Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims
Abstract
:1. Introduction
1.1. CBD as Lifestyle Product and Market Prevalence
1.2. CBD as a Medicinal Drug
1.3. Novel Food Status and Regulations
1.4. Concept/Aim
2. Methods
3. Molecular Targets of CBD and Putative (Pharmacological) Mode of Action
4. Absorption, Distribution, Metabolism, and Excretion (ADME)
5. Characterization of Adverse Health Effects of CBD
5.1. Liver
5.1.1. Animal Data
5.1.2. Human Data in Healthy Volunteers
Therapeutic Doses (1500 mg CBD/Person per Day)
Therapeutic Starting Dose or Lower (≤300 mg CBD/Person per Day)
5.2. Gastrointestinal Tract
5.3. Neurological, Psychiatric, and Psychological Effects
5.4. Endocrine System
5.4.1. Hypothalamic–Pituitary–Gonadal Axis
5.4.2. Thyroid Gland
5.4.3. Adrenal Glands
5.5. Reproductive System
5.6. Fertility
5.7. CBD Interaction with Drug Metabolism
5.8. Genotoxicity
6. Characterization of Beneficial Health Effects of CBD
6.1. Physical Performance
6.2. Cardiovascular System
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repeated dosing | ||||||||||||||
| Healthy human volunteers (n = 8/group); double-blind, placebo-controlled | 210 mg/day (3 mg/kg bw/day) or placebo | CBD isolated from hashish of undetermined age | Oral | 30 days |
| [101] | ||||||||
| Patients with epilepsy (n = 15); randomized, double-blind, placebo-controlled | 200–300 mg/day (2.9–4.3 mg/kg bw/day) | CBD isolated from hashish of undetermined age | Oral | 4.5 months |
|
| [101] | |||||||
| Patients with dystonic movement disorders (n = 5); preliminary open study | Oral doses of CBD rising from 100 to 600 mg/day (1.4–8.6 mg/kg bw/day) | Oral | 6 weeks |
|
| [102] | ||||||||
| Individuals with mild or moderate hypertension (n = 70); either untreated or receiving standard care therapy; randomized, placebo-controlled, triple-blind, crossover design | 225 to 300 mg split into three daily doses for the initial 2.5 weeks (3.2–4.3 mg/kg bw/day) and 375 to 450 mg split into three daily doses for the following 2.5 weeks (5.4–6.4 mg/kg bw/day) | DehydraTECHTM 2.0 CBD, a patented formulation with increased CBD bioavailability | Oral | 5 weeks |
|
| [103] | |||||||
| Single administration | ||||||||||||||
| Healthy young men (n = 13); double-blinded, placebo-controlled, crossover design | 45, 90 mg/day; (0.6, 1.3 mg/kg bw/day) | CBD capsules; 45/90 mg CBD; 150/300 mg organic multi-spectrum hemp oil | Oral | Acute |
| [105] | ||||||||
| 45, 90 mg/day (0.6, 1.3 mg/kg bw/day) | CBD encapsulated as TurboCBDTM; 45/90 mg CBD; 600/1200 mg American ginseng; 240/480 mg ginkgo biloba; 150/300 mg organic hemp oil | Oral | Acute |
|
| |||||||||
| Healthy volunteers (n = 40, 10/group); double-blind, placebo-controlled; 4 groups: placebo, CBD, diazepam (10 mg) or ipsapirone (5 mg) | 300 mg/day (4.3 mg/kg bw/day) | Not specified | Oral | acute |
| [106] |
6.3. Immune System
6.3.1. Immune Suppression
6.3.2. Antioxidative Activity
6.4. Nervous System
6.4.1. Positive Mood
6.4.2. Good Cognitive Functions
6.4.3. Neuroprotection
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive mood | ||||||||||||||
| Healthy volunteers (n = 17); randomized, double-blind, placebo-controlled, within-subject design | 200, 400, 800 mg/day (2.9, 5.7, 11.4 mg/kg bw/day) | Synthetic CBD ([+]-CBD isomer) (liquid solution) | Oral | Acute; 4 sessions over 4 weeks, separated by at least 5 days |
|
| [56] | |||||||
| Overweight, healthy volunteers (n = 65); randomized, double-blind, placebo-controlled | 15 mg/day (0.2 mg/kg bw/day) | Hemp-derived CBD (hemp oil extract in soft gel capsules) | Oral | Acute; 6 weeks |
|
| [155] | |||||||
| Healthy volunteers (n = 38); double-blind, placebo-controlled, within-subjects design | 300, 600, 900 mg (300 mg/mL solution) (4.3, 8.6, 12.9 mg/kg bw/day) | Not specified (liquid solution) | Oral | Acute; 4 sessions, separated by at least 1 week |
|
| [156] | |||||||
| Good cognitive functions | ||||||||||||||
| Healthy volunteers (n = 17); randomized, double-blind, placebo-controlled, crossover design | 15, 300, 1500 mg/day (0.2, 4.3, 21.4 mg/kg bw/day) | Synthetic CBD (100 mg/mL) in medium-chain triglyceride oil | Oral (administered together with a high-fat supplement) | Acute; four treatment sessions within 60 days (with a median washout period of 7.5 days) |
|
| [162] | |||||||
| Healthy volunteers (n = 48); randomized, double-blind, placebo-controlled | 50 mg/day (0.7 mg/kg bw/day) | Hemp-derived CDB | Oral | 8 weeks |
|
| [90] |
6.5. Anxiety
6.6. Stress Management
6.7. Relaxation
6.8. Sleep
6.9. Pain
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | ||||||||||||||
| Girls with significant somatoform psychological and chronic pain as a result of the human papillomavirus vaccine, 12–24 years old (n = 12); single-arm trial | 25 mg/day increasing to 150 mg/day (0.4–2.1 mg/kg bw/day) | CBD-enriched hemp oil | Oral | 12 weeks |
|
| [259] | |||||||
| Patients with a mean age of 64.5 years old (range, 58–75 years old), (n = 7); open-label, single-arm trial | Increasing doses of oral CBD (50 to 150 mg twice a day;(1.4–4.3 mg/kg bw/day) | CBD hemp oil | Oral | 3 weeks |
|
| [260] | |||||||
| Patients with multiple sclerosis, spinal cord injury, brachial plexus damage, and limb amputation due to neurofibromatosis (n = 24); randomized, double-blind, multigroup crossover design | 2.5–120 mg/24 h (0.04–1.7 mg/kg bw/day) | Whole-plant extracts of Δ9-THC (THC-rich CME), cannabidiol (CBD-rich CME) and a 1:1 preparation of the two (THC:CBD). | Sublingual spray | 2 weeks |
|
| [261] | |||||||
| 131 cancer patients taking opioids | 30 mg/day (0.4 mg/kg bw/day) | Hemp-derived CBD soft gels | Oral | 8 weeks |
|
| [254] | |||||||
| Patients with hand osteoarthritis or psoriatic arthritis experiencing moderate pain intensity despite therapy (n = 129); randomized, double-blind, placebo-controlled | 20–30 mg/day (0.3–0.4 mg/kg bw/day) | Synthetic CBD | Oral | 12 weeks |
| [255] | ||||||||
| Patients with painful polyneuropathy (n = 145); randomized, double-blind | 5–50 mg/day (0.07–0.7 mg/kg bw/day) | Not clear | Oral | 8 weeks |
| [256] | ||||||||
| Healthy men and women (18–30 years old); double-blind, crossover, balanced placebo 2 × 2 factorial design | 50 mg/day (0.7 mg/kg bw/day) | Hemp-derived CBD | Oral | Single administration |
|
| [257] | |||||||
| Healthy men and women (32 ± 8 years old) (n = 17); double-blind, placebo-controlled, crossover design | 200, 400 g, 800 mg/day (2.9, 5.7, 11.4 mg/kg bw/day) | Pure CBD | Oral | Single treatment |
|
| [56] | |||||||
| Male and female patients (34–60 years old) with acute, non-traumatic low back pain (n = 100); randomized, double- blinded, placebo-controlled clinical trial | 400 mg/day (5.7 mg/kg bw/day) | Synthetic CBD 99.9% purity | Oral | Single treatment |
|
| [258] | |||||||
| Healthy males (18–65 years old) (n = 40; n = 20 per group); randomized, double-blind, placebo-controlled, repeated-dose pilot study | 70 mg CBD (1 mg/kg bw/day) and 100 mg CBG per day | Water-soluble liquid. CBD oil nano-particularized, and then combined with other ingredients to maintain the suspension. | Oral | 3.5 days |
|
| [96] | |||||||
| Untrained men (21.85 ± 2.73 years old) (n = 13); double-blind, placebo- controlled, crossover design | 150 mg/day (2.14 mg/kg bw/day) | CBD oil | Oral | 3 days |
|
| [92] | |||||||
| Female patients with irritable bowel syndrome, 22–50 years old (n = 32); randomized, double-blinded, placebo- controlled crossover design | 50–300 mg/day (0.7–4.3 mg/kg bw/day) | CBD-containing chewing gum | Oral | 8 weeks |
|
| [232] |
6.10. Menstrual Discomfort
6.10.1. Studies on CBD and Menstrual Discomfort
6.10.2. Therapy of Endometriosis
7. Exposure Considerations
8. Risk Characterization
- ADI of 10 mg/day for a 70 kg person (corresponding to 0.14 mg/kg bw/day) [24].
- LOAEL for liver toxicity, 300 mg/day (corresponding to 4.3 mg/kg bw/day for a 70 kg person).
- Therapeutic dose range of 600–1000 mg/day (corresponding to approx. 9–14 mg/kg bw/day for a 70 kg person)
9. Benefit Characterization
10. Assessment—Risk–Benefit Integration
11. Conclusions
12. Research Needs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Website | Low (Micro) Dosage 1 [mg/day] | Medium (Standard) Dosage 1 [mg/day] | High (Macro) Dosage 1 [mg/day] | Recommended Dosage Range [mg/day] |
|---|---|---|---|---|
| https://flavorfix.com/cbd/cbd-dosage-chart-calculator/ 2 (accessed on 15 November 2024) | 180–909 | |||
| https://www.verywellmind.com/cbd-dosages-how-much-cbd-should-you-take-5078580 (accessed on 15 November 2024) | Anxiety: 300–600 Bowel disease: 10 Cancer-related pain: 50–600 Parkinson’s disease: 75–300 Poor sleep: 25 Psychosis: 600 | |||
| https://www.kurkliniken.de/blog/cbd-oel-richtig-dosieren-darauf-kommt-es-bei-der-anwendung-an.html (accessed on 15 November 2024) | 20–1500 | |||
| https://www.forbes.com/health/cbd/cbd-dosage/ (accessed on 15 November 2024) | Anxiety: 300–600 Select forms of epilepsy: starting at 2.5 mg/kg bw/twice daily Central neuropathic and cancer-related pain: maximum of 30 Opioid addiction: 400 or 800 Arthritis: maximum of 30 (or 250 if applied topically) | |||
| https://www.praktischarzt.de/ratgeber/cbd-oel/dosierung/ (accessed on 15 November 2024) | 0.5–20 | 20–100 | 400 | |
| https://naturalcbd.at/dosierung-von-cbd/ 3 (accessed on 15 November 2024) | 1–32 | 15–115 | 75–1500 | |
| https://www.cbd-vital.de/magazin/cbd-allgemein/cbd-dosierung (accessed on 15 November 2024) | 1–20 | 10–100 | 50–800 | |
| https://www.calconic.com/calculator-widgets/cbd-dosage-calculator 4 (accessed on 15 November 2024) | 10–20 | 15–30 | 25–50 | |
| https://cbd360.de/cbd-oel-dosierung/ (accessed on 15 November 2024) | 2–20 | 20–80 | 80–800 | |
| https://cbdsfinest.de/magazin/cbd-oel-dosierung-einnahme/ (accessed on 15 November 2024) | 0.5–20 | 10–100 | 50–800 | |
| https://www.benetui.com/de/magazin/wie-viel-mg-cbd-pro-tag-soll-ich-nehmen 4 (accessed on 15 November 2024) | 14 5 | 49 5 | 91 5 | Pain: 2.5–20 Anxiety/stress: 5–30 Sleep disorder: 40–160 Epilepsy: 200–300 |
| https://cannatrust.eu/wiki/cbd-dosierung/ 6 (accessed on 15 November 2024) | 10–25 | 30–75 | 60–150 | |
| https://cbd-deal24.de/was-ist-cbd/cbd-oel-dosierung/ (accessed on 15 November 2024) | 0.5–25 | 10–100 | 50–800 | |
| https://www.cbd-guru.co.uk/cbd-beginners-guide/?srsltid=AfmBOoqnpU082CprHEzOyFGyStiEb_HeWZ3VQa9vxEfcZ8Ro_Lybi1u8 (accessed on 15 November 2024) | 0.5–20 | 10–100 | 50–800 | |
| https://www.naturalwayscbd.com/blog/cbd-dosage-chart/ 7 (accessed on 15 November 2024) | 8–24 | 24–72 | 40–120 |
References
- FIP. Food and Feed Information Portal Database. Available online: https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search (accessed on 19 November 2024).
- EFSA. Statement on safety of cannabidiol as a novel food: Data gaps and uncertainties; Panel on Nutrition, Novel Foods and Food Allergens. EFSA J. 2022, 20, e07322. [Google Scholar]
- Lachenmeier, D.W.; Habel, S.; Fischer, B.; Herbi, F.; Zerbe, Y.; Bock, V.; Rajcic de Rezende, T.; Walch, S.G.; Sproll, C. Are adverse effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination? F1000Research 2022, 8, 1394. [Google Scholar] [CrossRef]
- Lindekamp, N.; Weigel, S.; Sachse, B.; Schäfer, B.; Rohn, S.; Triesch, N. Comprehensive analysis of 19 cannabinoids in commercial CBD oils: Concentrations, profiles, and safety implications. J. Consum. Prot. Food Saf. 2024, 19, 259–267. [Google Scholar] [CrossRef]
- Miller, O.S.; Elder, E.J., Jr.; Jones, K.J.; Gidal, B.E. Analysis of cannabidiol (CBD) and THC in nonprescription consumer products: Implications for patients and practitioners. Epilepsy Behav. 2022, 127, 108514. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Kilgore, M.; Babalonis, S. Cannabidiol (CBD) product contamination: Quantitative analysis of Δ9-tetrahydrocannabinol (Δ9-THC) concentrations found in commercially available CBD products. Drug Alcohol Depend. 2022, 237, 109522. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA J. 2015, 13, 4141. [Google Scholar] [CrossRef]
- Geppert, J.; Lietzow, J.; Hessel-Pras, S.; Kirsch, F.; Schäfer, B.; Sachse, B. Usage and health perception of cannabidiol-containing products among the population in Germany: A descriptive study conducted in 2020 and 2021. BMC Public Health 2023, 23, 2318. [Google Scholar] [CrossRef]
- Kraft, K.; Thomsen, M.; Schmidt, M. Cannabidiol: Food or drug? A positioning. J. Mod. Med. Chem. 2021, 9, 17–24. [Google Scholar] [CrossRef]
- Wheeler, M.; Merten, J.W.; Gordon, B.T.; Hamadi, H. CBD (cannabidiol) product attitudes, knowledge, and use among young adults. Subst. Use Misuse 2020, 55, 1138–1145. [Google Scholar] [CrossRef]
- Soleymanpour, M.; Saderholm, S.; Kavuluru, R. Therapeutic claims in cannabidiol (CBD) marketing messages on Twitter. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 9–12 December 2021; pp. 3083–3088. [Google Scholar] [CrossRef]
- Amann, L.; Kruse, E.; Lazard, A.J.; Reboussin, B.A.; Wagoner, K.G.; Romero-Sandoval, E.A. CBD retailers in NC promote CBD online to treat pain violating FDA rules about medical claims and offer low-CBD/high-price products. J. Pain Res. 2022, 15, 3847–3858. [Google Scholar] [CrossRef]
- Leas, E.C.; Moy, N.; McMenamin, S.B.; Shi, Y.; Benmarhnia, T.; Stone, M.D.; Trinidad, D.R.; White, M. Availability and promotion of cannabidiol (CBD) products in online Vape shops. Int. J. Environ. Res. Public Health 2021, 18, 6719. [Google Scholar] [CrossRef] [PubMed]
- Merten, J.W.; Gordon, B.T.; King, J.L.; Pappas, C. Cannabidiol (CBD): Perspectives from Pinterest. Subst. Use Misuse 2020, 55, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Moltke, J.; Hindocha, C. Reasons for cannabidiol use: A cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J. Cannabis Res. 2021, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Zenone, M.A.; Snyder, J.; Crooks, V. Selling cannabidiol products in Canada: A framing analysis of advertising claims by online retailers. BMC Public Health 2021, 21, 1285. [Google Scholar] [CrossRef]
- FDA. Cross Discipline Team Leader Review. Center for Drug Evaluation and Research. Application Number: 210365Orig1s000. Summary Review. Reference ID 4282210. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000SumR.pdf (accessed on 19 November 2024).
- EMA (European Medicine Agency). Assessment Report, Epidyolex, International Non-Proprietary Name: Cannabidiol. EMA/458106/2019. 2019. Available online: https://www.ema.europa.eu/en/documents/assessment-report/epidyolex-epar-public-assessment-report_en.pdf (accessed on 19 November 2024).
- EC. Information and Notices. Off. J. Eur. Union 2019, 62. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:C:2019:369:FULL&from=EN (accessed on 30 October 2019).
- Lachenmeier, D.W.; Rajcic de Rezende, T.; Habel, S.; Bock, V.; Sproll, C.; Walch, S.G. Current case law confirms novel food classification of hemp extracts and cannabidiol (CBD) in foods—Narcotic classification of cannabis foods remains unclear. Dtsch. Lebensm.-Rundsch. 2020, 116, 111–119. [Google Scholar] [CrossRef]
- Dietz, T.; Gerstenlauer, J.; Sproll, C.; Walch, S.G.; Lachenmeier, D.W. Cannabidiol (CBD) in Lebensmitteln—Beurteilung der Sicherheit: Gesundheitsschädlich bei über 300 mg/Tag. Dtsch. Lebensm.-Rundsch. 2022, 118, 446–453. [Google Scholar] [CrossRef]
- Gingrich, J.; Choudhuri, S.; Cournoyer, P.; Downey, J.; Muldoon Jacobs, K. Review of the oral toxicity of cannabidiol (CBD). Food Chem. Toxicol. 2023, 176, 113799. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Concludes That Existing Regulatory Frameworks for Foods and Supplements are Not Appropriate for Cannabidiol, Will Work with Congress on a New Way Forward. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-concludes-existing-regulatory-frameworks-foods-and-supplements-are-not-appropriate-cannabidiol (accessed on 19 November 2024).
- FSA. Food Standards Agency and Food Standards Scotland Update Consumer Advice for CBD. 2023. Available online: https://www.food.gov.uk/print/pdf/node/20686 (accessed on 12 October 2023).
- FSVO. Briefing Letter Cannabidiol (CBD) in Foods and the Effects on the Liver. Federal Food Safety and Veterinary Office. 2021. Available online: https://www.blv.admin.ch/dam/blv/en/dokumente/lebensmittel-und-ernaehrung/publikationen-forschung/briefing-letter-lebensmittel-lebereffekte.pdf.download.pdf/Briefing%20Letter%20Cannabidiol%20in%20Lebensmitteln%20und%20Lebereffekte%20DE.pdf (accessed on 19 November 2024).
- COT. Updated Position Paper on the Potential Risk of CBD in CBD Food Products. Committee On Toxicity. 2021. Available online: https://cot.food.gov.uk/sites/default/files/2021-08/CBD%20Position%20Paper%20updated%20July%202021.pdf (accessed on 19 November 2024).
- COT. Update CBD, Committee On Toxicity. February 2020. Available online: https://cot.food.gov.uk/sites/default/files/tox202002cbd.pdf (accessed on 19 November 2024).
- EFSA. Guidance on risk–benefit assessment of foods. EFSA Scientific Committee. EFSA J. 2024, 22, e8875. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2024.8875 (accessed on 28 November 2024).
- 6 Health Benefits of CBD Oil—And a Look at Side Effects. Available online: https://www.healthline.com/nutrition/cbd-oil-benefits#mental-health (accessed on 28 November 2024).
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharma. Tox. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Azevedo-Silva, J.; Fernandes, J.C. Beyond pain relief: A review on Cannabidiol potential in medical therapies. Pharmaceuticals 2023, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and cellular mechanisms of action of cannabidiol. Molecules 2023, 28, 5980. [Google Scholar] [CrossRef] [PubMed]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Polizio, A.H.; Abbate, A.; Toldo, S.; Mezzaroma, E. An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules 2024, 29, 473. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Beben, D.; Siwiela, O.; Szyjka, A.; Graczyk, M.; Rzepka, D.; Barg, E.; Moreira, H. Phytocannabinoids CBD, CBG, and their Derivatives CBD-HQ and CBG-A Induced In Vitro Cytotoxicity in 2D and 3D Colon Cancer Cell Models. Curr. Issues Mol. Biol. 2024, 46, 3626–3639. [Google Scholar] [CrossRef]
- Usami, N.; Yamamoto, I.; Watanabe, K. Generation of reactive oxygen species during mouse hepatic microsomal metabolism of cannabidiol and cannabidiol hydroxy-quinone. Life Sci. 2008, 83, 717–724. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wu, Q.; Li, Y.; Puig, M.; Moulin, F.; Choudhuri, S.; Gingrich, J.; Guo, L. Investigation of cannabidiol-induced cytotoxicity in human hepatic cells. Toxicology 2024, 506, 153884. [Google Scholar] [CrossRef] [PubMed]
- FDA. Combined Clinical and Statistical Review. Center for Drug Evaluation and Research. Application Number: 210365Orig1s000. Clinical Reviews. Reference ID 4277537. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000MedR.pdf (accessed on 19 November 2024).
- Atsmon, J.; Heffetz, D.; Deutsch, L.; Deutsch, F.; Sacks, H. Single-Dose Pharmacokinetics of Oral Cannabidiol Following Administration of PTL101: A New Formulation Based on Gelatin Matrix Pellets Technology. Clin. Pharmacol. Drug Dev. 2018, 7, 751–758. [Google Scholar] [CrossRef]
- Landmark, C.J.; Brandl, U. Pharmacology and drug interactions of cannabinoids. Epileptic Disord. 2020, 22, 16–22. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood-Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265. [Google Scholar] [CrossRef]
- Henderson, R.G.; Lefever, T.W.; Heintz, M.M.; Trexler, K.R.; Borghoff, S.J.; Bonn-Miller, M.O. Oral toxicity evaluation of cannabidiol. Food Chem. Toxicol. 2023, 176, 113778. [Google Scholar] [CrossRef]
- Henderson, R.G.; Welsh, B.T.; Rogers, J.M.; Borghoff, S.J.; Trexler, K.R.; Bonn-Miller, M.O.; Lefever, T.W. Reproductive and developmental toxicity evaluation of cannabidiol. Food Chem. Toxicol. 2023, 176, 113786. [Google Scholar] [CrossRef] [PubMed]
- Tallon, M.J.; Child, R. Subchronic oral toxicity assessment of a cannabis extract. Regul. Toxicol. Pharmacol. 2023, 144, 105496. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Church, R.J.; Li, J.; Knappertz, V. Cannabidiol and Abnormal Liver Chemistries in Healthy Adults: Results of a Phase I Clinical Trial. Clin. Pharmacol. Ther. 2021, 109, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Thai, C.; Tayo, B.; Critchley, D. A Phase 1 Open-Label, Fixed-Sequence Pharmacokinetic Drug Interaction Trial to Investigate the Effect of Cannabidiol on the CYP1A2 Probe Caffeine in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2021, 10, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Crockett, J.; Tayo, B.; Checketts, D.; Sommerville, K. Abrupt withdrawal of cannabidiol (CBD): A randomized trial. Epilepsy Behav. 2020, 104, 106938. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; Gunning, B.; Arenas Cabrera, C.M.; VanLandingham, K.; Crockett, J.; Critchley, D.; Wray, L.; Tayo, B.; Morrison, G.; Toledo, M. A Phase II Randomized Trial to Explore the Potential for Pharmacokinetic Drug-Drug Interactions with Stiripentol or Valproate when Combined with Cannabidiol in Patients with Epilepsy. CNS Drugs 2020, 34, 661–672. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V.; et al. Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.; Butler, J.; Ong, K.; Nguyen, T.H.; Cox, S.; Francis, B.; McIntosh, M.; Lilley, B. A Phase 1, Randomised, Placebo-Controlled, Dose Escalation Study to Investigate the Safety, Tolerability and Pharmacokinetics of Cannabidiol in Fed Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet 2020, 45, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Arout, C.A.; Haney, M.; Herrmann, E.S.; Bedi, G.; Cooper, Z.D. A placebo-controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. Br. J. Clin. Pharmacol. 2022, 88, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Zuardi, A.W.; Garrido, G.E.J.; Wichert-Ana, L.; Guarnieri, R.; Ferrari, L.; Azevedo-Marques, P.M.; Hallak, J.E.C.; McGuire, P.K.; Filho Busatto, G. Effects of cannabidiol (CBD) on regional cerebral blood flow. Public Am. Coll. Neuropsychopharmacol. 2004, 29, 417–426. [Google Scholar] [CrossRef]
- Rosenkrantz, H.; Esber, H.J. Cannabinoid-induced hormone changes in monkeys and rats. J. Toxicol. Environ. Health 1980, 6, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, H.; Fleischman, R.W.; Grant, R.J. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol. Appl. Pharmacol. 1981, 58, 118–131. [Google Scholar] [CrossRef]
- Carvalho, R.K.; Santos, M.L.; Souza, M.R.; Rocha, T.L.; Guimaraes, F.S.; Anselmo-Franci, J.A.; Mazaro-Costa, R. Chronic exposure to cannabidiol induces reproductive toxicity in male Swiss mice. J. Appl. Toxicol. 2018, 38, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.K.; Souza, M.R.; Santos, M.L.; Guimaraes, F.S.; Pobbe, R.L.H.; Andersen, M.L.; Mazaro-Costa, R. Chronic cannabidiol exposure promotes functional impairment in sexual behavior and fertility of male mice. Reprod. Toxicol. 2018, 81, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.K.; Rocha, T.L.; Fernandes, F.H.; Goncalves, B.B.; Souza, M.R.; Araujo, A.A.; Barbosa, C.C.; Silva, D.M.; Campos, H.M.; Tomazett, M.V.; et al. Decreasing sperm quality in mice subjected to chronic cannabidiol exposure: New insights of cannabidiol-mediated male reproductive toxicity. Chem. Biol. Interact. 2022, 351, 109743. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Maharao, N.; Paine, M.F.; Unadkat, J.D. Predicting the Potential for Cannabinoids to Precipitate Pharmacokinetic Drug Interactions via Reversible Inhibition or Inactivation of Major Cytochromes P450. Drug Metab. Dispos. 2020, 48, 1008–1017. [Google Scholar] [CrossRef]
- Bornheim, L.M.; Everhart, E.T.; Li, J.; Correia, M.A. Induction and genetic regulation of mouse hepatic cytochrome P450 by cannabidiol. Biochem. Pharmacol. 1994, 48, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef] [PubMed]
- ACNFP/COT (Advisory Committee on Novel Foods and Processes & Committee on Toxicity). Joint Position Paper from the Advisory Committee on Novel Foods and Processes (ACNFP) & Committee on Toxicity (COT) on Establishing a Provisional Acceptable Daily Intake (ADI) for Pure Form (≥98%) Cannabidiol (CBD) in Foods, Based on New Evidence. 2023. Available online: https://acnfp.food.gov.uk/JointpositionpaperfromACNFP%26COTonestablishingprovisionalADIforpureformCBDinfoods (accessed on 12 October 2023).
- FDA. Tertiary Pharmacology Review. Drug Approval Package: Epidiolex (Cannabidiol). Company: GW Research Ltd. Center for Drug Evaluation and Research. Application Number: 210365Orig1s000. Application Number: 210365Orig1s000. FDA Application Review Files. Non-ClinicalL Review(s). Reference ID: 4278212. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000PharmR.pdf (accessed on 27 April 2024).
- Henderson, R.G.; Welsh, B.T.; Trexler, K.R.; Bonn-Miller, M.O.; Lefever, T.W. Genotoxicity evaluation of cannabidiol. Regul. Toxicol. Pharmacol. 2023, 142, 105425. [Google Scholar] [CrossRef]
- Aljobaily, N.; Krutsinger, K.; Viereckl, M.J.; Joly, R.; Menlove, B.; Cone, B.; Suppes, A.; Han, Y. Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor. Nutrients 2023, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- WHO; IPCS. International Program on Chemical Safety. Environmental Health Criteria 240. Principles and Methods for the Risk Assessment of Chemicals in Food. A Joint Publication of the Food and Agriculture Organization of the United Nations and the World Health Organization. 2009. Available online: https://iris.who.int/bitstream/handle/10665/44065/WHO_EHC_240_eng.pdf (accessed on 19 November 2024).
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Crippa, J.A.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; de Lima Osório, F.; Loureiro, S.R.; dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; Pacheco, J.C.; et al. Efficacy and safety of cannabidiol plus standard care vs standard care alone for the treatment of emotional exhaustion and burnout among frontline health care workers during the COVID-19 pandemic: A randomized clinical trial. JAMA Netw. Open 2021, 4, e2120603. [Google Scholar] [CrossRef] [PubMed]
- FDA. Comment from GW Pharmaceuticals. Posted by the Food and Drug Administration on 19 July 2019. GW Pharmaceuticals’ Submission on Scientific Data and Information About Products Containing Cannabis or Cannabis-Derived Compounds. Document ID FDA-2019-N-1482-4257 Attachment 1. 2019. Available online: https://downloads.regulations.gov/FDA-2019-N-1482-4257/attachment_1.pdf (accessed on 19 November 2024).
- Hurd, Y.L.; Spriggs, S.; Alishayev, J.; Winkel, G.; Gurgov, K.; Kudrich, C.; Oprescu, A.M.; Salsitz, E. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Wolinski, K.; Plazinska, M.T.; Mikolajczak, P.; Ruchala, M. The effects of cannabinoids on the endocrine system. Endokrynol. Pol. 2018, 69, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Meah, F.; Lundholm, M.; Emanuele, N.; Amjed, H.; Poku, C.; Agrawal, L.; Emanuele, M.A. The effects of cannabis and cannabinoids on the endocrine system. Rev. Endocr. Metab. Disord. 2022, 23, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, J.H.; Cristofaro, P.; Ellingboe, J.; Benedikt, R.; Mello, N.K. Acute effects of marihuana on luteinizing hormone in menopausal women. Pharmacol. Biochem. Behav. 1985, 23, 765–768. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Jones, R.T.; Lerner, C.B. Depression of growth hormone and cortisol response to insulin-induced hypoglycemia after prolonged oral delta-9-tetrahydrocannabinol administration in man. J. Clin. Endocrinol. Metab. 1976, 42, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Gammon, C.M.; Freeman, G.M., Jr.; Xie, W.; Petersen, S.L.; Wetsel, W.C. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology 2005, 146, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Merari, A.; Barak, A.; Plaves, M. Effects of 1(2)—Tetrahydrocannabinol on copulation in the male rat. Psychopharmacologia 1973, 28, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.C.; Waalen, J.; Gerber, A.; Beutler, E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int. J. Obes. 2005, 29, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Vettor, R.; Pagano, C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pr. Res. Clin. Endocrinol. Metab. 2009, 23, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Ferk, F.; Misik, M.; Ropek, N.; Nersesyan, A.; Mejri, D.; Holzmann, K.; Lavorgna, M.; Isidori, M.; Knasmuller, S. Low doses of widely consumed cannabinoids (cannabidiol and cannabidivarin) cause DNA damage and chromosomal aberrations in human-derived cells. Arch. Toxicol. 2019, 93, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, J.S.; Silvers, W.S.; Fleegler, E.M.; Zeiger, R.S. Cannabis use in active athletes: Behaviors related to subjective effects. PLoS ONE 2019, 14, e0218998. [Google Scholar] [CrossRef]
- Ware, M.A.; Jensen, D.; Barrette, A.; Vernec, A.; Derman, W. Cannabis and the Health and Performance of the Elite Athlete. Clin. J. Sport Med. 2018, 28, 480–484. [Google Scholar] [CrossRef]
- Docter, S.; Khan, M.; Gohal, C.; Ravi, B.; Bhandari, M.; Gandhi, R.; Leroux, T. Cannabis Use and Sport: A Systematic Review. Sports Health 2020, 12, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Brisola-Santos, M.B.; Gallinaro, J.G.; Gil, F.; Sampaio-Junior, B.; Marin, M.C.; de Andrade, A.G.; Richter, K.P.; Glick, I.D.; Baltieri, D.A.; Castaldelli-Maia, J.M. Prevalence and correlates of cannabis use among athletes—A systematic review. Am. J. Addict. 2016, 25, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Sahinovic, A.; Irwin, C.; Doohan, P.T.; Kevin, R.C.; Cox, A.J.; Lau, N.S.; Desbrow, B.; Johnson, N.A.; Sabag, A.; Hislop, M.; et al. Effects of Cannabidiol on Exercise Physiology and Bioenergetics: A Randomised Controlled Pilot Trial. Sports Med. Open 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.A.; Kisiolek, J.N.; Ramani, A.; Townsend, R.; Rodriguez, E.; Butler, B.; Stewart, L.K. Effects of Oral Cannabidiol on Health and Fitness in Healthy Adults: An 8-Week Randomized Trial. Nutrients 2023, 15, 2664. [Google Scholar] [CrossRef] [PubMed]
- Hatchett, A.; Armstrong, K.; Hughes, B.; Parr, B. The Influence Cannabidiol on Delayed Onset of Muscle Soreness. Int. J. Phys. Educ. Sports Health 2020, 7, 89–94. [Google Scholar]
- Cochrane-Snyman, K.C.; Cruz, C.; Morales, J.; Coles, M. The Effects of Cannabidiol Oil on Noninvasive Measures of Muscle Damage in Men. Med. Sci. Sports Exerc. 2021, 53, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Crossland, B.W.; Rigby, B.R.; Duplanty, A.A.; King, G.A.; Juma, S.; Levine, N.A.; Clark, C.E.; Ramirez, K.P.; Varone, N.L. Acute Supplementation with Cannabidiol Does Not Attenuate Inflammation or Improve Measures of Performance following Strenuous Exercise. Healthcare 2022, 10, 1133. [Google Scholar] [CrossRef]
- Isenmann, E.; Veit, S.; Starke, L.; Flenker, U.; Diel, P. Effects of Cannabidiol Supplementation on Skeletal Muscle Regeneration after Intensive Resistance Training. Nutrients 2021, 13, 3028. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, E.A.T.; Veit, S.; Diel, P.R. Effects Of Cannabidiol Supplementation On The Skeletal Muscle Regeneration. Med. Sci. Sports Exerc. 2020, 52, 766. [Google Scholar] [CrossRef]
- Peters, E.N.; Yardley, H.; Harrison, A.; Eglit, G.M.L.; Antonio, J.; Turcotte, C.; Bonn-Miller, M.O. A randomized, double-blind, placebo-controlled, repeated-dose pilot study of the safety, tolerability, and preliminary effects of a cannabidiol (CBD)- and cannabigerol (CBG)-based beverage powder to support recovery from delayed onset muscle soreness (DOMS). J. Int. Soc. Sports Nutr. 2023, 20, 2280113. [Google Scholar] [CrossRef]
- Isenmann, E.; Veit, S.; Flenker, U.; Lesch, A.; Lachenmeier, D.W.; Diel, P. Influence of short-term chronic oral cannabidiol application on muscle recovery and performance after an intensive training protocol—A randomized double-blind crossover study. J. Int. Soc. Sports Nutr. 2024, 21, 2337252. [Google Scholar] [CrossRef]
- Kennedy, M. Cannabis, cannabidiol and tetrahydrocannabinol in sport: An overview. Intern. Med. J. 2022, 52, 1471–1477. [Google Scholar] [CrossRef]
- Burr, J.F.; Cheung, C.P.; Kasper, A.M.; Gillham, S.H.; Close, G.L. Cannabis and Athletic Performance. Sports Med. 2021, 51, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Karimian Azari, E.; Kerrigan, A.; O’Connor, A. Naturally Occurring Cannabinoids and their Role in Modulation of Cardiovascular Health. J. Diet. Suppl. 2020, 17, 625–650. [Google Scholar] [CrossRef]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Sandyk, R.; Snider, S.R. Open label evaluation of cannabidiol in dystonic movement disorders. Int. J. Neurosci. 1986, 30, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Dujic, G.; Kumric, M.; Vrdoljak, J.; Dujic, Z.; Bozic, J. Chronic Effects of Oral Cannabidiol Delivery on 24-h Ambulatory Blood Pressure in Patients with Hypertension (HYPER-H21-4): A Randomized, Placebo-Controlled, and Crossover Study. Cannabis Cannabinoid Res. 2023, 9, 979–989. [Google Scholar] [CrossRef]
- Kumric, M.; Dujic, G.; Vrdoljak, J.; Svagusa, K.; Kurir, T.T.; Supe-Domic, D.; Dujic, Z.; Bozic, J. CBD supplementation reduces arterial blood pressure via modulation of the sympatho-chromaffin system: A substudy from the HYPER-H21-4 trial. Biomed. Pharmacother. 2023, 160, 114387. [Google Scholar] [CrossRef] [PubMed]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlovic, D.; Dujic, Z.; Ainslie, P.N. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimaraes, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7, 82–88. [Google Scholar] [CrossRef]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef] [PubMed]
- Gong, H., Jr.; Tashkin, D.P.; Simmons, M.S.; Calvarese, B.; Shapiro, B.J. Acute and subacute bronchial effects of oral cannabinoids. Clin. Pharmacol. Ther. 1984, 35, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkotter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farre, M.; et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef] [PubMed]
- Borgwardt, S.J.; Allen, P.; Bhattacharyya, S.; Fusar-Poli, P.; Crippa, J.A.; Seal, M.L.; Fraccaro, V.; Atakan, Z.; Martin-Santos, R.; O’Carroll, C.; et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: Effects during response inhibition. Biol. Psychiatry 2008, 64, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Winton-Brown, T.T.; Allen, P.; Bhattacharyya, S.; Borgwardt, S.J.; Fusar-Poli, P.; Crippa, J.A.; Seal, M.L.; Martin-Santos, R.; Ffytche, D.; Zuardi, A.W.; et al. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: An FMRI study. Neuropsychopharmacology 2011, 36, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Crippa, J.A.; Bhattacharyya, S.; Borgwardt, S.J.; Allen, P.; Martin-Santos, R.; Seal, M.; Surguladze, S.A.; O’Carrol, C.; Atakan, Z.; et al. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry 2009, 66, 95–105. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A Systematic Review and Meta-Analysis of the Haemodynamic Effects of Cannabidiol. Front. Pharmacol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Fusar-Poli, P.; Borgwardt, S.; Martin-Santos, R.; Nosarti, C.; O’Carroll, C.; Allen, P.; Seal, M.L.; Fletcher, P.C.; Crippa, J.A.; et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: A neural basis for the effects of Cannabis sativa on learning and psychosis. Arch. Gen. Psychiatry 2009, 66, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’Carroll, C.M.; Seal, M.; Allen, P.; et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Hallak, J.E.; Dursun, S.M.; Bosi, D.C.; de Macedo, L.R.; Machado-de-Sousa, J.P.; Abrao, J.; Crippa, J.A.; McGuire, P.; Krystal, J.H.; Baker, G.B.; et al. The interplay of cannabinoid and NMDA glutamate receptor systems in humans: Preliminary evidence of interactive effects of cannabidiol and ketamine in healthy human subjects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Malcolm, R.J.; Babalonis, S.; Nuzzo, P.A.; Cooper, Z.D.; Bedi, G.; Gray, K.M.; McRae-Clark, A.; Lofwall, M.R.; Sparenborg, S. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 2016, 41, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrzab, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pedzinska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [PubMed]
- Pedzinska-Betiuk, A.; Weresa, J.; Schlicker, E.; Harasim-Symbor, E.; Toczek, M.; Kasacka, I.; Gajo, B.; Malinowska, B. Chronic cannabidiol treatment reduces the carbachol-induced coronary constriction and left ventricular cardiomyocyte width of the isolated hypertensive rat heart. Toxicol. Appl. Pharmacol. 2021, 411, 115368. [Google Scholar] [CrossRef]
- Borgen, L.; Davis, W. Cannabidiol (CBD) Attenuation of Effects of Delta-9-THC. In Pharmacologist; The American Society for Pharmacology and Experimental Therapeutics: Bethesda, MD, USA, 1973; Volume 15, p. 201. [Google Scholar]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Keown, O.; Astrand, A.; Lindblom, A.; Ryberg, E.; Hjorth, S.; Leslie, S.J.; Greasley, P.J.; Wainwright, C.L. Pharmacological profiling of the hemodynamic effects of cannabinoid ligands: A combined in vitro and in vivo approach. Pharmacol. Res. Perspect. 2015, 3, e00143. [Google Scholar] [CrossRef]
- Bright, T.P.; Farber, M.O.; Brown, D.J.; Lewis, S.C.; Forney, R.B. Cardiopulmonary effects of cannabidiol in anesthetized mongrel dogs. Toxicol. Appl. Pharmacol. 1975, 31, 520–526. [Google Scholar] [CrossRef]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol Affects the Bezold-Jarisch Reflex via TRPV1 and 5-HT(3) Receptors and Has Peripheral Sympathomimetic Effects in Spontaneously Hypertensive and Normotensive Rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.D.; Li, D.M. Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol. 1973, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Bockmann, S.; Hinz, B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells. Oncotarget 2018, 9, 34595–34616. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozlowska, H.; Kloza, M.; Sadowska, O.; Kozlowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Cornicelli, J.A.; Gilman, S.R.; Krom, B.A.; Kottke, B.A. Cannabinoids impair the formation of cholesteryl ester in cultured human cells. Arteriosclerosis 1981, 1, 449–454. [Google Scholar] [CrossRef]
- Formukong, E.A.; Evans, A.T.; Evans, F.J. The inhibitory effects of cannabinoids, the active constituents of Cannabis sativa L. on human and rabbit platelet aggregation. J. Pharm. Pharmacol. 1989, 41, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Sekar, K.; Pack, A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Research 2019, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Rachayon, M.; Jirakran, K.; Sodsai, P.; Klinchanhom, S.; Sughondhabirom, A.; Plaimas, K.; Suratanee, A.; Maes, M. In Vitro Effects of Cannabidiol on Activated Immune-Inflammatory Pathways in Major Depressive Patients and Healthy Controls. Pharmaceuticals 2022, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.M.; Vazquez, A.R.; Remijan, N.D.; Trotter, R.E.; McMillan, T.V.; Freedman, K.E.; Wei, Y.; Woelfel, K.A.; Arnold, O.R.; Wolfe, L.M.; et al. Evaluation of pharmacokinetics and acute anti-inflammatory potential of two oral cannabidiol preparations in healthy adults. Phytother. Res. 2020, 34, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.I.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neural Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef]
- Cassol, O.J., Jr.; Comim, C.M.; Silva, B.R.; Hermani, F.V.; Constantino, L.S.; Felisberto, F.; Petronilho, F.; Hallak, J.E.; De Martinis, B.S.; Zuardi, A.W.; et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010, 1348, 128–138. [Google Scholar] [CrossRef]
- Khaksar, S.; Bigdeli, M.; Samiee, A.; Shirazi-Zand, Z. Antioxidant and anti-apoptotic effects of cannabidiol in model of ischemic stroke in rats. Brain Res. Bull. 2022, 180, 118–130. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Zhang, Z.; Zhao, M.; Tong, C.; Cong, P.; Mao, S.; Zhao, Y.; Hou, M.; Piao, Y.; et al. Protective effect and mechanism of cannabidiol on myocardial injury in exhaustive exercise training mice. Chem. Biol. Interact. 2022, 365, 110079. [Google Scholar] [CrossRef]
- Cuba, L.F.; Salum, F.G.; Guimarães, F.S.; Cherubini, K.; Borghetti, R.L.; de Figueiredo, M.A.Z. Cannabidiol on 5-FU-induced oral mucositis in mice. Oral Dis. 2020, 26, 1483–1493. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Belgrave, B.E.; Bird, K.D.; Chesher, G.B.; Jackson, D.M.; Lubbe, K.E.; Starmer, G.A.; Teo, R.K. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology 1979, 64, 243–246. [Google Scholar] [CrossRef]
- Lees, R.; Hines, L.A.; Hindocha, C.; Baio, G.; Shaban, N.D.C.; Stothart, G.; Mofeez, A.; Morgan, C.J.A.; Curran, H.V.; Freeman, T.P. Effect of four-week cannabidiol treatment on cognitive function: Secondary outcomes from a randomised clinical trial for the treatment of cannabis use disorder. Psychopharmacology 2023, 240, 337–346. [Google Scholar] [CrossRef]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.P.; Karoly, H.C.; Ellingson, J.M.; Klawitter, J.; Sempio, C.; Squeri, J.E.; Bryan, A.D.; Bidwell, L.C.; Hutchison, K.E. Effects of cannabidiol in cannabis flower: Implications for harm reduction. Addict. Biol. 2022, 27, e13092. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.P.; Mueller, R.L.; Winiger, E.A.; Klawitter, J.; Sempio, C.; Williams, S.; Bryan, A.D.; Bidwell, L.C.; Hutchison, K.E. Cannabinoid Exposure and Subjective Effects of THC and CBD in Edible Cannabis Products. Cannabis Cannabinoid Res. 2024, 9, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Zamarripa, C.A.; Spindle, T.R.; Surujunarain, R.; Weerts, E.M.; Bansal, S.; Unadkat, J.D.; Paine, M.F.; Vandrey, R. Assessment of Orally Administered Delta9-Tetrahydrocannabinol When Coadministered with Cannabidiol on Delta9-Tetrahydrocannabinol Pharmacokinetics and Pharmacodynamics in Healthy Adults: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2254752. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.L.; Cesareo, K.R.; Raub, B.; Kedia, A.W.; Sandrock, J.E.; Kerksick, C.M.; Ziegenfuss, T.N. Effects of Hemp Extract on Markers of Wellness, Stress Resilience, Recovery and Clinical Biomarkers of Safety in Overweight, But Otherwise Healthy Subjects. J. Diet. Suppl. 2020, 17, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.L.; de Wit, H. Cannabidiol Does Not Dampen Responses to Emotional Stimuli in Healthy Adults. Cannabis Cannabinoid Res. 2017, 2, 105–113. [Google Scholar] [CrossRef]
- Colizzi, M.; Bhattacharyya, S. Does Cannabis Composition Matter? Differential Effects of Delta-9-tetrahydrocannabinol and Cannabidiol on Human Cognition. Curr. Addict. Rep. 2017, 4, 62–74. [Google Scholar] [CrossRef]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yucel, M.; Solowij, N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Crippa, J.A.; Allen, P.; Martin-Santos, R.; Borgwardt, S.; Fusar-Poli, P.; Rubia, K.; Kambeitz, J.; O’Carroll, C.; Seal, M.L.; et al. Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch. Gen. Psychiatry 2012, 69, 27–36. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Falkenberg, I.; Martin-Santos, R.; Atakan, Z.; Crippa, J.A.; Giampietro, V.; Brammer, M.; McGuire, P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology 2015, 40, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Woelfl, T.; Rohleder, C.; Mueller, J.K.; Lange, B.; Reuter, A.; Schmidt, A.M.; Koethe, D.; Hellmich, M.; Leweke, F.M. Effects of Cannabidiol and Delta-9-Tetrahydrocannabinol on Emotion, Cognition, and Attention: A Double-Blind, Placebo-Controlled, Randomized Experimental Trial in Healthy Volunteers. Front. Psychiatry 2020, 11, 576877. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.; Suraev, A.S.; Doohan, P.T.; Irwin, C.; Kevin, R.C.; Grunstein, R.R.; Hoyos, C.M.; McGregor, I.S. Effects of cannabidiol on simulated driving and cognitive performance: A dose-ranging randomised controlled trial. J. Psychopharmacol. 2022, 36, 1338–1349. [Google Scholar] [CrossRef]
- Batalla, A.; Bos, J.; Postma, A.; Bossong, M.G. The Impact of Cannabidiol on Human Brain Function: A Systematic Review. Front. Pharmacol. 2020, 11, 618184. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.A.P.; Green, S.F.; Hindocha, C.; Yamamori, Y.; Yim, J.L.L.; Jones, A.P.M.; Walker, H.R.; Tokarczuk, P.; Statton, B.; Howes, O.D.; et al. The effects of acute cannabidiol on cerebral blood flow and its relationship to memory: An arterial spin labelling magnetic resonance imaging study. J. Psychopharmacol. 2020, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S.M.; Mali, S.; Amin, P.D.; Oliveira, M. Neuroprotective potential of cannabidiol: Molecular mechanisms and clinical implications. J. Integr. Med. 2023, 21, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Neary, J.P. Neuroprotection Following Concussion: The Potential Role for Cannabidiol. Can. J. Neurol. Sci. 2020, 47, 289–300. [Google Scholar] [CrossRef]
- Beale, C.; Broyd, S.J.; Chye, Y.; Suo, C.; Schira, M.; Galettis, P.; Martin, J.H.; Yucel, M.; Solowij, N. Prolonged Cannabidiol Treatment Effects on Hippocampal Subfield Volumes in Current Cannabis Users. Cannabis Cannabinoid Res. 2018, 3, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaca, M.V.; Sonego, A.B.; Guimaraes, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Sagredo, O.; Pazos, M.R.; Garcia, C.; Pertwee, R.; Mechoulam, R.; Martinez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, O.; Gonzalez, S.; Aroyo, I.; Pazos, M.R.; Benito, C.; Lastres-Becker, I.; Romero, J.P.; Tolon, R.M.; Mechoulam, R.; Brouillet, E.; et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: Relevance for Huntington’s disease. Glia 2009, 57, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, O.; Ramos, J.A.; Decio, A.; Mechoulam, R.; Fernandez-Ruiz, J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur. J. Neurosci. 2007, 26, 843–851. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arencibia, M.; Gonzalez, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martinez, A.; Limon, I.D. Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martinez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: Role of 5HT(1A) and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Avraham, Y.; Grigoriadis, N.; Poutahidis, T.; Vorobiev, L.; Magen, I.; Ilan, Y.; Mechoulam, R.; Berry, E. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br. J. Pharmacol. 2011, 162, 1650–1658. [Google Scholar] [CrossRef]
- Magen, I.; Avraham, Y.; Ackerman, Z.; Vorobiev, L.; Mechoulam, R.; Berry, E.M. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 2010, 159, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.H.; Eckeli, A.L.; Zuardi, A.W.; Pena-Pereira, M.A.; Sobreira-Neto, M.A.; Sobreira, E.T.; Camilo, M.R.; Bergamaschi, M.M.; Schenck, C.H.; Hallak, J.E.; et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: A case series. J. Clin. Pharmacol. Ther. 2014, 39, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.H.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.; Crippa, J.A. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Crippa, J.A.; Hallak, J.E.; Pinto, J.P.; Chagas, M.H.; Rodrigues, G.G.; Dursun, S.M.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Maroon, J.; Bost, J. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Schröder, N.; da Silva, V.; Hallak, J.E.C.; Zuardi, A.W.; de Souza Crippa, J. Cannabidiol and neuroprotection: Evidence from preclinical studies. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Academic Press: London, UK, 2017; Chapter 83; pp. 802–812. [Google Scholar]
- Alali, S.; Riazi, G.; Ashrafi-Kooshk, M.R.; Meknatkhah, S.; Ahmadian, S.; Hooshyari Ardakani, M.; Hosseinkhani, B. Cannabidiol Inhibits Tau Aggregation In Vitro. Cells 2021, 10, 3521. [Google Scholar] [CrossRef]
- Martin-Moreno, A.M.; Reigada, D.; Ramirez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Scarante, F.F.; Joca, S.R.L.; Sales, A.J.; Gomes, F.V.; Sonego, A.B.; Rodrigues, N.S.; Galve-Roperh, I.; Guimaraes, F.S. Plastic and Neuroprotective Mechanisms Involved in the Therapeutic Effects of Cannabidiol in Psychiatric Disorders. Front. Pharmacol. 2017, 8, 269. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Diel, P. A warning against the negligent use of cannabidiol in professional and amateur athletes. Sports 2019, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanuš, L. The cannabinoid system: From the point of view of a chemist. In Marijuana and Madness: Psychiatry and Neurobiology; Castle, D., Murray, R., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 1–18. [Google Scholar]
- Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I.G. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology 1982, 76, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a potential treatment for anxiety and mood disorders: Molecular targets and epigenetic insights from preclinical research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.C.; McCartney, D.; Suraev, A.; McGregor, I.S. The safety and efficacy of low oral doses of cannabidiol: An evaluation of the evidence. Clin. Transl. Sci. 2023, 16, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimarães, F.S.; Crippa, J.A. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz. J. Psychiatry 2019, 41, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Pereira Junior, L.C.; Pereira, L.C.; Zimmermann, P.M.; Brum Junior, L.; Rechia, L.M.; Dias, I.; Hallak, J.E.; Campos, A.C.; Guimarães, F.S.; et al. Effect of two oral formulations of cannabidiol on responses to emotional stimuli in healthy human volunteers: Pharmaceutical vehicle matters. Braz. J. Psychiatry 2022, 44, 15–20. [Google Scholar] [CrossRef] [PubMed]
- de Faria, S.M.; de Morais Fabrício, D.; Tumas, V.; Castro, P.C.; Ponti, M.A.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.S.; Chagas, M.H.N. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J. Psychopharmacol. 2020, 34, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Masataka, N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef]
- Pacheco, J.C.; Souza, J.D.S.; Hallak, J.E.C.; Osório, F.d.L.; Campos, A.C.; Guimarães, F.S.; Zuardi, A.W.; Crippa, J.A.S. Cannabidiol as a treatment for mental health outcomes among health care workers during the coronavirus disease pandemic. J. Clin. Psychopharmacol. 2021, 41, 327–329. [Google Scholar] [CrossRef]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.S.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.D.S.; Zuardi, A.W.; Guimarães, F.S.; Osório, F.d.L.; Loureiro, S.R.; Campos, A.C.; Hallak, J.E.C.; Dos Santos, R.G.; Machado Silveira, I.L.; Pereira-Lima, K.; et al. Maintained anxiolytic effects of cannabidiol after treatment discontinuation in healthcare workers during the COVID-19 pandemic. Front. Pharmacol. 2022, 13, 856846. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Chagas, M.H.N.; de Oliveira, D.C.G.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef]
- Appiah-Kusi, E.; Petros, N.; Wilson, R.; Colizzi, M.; Bossong, M.G.; Valmaggia, L.; Mondelli, V.; McGuire, P.; Bhattacharyya, S. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology 2020, 237, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Li, E.; Rice, S.; Davey, C.G.; Ratheesh, A.; Adams, S.; Jackson, H.; Hetrick, S.; Parker, A.; Spelman, T.; et al. Cannabidiol for treatment-resistant anxiety disorders in young people: An open-label trial. J. Clin. Psychiatry 2022, 83, 21m14130. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Yamamori, Y.; Hindocha, C.; Jones, A.P.M.; Yim, J.L.L.; Walker, H.R.; Statton, B.; Wall, M.B.; Lees, R.H.; Howes, O.D.; et al. The acute effects of cannabidiol on emotional processing and anxiety: A neurocognitive imaging study. Psychopharmacology 2022, 239, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.; Lister, R.; Evans, N.; Antley, A.; Englund, A.; Murray, R.M.; Freeman, D.; Morrison, P.D. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J. Psychopharmacol. 2018, 32, 276–282. [Google Scholar] [CrossRef]
- Stanley, T.B.; Ferretti, M.L.; Bonn-Miller, M.O.; Irons, J.G. A double-blind, randomized, placebo-controlled test of the effects of cannabidiol on experiences of test anxiety among college students. Cannabis Cannabinoid Res. 2022, 8, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, M.K.; Lambros, A.M.; Smith, R.T.; Sagar, K.A.; El-Abboud, C.; Gruber, S.A. Clinical and cognitive improvement following full-spectrum, high-cannabidiol treatment for anxiety: Open-label data from a two-stage, phase 2 clinical trial. Commun. Med. 2022, 2, 139. [Google Scholar] [CrossRef] [PubMed]
- Gournay, L.R.; Ferretti, M.L.; Bilsky, S.; Vance, E.; Nguyen, A.M.; Mann, E.; Williams, P.; Leen-Feldner, E.W. The effects of cannabidiol on worry and anxiety among high trait worriers: A double-blind, randomized placebo controlled trial. Psychopharmacology 2023, 240, 2147–2161. [Google Scholar] [CrossRef]
- Das, R.K.; Kamboj, S.K.; Ramadas, M.; Yogan, K.; Gupta, V.; Redman, E.; Curran, H.V.; Morgan, C.J.A. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology 2013, 226, 781–792. [Google Scholar] [CrossRef]
- Gulbransen, G.; Xu, W.; Arroll, B. Cannabidiol prescription in clinical practice: An audit on the first 400 patients in New Zealand. BJGP Open 2020, 4, bjgpopen20X101010. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in anxiety and sleep: A large case series. Perm. J. 2019, 23, 18-041. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Opila-Lehman, J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm. J. 2016, 20, 16-005. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Stevenson, C.W.; Laviolette, S.R. Could cannabidiol be a treatment for coronavirus disease-19-related anxiety disorders? Cannabis Cannabinoid Res. 2021, 6, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol. Sci. 2020, 41, 3085–3098. [Google Scholar] [CrossRef] [PubMed]
- Kwee, C.M.B.; van Gerven, J.M.A.; Bongaerts, F.L.P.; Cath, D.C.; Jacobs, G.; Baas, J.M.P.; Groenink, L. Cannabidiol in clinical and preclinical anxiety research. A systematic review into concentration–effect relations using the IB-de-risk tool. J. Psychopharmacol. 2022, 36, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Shahinas, J. Dosage, efficacy and safety of cannabidiol administration in adults: A systematic review of human trials. J. Clin. Med. Res. 2020, 12, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Leen-Feldner, E.W.; Bynion, T.-M.; Gournay, R.; Bonn-Miller, M.O.; Feldner, M.T. Practical considerations for testing the effects of cannabidiol on human anxiety. J. Anxiety Disord. 2021, 82, 102429. [Google Scholar] [CrossRef]
- Tang, Y.; Tonkovich, K.L.; Rudisill, T.M. The effectiveness and safety of cannabidiol in non-seizure-related indications: A systematic review of published randomized clinical trials. Pharm. Med. 2022, 36, 353–385. [Google Scholar] [CrossRef] [PubMed]
- Chesney, E.; McGuire, P.; Freeman, T.P.; Strang, J.; Englund, A. Lack of evidence for the effectiveness or safety of over-the-counter cannabidiol products. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320954992. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A systematic review of cannabidiol dosing in clinical populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Vitetta, L.; Quezada, M.; Hall, S. Enhancing Endocannabinoid Control of Stress with Cannabidiol. J. Clin. Med. 2021, 10, 5852. [Google Scholar] [CrossRef]
- Karniol, I.G.; Shirakawa, I.; Kasinski, N.; Pfeferman, A.; Carlini, E.A. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur. J. Pharmacol. 1974, 28, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Spinella, T.C.; Burdeyny, V.; Oprea, A.; Perrot, T.S.; Barrett, S.P. The Impact of Cannabidiol Expectancy on Cortisol Responsivity in the Context of Acute Stress: Associations with Biological Sex. Cannabis Cannabinoid Res. 2024, 9, 1006–1014. [Google Scholar] [CrossRef]
- Spinella, T.C.; Stewart, S.H.; Naugler, J.; Yakovenko, I.; Barrett, S.P. Evaluating cannabidiol (CBD) expectancy effects on acute stress and anxiety in healthy adults: A randomized crossover study. Psychopharmacology 2021, 238, 1965–1977. [Google Scholar] [CrossRef]
- Bitencourt, R.M.; Takahashi, R.N. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: From bench research to confirmation in human trials. Front. Neurosci. 2018, 12, 502. [Google Scholar] [CrossRef]
- Stanciu, C.N.; Brunette, M.F.; Teja, N.; Budney, A.J. Evidence for use of cannabinoids in mood disorders, anxiety disorders, and PTSD: A systematic review. Psychiatr. Serv. 2021, 72, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C.; Cousijn, J.; Rall, M.; Bloomfield, M. The effectiveness of cannabinoids in the treatment of posttraumatic stress disorder (PTSD): A systematic review. J. Dual Diagn. 2020, 16, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the treatment of post-traumatic stress disorder: A case series. J. Altern. Complement. Med. 2019, 25, 392–397. [Google Scholar] [CrossRef]
- Telch, M.J.; Fischer, C.M.; Zaizar, E.D.; Rubin, M.; Papini, S. Use of Cannabidiol (CBD) oil in the treatment of PTSD: Study design and rationale for a placebo-controlled randomized clinical trial. Contemp. Clin. Trials 2022, 122, 106933. [Google Scholar] [CrossRef]
- van Orten-Luiten, A.B.; de Roos, N.M.; Majait, S.; Witteman, B.J.M.; Witkamp, R.F. Effects of Cannabidiol Chewing Gum on Perceived Pain and Well-Being of Irritable Bowel Syndrome Patients: A Placebo-Controlled Crossover Exploratory Intervention Study with Symptom-Driven Dosing. Cannabis Cannabinoid Res. 2022, 7, 436–444. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Relaxation-MeSH-NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh/?term=%22Relaxation%22%5BMeSH+Terms%5D (accessed on 19 November 2024).
- EFSA. Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698, 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2241. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to caffeine and theobromine in cocoa (Theobroma cacao L.) and enhancement of mood (ID 4276) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2269. [Google Scholar] [CrossRef]
- Bergeria, C.L.; Strickland, J.C.; Spindle, T.R.; Kalaba, M.; Satyavolu, P.U.; Feldner, M.; Vandrey, R.; Bonn-Miller, M.; Peters, E.N.; Weerts, E. A crowdsourcing survey study on the subjective effects of delta-8-tetrahydrocannabinol relative to delta-9-tetrahydrocannabinol and cannabidiol. Exp. Clin. Psychopharmacol. 2023, 31, 312–317. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the safety of hemp (Cannabis genus) for use as animal feed. EFSA J. 2011, 9, 2011. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Hall, W. Recreational cannabis: Sought-after effects, adverse effects, designer drugs, and harm minimization. In Handbook of Cannabis, 1st ed.; Pertwee, R.G., Ed.; Handbooks in psychopharmacology; Oxford University Press: Oxford, UK; New York, NY, USA, 2014; pp. 645–646. [Google Scholar]
- Alayli, A.F.G.; Kotz, D.; Kastaun, S. Recreational cannabidiol: Awareness, prevalence of use, and associated factors in a representative sample of the German population. Subst. Use Misuse 2022, 57, 1417–1424. [Google Scholar] [CrossRef]
- Miranda, A.; Peek, E.; Ancoli-Israel, S.; Young, J.W.; Perry, W.; Minassian, A. The Role of Cannabis and The Endocannabinoid System in Sleep Regulation and Cognition: A Review of Human and Animal Studies. Behav. Sleep Med. 2024, 22, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Suraev, A.; Grunstein, R.R.; Marshall, N.S.; D’Rozario, A.L.; Gordon, C.J.; Bartlett, D.J.; Wong, K.; Yee, B.J.; Vandrey, R.; Irwin, C.; et al. Cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) for chronic insomnia disorder (’CANSLEEP’ trial): Protocol for a randomised, placebo-controlled, double-blinded, proof-of-concept trial. BMJ Open 2020, 10, e034421. [Google Scholar] [CrossRef]
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem. Biodivers. 2007, 4, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Velzeboer, R.; Malas, A.; Boerkoel, P.; Cullen, K.; Hawkins, M.; Roesler, J.; Lai, W.W. Cannabis dosing and administration for sleep: A systematic review. Sleep 2022, 45, zsac218. [Google Scholar] [CrossRef]
- Ranum, R.M.; Whipple, M.O.; Croghan, I.; Bauer, B.; Toussaint, L.L.; Vincent, A. Use of Cannabidiol in the Management of Insomnia: A Systematic Review. Cannabis Cannabinoid Res. 2023, 8, 213–229. [Google Scholar] [CrossRef]
- Linares, I.M.P.; Guimaraes, F.S.; Eckeli, A.; Crippa, A.C.S.; Zuardi, A.W.; Souza, J.D.S.; Hallak, J.E.; Crippa, J.A.S. No Acute Effects of Cannabidiol on the Sleep-Wake Cycle of Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Front. Pharmacol. 2018, 9, 315. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Feldner, M.T.; Bynion, T.M.; Eglit, G.M.L.; Brunstetter, M.; Kalaba, M.; Zvorsky, I.; Peters, E.N.; Hennesy, M. A double-blind, randomized, placebo-controlled study of the safety and effects of CBN with and without CBD on sleep quality. Exp. Clin. Psychopharmacol. 2023, 32, 277–284. [Google Scholar] [CrossRef]
- Kisiolek, J.N.; Flores, V.A.; Ramani, A.; Butler, B.; Haughian, J.M.; Stewart, L.K. Eight Weeks of Daily Cannabidiol Supplementation Improves Sleep Quality and Immune Cell Cytotoxicity. Nutrients 2023, 15, 4173. [Google Scholar] [CrossRef]
- Saleska, J.L.; Bryant, C.; Kolobaric, A.; D’Adamo, C.R.; Colwell, C.S.; Loewy, D.; Chen, J.; Pauli, E.K. The Safety and Comparative Effectiveness of Non-Psychoactive Cannabinoid Formulations for the Improvement of Sleep: A Double-Blinded, Randomized Controlled Trial. J. Am. Nutr. Assoc. 2024, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Narayan, A.J.; Downey, L.A.; Rose, S.; Di Natale, L.; Hayley, A.C. Cannabidiol for moderate-severe insomnia: A randomized controlled pilot trial of 150 mg of nightly dosing. J. Clin. Sleep Med. 2024, 20, 753–763. [Google Scholar] [CrossRef]
- Corroon, J.; Kight, R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018, 3, 190–194. [Google Scholar] [CrossRef]
- NAS. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Medicine Division. National Academies of Sciences. Board on Population Health. Public Health Practice. Committee on the Health Effects of Marijuana. An Evidence Review Research Agenda. 2017. Available online: https://nap.nationalacademies.org/catalog/24625/the-health-effects-of-cannabis-and-cannabinoids-the-current-state (accessed on 19 November 2024).
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a treatment for arthritis and joint pain: An exploratory cross-sectional study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: A prospective cohort study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Arendt-Nielsen, L.; Duch, K.S.; Kristensen, S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: A randomized, double-blind, placebo-controlled trial. Pain 2022, 163, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, K.; Petersen, M.; Bach, F.W.; Heinesen, A.; Enggaard, T.P.; Almdal, T.P.; Holbech, J.V.; Vase, L.; Jensen, T.S.; Hansen, C.S.; et al. Oral capsules of tetra-hydro-cannabinol (THC), cannabidiol (CBD) and their combination in peripheral neuropathic pain treatment. Eur. J. Pain 2023, 27, 492–506. [Google Scholar] [CrossRef] [PubMed]
- De Vita, M.J.; Maisto, S.A.; Gilmour, C.E.; McGuire, L.; Tarvin, E.; Moskal, D. The effects of cannabidiol and analgesic expectancies on experimental pain reactivity in healthy adults: A balanced placebo design trial. Exp. Clin. Psychopharmacol. 2022, 30, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Bebee, B.; Taylor, D.M.; Bourke, E.; Pollack, K.; Foster, L.; Ching, M.; Wong, A. The CANBACK trial: A randomised, controlled clinical trial of oral cannabidiol for people presenting to the emergency department with acute low back pain. Med. J. Aust. 2021, 214, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, C.; Vadala, M. Short-Term Efficacy of CBD-Enriched Hemp Oil in Girls with Dysautonomic Syndrome after Human Papillomavirus Vaccination. Isr. Med. Assoc. J. 2017, 19, 79–84. [Google Scholar] [PubMed]
- Cunetti, L.; Manzo, L.; Peyraube, R.; Arnaiz, J.; Curi, L.; Orihuela, S. Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transpl. Proc. 2018, 50, 461–464. [Google Scholar] [CrossRef]
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin. Rehabil. 2003, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M. The Effects of Cannabidiol Isolate on Menstrual-Related Symptoms. Master’s Thesis, James Madison University (JMU), Harrisonburg, VA, USA, 2020. Available online: https://commons.lib.jmu.edu/masters202029/145 (accessed on 19 November 2024).
- Mohan, H.; Romano, R.; Andersen, A.; Vera, E.; Iwasaka-Neder, J.; Kajita, A.; de Sousa, C.; Mendes, V.; Huang, M.; Owoade, I. Efficacy of Cannabidiol Versus Ibuprofen in the Relief of Menstrual Pain in Females Living with Primary Dysmenorrhea: A phase II, Non-Inferiority trial. Princ. Pract. Clin. Res. 2022, 8, 68–76. [Google Scholar] [CrossRef]
- Clayton, P.; Hill, M.; Bogoda, N.; Subah, S.; Venkatesh, R. Palmitoylethanolamide: A Natural Compound for Health Management. Int. J. Mol. Sci. 2021, 22, 5305. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Vigano, P.; Mugione, A.; Panina-Bordignon, P.; Candiani, M. The molecular connections between the cannabinoid system and endometriosis. Mol. Hum. Reprod. 2012, 18, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, T.; Chicca, A.; Pellegata, D.; Bersinger, N.A.; Imboden, S.; Nirgianakis, K.; Gertsch, J.; Mueller, M.D. Association of endocannabinoids with pain in endometriosis. Pain 2022, 163, 193–203. [Google Scholar] [CrossRef]
- Lingegowda, H.; Williams, B.J.; Spiess, K.G.; Sisnett, D.J.; Lomax, A.E.; Koti, M.; Tayade, C. Role of the endocannabinoid system in the pathophysiology of endometriosis and therapeutic implications. J. Cannabis Res. 2022, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, N.; Nagabukuro, H.; Resuehr, D.; Zhang, G.; McAllister, S.L.; McGinty, K.A.; Mackie, K.; Berkley, K.J. Endocannabinoid involvement in endometriosis. Pain 2010, 151, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Cioffi, R.; Viganò, P.; Candiani, M.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Garavaglia, E.; Panina-Bordignon, P. Elevated Systemic Levels of Endocannabinoids and Related Mediators Across the Menstrual Cycle in Women with Endometriosis. Reprod. Sci. 2016, 23, 1071–1079. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Quattrone, F.; Pannese, M.; Ulisse, A.; Candiani, M.; Diaz-Alonso, J.; Velasco, G.; Panina-Bordignon, P. The cannabinoid receptor CB1 contributes to the development of ectopic lesions in a mouse model of endometriosis. Hum. Reprod. 2017, 32, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Allam, S.; Paris, E.; Lazcano, I.; Bitterman, P.; Basu, S.; O’Donnell, J.; Barua, A. Detection of Cannabinoid Receptor Expression by Endometriotic Lesions in Women with Endometriosis as an Alternative to Opioid-Based Pain Medication. J. Immunol. Res. 2022, 2022, 4323259. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Caudullo, S.; Raffone, E.; Macrí, F.; Interdonato, L.; Gugliandolo, E.; Interlandi, C.; et al. Molecular and Biochemical Mechanism of Cannabidiol in the Management of the Inflammatory and Oxidative Processes Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 5427. [Google Scholar] [CrossRef] [PubMed]
- Mistry, M.; Simpson, P.; Morris, E.; Fritz, A.K.; Karavadra, B.; Lennox, C.; Prosser-Snelling, E. Cannabidiol for the Management of Endometriosis and Chronic Pelvic Pain. J. Minim. Invasive Gynecol. 2022, 29, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wisotzki, E.; Franke, H.; Sproll, C.; Walch, S.G.; Lachenmeier, D.W. Updated Risk Assessment of Cannabidiol in Foods Based on Benchmark Dose-Response Modeling. Molecules 2024, 29, 4733. [Google Scholar] [CrossRef] [PubMed]
- Leinow, L.; Birnbaum, J. Heilen Mit CBD: Das Wissenschaftlich Fundierte Handbuch zur Medizinischen Anwendung von Cannabidiol; Riva Verlag: Munich, Germany, 2019. [Google Scholar]
- FDA. What You Should Know About Using Cannabis, Including CBD, When Pregnant or Breastfeeding | FDA (US FDA, 16.10.2019). 2019. Available online: https://www.fda.gov/consumers/consumer-updates/what-you-should-know-about-using-cannabis-including-cbd-when-pregnant-or-breastfeeding (accessed on 19 November 2024).
- FDA. What You Need to Know (And What We’re Working to Find Out) About Products Containing Cannabis or Cannabis-Derived Compounds, Including CBD. March 2020. Available online: https://www.fda.gov/consumers/consumer-updates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis (accessed on 19 November 2024).
- Lachenmeier, D.W.; Sproll, C.; Walch, S.G. Does Cannabidiol (CBD) in Food Supplements Pose a Serious Health Risk? Consequences of the European Food Safety Authority (EFSA) Clock Stop Regarding Novel Food Authorisation. Psychoactives 2023, 2, 66–75. [Google Scholar] [CrossRef]
- FSA. Safety Assessment: Synthetic Cannabidiol (CBD) as a Novel Food For Use in Food Supplements. 29 April 2024. Available online: https://www.food.gov.uk/sites/default/files/media/document/safety-assessment-synthetic-cannabidiol-as-a-novel-food.pdf (accessed on 19 November 2024).
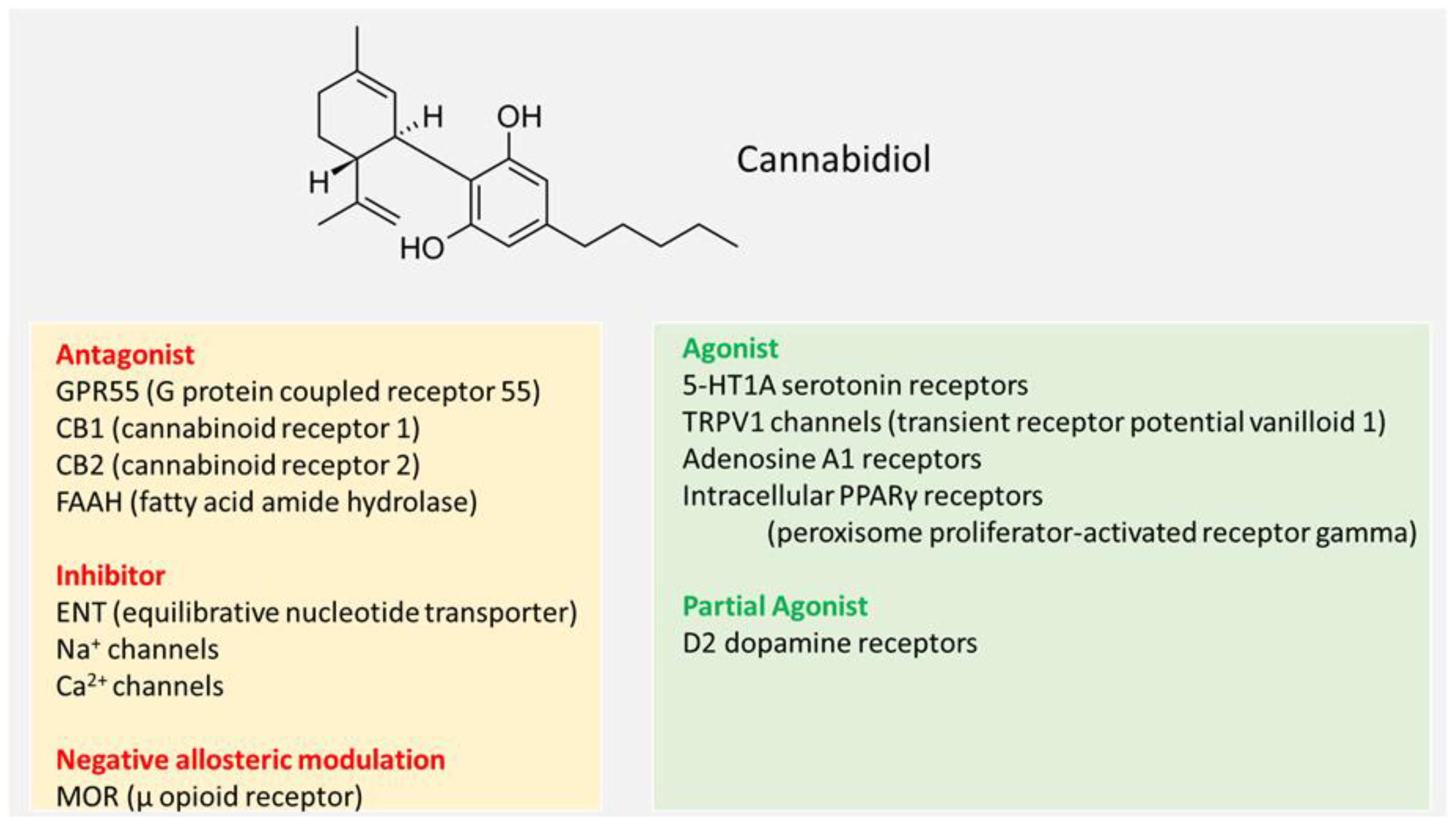
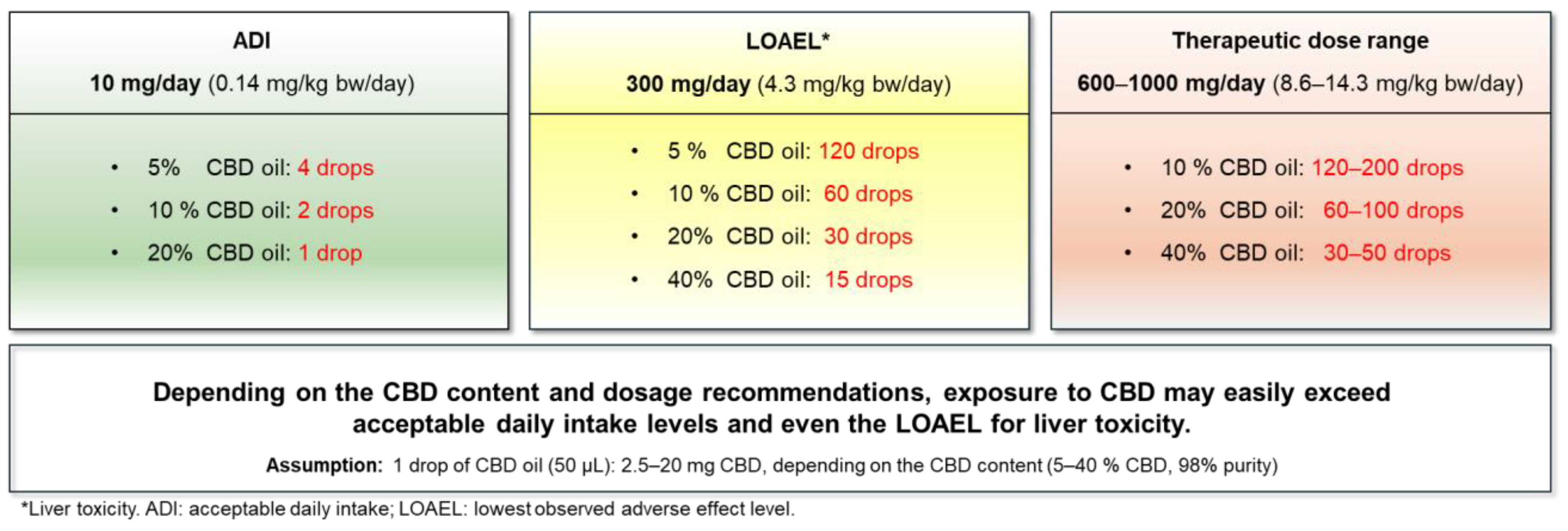
| Organ System/Endpoint | Adverse Effects | Dose Range/Conditions | References | |||
|---|---|---|---|---|---|---|
| Liver |
|
| [2,43,44,45,46,47,48] | |||
| Gastrointestinal |
|
| [46,49,50,51,52,53,54,55,56] | |||
| Neurological/Psychiatric |
|
| [2,18,48,54,57] | |||
| Endocrine |
|
| [2,18,43,44,45,58,59,60,61] | |||
| Reproduction/Fertility |
|
| [18,44,45,59,60,61,62] | |||
| Drug Metabolism |
|
| [2,17,63,64,65,66] | |||
| Genotoxicity |
|
| [2,18,67,68] |
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy men, 18–45 years old (n = 10); randomized, double-blind, placebo-controlled, crossover design | 300 mg/day (4.3 mg/kg bw/day) | Formulation of synthetic CBD | Oral | Single administration |
|
| [89] | |||||||
| Healthy men and women, 18–42 years old (n = 48); randomized, double-blind, placebo-controlled | 50 mg/day (0.7 mg/kg bw/day) | Hemp-derived CBD | Oral | 8 weeks |
|
| [90] | |||||||
| Trained men, 22–24 years old (n = 7–8 per group); not blinded | 16.67 mg/day (0.24 mg/kg bw/day) | CBD oil, no information on purity | Oral | Single administration |
|
| [91] | |||||||
| Untrained men, 21.85 ± 2.73 years old (n = 13); double-blinded, placebo controlled, crossover design | 150 mg/day (2.14 mg/kg bw/day) | CBD oil | Oral | 3 days |
|
| [92] | |||||||
| Female athletes 21.2 ± 1.8 years old (n = 24); double-blind, placebo-controlled, crossover design | 224–408 mg per application (5 mg/kg bw/day) | Not clear | Oral | 3 times in 10 h |
|
| [93] | |||||||
| Trained men and women, 24 ± 3 years old (n = 21); randomized, double-blind, placebo-controlled, study in a six-arm crossover design | 60 mg/person (0.86 mg/kg bw/day) | Plant derived | Oral | Single application |
| [94] | ||||||||
| Highly trained male weightlifters, 25 ± 3 years old (n = 12); double-blind, placebo-controlled, study in two-arm crossover design | 60 mg/person (0.86 mg/kg bw/day) | Plant derived | Oral | Single application |
|
| [95] | |||||||
| Healthy men, 18–65 years old (n = 40; 20 per group); randomized, double-blind, placebo-controlled, repeated-dose pilot study | 70 mg/person (1 mg/kg bw/day) and 100 mg CBG per day | Water soluble liquid. CBD oil nano-particularized and combined with other ingredients to maintain the suspension. | Oral | 3.5 days |
|
| [96] | |||||||
| Healthy men and women, 18–40 years old (n = 17, m = 15, f = 2); randomized, double-blind, placebo-controlled in a three-arm cross-over design | 60 mg/person (0.86 mg/kg bw/day) | CBD oil solubilized CBD | Oral | 6 days |
|
| [97] |
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy adults (n = 10); pilot, randomized, double-blind, parallel arm study | 30 mg/day (0.43 mg/kg bw/day) | Commercially available water- and lipid-soluble CBD powders. | Oral | Single application |
|
| [138] | |||||||
| Healthy men and women, 18–42 years old (n = 48); randomized, double-blind, placebo-controlled | 50 mg/day (0.7 mg/kg bw/day) | Hemp-derived CBD; no information about purity. | Oral | 8 weeks |
|
| [90] |
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy men and women (n = 60); randomized, double-blind, placebo-controlled | 100, 300, 900 mg/day (1.4, 4.3, 12.9 mg/kg bw/day) | 99.6% purity in corn oil filled in gelatin capsules | Oral | Single administration |
|
| [194] | |||||||
| Healthy men (n = 57); randomized, double-blind, placebo-controlled | 150, 300, 600 mg/day (2.1, 4.3, 8.6 mg/kg bw/day) | 99.9% purity in corn oil filled in gelatin capsules | Oral | Single administration |
|
| [195] | |||||||
| Healthy men (n = 45); randomized, double-blind, placebo-controlled | 150 mg/day (2.1 mg/kg bw/day) | 99.6% purity either as powder or dissolved in corn oil, filled in gelatin capsules | Oral | Single administration |
|
| [196] | |||||||
| Healthy men and women (n = 32); randomized, double-blind, placebo-controlled | 150, 300, 600 mg/day (2.1, 4.3, 8.6 mg/kg bw/day) | CBD isolate (< 0.3% THC), no purity stated, in MCT oil with 2% peppermint oil | Oral | Single administration |
|
| [207] | |||||||
| Male and female outpatients with moderate to severe anxiety (n = 14); open-label stage of a two-stage, phase 2 clinical trial (unblinded study) | About 30 mg/day (0.43 mg/kg bw/day) | Full-spectrum high CBD extract in MCT oil | Oral | 30 days |
|
| [208] | |||||||
| Men and women with elevated trait worry (n = 63); randomized, double-blind, placebo-controlled | 50 mg CBD/day (0.7 mg/kg bw/day) with 0.03 mg CBDV, 300 mg CBD/day (4.3 mg/kg bw/day) | CBD isolates in MCT oil in soft gel capsules | Oral | 2 weeks |
|
| [209] | |||||||
| Healthy men and women (n = 48); randomized, double-blind, placebo-controlled | 32 mg/day (0.46 mg/kg bw/day) | CBD (purity not specified) in ethanol vehicle | Inhalation | Single administration |
|
| [210] | |||||||
| Audit of patients (n = 400) seeking CBD prescriptions | 40–300 mg/day (0.6–4.3 mg/kg bw/day) | Pharmaceutical CBD oil (100 mg/mL in 25 mL bottles) | Oral | 3 weeks |
|
| [211] | |||||||
| Retrospective case series (n = 72) | 25 mg/day (0.34 mg/kg bw/day) | CBD (purity not specified) in capsule form | Oral | 3 months |
|
| [212] | |||||||
| Female patient, 10 years old (n = 1); case report | 12–25 mg/day (0.17–0.36 mg/kg bw/day) | Sublingual spray, capsules | Oral | 6 months |
|
| [213] |
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female patients with irritable bowel syndrome, 22–50 years old (n = 32); randomized, double-blinded, placebo-controlled, crossover design | 50 mg/day (0.7 mg/kg bw/day) | CBD-containing chewing gum | Oral | 8-week |
|
| [232] | |||||||
| 8 groups of 5 male volunteers (n = 40) | Single dose of 15 to 60 mg (0.2–0.9 mg/kg bw/day) with single dose of Δ9-THC | Substance solved in orange juice | Oral | Single dose |
| [224] | ||||||||
| Patients with clinical high risk for psychosis (n = 32) and healthy controls (n = 26) | 600 mg/day (8.6 mg/kg bw/day) | Capsules | Oral | 1 week |
| [203] | ||||||||
| Healthcare workers during the COVID-19 pandemic (n = 120) | 300 mg/day (150 mg twice per day) (4.3 mg/kg bw/day) | Dissolved in medium-chain triglyceride | Oral | 4 weeks |
|
| [72] | |||||||
| Healthcare workers (n = 13) during the COVID-19 pandemic (27 July–28 November 2020) | 330 mg CBD/day, (165 mg twice per day) (4.7 mg/kg bw/day) | Dissolved in medium-chain triglyceride | Oral | 4 weeks |
| [201] | ||||||||
| 11 patients with a diagnosis of PTSD; retrospective case series | Open-label, flexible dosing regimen: 25 to 100 mg CBD/day together with psychiatric medications and psychotherapy (0.3–1.4 mg/kg bw/day) | In oil or capsules | Oral | 8 weeks |
|
| [230] |
| Study Design | CBD Dose * | Source | Route | Duration | Effects | Comment/Limitations | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | ||||||||||||||
| Healthy men and women, 29 ± 8.5 years old (n = 26); randomized, double-blind, placebo-controlled, crossover design | 300 mg/day (4.3 mg/kg bw/day) | Pure compound, 99.9% purity | Oral | Single administration |
| [246] | ||||||||
| Healthy men and women, 18–55 years old (n = 296; n = 63 to 56 per group); randomized, double-blind, placebo-controlled | 20 mg CBN, (c) 20 mg CBN + 10 mg CBD (0.14 mg/kg bw/day), (d) 20 mg CBN + 20 mg CBD (0.3 mg/kg bw/day), or (e) 20 mg CBN + 100 mg CBD (1.4 mg/kg bw/day) | Gummies pure compounds | Oral | 7 days |
|
| [247] | |||||||
| Healthy men and women; 25.8 ± 6.1 years old (n = 28; n = 14 per group); double-blind, placebo-controlled intervention | 50 mg/day (0.7 mg/kg bw/day) | Purified hemp-derived CBD | Oral | 8 weeks |
|
| [248] | |||||||
| Healthy men and women, 45 ± 12 years old (n = 1793; n = 300 per group); 6 arm, randomized, double-blinded, controlled study | 6 groups combining 15 mg CBD/day (0.2 mg/kg bw/day) with other cannabinoids and/or melatonin: I. 15 mg CBD + 15 mg CBN + 5 mg melatonin II. 15 mg CBD + 15 mg CBN III. 15 mg CBD IV. 5 mg melatonin V. 15 mg CBD full spectrum + 15 mg CBN VI. 15 mg CBD + 15 mg CBN + 5 mg CBC | Not clear. Mixed quality (pure, from hemp extract/full spectrum) | Oral | 5 weeks |
|
| [249] | |||||||
| Healthy men and women, 18–45 years (n = 30; n = 15 per group); randomized, placebo-controlled, parallel design | 150 mg/day (2.1 mg/kg bw/day) | Pure CBD | Oral | 2 weeks |
|
| [250] |
| Dosage Type | CBD Dose (mg/day) | Possible Usage Pattern for CBD-Oils * |
|---|---|---|
| Micro dosage | 0.5–30 | 5% CBD-oil: < 1 drop–12 drops 10% CBD-oil: < 1 drop–6 drops 20% CBD-oil: << 1 drop–3 drops 40% CBD-oil: <<< 1 drop–1.5 drops |
| Standard dosage | 10–115 | 5% CBD-oil: 4–46 drops 10% CBD-oil: 2–23 drops 20% CBD-oil: 1–11.5 drops 40% CBD-oil: < 1 drop–6 drops |
| Macro dosage | 50–1500 | 5% CBD-oil: 20–600 drops 10% CBD-oil: 10–300 drops 20% CBD-oil: 5–150 drops 40% CBD-oil: 2.5–75 drops |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engeli, B.E.; Lachenmeier, D.W.; Diel, P.; Guth, S.; Villar Fernandez, M.A.; Roth, A.; Lampen, A.; Cartus, A.T.; Wätjen, W.; Hengstler, J.G.; et al. Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims. Nutrients 2025, 17, 489. https://doi.org/10.3390/nu17030489
Engeli BE, Lachenmeier DW, Diel P, Guth S, Villar Fernandez MA, Roth A, Lampen A, Cartus AT, Wätjen W, Hengstler JG, et al. Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims. Nutrients. 2025; 17(3):489. https://doi.org/10.3390/nu17030489
Chicago/Turabian StyleEngeli, Barbara E., Dirk W. Lachenmeier, Patrick Diel, Sabine Guth, Maria A. Villar Fernandez, Angelika Roth, Alfonso Lampen, Alexander T. Cartus, Wim Wätjen, Jan G. Hengstler, and et al. 2025. "Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims" Nutrients 17, no. 3: 489. https://doi.org/10.3390/nu17030489
APA StyleEngeli, B. E., Lachenmeier, D. W., Diel, P., Guth, S., Villar Fernandez, M. A., Roth, A., Lampen, A., Cartus, A. T., Wätjen, W., Hengstler, J. G., & Mally, A. (2025). Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims. Nutrients, 17(3), 489. https://doi.org/10.3390/nu17030489









