Abstract
Background: Post-COVID-19 irritable bowel syndrome (PCIBS) is a frequent finding and is frequently associated with enteral dysbiosis. This pilot study compared the effects of extracts from curcuma and boswellia on PCIBS and irritable bowel syndrome (IBS) in individuals who had never had a COVID-19 infection (controls). Methods: A total of 16 subjects with PCIBS and 28 controls with evidence of IBS gastrointestinal symptoms and with enteral dysbiosis were recruited and supplemented for 30 days with sunflower-lecithin-based formulations of extracts of Curcuma longa (500 mg) and Boswellia serrata (150 mg) b.i.d. and with low-FODMAP diet. Abdominal bloating, abdominal pain, enteral dysbiosis (as increased urinary indican), and the global assessment of efficacy (GAE) were evaluated at the end of the study. Results: In both cohorts, intra-cohort changes revealed a statistically significant (p < 0.05) reduction in bloating and abdominal pain. The GAE showed similar and relevant satisfactory rates in both groups. On the contrary, urinary indican values showed a significant decrease only in the IBS group. Conclusions: Supplementation with Curcuma and Boswellia has favorable effects on abdominal bloating and abdominal pain of subjects with PCIBS and with IBS, while enteral dysbiosis is significantly decreased only in patients with IBS. Additional studies are needed to confirm these preliminary findings and to clarify the reasons for the persistence of dysbiosis in PCIBS.
1. Introduction
COVID-19 infection is a disease mainly associated with severe acute respiratory failure. This infectious disease began in China in 2019 and has spread throughout the world in subsequent years. The World Health Organization stated that at the end of March 2024, over 774 million people had had this disease and more than seven million had died [1]
It has been demonstrated that SARS-CoV-2 may be found in the gastrointestinal tract (GIT) and that the angiotensin-converting enzyme 2 (ACE2) receptor is highly expressed throughout the GIT. Consequentially, SARS-CoV-2 may enter GIT cells via ACE2 receptors and damage the GIT organs [2]. SARS-CoV-2 was observed in the colonic tissues and feces of patients with COVID-19 [3]. In a meta-analysis of 60 studies comprising 4243 patients, the pooled prevalence of all gastrointestinal symptoms was 17.6% [4].
Weng and colleagues evaluated the long-term GIT pathological consequences of 117 patients hospitalized for COVID-19 infection [3]. Fifty-two (44%) patients reported gastrointestinal symptoms 90 days after discharge. The most frequent gastrointestinal symptoms were loss of appetite, nausea (18%), acid reflux (18%), diarrhea (15%), abdominal bloating (14%), belching (10%), vomiting (9%), and abdominal pain (7%). Various researchers, after having observed the appearance of new gastrointestinal symptoms at 6 months following COVID-19 infection, hypothesized the presence of a post-COVID-19 irritable bowel syndrome (PCIBS) [5,6].
A retrospective review of the clinical data of 147 COVID-19 patients found new GI symptoms in 16% of them, at a median follow-up time of 106 days [5]. The same study showed that 40% of the COVID-19 survivors reported new GI symptoms at 6 months [5]. These results have been confirmed by an international epidemiologic study on 614 COVID-19 patients, showing that at the 12-month follow-up, patients with COVID-19 had significantly higher rates of IBS than patients in the control group [7]. Moreover, gut dysbiosis has been described for at least 6 months in patients with PCIBS [8].
Recent reviews have evaluated the effects of various dietary supplements on gastrointestinal disorders, intestinal dysbiosis, and related symptoms [9,10,11]. Our research group demonstrated the presence of enteral dysbiosis in IBS patients with abdominal bloating as a prevalent finding and reported that supplementation with Curcuma and Boswellia extracts, as sunflower-lecithin-based formulations at different dosages, was efficacious in significantly reducing both the dysbiosis and abdominal bloating when combined with a low-FODMAP diet (LFD). This effect was significantly better when compared with a LFD alone [12].
These beneficial effects might result from the anti-inflammatory and gut antimicrobial modulation properties [12,13,14,15,16,17,18] exerted by Boswellia and Curcuma extracts [12,19,20]
Based on these findings, the purpose of this pilot study was to compare this cohort with subjects with IBS who had not previously shown signs of COVID-19 infection and to confirm the effects of the combination of Curcuma and Boswellia, both of which were formulated separately in phospholipids (as MerivaTM and CasperomeTM), along with an LFD regimen, in subjects with post COVID-19 infection with IBS-like symptoms, with small-bowel dysbiosis and abdominal bloating being the most relevant symptoms [21,22].
2. Materials and Methods
2.1. Study Design
This was a 30-day-supplementation case study observation in which two different groups of participants were compared, i.e., subjects with long COVID and IBS-like symptoms (the PCIBS group) and subjects with a diagnosis of IBS without previous COVID-19 infection (the control group). This two-cohort open-label study was conducted at the University of Pavia’s Department of Public Health in Italy. The study’s goal was to assess and contrast the safety and effectiveness of Curcuma and Boswellia extracts, prepared separately in phospholipids, in individuals with or without long-term COVID-19 infection with symptoms of IBS, abdominal bloating, and enteral dysbiosis. After receiving approval from the local independent ethics committee, the study was carried out in compliance with the ICH Guidelines for Good Clinical Practice and the Declaration of Helsinki (Ethic code number: 0912/09052020). Written informed consent was obtained from each participant. The study was conducted from 1 September 2021 to 10 June 2022.
2.2. Population
Male and female subjects, aged 18–75 years, with a diagnosis of PCIBS, 60–120 days after the end of their COVID-19 infection, were recruited, together with subjects of the same age with IBS without previous COVID-19 infection (the control group). The inclusion criteria for both PCIBS and IBS (Control) groups were as follows: (1) age: 18–75 years, male/female; (2) evidence of functional abdominal bloating/distention (FAB/D)-type IBS, according to Lacy et al. [11]; (3) presence of enteral dysbiosis, defined by the increase in urinary indican values with a normal skatole urinary concentration [12,23,24]; (4) absence of gastroenterological treatments in the last 15 days before starting the therapeutic intervention, except antispasmodics and anxiolytics.
The exclusion criteria for both PCIBS and controls were as follows: (1) normal urinary indican values or increased urinary skatole values; (2) subjects already on a LFD or other dietary restrictions, such as a gluten-free diet or lactose-free diet, within the past 6 months; (3) insulin-dependent diabetes; (4) known history of microscopic colitis, inflammatory bowel illness, diverticular disease, or celiac disease; (5) previous cholecystectomy or small-bowel or colonic surgery; (6) severe vomiting or bloody diarrhea; (7) hepatic disease (defined as altered values of liver function tests) or severe renal disease (defined as serum creatinine > 1.5 mg/dL).
2.3. Supplementation and Concomitant Medications
All subjects (in the PCIBS and control groups) included in the study received film-coated tablets twice daily for 30 days, each containing a combination of 500 mg of Curcuma longa L. as a sunflower-lecithin-based formulation (Meriva™) and 150 mg of Boswellia serrata extract as a sunflower-lecithin-based formulation (Casperome™), which were kindly provided by Indena S.p.A., Milan, Italy. Meriva is a food-grade lecithin formulation of curcumin in 500 mg film-coated tablets, containing a standardized amount of 100 mg of highly bioavailable curcuminoids [21]. Casperome is a delivery form of a highly standardized Boswellia serrata extract and soy lecithin in a 1:1 ratio. [22]. The choice of dose was based on previous clinical experiences with Curcuma and Boswellia extracts and the daily suggested dosage of each single botanical ingredient [12,25].
The ratio of the amount of supplement consumed (as shown by the pills that were returned) to the anticipated consumption for each participant throughout the actual supplementation period was used to estimate the supplementation compliance. All participants (in the PCIBS and control groups) received dietary guidance from the same dietician regarding a lLFD [26]. Concurrent administration of any other medications for the treatment of digestive disorders that might influence the results or interfere with the study supplementation was prohibited. These medications included anxiolytics, short-acting analgesics (like paracetamol), and short-acting spasmolytics (like butylscopolaminium bromide, dihydrate phloroglucinol, and derivatives).
2.4. Clinical Evaluation
On the questionnaire, participants were asked whether they felt “bloated/uncomfortably full”. There were four options to choose from: none (symptom did not occur) (score: 0); mild (symptom occurred but did not interfere with usual activities) (score: 1); moderate (occurrence of symptom somewhat interfered with usual activities) (score: 2); or severe (occurrence of symptom resulted in an inability to perform usual activities) (score: 3) [27]. An established visual analog scale for measuring pain was used to determine the level of the abdominal pain (0 being “no pain” and 10 being “most severe pain”) [28,29].
Urinary indican and skatole levels were measured for each patient seven days before study participation and at the conclusion of the study to determine intestinal dysbiosis (29). Skatole and indican levels in the urine were regarded as normal when they were less than 10 µg/L and 10 mg/L, respectively [30]. Every participant completed a global assessment of efficacy (GAE) using a 4-point scale at the conclusion of the study with 1 denoting “ineffective”; 2 being “moderately effective/slight improvement in complaints”; 3 denoting “effective/marked improvement in symptoms”; and 4 being “very effective/as good as no symptoms”, according to Kruis and colleagues [31,32]. Serum and urine samples were taken for safety monitoring at every appointment, and vital signs were examined. Analyses were conducted in the laboratory and compared to the usual ranges. During the 30-day supplementation period, participants were told to report any discomfort as soon as possible, as this was considered to be an adverse event. At the last appointment, adverse events were also examined.
2.5. Study Endpoints
The reduction in the severity of stomach bloating was the primary outcome. The following were secondary endpoints: (1) the change in urine indican levels; (2) the change in the intensity of stomach discomfort; and (3) the GAE, as determined by the participants at the conclusion of the research. Laboratory findings, vital signs, and adverse occurrences were all considered safety endpoints.
2.6. Statistical Analysis
Baseline data have been presented as the mean values ± standard deviation of the mean (SD) unless otherwise indicated. The normal distribution of the variables was checked using the Shapiro–Wilk test and using Q–Q graphs. Baseline differences in demographic and clinical characteristics between the two groups (PCIBS and control) were examined using independent t-tests.
The homogeneity of the variances was estimated by using Levene’s test. A one-factor covariance ANCOVA test was used for continuous variables to determine differences between the two cohorts.
The same model was used to compare changes in the variables regarding the effects between the two cohorts: the effect was quantified by no infection (control) minus infection (PCIBS), adjusting for age and gender. Correlations between changes pre- and post-study in the PCIBS and control groups have been estimated by the Pearson’s correlation coefficient when the assumptions of normality were met and by the Spearman’s correlation coefficient when the assumptions of normality were not met. A p < 0.05 value was considered significant. The Statistical Package for the Social Sciences version 28 software was used to perform the statistical analysis (SPSS Inc., Chicago, IL, USA).
3. Results
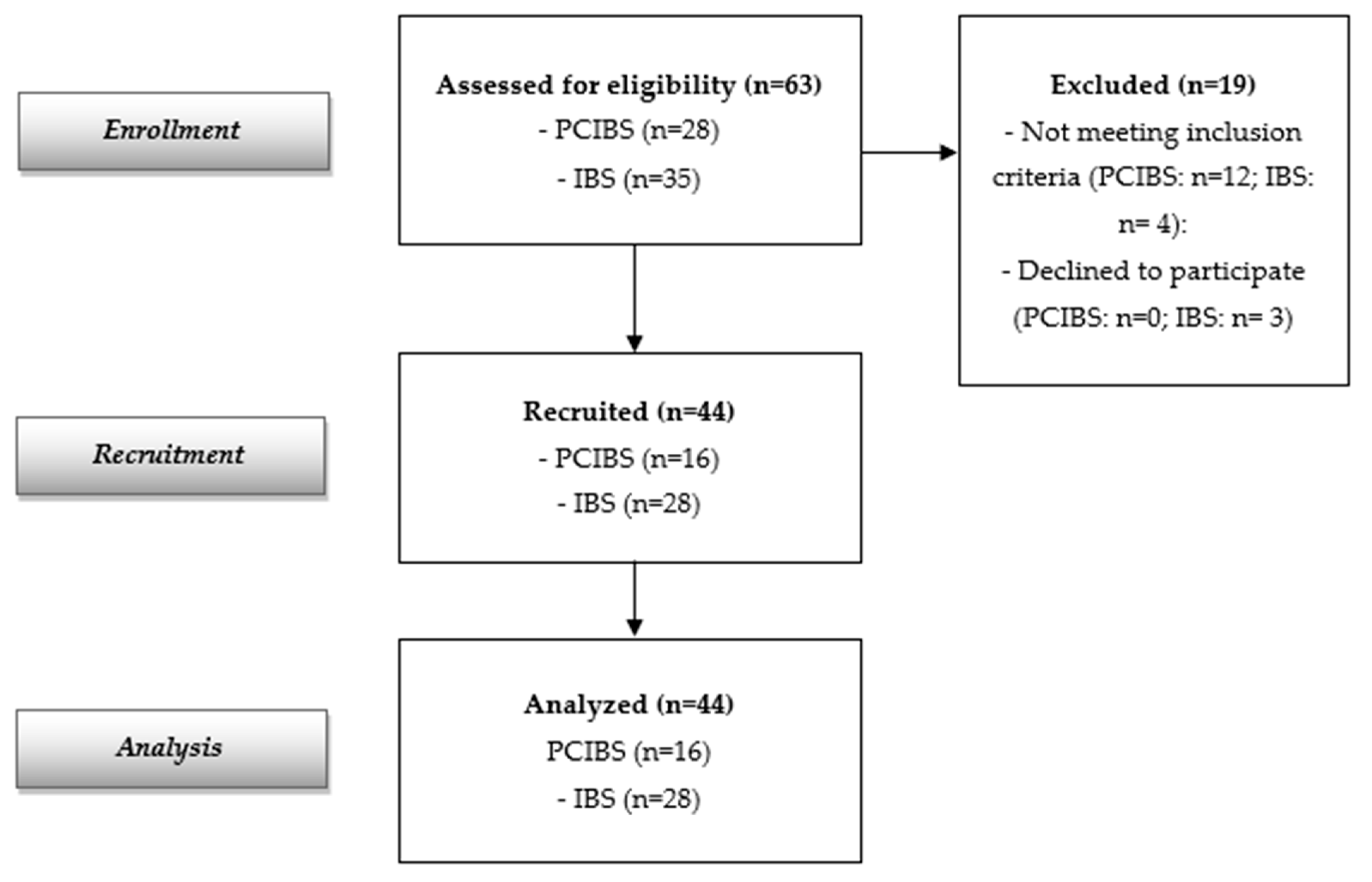
The flow diagram reported in Figure 1 indicates 63 screened participants (28 with PCIBS and 35 subjects with IBS without previous COVID-19 infection). Forty-four subjects were recruited and analyzed (16 with PCIBS and 28 subjects with IBS without previous COVID-19 infection, considered as the control group). The recruited population at baseline is described in Table 1. The data show a total of 44 recruited adults, 28 females and 16 males (mean age: 44.59 ± 16.20 years); 16 were in the PCIBS group and 28 were in the control group. All of them completed the study. Baseline demographic and clinical characteristics were similar in both groups. Only the abdominal bloating score differed at baseline between the two cohorts, although this was the major symptom in both groups.
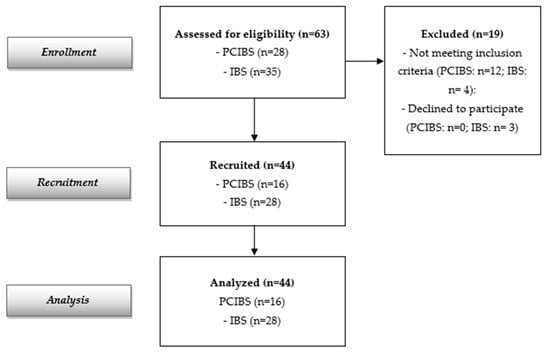
Figure 1.
Flow diagram of the study.

Table 1.
Descriptive statistics of the sample at baseline.
No adverse effects related to the supplementation were reported during the study.
Participants in the control group had less diarrhea (39.3%) compared with the PCIBS group at baseline (62.5%). Regarding the incidence of constipation, the two cohorts showed similar data (control group: 35.7% versus PCIBS group: 25%), and there has been no evidence of a statistically significant correlation between the two groups or of constipation or diarrhea.
The mean difference changes in the primary and secondary outcomes after supplementation are reported in Table 2.

Table 2.
Mean difference changes in primary and secondary outcomes at the end of study.
Intra-cohort changes showed a statistically significant (p < 0.05) decrease in bloating in both cohorts. Also, the abdominal pain decreased significantly in both groups. On the contrary, urinary indican values showed a significant decrease only in the control group (subjects with IBS without previous COVID-19 infection).
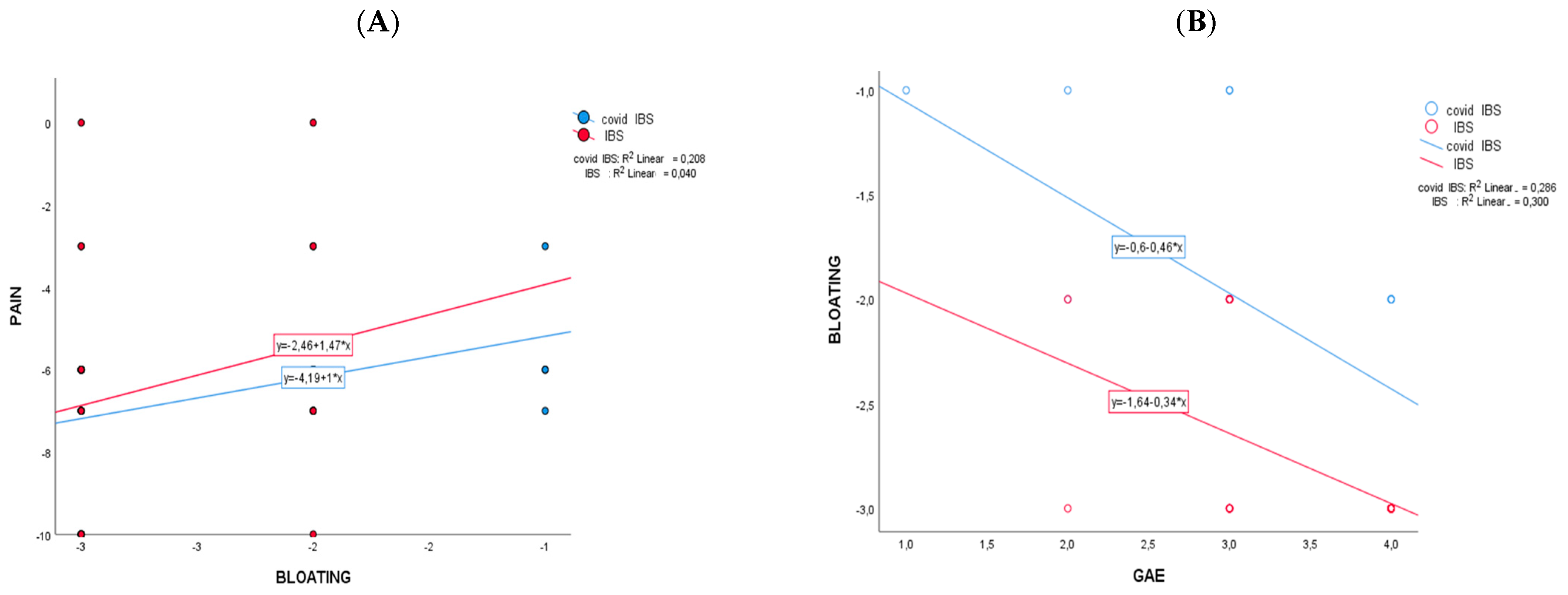
The comparison between the two cohorts (net effect in control minus PCIBS group) showed that the changes differ significantly for bloating (−0.899; CI95%: −1.270; −0.528; p < 0.001) and for urinary indican values (−44.090; CI95%: −74.551; −13.629; p = 0.006). No significant changes between the cohorts have been recorded for abdominal pain (Table 2) and GAE (Table 3). After supplementation (Table 3), 81.3% of participants in the PCIBS group has a GAE equal to or higher than grade 3, as a sum of grade 3 (“effective/marked improvement in symptoms”) and grade 4 (“very effective/as good as no symptoms”); among the control group, the value was 92.8%. Figure 2 reports the results of the Pearson correlation analysis of mean difference changes (t1−t0) with the intervention regarding the statistically significant markers of Table 2. The correlation scatterplot showed that in the PCIBS cohort, the decrease over time in pain was significantly correlated with the decrease in abdominal bloating (r = 0.456; p > 0.01) (Figure 2A). A negative statistically significant association was recorded in both cohorts between a decrease in bloating over time and an increase in the GAE score at the end of the study (r2 = −0.547, control group; and r2 = −0.532, PCIBS group; p > 0.01) (Figure 2B).

Table 3.
Frequencies of GAE (global assessment of efficacy) at the end of study.
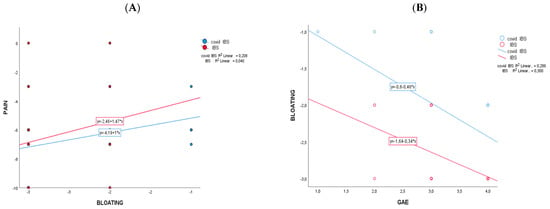
Figure 2.
Pearson correlations between Δ-changes (end vs. baseline) on the two cohorts that changed significantly. In (A) is pain versus bloating and in (B) is bloating versus GAE.
4. Discussion
This study demonstrates that supplementation with Curcuma and Boswellia extracts combined with a low-FODMAP diet is correlated with a significant decrease in bloating in subjects with long COVID and FAB/D-type IBS-like symptoms (PCIBS); this is similar to what was found in subjects with FAB/D-type IBS without previous COVID-19 viral infection [11]. These findings prove that supplementation of a rational premix combination of Curcuma phospholipids and Boswellia phospholipids is efficacious in achieving the primary endpoint of the study, that is, the decrease in abdominal bloating in subjects with FAB/D-type IBS, with or without previous COVID-19 infection.
In our trial, positive effects were also been found when abdominal pain was evaluated. A significant relief of abdominal pain was observed in the PCIBS and control groups (IBS without previous COVID-19 infection).
These results suggest that the combination of Curcuma and Boswellia extracts may be a promising supplement to treat abdominal bloating and abdominal pain in subjects with PCIBS and in subjects with IBS without previous COVID-19 infection.
A previous study of subjects with IBS without previous COVID-19 infection showed that these results are specifically due to the Curcuma and Boswellia phospholipid-based supplement [12]. In that clinical observation, the control group with IBS followed only an LFD regimen with significantly lower efficacy compared to the IBS group supplemented with Curcuma and Boswellia extracts in association with an LFD [12].
The favorable outcome of this rational botanical supplementation is confirmed by the participants’ global assessment of efficacy that showed a similarly positive outcome in both of the groups. At the end of the study, 81.3% of participants in the PCIBS group were classified as grade 3 (“effective/marked improvement in symptoms”) or grade 4 (“very effective/as good as no symptoms”) in terms of the GAE, and this result was similarly obtained by 92.8% of participants in the control group (subjects with IBS without previous COVID-19 infection).
A correlation analysis confirmed the relevant importance of treating abdominal bloating by showing that in both the cohorts, the decrease over time in pain was significantly correlated with the decrease in bloating. In addition, a statistically significant association was recorded in both cohorts between a decrease in bloating over time and an increase in the GAE score at the end of study. Additional studies are needed to confirm this preliminary observation and to evaluate the long-term effect of this treatments in subjects with IBS.
Post-infectious IBS is a well-known clinical finding. Stewart was the first to describe this phenomenon in 1950 [33]. In these cases, gastrointestinal symptoms may persist following clearance of an infecting intestinal pathogen. The COVID-19 pandemic has overwhelmed healthcare services since 2019 and acute gastrointestinal symptoms have been frequently found in addition to the usual respiratory problems [34]. Moreover, prospective studies showed the appearance and persistence of symptoms compatible with IBS diagnostic criteria various months after serological negativization of the infection [3,6,35].
A recent review hypothesized various mechanisms for the pathogenesis of post-acute-COVID-19 irritable bowel syndrome (PCIBS), including persistent inflammation, autoimmunity, viral antigen persistence, altered cytokine production, prior mental health conditions, maladaptive neuro-immune interactions, and alteration to the fecal microbiome [36]. Multiple evidences suggest that oxidative stress plays a critical role in the pathophysiology of COVID-19 infection and the long-COVID condition, and that after the acute phase of COVID-19 infection, the disease is dominated by immune–pathological pro-inflammatory elements [37,38]. The anti-inflammatory effect of both Curcuma and Boswellia extracts could at least, in part, explain the benefit of this supplementation in subjects with long-COVID-19 and IBS-like symptoms [13,39,40,41,42].
Various factors that promote epithelial barrier damage, intestinal inflammation, gut dysfunction, and intestinal dysbiosis (such as antibiotics and other pharmacological treatments of the acute phase of COVID-19 infection, gut–lung axis impairment, disease-related psychological stress, as well as the virus itself) could be involved in the pathogenic process of PCIBS [43].
As has previously been reported, Qin Liu and colleagues showed that gut dysbiosis persists for at least 6 months in patients with post-acute-COVID-19 syndrome [8]. The gut microbiomes of these patients are characterized by higher levels of Ruminococcus gnavus and Bacteroides vulgatus, and lower levels of Faecalibacterium prausnitzii. Butyrate-producing bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, showed the largest inverse correlations with post-acute-COVID-19 syndrome at 6 months [8]. Based on these observations, the possible presence of small-bowel dysbiosis was evaluated in the enrollment phase of this study and was confirmed in 24 out of 28 post-COVID-19 patients with IBS-like symptoms (85.7%). Supplementation with a combination of Curcuma phospholipids and Boswellia phospholipids produced a significant difference when the effect on urinary indican of subjects with PCIBS and subjects with IBS without previous COVID-19 infection were compared. As a matter of fact, a significant reduction in urinary indican, as the marker of small-bowel dysbiosis, was observed only in subjects with IBS without COVID-19 infection. Meanwhile subjects with PCIBS did not show significant urinary indican changes after supplementation. In addition, when the two cohorts were compared, the indican difference achieved a statistically significant value. These data are correlated with the previous results reported by Qi Su and colleagues, who showed that in subjects with post-acute-COVID-19 gastrointestinal symptoms, gut dysbiosis may linger beyond one year after SARS-CoV-2 clearance [44]. To date, there has been no clarity on the reason for this behavior. It was reported that Curcuma extracts favor beneficial bacterial strains in the gut microbiota [45]. Interestingly, two distinct phenomena that may be related to curcumin activity are produced by the interaction between curcumin and gut flora. Curcumin’s beneficial modulation of intestinal microflora and the gut microbiota’s biotransformation of curcumin serve as examples of these two phenomena [45]. In addition, Boswellia serrata resin has been added as a supplement to rabbit diets at different dosages to obtain changes in the fecal microbiota. Relevant changes were found in the cecal microbiota of rabbits treated with Boswellia serrata, with a significant reduction in bacterial counts and, in particular, a decrease in Salmonella enteritidis and Escherichia coli, compared to the untreated control group. These results could be due to the high polyphenol content of Boswellia serrata extracts and to the presence of boswellic acids, which have a powerful antimicrobial effect [46]. Therefore, both Curcuma longa and Boswellia serrata extracts may act favorably on gut microbiota and intestinal dysbiosis, as clearly demonstrated in the subjects with IBS without previous COVID-19 infection who were evaluated in this study. On the contrary, this did not occur in subjects with PCIBS, thus confirming the need of additional research on the pathogenesis and biological characteristics of PCIBS. Even though the intra-cohort analysis showed a significant reduction in bloating after supplementation in both cohorts, the effect of supplementation was more prominent in the control group as compared to the PCIBS cohort, and this could possibly be influenced by the persistence of dysbiosis in the latter group, as shown also by Meringer and Mehandru [36].
Phytosome™ technology is a food-grade delivery system in the form of a lecithin-based solid dispersion of botanical ingredients with the aim of improving their solubility in gastro-intestinal fluids and promoting their effectiveness. Bresciani et al. recently showed that the formulation of phytosomes significantly affected the biotransformation of curcuminoids because the fecal human microbiota fermented lecithin–curcuminoids, which resulted in the more effective production of curcuminoid catabolites [47]. In our study, the Phytosome technology has been used in both Curcuma and Boswellia extracts and this could have promoted the favorable clinical outcome, due to a potential benefit of the highly bioavailable extracts.
This study aimed to explore whether a shared intervention (Curcuma/Boswellia + a low-FODMAP diet) could alleviate overlapping symptoms (bloating, pain) in post-COVID-19 IBS (PCIBS) and traditional IBS, despite differing origins. While both groups showed symptomatic improvement, the lack of urinary indican reduction in people with PCIBS suggests divergent dysbiosis mechanisms compared to those with traditional IBS, implying that while anti-inflammatory effects may address shared symptoms, PCIBS may require etiology-specific strategies for dysbiosis. The comparison highlights the intervention’s potential for symptom relief across etiologies but underscores the need for further research to unravel the specific pathophysiology of PCIBS.
5. Limitations
Various limitations of our preliminary study need to be considered. One of the study’s weaknesses is the absence of a post-COVID-19 randomized control group with IBS-like symptoms who received a placebo and another group who received only an LFD. An additional limitation is that a group of patients with PCIBS with normal urinary indican values has not been considered. Moreover, none of the study groups’ adherence to an LFD has been examined. This is important mostly because the LFD is considered to be the first-line standard of care in the management of GI symptoms in IBS patients. However, further studies should possibly address those aspects.
6. Conclusions
In conclusion, this study shows positive effects of supplementation with Curcuma longa and Boswellia serrata extracts (as Curcuma phospholipids and Boswellia phospholipids) on the abdominal bloating and abdominal pain of subjects with post-acute-COVID-19 IBS-like symptoms. These results are similar to those observed in subjects with IBS without previous COVID-19 infection. In addition, the results of this investigation may indicate that small-bowel dysbiosis is a frequent finding in people with post-acute-COVID-19 infection and that it remains following supplementation with extracts of Curcuma longa and Boswellia serrata, but it drastically diminishes in participants with IBS without previous COVID-19 infection. Further research is required to validate these results, elucidate the pathophysiology of post-acute PCIBS, and assess the causes of the persistence of small-bowel dysbiosis following supplementation with extracts of Boswellia serrata and Curcuma longa.
Author Contributions
Conceptualization, A.G.; methodology, A.G. and MR.; software, S.P.; validation, AG. and M.R.; formal analysis S.P.; investigation, A.G.; resources, A.G. and M.R.; data curation, A.G. and C.G.; writing—original draft preparation, A.G.; writing—review and editing, A.G., M.R. and S.P.; visualization, M.R.; supervision, C.G. and G.C.B.; project administration, A.G.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice, following approval from the Local Independent Ethics Committee (Ethic code number: 0912/09052020, approved on 9 May 2020).
Informed Consent Statement
Written informed consent was obtained from the participants included in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data supporting this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the participants for participating in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Available online: https://www.who.int/health-topics/coronavirus (accessed on 30 March 2024).
- Kaafarani, H.M.A.; El Moheb, M.; Hwabejire, J.O.; Naar, L.; Christensen, M.A.; Breen, K.; Gaitanidis, A.; Alser, O.; Mashbari, H.; Bankhead-Kendall, B.; et al. Gastrointestinal Complications in Critically Ill Patients With COVID-19. Ann. Surg. 2020, 272, e61–e62. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Li, Y.; Li, J.; Shen, L.; Zhu, L.; Liang, Y.; Lin, X.; Jiao, N.; Cheng, S.; Huang, Y.; et al. Gastrointestinal Sequelae 90 Days after Discharge for COVID-19. Lancet Gastroenterol. Hepatol. 2021, 6, 344–346. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-Analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Blackett, J.W.; Li, J.; Jodorkovsky, D.; Freedberg, D.E. Prevalence and Risk Factors for Gastrointestinal Symptoms after Recovery from COVID-19. Neurogastroenterol. Motil. 2022, 34, e14251. [Google Scholar] [CrossRef]
- Cooney, J.; Appiahene, P.; Findlay, R.; Al-Hillawi, L.; Rafique, K.; Laband, W.; Shandro, B.; Poullis, A. COVID-19 Infection Causing Residual Gastrointestinal Symptoms—A Single UK Centre Case Series. Clin. Med. 2022, 22, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Cacciari, G.; Falangone, F.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Post COVID-19 Irritable Bowel Syndrome. Gut 2023, 72, 484–492. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Donato, K.; Bertelli, M. Dietary Supplements for Intestinal Inflammation. J. Prev. Med. Hyg. 2022, 63, E214–E220. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Li, L.; Liu, W.; Sun, M. A Brief Review of Nutraceutical Ingredients in Gastrointestinal Disorders: Evidence and Suggestions. Int. J. Mol. Sci. 2020, 21, 1822. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, A.; Riva, A.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Peroni, G.; Rondanelli, M. Beneficial Effects on Abdominal Bloating with an Innovative Food-Grade Formulation of Curcuma Longa and Boswellia Serrata Extracts in Subjects with Irritable Bowel Syndrome and Small Bowel Dysbiosis. Nutrients 2022, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.Z. Boswellia Serrata, a Potential Antiinflammatory Agent: An Overview. Indian. J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Maraghehpour, B.; Khayamzadeh, M.; Najafi, S.; Kharazifard, M. Traditionally Used Herbal Medicines with Antibacterial Effect on Aggegatibacter Actinomycetemcomitans: Boswellia Serrata and Nigella Sativa. J. Indian Soc. Periodontol. 2016, 20, 603–607. [Google Scholar] [CrossRef]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S.; Shah, B.A.; Taneja, S.C. Antistaphylococcal and Biofilm Inhibitory Activities of Acetyl-11-Keto-β-Boswellic Acid from Boswellia Serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-Inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial Effects of Curcumin: An in Vitro Minimum Inhibitory Concentration Study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin Reverses Impaired Hippocampal Neurogenesis and Increases Serotonin Receptor 1A MRNA and Brain-Derived Neurotrophic Factor Expression in Chronically Stressed Rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Li, J.; Wang, R.; Xie, X.; Yu, X.; Pan, J.; Xu, Y.; Zheng, L. The Effect of Curcumin on the Brain-Gut Axis in Rat Model of Irritable Bowel Syndrome: Involvement of 5-HT-Dependent Signaling. Metab. Brain Dis. 2015, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Riva, A.; Morazzoni, P.; Artaria, C.; Allegrini, P.; Meins, J.; Savio, D.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. A Single-Dose, Randomized, Cross-over, Two-Way, Open-Label Study for Comparing the Absorption of Boswellic Acids and Its Lecithin Formulation. Phytomedicine 2016, 23, 1375–1382. [Google Scholar] [CrossRef]
- Hendrikx, T.; Schnabl, B. Indoles: Metabolites Produced by Intestinal Bacteria Capable of Controlling Liver Disease Manifestation. J. Intern. Med. 2019, 286, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bralley, J.A. Clinical Applications of Urinary Organic Acids. Part 2. Dysbiosis Markers. Altern. Med. Rev. 2008, 13, 292–306. [Google Scholar] [PubMed]
- Giacosa, A.; Riva, A.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Gasparri, C.; Faliva, M.A.; Peroni, G.; Perna, S.; et al. Symptomatic Uncomplicated Diverticular Disease Management: An Innovative Food-Grade Formulation of Curcuma Longa and Boswellia Serrata Extracts. Drugs Context 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.W.; Juraschek, S.P.; Appel, L.J.; Miller, E.R.; Mueller, N.T. Effects of the DASH Diet and Sodium Intake on Bloating: Results From the DASH–Sodium Trial. Am. J. Gastroenterol. 2019, 114, 1109–1115. [Google Scholar] [CrossRef]
- El Sherif, F.A.; Othman, A.H.; Abd El-Rahman, A.M.; Taha, O. Effect of Adding Intrathecal Morphine to a Multimodal Analgesic Regimen for Postoperative Pain Management after Laparoscopic Bariatric Surgery: A Prospective, Double-Blind, Randomized Controlled Trial. Br. J. Pain 2016, 10, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, A.J.M. Treatment of symptomatic diverticular disease with high fibre diet. Lancet 1977, 309, 664–666. [Google Scholar] [CrossRef]
- Lombardi, F.; Fiasca, F.; Minelli, M.; Maio, D.; Mattei, A.; Vergallo, I.; Cifone, M.G.; Cinque, B.; Minelli, M. The Effects of Low-Nickel Diet Combined with Oral Administration of Selected Probiotics on Patients with Systemic Nickel Allergy Syndrome (SNAS) and Gut Dysbiosis. Nutrients 2020, 12, 1040. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E. Musosal Tumour Necrosis Factor α in Diverticular Disease of the Colon Is Overexpressed with Disease Severity. Color. Dis. 2012, 14, e258–e263. [Google Scholar] [CrossRef]
- Kruis, W.; Meier, E.; Schumacher, M.; Mickisch, O.; Greinwald, R.; Mueller, R. Randomised Clinical Trial: Mesalazine (Salofalk Granules) for Uncomplicated Diverticular Disease of the Colon—A Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2013, 37, 680–690. [Google Scholar] [CrossRef]
- Stewart, G.T. Post-Dysenteric Colitis. Br. Med. J. 1950, 1, 405–409. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Liao, H.; Marley, G.; Wu, D.; Tang, W. Epidemiologic, Clinical, and Laboratory Findings of the COVID-19 in the Current Pandemic: Systematic Review and Meta-Analysis. BMC Infect. Dis. 2020, 20, 640. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Meringer, H.; Mehandru, S. Gastrointestinal Post-Acute COVID-19 Syndrome. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 345–346. [Google Scholar] [CrossRef] [PubMed]
- García, L.F. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front. Immunol. 2020, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.J.; Crooks, C.; Naja, M.; Ledlie, A.; Goulden, B.; Liddle, T.; Khan, E.; Mehta, P.; Martin-Gutierrez, L.; Waddington, K.E.; et al. COVID-19-Associated Hyperinflammation and Escalation of Patient Care: A Retrospective Longitudinal Cohort Study. Lancet Rheumatol. 2020, 2, e594–e602. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.; Wahl, M. Pharmacology of Curcuma Longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Boswellic Acids in Chronic Inflammatory Diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Ponziani, F.R.; Bibbò, S.; Segal, J.P.; Cammarota, G.; Gasbarrini, A. COVID-19 as a Trigger of Irritable Bowel Syndrome: A Review of Potential Mechanisms. World J. Gastroenterol. 2021, 27, 7433–7445. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Lau, R.I.; Liu, Q.; Chan, F.K.L.; Ng, S.C. Post-Acute COVID-19 Syndrome and Gut Dysbiosis Linger beyond 1 Year after SARS-CoV-2 Clearance. Gut 2023, 72, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Ismail, I.E.; Abdelnour, S.A.; Shehata, S.A.; Abd El-Hack, M.E.; El-Edel, M.A.; Taha, A.E.; Schiavitto, M.; Tufarelli, V. Effect of Dietary Boswellia Serrata Resin on Growth Performance, Blood Biochemistry, and Cecal Microbiota of Growing Rabbits. Front. Vet. Sci. 2019, 6, 471. [Google Scholar] [CrossRef]
- Bresciani, L.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; Del Rio, D. The Effect of Formulation of Curcuminoids on Their Metabolism by Human Colonic Microbiota. Molecules 2020, 25, 940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).