Minerals and Human Health: From Deficiency to Toxicity
Abstract
:1. Introduction
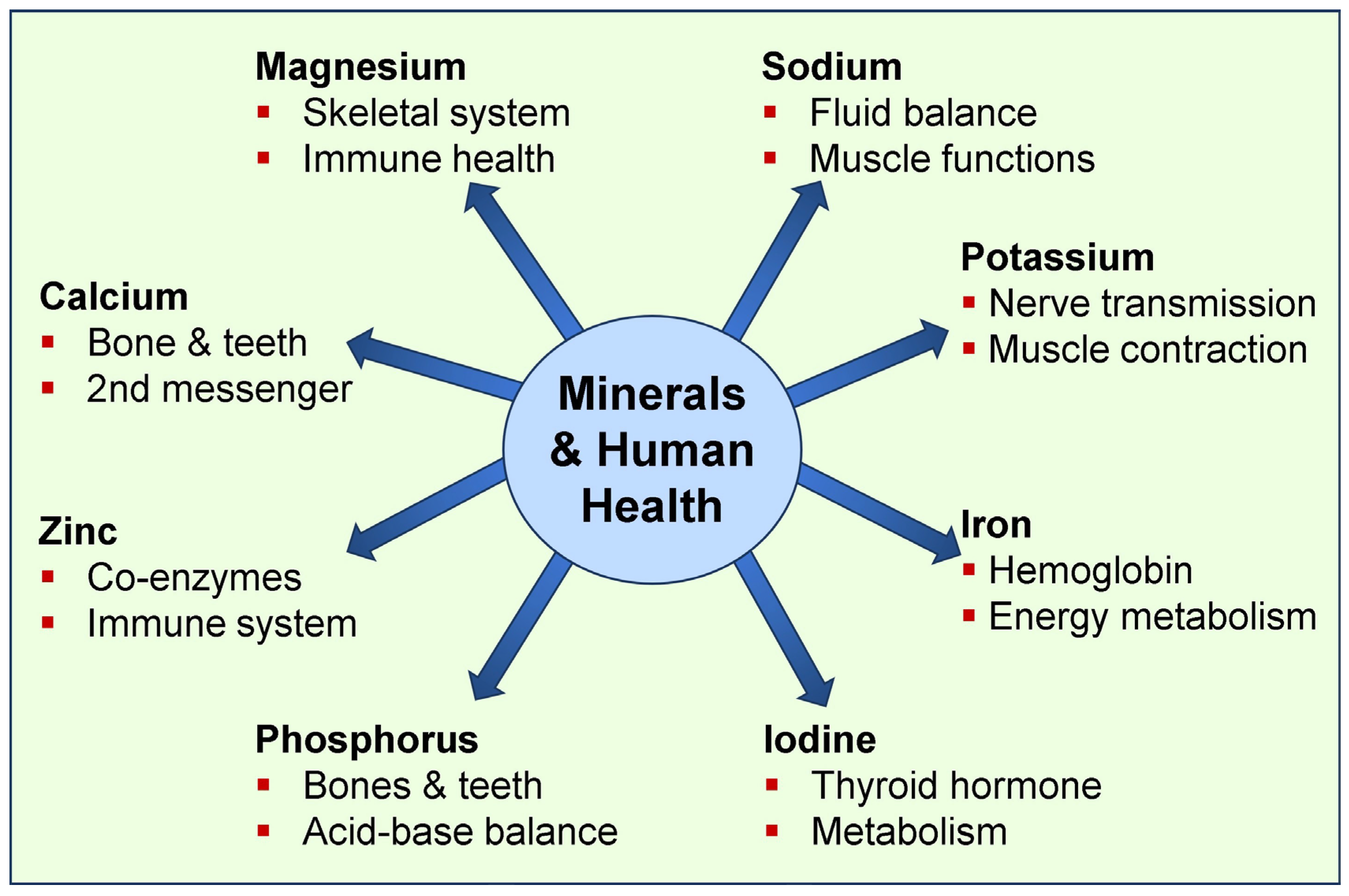
1.1. The Importance of Micro-Minerals in Physiological Functions
1.2. Broader Physiological Functions of Macro-Minerals
2. Calcium—From Physiology to Pathology
2.1. The Impact of Global Calcium Deficiency
2.2. Causes of Hypocalcemia
2.3. Treatment of Hypocalcemia
2.4. Causes of Hypercalcemia
2.5. Treatment of Hypercalcemia
3. Magnesium—From Physiology to Pathology
3.1. Effects of Hypermagnesemia
3.2. Treatment of Hypo- and Hypermagnesemia
4. Effects of the Ca–Mg Balance in Physiology
4.1. Healthy Balance of Ca:Mg Ratio
4.2. Ca-to-Mg Ratio for Physiological Functions
4.3. Ca-to-Mg Ratio also Affects Non-Mineral Functions
4.4. Importance of the Ca-to-Mg Ratio for Vitamin D and CTR Functions
4.5. Regulation of Ca and Phosphate Through Parathyroid Hormone
4.6. Importance of the Ca-to-Mg Ratio in Non-Mineral Disorders
5. Phosphate and Human Health
5.1. Phosphate Interactions with Other Minerals
5.2. Causes of Hyperphosphatemia
5.3. Treatment of Hyperphosphatemia
5.4. Causes of Hypophosphatemia
5.5. Treatment of Hypophosphatemia
6. Additional Functions of Common Minerals
6.1. Effects of Untreated Mineral Deficiencies
6.2. Effects of Other Trace Minerals on Human Health
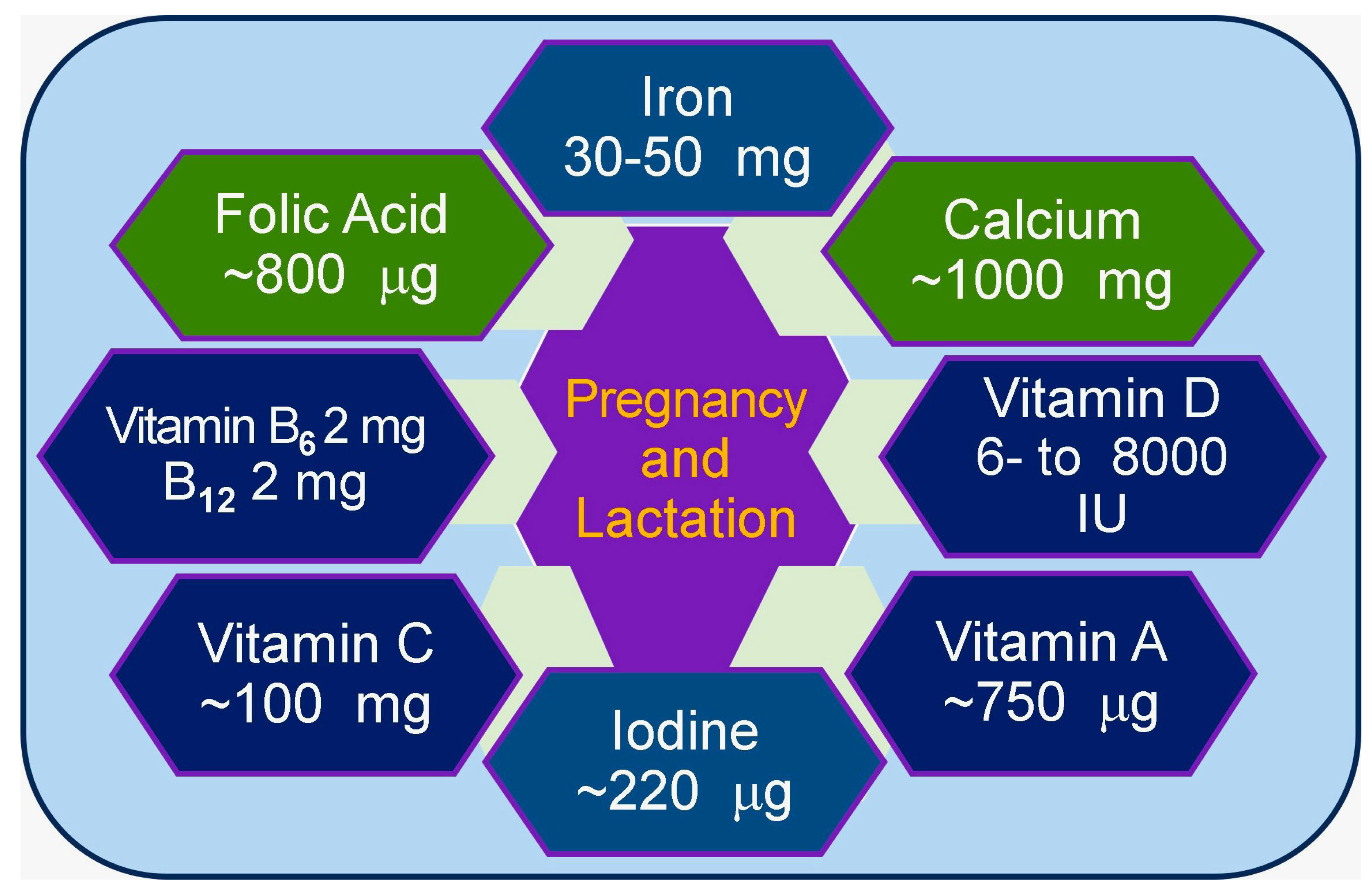
6.3. Importance of Minerals During Pregnancy and Lactation
6.4. Abnormal Mineral Metabolism Secondary to Genetic Abnormalities
6.5. Strategies to Reduce the Burden of Mineral Deficiencies
7. Mineral Toxicity and Human Health
8. Discussion
9. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Faba, L.; Gasa, J.; Tokach, M.D.; Varella, E.; Sola-Oriol, D. Effects of supplementing organic microminerals and methionine during the rearing phase of replacement gilts on lameness, growth, and body composition. J. Anim. Sci. 2018, 96, 3274–3287. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.M.A.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.K.M.; Islam, M.S.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990-2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef]
- Mogire, R.M.; Muriuki, J.M.; Morovat, A.; Mentzer, A.J.; Webb, E.L.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Cutland, C.L.; Sirima, S.B.; et al. Vitamin D Deficiency and Its Association with Iron Deficiency in African Children. Nutrients 2022, 14, 1372. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef]
- Chao, H.C. Zinc Deficiency and Therapeutic Value of Zinc Supplementation in Pediatric Gastrointestinal Diseases. Nutrients 2023, 15, 4093. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef]
- Huwiler, V.V.; Maissen-Abgottspon, S.; Stanga, Z.; Mühlebach, S.; Trepp, R.; Bally, L.; Bano, A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 295–313. [Google Scholar] [CrossRef]
- Sadler, R.A.; Mallard, B.A.; Shandilya, U.K.; Hachemi, M.A.; Karrow, N.A. The Immunomodulatory Effects of Selenium: A Journey from the Environment to the Human Immune System. Nutrients 2024, 16, 3324. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Erem, S.; Atfi, A.; Razzaque, M.S. Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol. 2019, 193, 105400. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joris, P.J.; Minović, I.; Post, A.; van Beek, A.P.; de Borst, M.H.; Mensink, R.P.; Bakker, S.J.L. Long-term magnesium supplementation improves glucocorticoid metabolism: A post-hoc analysis of an intervention trial. Clin. Endocrinol. 2021, 94, 150–157. [Google Scholar] [CrossRef]
- Stocco, D.M.; Wang, X.; Jo, Y.; Manna, P.R. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Amos, A.; Razzaque, M.S. Zinc and its role in vitamin D function. Curr. Res. Physiol. 2022, 5, 203–207. [Google Scholar] [CrossRef]
- Michigami, T.; Yamazaki, M.; Razzaque, M.S. Extracellular Phosphate, Inflammation and Cytotoxicity. Adv. Exp. Med. Biol. 2022, 1362, 15–25. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Sitara, D.; Taguchi, T.; St-Arnaud, R.; Lanske, B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006, 20, 720–722. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Lanske, B. Hypervitaminosis D and premature aging: Lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 2006, 12, 298–305. [Google Scholar] [CrossRef]
- Vervloet, M. Modifying Phosphate Toxicity in Chronic Kidney Disease. Toxins 2019, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Lanske, B.; Razzaque, M.S. Mineral metabolism and aging: The fibroblast growth factor 23 enigma. Curr. Opin. Nephrol. Hypertens. 2007, 16, 311–318. [Google Scholar] [CrossRef]
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- LeBrasseur, N. Calcium for strong clotting. J. Cell Biol. 2003, 160, 980. [Google Scholar] [CrossRef]
- Pepe, J.; Colangelo, L.; Biamonte, F.; Sonato, C.; Danese, V.C.; Cecchetti, V.; Occhiuto, M.; Piazzolla, V.; De Martino, V.; Ferrone, F.; et al. Diagnosis and management of hypocalcemia. Endocrine 2020, 69, 485–495. [Google Scholar] [CrossRef]
- Mahadevan, S.; Kumaravel, V.; Bharath, R. Calcium and bone disorders in pregnancy. Indian J. Endocrinol. Metab. 2012, 16, 358–363. [Google Scholar] [CrossRef]
- Walker, M.D.; Shane, E. Hypercalcemia: A Review. Jama 2022, 328, 1624–1636. [Google Scholar] [CrossRef]
- Rejnmark, L.; Gosmanova, E.O.; Khan, A.A.; Makita, N.; Imanishi, Y.; Takeuchi, Y.; Sprague, S.; Shoback, D.M.; Kohlmeier, L.; Rubin, M.R.; et al. Palopegteriparatide treatment Improves renal function in adults with chronic hypoparathyroidism: 1-year results from the phase 3 PaTHway trial. Adv. Ther. 2024, 41, 2500–2518. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Significance of plasma PTH-rp in patients with hypercalcemia of malignancy treated with bisphosphonate. Cancer 1994, 73, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R.; Bhuiyan, A.; Alam, A.; Chowdhury, M.K. Treatment utcomes of hypercalcemia of malignancy among advanced cancer patients attending palliative care Unit of a tertiary care hospital in Bangladesh: An observational study. Health Sci. Rep. 2024, 7, e70247. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, H.; Glezerman, I.G. Hypercalcemia of malignancy and new treatment options. Ther. Clin. Risk Manag. 2015, 11, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Li, H.; Wimalawansa, S.J. Cancer-associated hypercalcemia and parathyroid hormone-related peptide: A new peptide with diverse roles. Reg. Pept. Lett. 1997, 7, 39–42. [Google Scholar]
- El-Hajj Fuleihan, G.; Clines, G.A.; Hu, M.I.; Marcocci, C.; Murad, M.H.; Piggott, T.; Van Poznak, C.; Wu, J.Y.; Drake, M.T. Treatment of Hypercalcemia of Malignancy in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2023, 108, 507–528. [Google Scholar] [CrossRef]
- Fatima, G.; Dzupina, A.; Alhmadi, H.B.; Magomedova, A.; Siddiqui, Z.; Mehdi, A.; Hadi, N. Magnesium Matters: A Comprehensive Review of Its Vital Role in Health and Diseases. Cureus 2024, 16, e71392. [Google Scholar] [CrossRef]
- Fanni, D.; Gerosa, C.; Nurchi, V.M.; Manchia, M.; Saba, L.; Coghe, F.; Crisponi, G.; Gibo, Y.; Van Eyken, P.; Fanos, V.; et al. The role of magnesium in pregnancy and in fetal programming of adult diseases. Biol. Trace Elem. Res. 2021, 199, 3647–3657. [Google Scholar] [CrossRef]
- Ayuk, J.; Gittoes, N.J. Contemporary view of the clinical relevance of magnesium homeostasis. Ann. Clin. Biochem. 2014, 51, 179–188. [Google Scholar] [CrossRef]
- Oost, L.J.; Tack, C.J.; de Baaij, J.H.F. Hypomagnesemia and Cardiovascular Risk in Type 2 Diabetes. Endocr. Rev. 2023, 44, 357–378. [Google Scholar] [CrossRef]
- Mawri, S.; Gildeh, E.; Joseph, N.; Rabbani, B.; Zweig, B. Cardiac Dysrhythmias and Neurological Dysregulation: Manifestations of Profound Hypomagnesemia. Case Rep. Cardiol. 2017, 2017, 6250312. [Google Scholar] [CrossRef]
- Hansen, B.A.; Bruserud, O. Hypomagnesemia in critically ill patients. J. Intensive Care 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: A review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current state of the science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Munoz Martin, A.J. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.M.; Spinler, S.A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 2021, 31, 143–160. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef]
- Nagele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Di Mario, F.; Regolisti, G.; Greco, P.; Maccari, C.; Superchi, E.; Morabito, S.; Pistolesi, V.; Fiaccadori, E. Prevention of hypomagnesemia in critically ill patients with acute kidney injury on continuous kidney replacement therapy: The role of early supplementation and close monitoring. J. Nephrol. 2021, 34, 1271–1279. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in infectious diseases in older people. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef]
- Gunay, S.; Caliskan, S.; Sigirli, D. Relationship of magnesemia with myocardial damage and mortality in patients with COVID-19. Magnes. Res. 2021, 34, 93–102. [Google Scholar] [CrossRef]
- Aal-Hamad, A.H.; Al-Alawi, A.M.; Kashoub, M.S.; Falhammar, H. Hypermagnesemia in clinical practice. Medicina 2023, 59, 1190. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Shimada, N.; Kanzaki, M.; Ikegami, T.; Fukuoka, T.; Fukushima, M.; Asano, K. The characteristics of patients with hypermagnesemia who underwent emergency hemodialysis. Acute Med. Surg. 2018, 5, 222–229. [Google Scholar] [CrossRef]
- Tosto, F.; Magro, G.; Laterza, V.; Romozzi, M. Neurological manifestations of hypermagnesemia: A narrative review. Acta Neurol. Belg. 2024. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef]
- Dakal, T.C. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19. Immunobiology 2021, 226, 152021. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Alemzadeh, E.; Ziaee, M.; Abedi, A.; Salehiniya, H. The effect of low serum calcium level on the severity and mortality of COVID patients: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2021, 9, 1219–1228. [Google Scholar] [CrossRef]
- Raesi, A.; Saedi Dezaki, E.; Moosapour, H.; Saeidifard, F.; Habibi, Z.; Rahmani, F.; Kheiri, S.; Taheri, E. Hypocalcemia in COVID-19: A prognostic marker for severe disease. Iran. J. Pathol. 2021, 16, 144–153. [Google Scholar] [CrossRef]
- Yogi, A.; Callera, G.E.; Antunes, T.T.; Tostes, R.C.; Touyz, R.M. Vascular biology of magnesium and its transporters in hypertension. Magnes. Res. 2010, 23, S207–S215. [Google Scholar] [CrossRef]
- Sun, J.K.; Zhang, W.H.; Zou, L.; Liu, Y.; Li, J.J.; Kan, X.H.; Dai, L.; Shi, Q.K.; Yuan, S.T.; Yu, W.K.; et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging 2020, 12, 11287–11295. [Google Scholar] [CrossRef]
- Souza, A.C.R.; Vasconcelos, A.R.; Dias, D.D.; Komoni, G.; Name, J.J. The integral role of magnesium in muscle integrity and aging: A comprehensive review. Nutrients 2023, 15, 5127. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Ruan, Z.; Li, L.; Wu, J.; Wang, B. Nonlinear relationship between dietary calcium and magnesium intake and peripheral neuropathy in the general population of the United States. Front. Nutr. 2023, 10, 1217465. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Rosanoff, A.; Dai, Q.; Saldanha, L.G.; Potischman, N.A. Perspective: Characterization of dietary supplements containing calcium and magnesium and their respective ratio-Is a rising ratio a cause for concern? Adv. Nutr. 2021, 12, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shu, X.O.; Deng, X.; Xiang, Y.B.; Li, H.; Yang, G.; Shrubsole, M.J.; Ji, B.; Cai, H.; Chow, W.H.; et al. Modifying effect of calcium/magnesium intake ratio and mortality: A population-based cohort study. BMJ Open 2013, 3, e002111. [Google Scholar] [CrossRef]
- Yang, L.; Frindt, G.; Palmer, L.G. Magnesium modulates ROMK channel-mediated potassium secretion. J. Am. Soc. Nephrol. 2010, 21, 2109–2116. [Google Scholar] [CrossRef]
- Sontia, B.; Touyz, R.M. Magnesium transport in hypertension. Pathophysiology 2007, 14, 205–211. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Zietse, R. Disorders of calcium and magnesium balance: A physiology-based approach. Pediatr. Nephrol. 2013, 28, 1195–1206. [Google Scholar] [CrossRef]
- Suzuki, Y.; Bürzle, M.; Hediger, M.A. 369Physiology and pathology of calcium and magnesium transport. In The Spectrum of Mineral and Bone Disorders in Chronic Kidney Disease, Olgaard, K., Salusky, I.B., Silver, J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 369–377. [Google Scholar]
- Adomako, E.A.; Yu, A.S.L. Magnesium Disorders: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 803–815. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Panahi-Azar, A.; Sheybani-Arani, M.; Morovatshoar, R.; Mirzadeh, M.; Salimi Asl, A.; Naghdipour Mirsadeghi, M.; Khajavi-Mayvan, F. Vitamins, minerals and their maternal levels’ role in brain development: An updated literature-review. Clin. Nutr. ESPEN 2024, 63, 31–45. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, nutrition, detection, and social impact of diseases linked to Its deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Cauley, J.A.; Chlebowski, R.T.; Wactawski-Wende, J.; Robbins, J.A.; Rodabough, R.J.; Chen, Z.; Johnson, K.C.; O’Sullivan, M.J.; Jackson, R.D.; Manson, J.E. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: The Women’s Health Initiative. J. Womens Health 2013, 22, 915–929. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H. New insights into renal calcium-sensing receptor activation. Curr. Opin. Nephrol. Hypertens. 2024, 33, 433–440. [Google Scholar] [CrossRef]
- Tian, L.; Andrews, C.; Yan, Q.; Yang, J.J. Molecular regulation of calcium-sensing receptor (CaSR)-mediated signaling. Chronic Dis. Transl. Med. 2024, 10, 167–194. [Google Scholar] [CrossRef]
- Fouhy, L.E.; Mangano, K.M.; Zhang, X.; Hughes, B.D.; Tucker, K.L.; Noel, S.E. Association between a Calcium-to-Magnesium Ratio and Osteoporosis among Puerto Rican Adults. J. Nutr. 2023, 153, 2642–2650. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential nutrient Interactions: Does low or suboptimal magnesium status interact with vitamin D and/or calcium status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef]
- Yücel, K.; Gürbüz, A.F. Evaluation of calcium/magnesium ratio in patients with type 2 diabetes mellitus. Turk J. Biochem. 2023, 48, 327–334. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Mercado, M.; Rodriguez-Moran, M.; Ramirez-Renteria, C.; Martinez-Aguilar, G.; Marrero-Rodriguez, D.; Ferreira-Hermosillo, A.; Simental-Mendia, L.E.; Remba-Shapiro, I.; Gamboa-Gomez, C.I.; et al. Magnesium-to-calcium ratio and mortality from COVID-19. Nutrients 2022, 14, 1686. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Q.; Zhang, H.; Xu, Z.; Wang, X.; Pang, J.; Ma, J.; Ling, W.; Li, D. Associations of serum magnesium levels and calcium-magnesium ratios with mortality in patients with coronary artery disease. Diabetes Metab. 2020, 46, 384–391. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Roman, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugan-Miguelsanz, J.M. Magnesium status and Ca/Mg ratios in a series of children and adolescents with chronic diseases. Nutrients 2022, 14, 2941. [Google Scholar] [CrossRef]
- Azem, R.; Daou, R.; Bassil, E.; Anvari, E.M.; Taliercio, J.J.; Arrigain, S.; Schold, J.D.; Vachharajani, T.; Nally, J.; Na Khoul, G.N. Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol. 2020, 21, 49. [Google Scholar] [CrossRef]
- La Carrubba, A.; Veronese, N.; Di Bella, G.; Cusumano, C.; Di Prazza, A.; Ciriminna, S.; Ganci, A.; Naro, L.; Dominguez, L.J.; Barbagallo, M.; et al. Prognostic Value of Magnesium in COVID-19: Findings from the COMEPA Study. Nutrients 2023, 15, 830. [Google Scholar] [CrossRef] [PubMed]
- DeLuccia, R.; Cheung, M.; Ng, T.; Ramadoss, R.; Altasan, A.; Sukumar, D. Calcium to magnesium Ratio higher than optimal across age groups. Curr. Dev. Nutr. 2019, 3, nzz034-P10. [Google Scholar] [CrossRef]
- Ciosek, Z.; Kot, K.; Kosik-Bogacka, D.; Lanocha-Arendarczyk, N.; Rotter, I. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Steck, S.E.; Omofuma, O.O.; Su, L.J.; Maise, A.A.; Woloszynska-Read, A.; Johnson, C.S.; Zhang, H.; Bensen, J.T.; Fontham, E.T.H.; Mohler, J.L.; et al. Calcium, magnesium, and whole-milk intakes and high-aggressive prostate cancer in the North Carolina-Louisiana Prostate Cancer Project (PCaP). Am. J. Clin. Nutr. 2018, 107, 799–807. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Weiss, S.T.; Hollis, B.W. Integrating Endocrine, Genomic, and Extra-Skeletal Benefits of Vitamin D into National and Regional Clinical Guidelines. Nutrients 2024, 16, 3969. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Controlling chronic diseases and acute Infections with vitamin D sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism and function in the skin. Mol. Cell Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, T.; O’Connor, C.; Barlow, J.W.; Walsh, J.; Scalabrino, G.; Xu, F.; Sheridan, H. The biological responses of vitamin K2: A comprehensive review. Food Sci. Nutr. 2023, 11, 1634–1656. [Google Scholar] [CrossRef]
- Hariri, E.; Kassis, N.; Iskandar, J.P.; Schurgers, L.J.; Saad, A.; Abdelfattah, O.; Bansal, A.; Isogai, T.; Harb, S.C.; Kapadia, S. Vitamin K(2)-a neglected player in cardiovascular health: A narrative review. Open Heart 2021, 8, e001715. [Google Scholar] [CrossRef]
- Alharazy, S.; Robertson, M.D.; Lanham-New, S.; Naseer, M.I.; Chaudhary, A.G.; Alissa, E. Directly measured free and total 25-hydroxyvitamin D levels in relation to metabolic health in multi-ethnic postmenopausal females in Saudi Arabia. Endocr. Connect. 2021, 10, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Enhancing the design of nutrient clinical trials for disease prevention: A focus on vitamin D: A systematic review. In Nutrition Reviews; Oxford University Press: Oxford, UK, 2025; pp. 1–41. [Google Scholar] [CrossRef]
- Yao, P.; Lu, L.; Hu, Y.; Liu, G.; Chen, X.; Sun, L.; Ye, X.; Zheng, H.; Chen, Y.; Hu, F.B.; et al. A dose-response study of vitamin D3 supplementation in healthy Chinese: A 5-arm randomized, placebo-controlled trial. Eur. J. Nutr. 2016, 55, 383–392. [Google Scholar] [CrossRef]
- Grant, W.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R. Vitamin D: Evidence-based health benefits and recommendations for population guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
- Sun, M.; Wu, X.; Yu, Y.; Wang, L.; Xie, D.; Zhang, Z.; Chen, L.; Lu, A.; Zhang, G.; Li, F. Disorders of calcium and phosphorus metabolism and the proteomics/metabolomics-based research. Front. Cell Dev. Biol. 2020, 8, 576110. [Google Scholar] [CrossRef]
- Ducy, P. A central regulation of PTH secretion and function. Neuron 2023, 111, 1847–1849. [Google Scholar] [CrossRef]
- Quinn, S.J.; Thomsen, A.R.; Egbuna, O.; Pang, J.; Baxi, K.; Goltzman, D.; Pollak, M.; Brown, E.M. CaSR-mediated interactions between calcium and magnesium homeostasis in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E724–E733. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Biology of vitamin D. J. Steroids Horm. Sci. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Guo, J.H.; Zhang, X.S.; Vogt, R.D.; Xiao, J.S.; Zhao, D.W.; Xiang, R.J.; Luo, J.H. Evaluating controlling factors to Al(i)/(Ca + Mg) molar ratio in acidic soil water, southern and southwestern China: Multivariate approach. Environ. Monit. Assess. 2007, 129, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: An essential component for skeletal health. Ann. N. Y. Acad. Sci. 2011, 1240, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Dunuweera, R.; Shimomura, R.M.G.; Priyankarage, J.V.; Jayasingha, P.; Wimalawansa, S.J. Chronic kidney disease of multifunctional origin (CKDmfo) prevailing in Sri Lanka: Re-evaluated. World J. Pharma. Res. 2017, 6, 33–66. [Google Scholar]
- Wimalawansa, S.J. Effect of Water Hardness on Non-Communicable Diseases, Including Chronic Kidney Disease of Multifactorial Origin (CKDmfo/CKDuo). J. Environ. Health Sci. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Dissanayake, C.B. Nanocrystal-induced chronic tubular-nephropathy in tropical countries: Diagnosis, mitigation, and eradication. Eur. J. Med. Res. 2023, 28, 221. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Dissanayake, C.B. Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments 2019, 7, 2. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Molecular and cellular toxicity of fluoride in mystery, tubulointerstitial chronic kidney disease: A systematic review. Rev. Environ. Sci. Bio/Technol. 2019, 19, 117–147. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Public health interventions for chronic diseases: Cost-benefit modelizations for eradicating chronic kidney disease of multifactorial origin (CKDmfo/CKDu) from tropical countries. Heliyon 2019, 5, e02309. [Google Scholar] [CrossRef]
- Razzaque, M.S. Interactions between FGF23 and vitamin D. Endocr. Connect. 2022, 11, e220239. [Google Scholar] [CrossRef]
- Hong, S.H.; Park, S.J.; Lee, S.; Kim, S.; Cho, M.H. Biological effects of inorganic phosphate: Potential signal of toxicity. J. Toxicol. Sci. 2015, 40, 55–69. [Google Scholar] [CrossRef]
- Razzaque, M.S. Phosphate metabolism: From physiology to toxicity. Adv. Exp. Med. Biol. 2022, 1362, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Clavel-Chapelon, F.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. High dietary phosphorus intake is associated with an increased risk of type 2 diabetes in the large prospective E3N cohort study. Clin. Nutr. 2018, 37, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.I.; Oh, J.; Razzaque, M.S. Common dietary sources of natural and artificial phosphate in food. Adv. Exp. Med. Biol. 2022, 1362, 99–105. [Google Scholar] [CrossRef]
- Itkonen, S.T.; Lamberg-Allardt, C. Phosphorus—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef]
- Gross, P.; Six, I.; Kamel, S.; Massy, Z.A. Vascular toxicity of phosphate in chronic kidney disease: Beyond vascular calcification. Circ. J. 2014, 78, 2339–2346. [Google Scholar] [CrossRef]
- Mironov, N.; Haque, M.; Atfi, A.; Razzaque, M.S. Phosphate dysregulation and metabolic syndrome. Nutrients 2022, 14, 4477. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Isaka, Y. Effects of magnesium on the phosphate Toxicity in chronic kidney disease: Time for intervention studies. Nutrients 2017, 9, 112. [Google Scholar] [CrossRef]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef]
- Geerse, D.A.; Bindels, A.J.; Kuiper, M.A.; Roos, A.N.; Spronk, P.E.; Schultz, M.J. Treatment of hypophosphatemia in the intensive care unit: A review. Crit. Care 2010, 14, R147. [Google Scholar] [CrossRef]
- Kilpatrick, R.D.; Danese, M.D.; Belozeroff, V.; Smirnakis, K.; Goodman, W.G.; Rothman, K.J. The association of vitamin D use with hypercalcemia and hyperphosphatemia in hemodialysis patients: A case-crossover study. Pharmacoepidemiol. Drug Saf. 2011, 20, 914–921. [Google Scholar] [CrossRef]
- Bianchi, S.; Aucella, F.; De Nicola, L.; Genovesi, S.; Paoletti, E.; Regolisti, G. Management of hyperkalemia in patients with kidney disease: A position paper endorsed by the Italian Society of Nephrology. J. Nephrol. 2019, 32, 499–516. [Google Scholar] [CrossRef]
- Amanzadeh, J.; Reilly, R.F., Jr. Hypophosphatemia: An evidence-based approach to its clinical consequences and management. Nat. Clin. Pr. Nephrol. 2006, 2, 136–148. [Google Scholar] [CrossRef]
- Ali, A.A.H. Overview of the vital roles of macro minerals in the human body. J. Trace Elem. Miner. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S.; Robbins, P.A. The interplay between iron and oxygen homeostasis with a particular focus on the heart. J. Appl. Physiol. (1985) 2017, 123, 967–973. [Google Scholar] [CrossRef]
- Hirota, K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs). Free Radic. Biol. Med. 2019, 133, 118–129. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Soliman, A.T.; Alaaraj, N.; Noor, H.; Alyafei, F.; Ahmed, S.; Shaat, M.; Itani, M.; Elalaily, R.; Soliman, N. Nutritional interventions during adolescence and their possible effects. Acta Biomed. 2022, 93, e2022087. [Google Scholar] [CrossRef]
- Mireku, M.O.; Davidson, L.L.; Boivin, M.J.; Zoumenou, R.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. Prenatal Iron Deficiency, Neonatal Ferritin, and Infant Cognitive Function. Pediatrics 2016, 138, e20161319. [Google Scholar] [CrossRef]
- Jauregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Croce, L.; Chiovato, L.; Tonacchera, M.; Petrosino, E.; Tanda, M.L.; Moleti, M.; Magri, F.; Olivieri, A.; Pearce, E.N.; Rotondi, M. Iodine status and supplementation in pregnancy: An overview of the evidence provided by meta-analyses. Rev. Endocr. Metab. Disord. 2023, 24, 241–250. [Google Scholar] [CrossRef]
- Kabthymer, R.H.; Shaka, M.F.; Ayele, G.M.; Malako, B.G. Systematic review and meta-analysis of iodine deficiency and its associated factors among pregnant women in Ethiopia. BMC Pregnancy Childbirth 2021, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. The evidence linking zinc deficiency with children’s cognitive and motor functioning. J. Nutr. 2003, 133, 1473S–1476S. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Mhalla, Y.; Trabelsi, I.; Sekma, A.; Youssef, R.; Bel Haj Ali, K.; Ben Soltane, H.; Yacoubi, H.; Msolli, M.A.; Stambouli, N.; et al. Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. Clin. Infect. Dis. 2023, 76, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. COVID-19 Pandemic: Can Maintaining Optimal Zinc Balance Enhance Host Resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. COVID-19 pandemic: Can zinc supplementation provide an additional shield against the infection? Comput. Struct. Biotechnol. J. 2021, 19, 1371–1378. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Wang, M.; Deng, H.; Yang, W. Correlation between helicobacter pylori infection and iron deficiency in children. Pak. J. Med. Sci. 2022, 38, 1188–1192. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Coban, S.O.; Atfi, A.; Razzaque, M.S. The role of magnesium in pancreatic beta-cell function and homeostasis. Front. Nutr. 2024, 11, 1458700. [Google Scholar] [CrossRef]
- Mahdi, G.S. Chromium deficiency might contribute to insulin resistance, type 2 diabetes mellitus, dyslipidaemia, and atherosclerosis. Diabet. Med. 1996, 13, 389–390. [Google Scholar] [CrossRef]
- Bai, J.; Xun, P.; Morris, S.; Jacobs, D.R., Jr.; Liu, K.; He, K. Chromium exposure and incidence of metabolic syndrome among American young adults over a 23-year follow-up: The CARDIA Trace Element Study. Sci. Rep. 2015, 5, 15606. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Morris, J.S.; Park, K. Toenail Chromium Concentration and Metabolic Syndrome among Korean Adults. Int. J. Environ. Res. Public. Health 2018, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Shaju, R.; Atfi, A.; Razzaque, M.S. Zinc and Diabetes: A Connection between Micronutrient and Metabolism. Cells 2024, 13, 1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.F.; Hao, J.H.; Chen, Y.H.; Su, P.Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.Y.; Zhang, C.; et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef]
- Frimpong, E.; Ankapong, E.; Boakye, K.O.; Ansah, E.; Gyamfi, O.; Barnes, B.; Dodd, M.; Darko, G. Uptake and in-vitro bioaccessibility of toxic metals in cocoa beans: Human health risks. Environ. Geochem. Health 2024, 47, 33. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in Pregnancy and Lactation: A Review Article. J. Clin. Diagn. Res. 2017, 11, Qe01–Qe05. [Google Scholar] [CrossRef]
- Grossklaus, R.; Liesenkötter, K.P.; Doubek, K.; Völzke, H.; Gaertner, R. Iodine Deficiency, Maternal Hypothyroxinemia and Endocrine Disrupters Affecting Fetal Brain Development: A Scoping Review. Nutrients 2023, 15, 2249. [Google Scholar] [CrossRef]
- Makrides, M.; Crosby, D.D.; Bain, E.; Crowther, C.A. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev. 2014, 2014, CD000937. [Google Scholar] [CrossRef]
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimarães, R.C.A.; Pott, A.; Hiane, P.A.; Freitas, K.C. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef] [PubMed]
- Hansu, K.; Cikim, I.G. Vitamin and mineral levels during pregnancy. Rev. Assoc. Med. Bras. (1992) 2022, 68, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.Y.; Miller, W.L.; Portale, A.A. Novel gene mutations in patients with 1alpha-hydroxylase deficiency that confer partial enzyme activity in vitro. J. Clin. Endocrinol. Metab. 2002, 87, 2424–2430. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Gole, E.; Melachroinou, K.; Meristoudis, C.; Siahanidou, T.; Papadimitriou, A. Identification and Functional Characterization of a Calcium-Sensing Receptor Mutation in an Infant with Familial Hypocalciuric Hypercalcemia. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Sandoval-Cooper, M.J.; Tenenhouse, H.S.; Castellino, F.J. A missense mutation in the sodium phosphate co-transporter Slc34a1 impairs phosphate homeostasis. J. Am. Soc. Nephrol. 2008, 19, 1753–1762. [Google Scholar] [CrossRef]
- Yu, X.; White, K.E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor. Rev. 2005, 16, 221–232. [Google Scholar] [CrossRef]
- Razzaque, M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef]
- Nanba, K.; Usui, T.; Nakamura, M.; Toyota, Y.; Hirota, K.; Tamanaha, T.; Kawashima, S.T.; Nakao, K.; Yuno, A.; Tagami, T.; et al. A novel GATA3 nonsense mutation in a newly diagnosed adult patient of hypoparathyroidism, deafness, and renal dysplasia (HDR) syndrome. Endocr. Pract. 2013, 19, e17–e20. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Cioppi, F.; Maraghelli, D.; Cavalli, T.; Tonelli, F.; Brandi, M.L. Bone and Mineral Metabolism Phenotypes in MEN1-Related and Sporadic Primary Hyperparathyroidism, before and after Parathyroidectomy. Cells 2021, 10, 1895. [Google Scholar] [CrossRef]
- Robinson-Cohen, C. Genetic variants of mineral metabolism in health and disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 387–393. [Google Scholar] [CrossRef]
- Avnee; Sood, S.; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An approach to eradicate micronutrient deficiency. Front. Nutr. 2023, 10, 1233070. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Rational food fortification programs to alleviate micronutrient deficiencies. J. Food Process. Technol. 2013, 4, 257–267. [Google Scholar] [CrossRef]
- Yang, Z.; Laillou, A.; Smith, G.; Schofield, D.; Moench-Pfanner, R. A review of vitamin D fortification: Implications for nutrition programming in Southeast Asia. Food Nutr. Bull. 2013, 34, S81–S89. [Google Scholar] [CrossRef]
- Smith, G.; Wimalawansa, S.J. Reconciling the irreconcilable: Micronutrients in clinical nutrition and public health. Vitam. Miner. 2015, 4, 1–4. [Google Scholar] [CrossRef]
- Silva, P.; Araujo, R.; Lopes, F.; Ray, S. Nutrition and food literacy: Framing the challenges to health communication. Nutrients 2023, 15, 4708. [Google Scholar] [CrossRef]
- Barker, M.; Dombrowski, S.U.; Colbourn, T.; Fall, C.H.D.; Kriznik, N.M.; Lawrence, W.T.; Norris, S.A.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef]
- Wu, H.; Bai, M.; Li, X.; Xing, Y.; Sun, S. Diagnosis and treatment of brain injury complicated by hypernatremia. Front. Neurol. 2022, 13, 1026540. [Google Scholar] [CrossRef]
- An, J.N.; Lee, J.P.; Jeon, H.J.; Kim, D.H.; Oh, Y.K.; Kim, Y.S.; Lim, C.S. Severe hyperkalemia requiring hospitalization: Predictors of mortality. Crit. Care 2012, 16, R225. [Google Scholar] [CrossRef]
- Costa, D.; Patella, G.; Provenzano, M.; Ielapi, N.; Faga, T.; Zicarelli, M.; Arturi, F.; Coppolino, G.; Bolignano, D.; De Sarro, G.; et al. Hyperkalemia in CKD: An overview of available therapeutic strategies. Front. Med. 2023, 10, 1178140. [Google Scholar] [CrossRef]
- Juurlink, D.N.; Tenenbein, M.; Koren, G.; Redelmeier, D.A. Iron poisoning in young children: Association with the birth of a sibling. CMAJ 2003, 168, 1539–1542. [Google Scholar]
- Kozaki, K.; Egawa, H.; Garcia-Kennedy, R.; Cox, K.L.; Lindsay, J.; Esquivel, C.O. Hepatic failure due to massive iron ingestion successfully treated with liver transplantation. Clin. Transpl. 1995, 9, 85–87. [Google Scholar] [CrossRef]
- Chandran, J.; Sanketh, R.; Vyasam, S.; Chrysolyte, A.; Ebenezer, K. Accidental iron poisoning in children—Experience from a teaching institution. J. Fam. Med. Prim. Care 2023, 12, 2520–2523. [Google Scholar] [CrossRef]
- Sankar, J.; Shukla, A.; Khurana, R.; Dubey, N. Near fatal iron intoxication managed conservatively. BMJ Case Rep. 2013, 2013, bcr2012007670. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Vallamkondu, J.; Corgiat, E.B.; Gill, K.D. Understanding aspects of aluminum exposure in Alzheimer’s disease development. Brain Pathol. 2016, 26, 139–154. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Niklinski, J. Cadmium toxicity and health effects-A brief summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Bandara, J.M.; Wijewardena, H.V.; Liyanege, J.; Upul, M.A.; Bandara, J.M. Chronic renal failure in Sri Lanka caused by elevated dietary cadmium: Trojan horse of the green revolution. Toxicol. Lett. 2010, 198, 33–39. [Google Scholar] [CrossRef]
- Umair, M.; Alfadhel, M. Genetic disorders associated with metal metabolism. Cells 2019, 8, 1598. [Google Scholar] [CrossRef]
- Brown, A.S.; Gwinn, M.; Cogswell, M.E.; Khoury, M.J. Hemochromatosis-associated morbidity in the United States: An analysis of the National Hospital Discharge Survey, 1979–1997. Genet. Med. 2001, 3, 109–111. [Google Scholar] [CrossRef]
- Porter, J.L.; Rawla, P. Hemochromatosis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- de Bie, P.; van de Sluis, B.; Burstein, E.; van de Berghe, P.V.; Muller, P.; Berger, R.; Gitlin, J.D.; Wijmenga, C.; Klomp, L.W. Distinct Wilson’s disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology 2007, 133, 1316–1326. [Google Scholar] [CrossRef]
- Lorincz, M.T. Wilson disease and related copper disorders. Handb. Clin. Neurol. 2018, 147, 279–292. [Google Scholar] [CrossRef]
- Charkiewicz, A.E. Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements? Curr. Issues Mol. Biol. 2024, 46, 8441–8463. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Prasad, A.N. Menkes disease: What a multidisciplinary approach can do. J. Multidiscip. Healthc. 2016, 9, 371–385. [Google Scholar] [CrossRef] [PubMed]


| Minerals | Sources | Main Physiological Functions | Potential Human Toxicity |
|---|---|---|---|
| Boron | Fruits (avocados, raisins, peaches, apples, grapes, oranges, bananas); vegetables and legumes (broccoli, potatoes, carrots, celery); nuts and seeds (peanuts, almonds, Brazil nuts and hazelnuts) | It affects the metabolism of steroid hormones (estrogen, testosterone, and vitamin D), improves cognitive performance in older adults, and improves antioxidant activity and wound healing. | Skin reactions (rashes and dermatitis) and neurological symptoms (headaches, restlessness, and convulsions) affect fertility and male reproductive organs |
| Calcium | Milk and milk products; canned fish with bones (salmon and sardines); fortified tofu and soy beverage; greens (broccoli and mustard greens); legumes | Important for bone and teeth health; second messenger. | Kidney stones and nephrocalcinosis, abnormal heart rhythms, and vascular calcification |
| Chloride | Table salt, soy sauce, and processed foods | Maintaining pH levels in the body (particularly in the blood) is essential for producing hydrochloric acid (in the stomach), facilitating the transmission of nerve impulses, and maintaining proper fluid balance and blood pressure. | Impair sodium or potassium metabolism |
| Chromium | Whole grains (bread and cereals, oatmeal, and barley); seafood (mussels, oysters, and shrimp); fruits (apples, bananas, and grapes); lean meats (beef, turkey, and chicken); vegetables (broccoli, green beans, potatoes, and asparagus) | Blood sugar regulation is achieved by improving insulin sensitivity; protein metabolism is achieved through the breakdown and absorption of proteins; and fatty acid and cholesterol synthesis is stimulated. | Skin issues (contact dermatitis, skin ulcers, and sensitization), liver and kidney damage; increased risk of lung cancer |
| Cobalt | Fish and shellfish (oysters, clams, and mussels), meat (mainly liver and kidney), milk and dairy products, legumes, beans and nuts | Essential components of vitamin B12 (cobalamin), erythropoiesis, cofactor for methyl malonyl-CoA mutase, and methionine synthase. | Chronic exposure may lead to asthma-like symptoms, toxic cardiomyopathy, cognitive decline, and polycythemia |
| Copper | Legumes, nuts and seeds, whole grains, organ meats, and drinking water | Many enzymes are needed for iron metabolism. | Liver damage, kidney failure, neurological effects (mood changes, depression, anxiety, irritability, and difficulty focusing), hematological effects (hemolytic anemia) |
| Fluoride | Beverages (black tea and coffee, grape juice, chocolate, and almond milk); fruits (grapes and raisins, apples, strawberries, bananas, peaches, watermelon, and cherries); vegetables (spinach, potatoes, carrots, and asparagus); seafood (shrimp, crab, and oysters) | Preventing and reversing dental caries by strengthening tooth enamel, contributing to the mineralization and strength of skeletal tissues, increases the stability and crystallinity of bone apatite structures. | Dental fluorosis (white chalky opacities on tooth enamel, brownish discoloration or pigmentation and pitting of tooth); skeletal fluorosis (increased bone density and decreased elasticity, joint pain and decreased mobility, and increased risk of fractures) |
| Iodine | Seafood, foods grown in iodine-rich soil, iodized salt, bread, and dairy products | Iodine is present in the thyroid hormone, which helps regulate growth, development, and metabolism. | Thyroid dysfunction (hypothyroidism, hyperthyroidism, thyroiditis, or increased risk of thyroid cancer); neurological effects (delirium, seizures, and stupor) |
| Iron | Organ meats, red meats, fish, poultry, shellfish (especially clams), egg yolks, legumes, dried fruits, dark leafy greens, and iron-enriched breads and cereals | Part of a molecule (hemoglobin) found in red blood cells that carries oxygen in the body needed for energy metabolism. | Liver damage (chronic liver disease, cirrhosis, and increased risk of hepatocellular carcinoma); cardiac effects (heart failure and arrhythmias); neurological effects (potential acceleration of neurodegenerative diseases) |
| Magnesium | Nuts and seeds, legumes, leafy green vegetables, seafood, and chocolate | Maintain skeletal system and immune system health. | Gastrointestinal effects (diarrhea and abdominal discomfort); cardiovascular effects (hypotension, bradycardia, and heart blocks); neuromuscular effects (muscle weakness and paralysis) |
| Manganese | Nuts and seeds (hazelnuts, pecans, and pine nuts); legumes (chickpeas, soybeans, and lentils); shellfish (mussels, oysters, and clams) | A key component of the antioxidant enzyme superoxide dismutase (SOD); it plays a role in blood clotting and hemostasis; acts as a cofactor for various enzymes. | Cognitive impairment, increased susceptibility to respiratory tract infections, and slurred speech |
| Molybdenum | Legumes (black-eyed peas, lima beans, lentils, and pinto beans); whole grains (oats, barley, and brown rice); dairy products (milk, yogurt, and cheese); vegetables (spinach, potatoes, and asparagus) | The enzyme cofactor for xanthine oxidase and aldehyde oxidase plays a role in the liver’s phase I and II detoxification pathways. | Joint pain and gout-like symptoms, anemia, and neurological effects (seizures and hallucinations) |
| Phosphorus | Meat, fish, poultry, eggs, and milk | Important for healthy bones and teeth; maintains acid-base balance. | Cardiovascular calcification, impaired renal functions, and dysregulation in bone metabolism |
| Potassium | Meats, milk, fresh fruits and vegetables, whole grains, and legumes | Needed for proper fluid balance, nerve transmission, and muscle contraction. | Cardiovascular effects (palpitations, arrhythmias, and potential heart attack); neurological effects (fatigue, headache, delirium, or seizures); muscle-related effects (weakness, pain, and in severe cases, and paralysis) |
| Selenium | Meat, seafood, and grains | A key component of antioxidant enzymes, particularly glutathione peroxidases, is important for male fertility and spermatogenesis in regulating thyroid hormones. Selenium-containing enzymes help make DNA and protect against cell damage. | Dermatitis, alopecia, nail discoloration, peripheral neuropathy, decreased cognitive function, and cardiovascular issues (tachycardia and palpitations) |
| Silicon | Legumes (soybeans, tofu, and red lentils); nuts and seeds (almonds, peanuts, and sunflower seeds); whole grains (oats, barley, and brown rice) | It plays a role in maintaining the structural integrity and elasticity of skin, hair, and nails, crucial for synthesizing and stabilizing collagen. | Excessive exposure can cause silicosis (a progressive and irreversible lung disease); exposure to silica dust can also increase the risk of lung cancer, chronic obstructive pulmonary disease (COPD), and tuberculosis; long-term exposure increases the risk of autoimmune diseases. |
| Sodium | Table salt, soy sauce, and processed foods | Plays a key role in regulating blood pressure by influencing blood volume and vascular tone; critical for the conduction of nerve impulses, allowing proper communication between nerve cells; essential for normal muscle contraction and relaxation; involved in the transport of various nutrients across cell membranes, including glucose, amino acids, and phosphate. | Neurological effects (confusion, seizures, coma, potential cerebrovascular damage, thirst and dehydration, muscle weakness, and pain) |
| Zinc | Meats, fish, poultry, whole grains, and vegetables | They are needed for making protein and genetic material and have a role in taste perception, wound healing, normal fetal development, sperm production, normal growth and sexual maturation, and immune system health. | Copper deficiency (resulting in anemia and neutropenia); impaired immune function; and neurological effects (lethargy, dizziness, and, in severe cases, convulsions) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. https://doi.org/10.3390/nu17030454
Razzaque MS, Wimalawansa SJ. Minerals and Human Health: From Deficiency to Toxicity. Nutrients. 2025; 17(3):454. https://doi.org/10.3390/nu17030454
Chicago/Turabian StyleRazzaque, Mohammed S., and Sunil J. Wimalawansa. 2025. "Minerals and Human Health: From Deficiency to Toxicity" Nutrients 17, no. 3: 454. https://doi.org/10.3390/nu17030454
APA StyleRazzaque, M. S., & Wimalawansa, S. J. (2025). Minerals and Human Health: From Deficiency to Toxicity. Nutrients, 17(3), 454. https://doi.org/10.3390/nu17030454








