Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes—Underlying Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Materials and Method
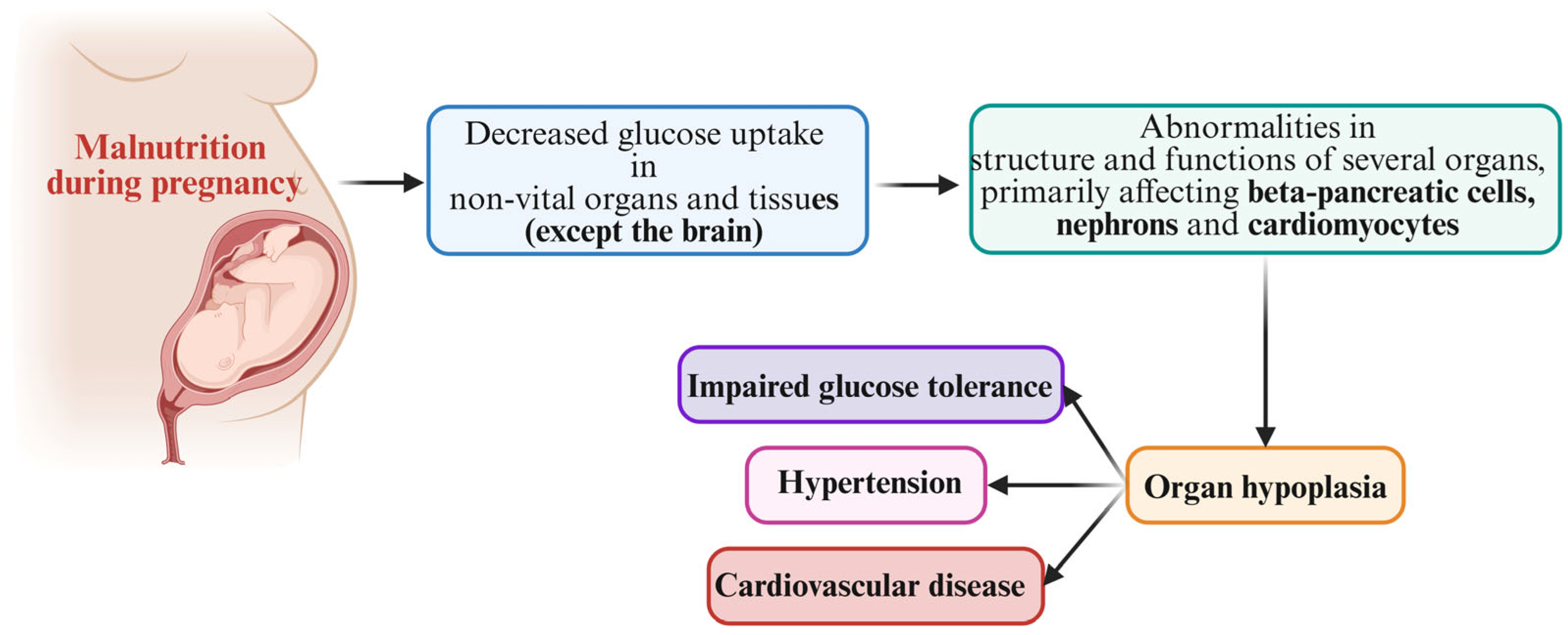
3. Hypothesis on Fetal Growth Restriction and Developmental Origins of Adult Disease
4. Mismatch Concept
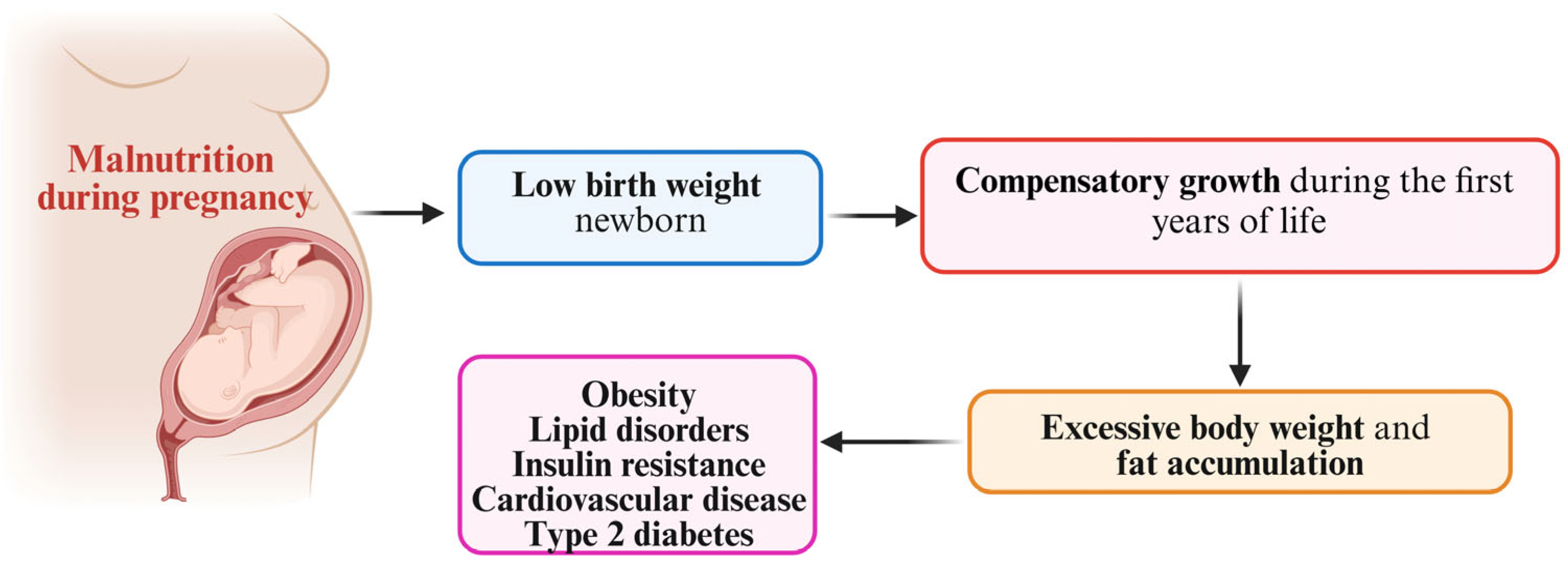
5. The Catch-Up Growth Hypothesis
6. Catch-Up Fat Phenotype and Body Composition in Fetal Growth Restriction-Affected Individuals
7. Fetal Growth Restriction and Brain–Gut Axis
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152 (Suppl. S1), 3–57. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Bujold, E. Early Detection and Prevention of Intrauterine Growth Restriction and Its Consequences. JAMA Pediatr. 2020, 174, 749–750. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society forMaternal-FetalMedicin. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, e97–e109. [Google Scholar] [CrossRef]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: Comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef] [PubMed]
- Lubchenco, L.O.; Hansman, C.; Boyd, E. Intrauterine growth as estimated from live born birth-weight data 24–42 weeks of gestation. Pediatrics 1963, 32, 793–823. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Munoz, F.M.; Bardají, A.; Boghossian, N.S.; Khalil, A.; Mousa, H.; Nesin, M.; Nisar, M.I.; Pool, V.; Spiegel, H.M.L.; et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 2017, 35 Pt A, 6518–6528. [Google Scholar]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Highlighting the trajectory from intrauterine growth restriction to future obesity. Front. Endocrinol. 2022, 13, 1041718. [Google Scholar] [CrossRef]
- Darendeliler, F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101260. [Google Scholar] [CrossRef]
- Lee, P.A.; Chernausek, S.D.; Hokken-Koelega, A.C.; Czernichow, P. International Small for Gestational Age Advisory Board consensus development conference statement: Management of short children born small for gestational age, 2001. Pediatrics 2003, 111 Pt 1, 1253–1261. [Google Scholar] [CrossRef]
- Osuchukwu, O.O.; Reed, D.J. Small for Gestational Age. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563247/ (accessed on 23 January 2022).
- Slancheva, B.; Mumdzhiev, H. Small for gestational age newborns--definition, etiology and neonatal treatment. Akush. Ginekol. 2022, 52, 25–32. [Google Scholar]
- Ludvigsson, J.F.; Lu, D.; Hammarström, L.; Cnattingius, S.; Fang, F. Small for gestational age and risk of childhood mortality: A Swedish population study. PLoS Med. 2018, 15, e1002717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Ergaz, Z.; Avgil, M.; Ornoy, A. Intrauterine growth restriction-etiology and consequences: What do we know about the human situation and experimental animal models? Reprod. Toxicol. 2005, 20, 301–322. [Google Scholar] [CrossRef]
- Kesavan, K.; Devaskar, S.U. Intrauterine Growth Restriction: Postnatal Monitoring and Outcomes. Pediatr. Clin. N. Am. 2019, 66, 403–423. [Google Scholar] [CrossRef]
- Armengaud, J.B.; Yzydorczyk, C.; Siddeek, B.; Peyter, A.C.; Simeoni, U. Intrauterine growth restriction: Clinical consequences on health and disease at adulthood. Reprod. Toxicol. 2021, 99, 168–176. [Google Scholar] [CrossRef]
- Varvarigou, A.A. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J. Pediatr. Endocrinol. Metab. 2010, 23, 215–224. [Google Scholar] [CrossRef]
- Díaz-Ortega, J.L.; Yupari-Azabache, I.L.; Caballero Vidal, J.A.; Conde-Parada, N.E.; Rojas Gamboa, A.F. Criteria in the Diagnosis of Metabolic Syndrome in Children: A Scoping Review. Diabetes Metab. Syndr. Obes. 2023, 16, 3489–3500. [Google Scholar] [CrossRef]
- Baird, J.F.; Fisher, D.; Lucas, P.; Kleijnen, J.; Roberts, H.; Law, C. Being big or growing fast: Systematic review of size and growth in infancy and later obesity. BMJ 2005, 331, 929. [Google Scholar] [CrossRef]
- Brisbois, T.D.; Farmer, A.P.; McCargar, L.J. Early markers of adult obesity: A review. ObesRev 2012, 13, 347–367. [Google Scholar] [CrossRef]
- Monasta, L.; Batty, G.D.; Cattaneo, A.; Lutje, V.; Ronfani, L.; Van Lenthe, F.J. Early-life determinants of overweight and obesity: A review of systematic reviews. Obes. Rev. 2010, 11, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Neel, J.V. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 1962, 14, 353–362. [Google Scholar] [PubMed]
- Speakman, J. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: The ‘drifty gene’ hypothesis. Int. J. Obes. 2008, 32, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 30, 1111. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Mierzynski, R.; Dluski, D.; Darmochwal-Kolarz, D.; Poniedziałek-Czajkowska, E.; Leszczynska-Gorzelak, B.; Kimber-Trojnar, Z.; Agnieszka-Wankowicz, A.; Oleszczuk, J. Intra-uterine Growth Retardation as a Risk Factor of Postnatal Metabolic Disorders. Curr. Pharm. Biotechnol. 2016, 17, 587–596. [Google Scholar] [CrossRef]
- Morrison, J.L.; Duffield, J.A.; Muhlhausler, B.S.; Gentili, S.; McMillen, I.C. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr. Nephrol. 2010, 25, 669–677. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Developmental programming of health and disease. Proc. Nutr. Soc. 2006, 65, 97–105. [Google Scholar] [CrossRef]
- Joss-Moore, L.A.; Lane, R.H. The developmental origins of adult disease. Curr. Opin. Pediatr. 2009, 21, 230–234. [Google Scholar] [CrossRef]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.J. Tandem repeats mediating genetic plasticity in health and disease. Nat. Rev. Genet. 2018, 19, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Baccarelli, A. Environmental epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Sebert, S.P.; Hyatt, M.A.; Budge, H. Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 604–610. [Google Scholar] [CrossRef]
- Vu-Hong, T.A.; Durand, E.; Deghmoun, S.; Boutin, P.; Meyre, D.; Chevenne, D.; Czernichow, P.; Froguel, P.; Levy-Marchal, C. The INS VNTR locus does not associate with smallness for gestational age (SGA) but interacts with SGA to increase insulin resistance in young adults. J. Clin. Endocrinol. Metab. 2006, 91, 2437–2440. [Google Scholar] [CrossRef][Green Version]
- Park, J.H.; Stoffers, D.A.; Nicholls, R.D.; Simmons, R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Investig. 2008, 118, 2316–2324. [Google Scholar] [CrossRef]
- Blondeau, B.; Avril, I.; Duchene, B.; Bréant, B. Endocrine pancreas development is altered in foetuses from rats previously showing intrauterine growth retardation in response to malnutrition. Diabetologia 2022, 45, 394–401. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 2004, 20, 63–68. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef]
- McGarvey, S.T. Obesity in Samoans and a perspective on its etiology in Polynesians. Am. J. Clin. Nutr. 1991, 53, 1586S–1594S. [Google Scholar] [CrossRef]
- Gosling, A.L.; Buckley, H.R.; Matisoo-Smith, E.; Merriman, T.R. Pacific Populations, Metabolic Disease and ‘Just-So Stories’: A Critique of the ‘Thrifty Genotype’ Hypothesis in Oceania. Ann. Hum. Genet. 2015, 79, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Minster, R.L.; Hawley, N.L.; Su, C.T.; Sun, G.; Kershaw, E.E.; Cheng, H.; Buhule, O.D.; Lin, J.; Reupena, M.A.S.; Viali, S.I.; et al. A thrifty variant in CREBRF strong influences body mass index in Samoans. Nat. Genet. 2016, 48, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Tiebe, M.; Lutz, M.; De La Garza, A.; Buechling, T.; Boutros, M.; Teleman, A.A. REPTOR and REPTOR-BP Regulate Organismal Metabolism and Transcription Downstream of TORC1. Dev. Cell 2015, 33, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Stocker, H. Stress relief downstream of TOR. Dev. Cell 2015, 33, 245–246. [Google Scholar] [CrossRef][Green Version]
- Fu, H.; Hawley, N.L.; Carlson, J.C.; Russell, E.M.; Pomer, A.; Cheng, H.; Naseri, T.; Reupena, M.S.; Deka, R.; Choy, C.C.; et al. The missense variant, rs373863828, in CREBRF plays a role in longitudinal changes in body mass index in Samoans. Obes. Res. Clin. Pract. 2022, 3, 220–227. [Google Scholar] [CrossRef]
- Arslanian, K.J.; Fidow, U.T.; Atanoa, T.; Unasa-Apelu, F.; Naseri, T.; Wetzel, A.I.; Pomer, A.; Duckham, R.L.; McGarvey, S.T.; Strayer, J.A.; et al. A missense variant in CREBRF, rs373863828, is associated with fat-free mass, not fat mass in Samoan infants. Int. J. Obes. 2005, 45, 45–55. [Google Scholar] [CrossRef]
- Neel, J.V. The “thrifty genotype” in 1998. Nutr. Rev. 1999, 57 Pt 2, S2–S9. [Google Scholar] [CrossRef]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Debal, D.; D’Udine, B.; Foley, R.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef]
- Dulloo, A.G. Regulation of fat storage via suppressed thermogenesis: A thrifty phenotype that predisposes individuals with catch-up growth to insulin resistance and obesity. Horm. Res. 2006, 65 (Suppl. 3), 90–97. [Google Scholar] [CrossRef]
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739, Erratum in Pediatr. Res. 1996, 39, 175. [Google Scholar] [CrossRef]
- Karlberg, J.; Albertsson-Wikland, K.; Kwan, C.W.; Chan, F.Y. Early spontaneous catch-up growth. J. Pediatr. Endocrinol. Metab. 2002, 15 (Suppl. 5), 1243–1255. [Google Scholar] [PubMed]
- Argente, J.; Mehls, O.; Barrios, V. Growth and body composition in very young SGA children. Pediatr. Nephrol. 2010, 25, 679–685. [Google Scholar] [CrossRef]
- Beltrand, J.; Nicolescu, R.; Kaguelidou, F.; Verkauskiene, R.; Sibony, O.; Chevenne, D.; Claris, O.; Lévy-Marchal, C. Catch-up growth following fetal growth restriction promotes rapid restoration of fat mass but without metabolic consequences at one year of age. PLoS ONE 2009, 4, e5343. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.L.; Overpeck, M.D.; McGlynn, A.; Kuczmarski, R.J.; Maurer, K.R.; Davis, W.W. Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics 1999, 104, e33. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Joseph, D.V.; Bankart, M.J.; Petersen, S.A.; Wailoo, M.P. Fetal growth restriction: Relation to growth and obesity at the age of 9 years. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F479–F483. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Forsen, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef]
- Cianfarani, S.; Germani, D.; Branca, F. Low birth weight and adult insulin resistance: The ‘catch-up growth’ hypothesis. Arch. Dis. Child. Fetal Neonatal 1999, 81, F71–F73. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Forsen, T.; Tuomilehto, J.; Winter, P.D.; Osmond, C.; Barker, D.J. Catch-up growth in childhood and death from coronary heart disease: Longitudinal study. BMJ 1999, 318, 427–431. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middleincome countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Popkin, B.M.; Richards, M.K.; Monteiro, C.A. Stunting is associated with overweight in children of four nations that are undergoing nutrition transition. J. Nutr. 1996, 126, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, A.L.; Martins, P.; Hoffman, D.; Roberts, S.B. The link between childhood undernutrition and risk of chronic diseases in adulthood: A case study of Brazil. Nutr. Rev. 2003, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Barros, F.C. The catch-up dilemma—Relevance of Leitch’s ‘low-high’ pig to child growth in developing countries. Int. J. Epidemiol. 2001, 30, 217–220. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Jacquet, J.; Seydoux, J.; Montani, J.P. The thrifty ‘catch-up fat’ phenotype: Its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int. J. Obes. 2006, 30 (Suppl. 4), S23–S35. [Google Scholar] [CrossRef]
- Manapurath, R.; Gadapani, B.; Pereira-da-Silva, L. Body Composition of Infants Born with Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1085. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, M.; Rotteveel, J.; Lafeber, H.N.; van Weissenbruch, M.M. Lean mass and fat mass accretion between term age and 6 months post-term in growth-restricted preterm infants. Eur. J. Clin. Nutr. 2014, 68, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Calek, E.; Binder, J.; Palmrich, P.; Eibensteiner, F.; Thajer, A.; Kainz, T.; Harreiter, K.; Berger, A.; Binder, C. Effects of Intrauterine Growth Restriction (IUGR) on Growth and Body Composition Compared to Constitutionally Small Infants. Nutrients 2023, 15, 4158. [Google Scholar] [CrossRef]
- Colle, E.; Schiff, D.; Andrew, G.; Bauer, C.B.; Fitzhardinge, P. Insulin responses during catch-up growth of infants who were small for gestational age. Pediatrics 1976, 57, 363–371. [Google Scholar] [CrossRef]
- Ezzahir, N.; Alberti, C.; Deghmoun, S.; Zaccaria, I.; Czernichow, P.; Lévy-Marchal, C.; Jaquet, D. Time course of catch-up in adiposity influences adult anthropometry in individuals who were born small for gestational age. Pediatr. Res. 2005, 58, 243–247. [Google Scholar] [CrossRef]
- Mericq, V.; Ong, K.K.; Bazaes, R.; Peña, V.; Avila, A.; Salazar, T.; Soto, N.; Iñiguez, G.; Dunger, D.B. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 2005, 48, 2609–2614. [Google Scholar] [CrossRef]
- Soto, N.; Bazaes, R.A.; Pena, V.; Salazar, T.; Avila, A.; Iñiguez, G.; Ong, K.K.; Dunger, D.B.; Mericq, M.V. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: Results from a prospective cohort. J. Clin. Endocrinol. Metab. 2003, 88, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Selz, R.; Tappy, L.; Theintz, G.E. Metabolism of oral glucose in children born small for gestational age: Evidence for an impaired whole body glucose oxidation. Metabolism 2004, 53, 847–8851. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Ong, K.; Dunger, D.B.; de Zegher, F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 2006, 91, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Balomenou, F.; Rallis, D.; Evangelou, F.; Zisi, A.; Balomenou, K.; Tsekas, N.; Tzoufi, M.; Siomou, E.; Giapros, V. Is small for gestational age status independently correlated with body composition during childhood? Eur. J. Pediatr. 2023, 182, 661–668. [Google Scholar] [CrossRef]
- Labayen, I.; Moreno, L.A.; Blay, M.G.; Blay, V.A.; Mesana, M.I.; González-Gross, M.; Bueno, G.; Sarría, A.; Bueno, M. Early programming of body composition and fat distribution in adolescents. J. Nutr. 2006, 136, 147–152. [Google Scholar] [CrossRef]
- Rasmussen, E.L.; Malis, C.; Jensen, C.B.; Storgaard, H.; Poulsen, P.; Pilgaard, K.; Schou, J.H.; Madsbad, S.; Astrup, A.; Vaag, A. Altered fat tissue distribution in young adult men who had low birth weight. Diabetes Care 2005, 28, 151–153. [Google Scholar] [CrossRef]
- Rolfe Ede, L.; Loos, R.J.; Druet, C.; Stolk, R.P.; Ekelund, U.; Griffin, S.J.; Forouhi, N.G.; Wareham, N.J.; Ong, K.K. Association between birth weight and visceral fat in adults. Am. J. Clin. Nutr. 2010, 92, 347–352. [Google Scholar] [CrossRef]
- Modi, N.; Thomas, E.L.; Harrington, T.A.; Uthaya, S.; Dore, C.J.; Bell, J.D. Determinants of adiposity during preweaning postnatal growth in appropriately grown and growth-restricted term infants. Pediatr. Res. 2006, 60, 345–348. [Google Scholar] [CrossRef]
- Vaag, A.; Jensen, C.B.; Poulsen, P. Metabolic aspects of insulin resistance in individuals born small for gestational age. Horm. Res. 2006, 65 (Suppl. 3), 137–143. [Google Scholar] [CrossRef]
- Byberg, L.; McKeigue, P.M.; Zethelius, B.; Lithell, H.O. Birth weight and the insulin resistance syndrome: Association of low birth weight with truncal obesity and raised plasminogen activator inhibitor-1 but not with abdominal obesity or plasma lipid disturbances. Diabetologia 2000, 43, 54–60. [Google Scholar] [CrossRef]
- Papandreou, D.; Karavolias, C.; Arvaniti, F.; Kafeza, E.; Sidawi, F. Fasting Ghrelin Levels Are Decreased in Obese Subjects and Are Significantly Related With Insulin Resistance and Body Mass Index. Open Access Maced. J. Med. Sci. 2017, 5, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Monti, V.; Carlson, J.J.; Hunt, S.C.; Adams, T.D. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J. Am. Diet. Assoc. 2006, 106, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Nogueiras, R.; Broglio, F.; D’Alessio, D.; Tschop, M.H. Ghrelin, obesity and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 705–712. [Google Scholar] [CrossRef]
- James, R.J.; Drewett, R.F.; Cheetham, T.D. Low cord ghrelin levels in term infants are associated with slow weight gain over the first 3 months of life. J. Clin. Endocrinol. Metab. 2004, 89, 3847–3850. [Google Scholar] [CrossRef][Green Version]
- Bucur-Grosu, M.L.; Avasiloaiei, A.; Moscalu, M.; Dimitriu, D.C.; Păduraru, L.; Stamatin, M. Desacylated ghrelin and leptin in the cord blood of small-for-gestational-age newborns with intrauterine growth restriction. Acta Endocrinol. 2019, 15, 305–310. [Google Scholar]
- Barazzoni, R.; Zanetti, M.; Ferreira, C.; Vinci, P.; Pirulli, A.; Mucci, M.; Dore, F.; Fonda, M.; Ciocchi, B.; Cattin, L.; et al. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3935–3940. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Gil, M.J.; Becerril, S.; Sáinz, N.; Silva, C.; Salvador, J.; Colina, I.; Frühbeck, G. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int. J. Obes. 2009, 33, 541–552. [Google Scholar] [CrossRef]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–66. [Google Scholar] [CrossRef]
- Brennan, A.M.; Mantzoros, C.S. Drug Insight: The role of leptin in human physiology and pathophysiology–emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef]
- Bellone, S.; Prodam, F.; Savastio, S.; De Rienzo, F.; Demarchi, I.; Trovato, L.; Petri, A.; Rapa, A.; Aimaretti, G.; Bona, G. Acylated and unacylated ghrelin levels in normal weight and obese children: Influence of puberty and relationship with insulin, leptin and adiponectin levels. J. Endocrinol. Investig. 2012, 35, 191–197. [Google Scholar]
- Zhang, C.S.; Wang, L.X.; Wang, R.; Liu, Y.; Song, L.M.; Yuan, J.H.; Wang, B.; Dong, J. The Correlation Between Circulating Ghrelin and Insulin Resistance in Obesity: A Meta-Analysis. Front. Physiol. 2018, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Rambhojan, C.; Bouaziz-Amar, E.; Larifla, L.; Deloumeaux, J.; Clepier, J.; Plumasseau, J.; Lacorte, J.M.; Foucan, L. Ghrelin, adipokines, metabolic factors in relation with weight status in school-children and results of a 1-year lifestyle intervention program. Nutr. Metab. 2015, 12, 43. [Google Scholar] [CrossRef]
- Lewis, K.A.; Brown, S.A. Searching for Evidence of an Anti-Inflammatory Diet in Children: A Systematic Review of Randomized Controlled Trials for Pediatric Obesity Interventions With a Focus on Leptin, Ghrelin, and Adiponectin. Biol. Res. Nurs. 2017, 19, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Hassink, S.G.; Sheslow, D.V.; de Lancey, E.; Opentanova, I.; Considine, R.V.; Caro, J.F. Serum leptin in children with obesity: Relationship to gender and development. Pediatrics 1996, 98, 201–203. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Rifas-Shiman, S.L.; Williams, C.J.; Fargnoli, J.L.; Kelesidis, T.; Gillman, M.W. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: A prospective cohort study. Pediatrics 2009, 123, 682–689. [Google Scholar] [CrossRef]
- Stefaniak, M.; Dmoch-Gajzlerska, E. Maternal Serum and Cord Blood Leptin Concentrations at Delivery in Normal Pregnancies and in Pregnancies Complicated by Intrauterine Growth Restriction. Obes. Facts 2022, 15, 62–69. [Google Scholar] [CrossRef]
- Jaquet, D.; Leger, J.; Tabone, M.D.; Czernichow, P.; Levy-Marchal, C. High serum leptin concentrations during catch-up growth of children born with intrauterine growth retardation. J. Clin. Endocrinol. Metab. 1999, 84, 1949–1953. [Google Scholar] [CrossRef]
- Martinez-Aguayo, A.; Capurro, T.; Pena, V.; Peña, V.; Iñiguez, G.; Hernández, M.I.; Avila, A.; Salazar, T.; Asenjo, S.; Mericq, V. Comparison of leptin levels, body composition and insulin sensitivity and secretion by OGTT in healthy, early pubertal girls born at either appropriate- or small-for-gestational age. Clin. Endocrinol. 2007, 67, 526–532. [Google Scholar] [CrossRef]
- Shigemura, N.; Ohta, R.; Kusakabe, Y.; Miura, H.; Hino, A.; Koyano, K.; Nakashima, K.; Ninomiya, Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 2004, 145, 839–847. [Google Scholar] [CrossRef]
- Achard, V.; Boullu-Ciocca, S.; Desbriére, R.; Grino, M. Perinatal programming of central obesity and the metabolic syndrome: Role of glucocorticoids. Metab. Syndr. Relat. Disord. 2006, 4, 129–137. [Google Scholar] [CrossRef]
- Heksch, R.; Kamboj, M.; Anglin, K.; Obrynba, K. Review of Prader-Willi syndrome: The endocrine approach. Transl. Pediatr. 2017, 6, 274–285. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Holland, A.J.; Butler, J.V.; Whittington, J.E. Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int. J. Obes. 2012, 36, 1564–1570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wieting, J.; Jahn, K.; Buchholz, V.; Lichtinghagen, R.; Deest-Gaubatz, S.; Bleich, S.; Eberlein, C.K.; Deest, M.; Frieling, H. Alteration of serum leptin and LEP/LEPR promoter methylation in Prader-Willi syndrome. Psychoneuroendocrinology 2022, 143, 105857. [Google Scholar] [CrossRef] [PubMed]
- Feigerlová, E.; Diene, G.; Conte-Auriol, F.; Molinas, C.; Gennero, I.; Salles, J.-P.; Arnaud, C.; Tauber, M. Hyperghrelinemia precedes obesity in Prader–Willi syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 2800–2805. [Google Scholar] [CrossRef]
- Crinò, A.; Fintini, D.; Bocchini, S.; Grugni, G. Obesity management in Prader–Willi syndrome: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 579–593. [Google Scholar] [CrossRef]
- Madeo, S.F.; Zagaroli, L.; Vandelli, S.; Calcaterra, V.; Crinò, A.; De Sanctis, L.; Faienza, M.F.; Fintini, D.; Guazzarotti, L.; Licenziati, M.R.; et al. Endocrine features of Prader-Willi syndrome: A narrative review focusing on genotype-phenotype correlation. Front. Endocrinol. 2024, 15, 1382583. [Google Scholar] [CrossRef]
- Wüst, S.; Entringer, S.; Federenko, I.S.; Schlotz, W.; Hellhammer, D.H. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology 2005, 30, 591–598. [Google Scholar] [CrossRef]
- Piazza, P.V.; Le Moal, M. Glucocorticoids as a biological substrate of reward: Physiological and pathophysiological implications. Brain Res. Brain Res. Rev. 1997, 25, 359–372. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Portella, A.K.; Silveira, P.P.; Bettiol, H.; Agranonik, M.; Silva, A.A.; Goldani, M.Z. Severe intrauterine growth restriction is associated with higher spontaneous carbohydrate intake in young women. Pediatr. Res. 2009, 65, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, K.; Guo, J.; Chen, J.; Chen, M.; Xie, Z.; Chen, P.; Wu, B.; Lin, N. Small for gestational age is a risk factor for thyroid dysfunction in preterm newborns. BMC Pediatr. 2020, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.; Laura, F.; Sara, N.; Salvatore, G. Thyroid function in small for gestational age newborns: A review. J. Clin. Res. Pediatr. Endocrinol. 2013, 5 (Suppl. 1), 2–7. [Google Scholar] [PubMed]
- González, I.F.; Maeso-Méndez, S.; Miranda, A.S.; Del Hoyo Moracho, M.; Blázquez, I.L.; López, I.D. Differences in thyroid function between small for gestational age and those with appropriate weight for gestational age. Is thyroid function normal in small for gestational age newborns? An. Pediatr. 2021, 95, 330–335. [Google Scholar] [CrossRef]
- Cianfarani, S.; Maiorana, A.; Geremia, C.; Scirè, G.; Spadoni, G.L.; Germani, D. Blood glucose concentrations are reduced in children born small for gestational age (SGA), and thyroid-stimulating hormone levels are increased in SGA with blunted postnatal catch-up growth. J. Clin. Endocrinol. Metab. 2003, 88, 2699–2705. [Google Scholar] [CrossRef]
- Uchiyama, A.; Watanabe, H.; Nakanishi, H.; Totsu, S. Small for gestational age is a risk factor for the development of delayed thyrotropin elevation in infants weighing less than 2000 g. Clin. Endocrinol. 2018, 89, 431–436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam-Raileanu, A.; Miron, I.; Lupu, A.; Bozomitu, L.; Sasaran, M.O.; Russu, R.; Rosu, S.T.; Nedelcu, A.H.; Salaru, D.L.; Baciu, G.; et al. Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes—Underlying Mechanisms and Clinical Implications. Nutrients 2025, 17, 555. https://doi.org/10.3390/nu17030555
Adam-Raileanu A, Miron I, Lupu A, Bozomitu L, Sasaran MO, Russu R, Rosu ST, Nedelcu AH, Salaru DL, Baciu G, et al. Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes—Underlying Mechanisms and Clinical Implications. Nutrients. 2025; 17(3):555. https://doi.org/10.3390/nu17030555
Chicago/Turabian StyleAdam-Raileanu, Anca, Ingrith Miron, Ancuta Lupu, Laura Bozomitu, Maria Oana Sasaran, Ruxandra Russu, Solange Tamara Rosu, Alin Horatiu Nedelcu, Delia Lidia Salaru, Ginel Baciu, and et al. 2025. "Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes—Underlying Mechanisms and Clinical Implications" Nutrients 17, no. 3: 555. https://doi.org/10.3390/nu17030555
APA StyleAdam-Raileanu, A., Miron, I., Lupu, A., Bozomitu, L., Sasaran, M. O., Russu, R., Rosu, S. T., Nedelcu, A. H., Salaru, D. L., Baciu, G., Mihai, C. M., Chisnoiu, T., Beser, O. F., & Lupu, V. V. (2025). Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes—Underlying Mechanisms and Clinical Implications. Nutrients, 17(3), 555. https://doi.org/10.3390/nu17030555






