa, B. Lipoprotein(a) Response to Dietary Saturated Fat Reduction: Relationship to Apolipoprotein(a) Size Polymorphism in African Americans. Nutrients 2025, 17, 426. https://doi.org/10.3390/nu17030426
Law HG, Myagmarsuren M, Bang H, Zhang W, Lefevre M, Berglund L, Enkhmaa B. Lipoprotein(a) Response to Dietary Saturated Fat Reduction: Relationship to Apolipoprotein(a) Size Polymorphism in African Americans. Nutrients. 2025; 17(3):426. https://doi.org/10.3390/nu17030426
Chicago/Turabian StyleLaw, Hayley G., Munkhtuya Myagmarsuren, Heejung Bang, Wei Zhang, Michael Lefevre, Lars Berglund, and Byambaa Enkhmaa. 2025. "Lipoprotein(a) Response to Dietary Saturated Fat Reduction: Relationship to Apolipoprotein(a) Size Polymorphism in African Americans" Nutrients 17, no. 3: 426. https://doi.org/10.3390/nu17030426
APA StyleLaw, H. G., Myagmarsuren, M., Bang, H., Zhang, W., Lefevre, M., Berglund, L., & Enkhmaa, B. (2025). Lipoprotein(a) Response to Dietary Saturated Fat Reduction: Relationship to Apolipoprotein(a) Size Polymorphism in African Americans. Nutrients, 17(3), 426. https://doi.org/10.3390/nu17030426






