Antioxidant Intake and Ovarian Reserve in Women Attending a Fertility Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Antral Follicle Count Measurement
2.3. Antioxidant Intake Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, X.; Zhang, S.; Guo, C.; Li, J.; Mi, Y.; Zhang, C. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging 2018, 10, 2016–2036. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Shen, M.; Lin, F.; Zhang, J.; Tang, Y.; Chen, W.K.; Liu, H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Cruz, R.; Pinto, B.; Costa, L.; Felgueira, E.; Oliveira, P.; Casal, S.; Rebelo, I. Retinoic acid (all-trans) presents antioxidant properties within human ovary and reduces progesterone production by human granulosa cells. Syst. Biol. Reprod. Med. 2023, 69, 129–141. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Division of Reproductive Health. Available online: http://nccd.cdc.gov/drh_art (accessed on 15 July 2023).
- Moslehi, N.; Mirmiran, P.; Azizi, F.; Tehrani, F.R. Do dietary intakes influence the rate of decline in anti-Mullerian hormone among eumenorrheic women? A population-based prospective investigation. Nutr. J. 2019, 18, 83. [Google Scholar] [CrossRef]
- Shang, Y.; Song, N.; He, R.; Wu, M. Antioxidants and Fertility in Women with Ovarian Aging: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100273. [Google Scholar] [CrossRef]
- Rodríguez-Varela, C.; Labarta, E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Saleh, R.; Sallam, H.; Elsuity, M.A.; Dutta, S.; Sengupta, P.; Nasr, A. Antioxidant therapy for infertile couples: A comprehensive review of the current status and consideration of future prospects. Front. Endocrinol. 2025, 15, 1503905. [Google Scholar] [CrossRef] [PubMed]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 8, CD007807. [Google Scholar] [PubMed]
- Li, M.-C.; Nassan, F.L.; Chiu, Y.-H.; Mínguez-Alarcón, L.; Williams, P.L.; Souter, I.; Hauser, R.; Chavarro, J.E.; EARTH Study Team. Intake of Antioxidants in Relation to Infertility Treatment Outcomes with Assisted Reproductive Technologies. Epidemiology 2019, 30, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Panti, A.A.; Shehu, C.E.; Saidu, Y.; Tunau, K.A.; Nwobodo, E.I.; Jimoh, A.; Bilbis, L.S.; Umar, A.B.; Hassan, M. Oxidative stress and outcome of antioxidant supplementation in patients with polycystic ovarian syndrome (PCOS). Int. J. Reprod. Contracept. Obstet. Gynecol. 2018, 7, 1667. [Google Scholar] [CrossRef]
- Youssef, M.A.F.M.; Abdelmoty, H.I.; Elashmwi, H.A.; Abduljawad, E.M.; Elghamary, N.; Magdy, A.; Mohesen, M.N.; Abdella, R.M.A.; Abdel Bar, M.; Gouda, H.M.; et al. Oral antioxidant supplementation for women with unexplained infertility undergoing ICSI/IVF: Randomized controlled trial. Hum. Fertil. 2015, 18, 38–42. [Google Scholar] [CrossRef]
- Safiyeh, F.D.; Mojgan, M.; Parviz, S.; Sakineh, M.A.; Behnaz, S.O. The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: A randomized controlled clinical trial. Complement. Ther. Med. 2021, 56, 102533. [Google Scholar] [CrossRef]
- Fujihara, M.; Yamamizu, K.; Comizzoli, P.; Wildt, D.E.; Songsasen, N. Retinoic acid promotes in vitro follicle activation in the cat ovary by regulating expression of matrix metalloproteinase 9. PLoS ONE 2018, 13, e0202759. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Cano, A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol. Reprod. Dev. 2002, 61, 385–397. [Google Scholar] [CrossRef]
- Han, Q.; Chen, Z.J.; Du, Y. Dietary supplementation for female infertility: Recent advances in the nutritional therapy for premature ovarian insufficiency. Front. Microbiol. 2022, 13, 1001209. [Google Scholar] [CrossRef]
- Turkler, C.; Onat, T.; Yildirim, E.; Kaplan, S.; Yazici, G.N.; Mammadov, R.; Sunar, M. An experimental study on the use of lycopene to prevent infertility due to acute oxidative ovarian damage caused by a single high dose of methotrexate. Adv. Clin. Exp. Med. 2020, 29, 5–11. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Chiu, Y.-H.; Souter, I.; Williams, P.L.; Calafat, A.M.; Hauser, R.; Chavarro, J.E.; EARTH Study team. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reprod. Toxicol. 2016, 65, 104–112. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Subar, A.F.; Sampson, L.K.; Willett, W.C. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am. J. Epidemiol. 2017, 185, 570–584. [Google Scholar] [CrossRef]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Rood, J.C.; Harnack, L.J.; Sampson, L.K.; et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared with Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am. J. Epidemiol. 2018, 187, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- U. S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25. Nutrient Data Laboratory Home Page. 2012. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 15 July 2023).
- Institute of Medicine. Panel on Micronutrients, Institute of Medicine (U.S.). Food and Nutrition Board. DRI, Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- National Institutes of Health. Office of Dietary Supplements. Vitamin A. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 12 March 2020).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Hertzmark, E.; Li, R.; Hong, B.; Spiegelman, D.; The SAS GLMCURV9 Macro. Yale Medicine. Available online: https://files-profile.medicine.yale.edu/documents/5c89b92a-4fae-4a34-aa5c-c23b2aaded4e (accessed on 10 January 2025).
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef]
- Mumford, S.L.; Browne, R.W.; Schliep, K.C.; Schmelzer, J.; Plowden, T.C.; Michels, K.A.; Sjaarda, L.A.; Zarek, S.M.; Perkins, N.J.; Messer, L.C.; et al. Serum Antioxidants Are Associated with Serum Reproductive Hormones and Ovulation among Healthy Women. J. Nutr. 2016, 146, 98–106. [Google Scholar] [CrossRef]
- Pearce, K.; Tremellen, K. Influence of nutrition on the decline of ovarian reserve and subsequent onset of natural menopause. Hum. Fertil. 2016, 19, 173–179. [Google Scholar] [CrossRef]
- Marques, C.S.; Reis Lima, M.J.; Oliveira, J.; Teixeira-Lemos, E. Tomato Lycopene: Functional Proprieties and Health Benefits. Int. J. Agric. Biol. Eng. 2015, 9, 1089–1099. [Google Scholar]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Uçar, S.; Pandir, D. Furan induced ovarian damage in non-diabetic and diabetic rats and cellular protective role of lycopene. Arch. Gynecol. Obstet. 2017, 296, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Kulhan, N.G.; Kulhan, M.; Turkler, C.; Ata, N.; Kiremitli, T.; Kiremitli, S.; Keskin Cimen, F.; Suleyman, H.; Toprak, V. Effect of lycopene on oxidative ovary-damage induced by cisplatin in rats. Gen. Physiol. Biophys. 2019, 38, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rakha, S.I.; Ateya, A.I.; Safhi, F.A.; Abdellatif, A.M. Ameliorative effect of lycopene on follicular reserve depletion, oxidative damage, apoptosis rate, and hormonal profile during repeated superovulations in mice. Vet. Sci. 2024, 11, 414. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Association of diet with the onset of menopause in Japanese women. Am. J. Epidemiol. 2000, 152, 863–867. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Inaba, S.; Kawakami, N.; Shimizu, H. Association of diet and other lifestyle with onset of menopause in Japanese women. Maturitas 1998, 29, 105–113. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Rasool, S.; Shah, D. Fertility with early reduction of ovarian reserve: The last straw that breaks the Camel’s back. Fertil. Res. Pract. 2017, 3. [Google Scholar] [CrossRef]
- Damdimopoulou, P.; Chiang, C.; Flaws, J.A. Retinoic acid signaling in ovarian folliculogenesis and steroidogenesis. Reprod. Toxicol. 2019, 87, 32–41. [Google Scholar] [CrossRef]
- Aksoy, H.; Cinar, L.; Acmaz, G.; Aksoy, U.; Aydin, T.; Vurdem, U.E.; Oz, L.; Karadag, O.I.; Kartal, D. The effect of isotretinoin on ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with acne. Gynecol. Obstet. Investig. 2015, 79, 78–82. [Google Scholar] [CrossRef]
- Sikar Aktürk, A.; Abalı, R.; Yüksel, M.A.; Güzel, E.Ç.; Güzel, S.; Kıran, R. The effects of isotretinoin on the ovarian reserve of females with acne. Gynecol. Endocrinol. 2014, 30, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Can, P.; Kocatürk, E.; Mihmanlı, V.; Sucu, V.; Degirmentepe, E.; Kızıltaç, U. The evaluation of ovarian reserve and menstrual irregularities in female patients treated with systemic isotretinoin. Turk. Arch. Dermatol. Venerol. 2020, 54, 79–84. [Google Scholar] [CrossRef]
- Öztürk, S.; Öztürk, T.; Ucak, H.; Erden, I.; Demir, B.; Kayalı, A.; Cicek, D. Evaluation of ovarian reserve and function in female patients treated with oral isotretinoin for severe acne: An exploratory study. Cutan. Ocul. Toxicol. 2015, 34, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Abali, R.; Yuksel, M.A.; Aktas, C.; Celik, C.; Guzel, S.; Erfan, G.; Sahin, O. Decreased ovarian reserve in female Sprague-Dawley rats induced by isotretinoin (retinoic acid) exposure. Reprod. Biomed. Online 2013, 27, 184–191. [Google Scholar] [CrossRef]
- National Institutes of Health. Office of Dietary Supplements. Nutrient Recommendations and Databases. US Department of Health and Human Services, Bethesda. 2023. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 15 July 2023).
- Souter, I.; Chiu, Y.-H.; Batsis, M.; Afeiche, M.C.; Williams, P.L.; Hauser, R.; Chavarro, J.E.; EARTH Study Team. The association of protein intake (amount and type) with ovarian antral follicle counts among infertile women: Results from the EARTH prospective study cohort. BJOG 2017, 124, 1547–1555. [Google Scholar] [CrossRef]
- Appt, S.E.; Chen, H.; Goode, A.K.; Hoyer, P.B.; Clarkson, T.B.; Adams, M.R.; Wilson, M.E.; Franke, A.A.; Kaplan, J.R. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys (Macaca fascicularis). Menopause 2010, 17, 741–748. [Google Scholar] [CrossRef]
- Purdue-Smithe, A.C. Vitamin D, Calcium, and Dairy Consumption and Risk of Early Menopause. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2018. [Google Scholar]
- Rostami Dovom, M.; Noroozzadeh, M.; Mosaffa, N.; Zadeh-Vakili, A.; Piryaei, A.; Ramezani Tehrani, F. Induced premature ovarian insufficiency by using D galactose and its effects on reproductive profiles in small laboratory animals: A systematic review. J. Ovarian Res. 2019, 12, 96. [Google Scholar] [CrossRef]
- Moslehi, N.; Marzbani, R.; Rezadoost, H.; Mirmiran, P.; Ramezani Tehrani, F.; Azizi, F. Serum metabolomics study of the association between dairy intake and the anti-müllerian hormone annual decline rate. Nutr. Metab. 2021, 18, 66. [Google Scholar] [CrossRef]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Dos Santos, C.N.; Pinto, P.; Re, R.; Rémond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef]
- Anderson, C.; Park, Y.M.; Stanczyk, F.Z.; Sandler, D.P.; Nichols, H.B. Dietary factors and serum antimüllerian hormone concentrations in late premenopausal women. Fertil. Steril. 2018, 110, 1145–1153. [Google Scholar] [CrossRef]
- Willett, W.C. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- U. S. Census Bureau. QuickFacts; U.S. Department of Commerce: Washington, DC, USA, 2025. Available online: https://www.census.gov/quickfacts/ (accessed on 12 January 2025).
- National Center for Health Statistics. FastStats; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2025. Available online: https://www.cdc.gov/nchs/fastats/default.htm (accessed on 12 January 2025).
- Stephen, E.H.; Chandra, A. Use of Infertility Services in the United States: 1995. Fam. Plann. Perspect. 2000, 32, 132–137. [Google Scholar] [CrossRef]
- Fraser, A.; McNally, W.; Sattar, N.; Anderson, E.L.; Lashen, H.; Fleming, R.; Lawlor, D.A.; Nelson, S.M. Prenatal exposures and anti-Mullerian hormone in female adolescents: The Avon Longitudinal Study of Parents and Children. Am. J. Epidemiol. 2013, 178, 1414–1423. [Google Scholar] [CrossRef]

| Characteristics | Total | ||
|---|---|---|---|
| Age (at study entry), years | 35 (32–38) | ||
| Ever smoked | 144 (25.4) | ||
| White | 470 (82.9) | ||
| College degree or higher | 527 (92.9) | ||
| BMI, kg/m2 | 23.2 (21.2–26.0) | ||
| Physical activity, hours/week | 5 (2.5–9.9) | ||
| Total energy intake, Kcal/day | 1647.7 (1335.4–2023.6) | ||
| Alcohol, g/day | 4.7 (1.4–12.4) | ||
| Caffeine, mg/day | 103.6 (43.7–171.9) | ||
| Folate intake, DFE mg/day | 1457.8 (775.8–2095.0) | ||
| Vitamin B12 mcg/day | 11.3 (8.6–15.6) | ||
| Vitamin A, mcg/day | 1810.5 (1283.4–2214.8) | ||
| Vitamin C, mg/day | 174.9 (120.4–236.8) | ||
| Vitamin E, mg/day | 19.9 (14.3–24.1) | ||
| Retinol, mcg/day | 1202.5 (794.5–1554.1) | ||
| Total carotenoids, mcg/day | 14,418.0 (10,747.0–19,274.0) | ||
| Alpha-Carotene, mcg/day | 549.8 (293.0–933.1) | ||
| Beta-Carotene, mcg/day | 5816.5 (3802.2–7827.8) | ||
| Beta Cryptoxanthin, mcg/day | 83.8 (50.9–145.5) | ||
| Lycopene, mcg/day | 3724.3 (2640.5–5378.3) | ||
| Lutein and zeaxanthin, mcg/day | 3264.0 (2293.0–4920.3) | ||
| Prior pregnancy | 250 (44.1) | ||
| Prior infertility exam | 474 (83.6) | ||
| Prior fertility treatment | 336 (59.3) | ||
| Day 3 FSH, IU/ml | 6.9 (5.9–8.4) | ||
| Infertility diagnosis | |||
| Male factor | 138 (24.3) | ||
| Female factor | |||
| DOR | 53 (9.3) | ||
| Endometriosis | 20 (3.5) | ||
| Ovulatory | 54 (9.5) | ||
| Tubal | 27 (4.8) | ||
| Uterine | 10 (1.8) | ||
| Unexplained | 265 (46.7) | ||
| Antioxidant Intake | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Trend | |
|---|---|---|---|---|---|---|
| Vitamin A **, mcg/day | ||||||
| n (range) | 141 (327.44–1279.18) | 142 (1283.41–1809.57) | 142 (1810.5–2212.89) | 142 (2214.77–12,473.66) | ||
| AFC (95% CI) | 13.3 (12.6–14.0) | 13.5 (12.8–14.2) | 13.1 (12.4–13.8) | 13.3 (12.6–14.1) | 0.88 | |
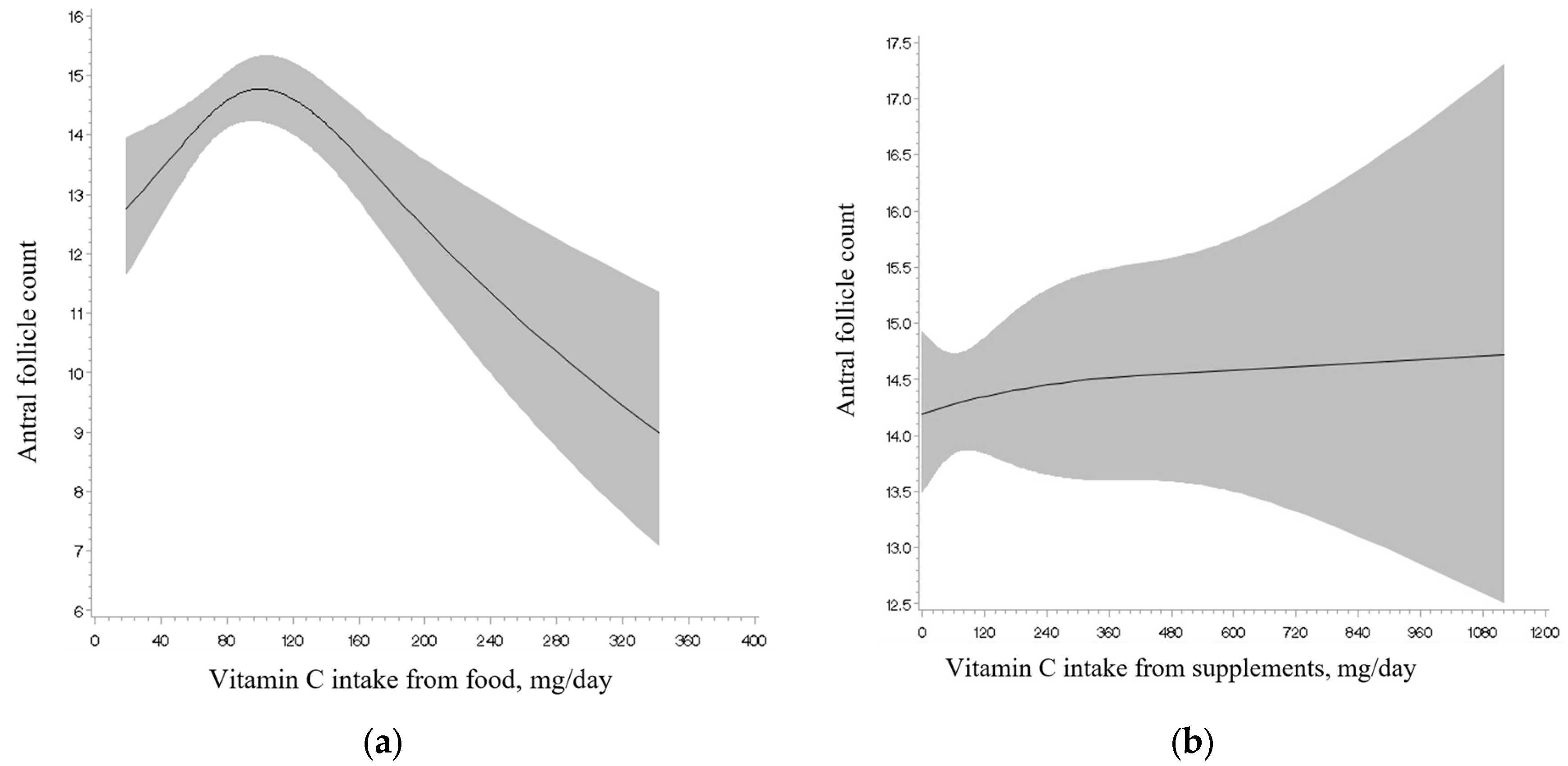
| Vitamin C, mg/day | ||||||
| n (range) | 141 (25.57–120.22) | 142 (120.41–174.68) | 142 (174.90–236.82) | 142 (236.83–1546.70) | ||
| AFC (95% CI) | 12.9 (12.2–13.7) | 13.6 (13.0–14.3) | 13.6 (12.9–14.4) | 13.0 (12.3–13.7) | 0.65 | |
| Vitamin E, mg/day | ||||||
| n (range) | 141 (3.18–14.28) | 142 (14.29–19.92) | 142 (19.95–24.11) | 142 (24.12–354.93) | ||
| AFC (95% CI) | 13.0 (12.3–13.8) | 12.8 (12.1–13.5) | 13.8 (13.2–14.6) | 13.5 (12.7–14.2) | 0.21 | |
| Preformed retinol, mcg/day | ||||||
| n (range) | 141 (100.33–784.86) | 142 (794.49–1202.40) | 142 (1202.54–1553.03) | 142 (1554.05–10,228.47) | ||
| AFC (95% CI) | 13.8 (13.0–14.5) | 13.1 (12.5–13.9) | 13.6 (13.0–14.4) | 12.8 (12.1–13.5) * | 0.07 | |
| Total carotenoids, mcg/day | ||||||
| n (range) | 141 (2428.63–10,713.90) | 142 (10,746.57–14,408.07) | 142 (14,418.35–19,236.59) | 142 (19,274.22–51,287.62) | ||
| AFC (95% CI) | 13.3 (12.6–14.0) | 13.3 (12.6–14.0) | 13.1 (12.4–13.8) | 13.4 (12.7–14.1) | 0.79 | |
| Alpha-Carotene, mcg/day | ||||||
| n (range) | 141 (5.64–292.57) | 142 (292.96–549.41) | 142 (549.77–931.61) | 142 (933.07–4300.58) | ||
| AFC (95% CI) | 13.3 (12.6–14.1) | 13.8 (13.0–14.5) | 12.8 (12.1–13.5) | 13.4 (12.7–14.2) | 0.84 | |
| Beta-Carotene, mcg/day | ||||||
| n (range) | 141 (724.14–3796.19) | 142 (3802.24–5801.09) | 142 (5816.52–7824.56) | 142 (7827.80–30,102.44) | ||
| AFC (95% CI) | 13.0 (12.3–13.8) | 13.4 (12.7–14.1) | 13.0 (12.3–13.7) | 13.9 (13.0–14.8) | 0.24 | |
| Beta-Cryptoxanthin, mcg/day | ||||||
| n (range) | 141 (5.13–50.87) | 142 (50.90–83.77) | 142 (83.81–145.08) | 142 (145.51–693.61) | ||
| AFC (95% CI) | 13.4 (12.8–14.2) | 13.0 (12.3–13.7) | 13.4 (12.7–14.1) | 13.3 (12.7–14.0) | 0.91 | |
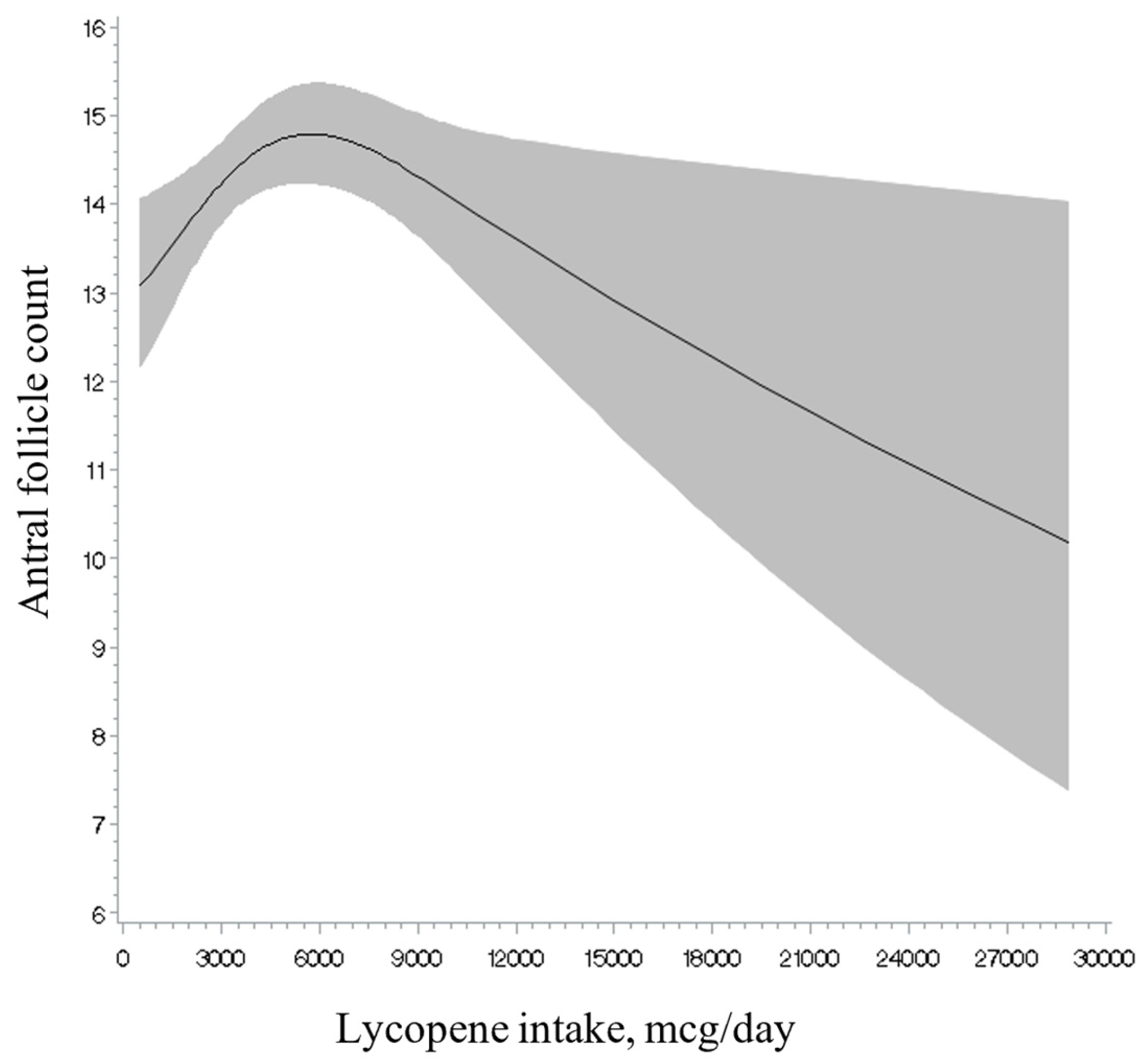
| Lycopene, mcg/day | ||||||
| n (range) | 141 (533.19–2622.20) | 142 (2640.49–3708.37) | 142 (3724.29–5371.89) | 142 (5378.27–28,885.46) | ||
| AFC (95% CI) | 13.3 (12.7–14.0) | 12.6 (11.9–13.2) | 13.6 (12.9–14.3) | 13.8 (13.1–14.5) | 0.06 | |
| Lutein and zeaxanthin, mcg/day | ||||||
| n (range) | 141 (565.34–2292.55) | 142 (2292.97–3261.47) | 142 (3263.99–4902.56) | 142 (4920.30–21,623.60) | ||
| AFC (95% CI) | 13.2 (12.5–13.9) | 13.4 (12.7–14.0) | 13.5 (12.8–14.2) | 13.2 (12.5–13.9) | 0.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Cárceles, A.B.; Souter, I.; Li, M.-C.; Mitsunami, M.; Dimitriadis, I.; Ford, J.B.; Mínguez-Alarcón, L.; Chavarro, J.E., on behalf of the EARTH Study Team. Antioxidant Intake and Ovarian Reserve in Women Attending a Fertility Center. Nutrients 2025, 17, 554. https://doi.org/10.3390/nu17030554
Maldonado-Cárceles AB, Souter I, Li M-C, Mitsunami M, Dimitriadis I, Ford JB, Mínguez-Alarcón L, Chavarro JE on behalf of the EARTH Study Team. Antioxidant Intake and Ovarian Reserve in Women Attending a Fertility Center. Nutrients. 2025; 17(3):554. https://doi.org/10.3390/nu17030554
Chicago/Turabian StyleMaldonado-Cárceles, Ana B., Irene Souter, Ming-Chieh Li, Makiko Mitsunami, Irene Dimitriadis, Jennifer B. Ford, Lidia Mínguez-Alarcón, and Jorge E. Chavarro on behalf of the EARTH Study Team. 2025. "Antioxidant Intake and Ovarian Reserve in Women Attending a Fertility Center" Nutrients 17, no. 3: 554. https://doi.org/10.3390/nu17030554
APA StyleMaldonado-Cárceles, A. B., Souter, I., Li, M.-C., Mitsunami, M., Dimitriadis, I., Ford, J. B., Mínguez-Alarcón, L., & Chavarro, J. E., on behalf of the EARTH Study Team. (2025). Antioxidant Intake and Ovarian Reserve in Women Attending a Fertility Center. Nutrients, 17(3), 554. https://doi.org/10.3390/nu17030554






