Malnutrition Risk in Older Adults: Evaluating the Diagnostic Relevance of Serum Biomarkers: SIRT-1, CCK-8, Melatonin, and Total Antioxidant Capacity (TAC)
Abstract
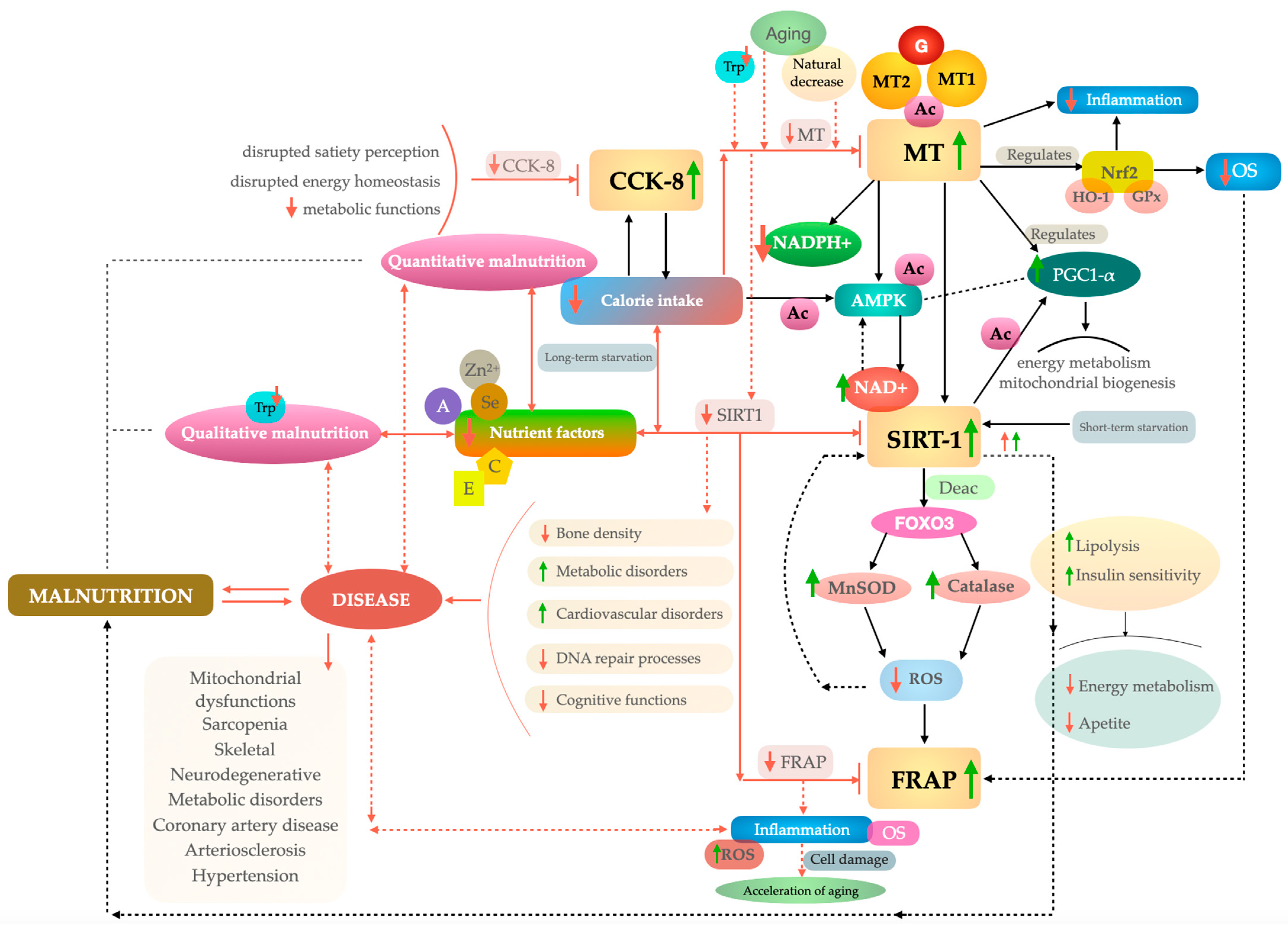
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Functional Assessment
2.3. Assessment of Frailty
- A subjective experience of diminished strength or fatigue;
- Feeling of fatigue;
- Weight loss (at least 5 kg in a year);
- Slowing gait speed;
- Low physical activity.
2.4. Determination of SIRT-1, Melatonin, and CCK-8 in Serum
2.5. Total Antioxidant Capacity (TAC)
2.6. Statistical Analysis
3. Results
3.1. Identification of Optimal Thresholds for SIRT-1, Cholecystokinin-8, Melatonin, and TAC in Assessing Malnutrition Risk
3.2. Estimation of Correlations Among Biochemical and Molecular Biomarkers
3.3. Evaluation of Assayed Serum Biomarkers Across Smoking Status and Clinical Comorbidities
3.4. Correlations Between Assayed Biomarkers and Functional and Body Composition Assessments
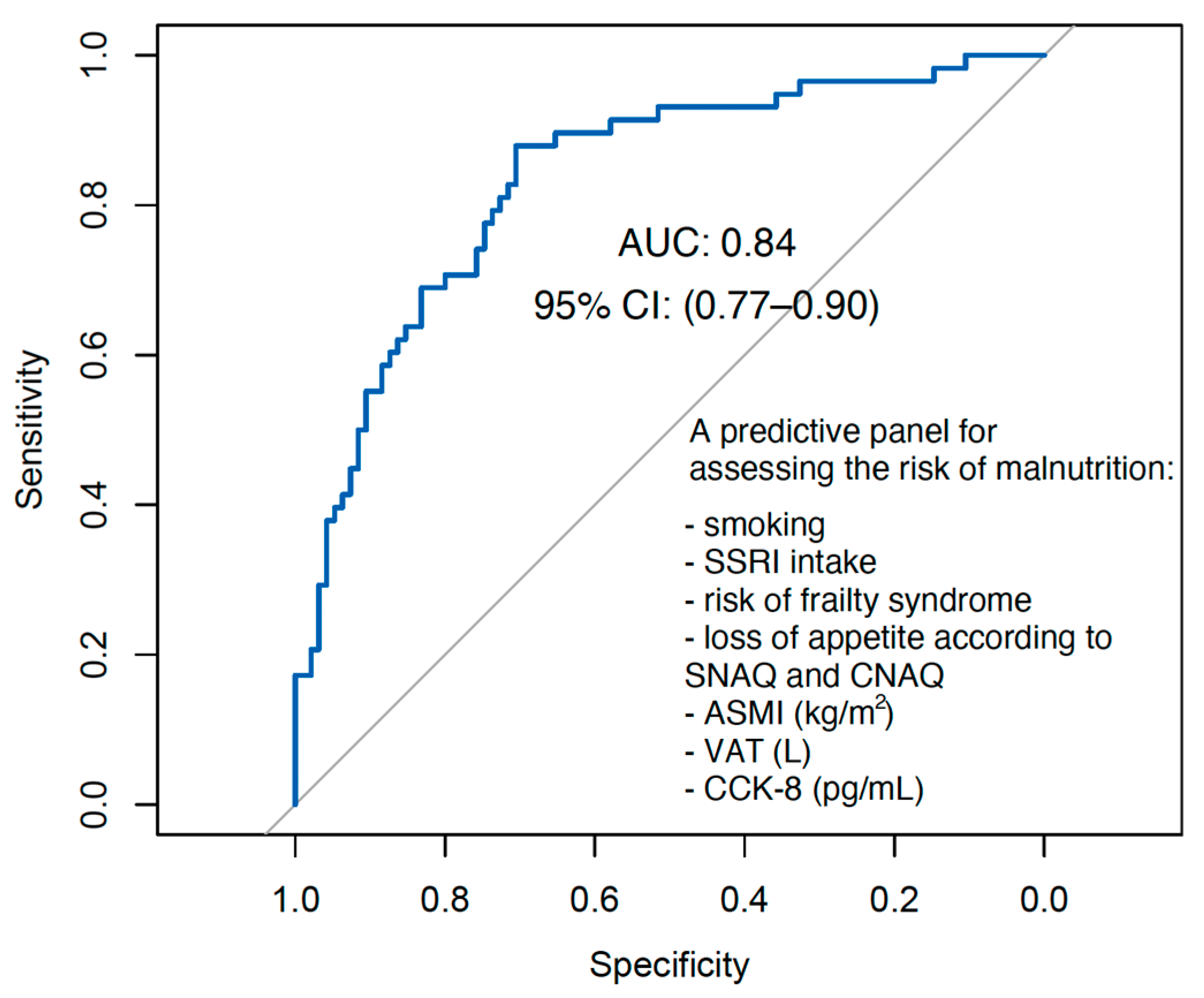
3.5. Identification of Determinants of Malnutrition Risk Among Older People Using a Multivariate Analysis
3.5.1. Model Development and Predictor Selection Procedure
3.5.2. Hosmer–Lemeshow Test Results
3.5.3. Cross-Validation Results
3.5.4. Multicollinearity Testing
3.6. Interpretation of the Regression Coefficient
3.7. Adjusted Biomarker Effects in Multivariable Models: Accounting for Covariates and Confounding Factors
3.8. Evaluating Predictive Accuracy: Discriminatory Performance of the Logistic Regression Model for Malnutrition Risk
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Optimal Cut-Off Point | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| SIRT-1 | ≥0.56 ng/mL | 0.50 | 0.79 | 0.32 | 0.55 |
| CCK-8 | ≥168.55 pg/mL | 0.59 | 0.57 | 0.61 | 0.58 |
| Melatonin | ≥242.90 pg/mL | 0.42 | 0.95 | 0.09 | 0.46 |
| TAC | ≥1682.17 µmol/L | 0.63 | 0.05 | 0.98 | 0.42 |
| Parameter | SIRT-1 | CCK-8 | TAC |
|---|---|---|---|
| SIRT-1 | - | - | Rho = 0.02 padj = 1.000 |
| CCK-8 | Rho = 0.12 padj = 0.619 | - | Rho = 0.10 padj = 0.873 |
| Melatonin | Rho = 0.18 padj = 0.172 | Rho = 0.06 padj = 1.000 | Rho = −0.05 padj = 1.000 |
| Characteristic | N | Overall Sample * | Risk of Malnutrition | p * | |
|---|---|---|---|---|---|
| Yes (n = 58) * | No (n = 95) * | ||||
| GS, kg | 153 | 20.10 (15.50, 25.75) | 20.28 (15.40, 25.00) | 20.05 (16.13, 28.00) | 0.446 |
| FS | 153 | 52.00 (33.99%) | 35.00 (60.34%) | 17.00 (17.89%) | <0.001 ** |
| RD | 153 | 33.00 (21.57%) | 21.00 (36.21%) | 12.00 (12.63%) | 0.001 ** |
| LA | 153 | 85.00 (55.56%) | 45.00 (77.59%) | 40.00 (42.11%) | <0.001 ** |
| PS | 153 | 15.00 (9.80%) | 11.00 (18.97%) | 4.00 (4.21%) | 0.003 ** |
| ASMI, kg/m2 | 153 | 7.66 (6.86, 8.96) | 7.27 (6.62, 8.63) | 7.85 (7.13, 9.13) | 0.025 |
| TBW, L | 153 | 32.63 (29.20, 37.98) | 31.11 (28.09, 35.78) | 34.00 (29.99, 38.35) | 0.050 |
| TEE | 153 | 2204.44 (2028.60, 2410.78) | 2173.83 (1996.63, 2308.88) | 2223.33 (2045.05, 2446.89) | 0.104 |
| REE | 153 | 1348.71 (1255.16, 1480.11) | 1297.85 (1241.09, 1442.25) | 1364.15 (1286.95, 1488.71) | 0.061 |
| FFMI kg/m2 | 153 | 16.80 (15.45, 18.90) | 16.43 (14.96, 18.48) | 17.17 (15.65, 19.10) | 0.063 |
| FMI kg/m2 | 153 | 11.00 (8.73, 13.48) | 10.87 (8.38, 13.32) | 11.24 (8.80, 13.53) | 0.443 |
| PA | 153 | 5.31 (5.02, 5.81) | 5.15 (4.95, 5.57) | 5.48 (5.07, 5.95) | 0.010 |
| VAT, L | 153 | 1.86 (1.31, 2.48) | 1.78 (1.29, 2.53) | 1.89 (1.31, 2.43) | 0.683 |
Appendix B
| Exposure | Malnutrition Risk | Nobs | R2tjur | ||
|---|---|---|---|---|---|
| OR | CI 95% | p | |||
| SIRT-1 Centered at Mdn = 0.92 ng/mL | 1.03 | 0.719–1.406 | 0.857 | 153 | 0.265 |
| CCK-8 Centered at Mdn = 160.53 pg/mL | 1.01 | 0.996–1.012 | 0.065 | 153 | 0.190 |
| Melatonin Centered at Mdn = 431.02 pg/mL | 1.00 | 0.998–1.002 | 0.997 | 153 | 0.115 |
| TAC Centered at Mdn = 1165.50 µmol/L | 1.00 | −0.003–0.010 | 0.286 | 153 | 0.087 |
References
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Elia, M. Defining, recognizing, and reporting malnutrition. Int. J. Low. Extrem. Wounds 2017, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Camprubi-Robles, M.; Bear, D.; Cederholm, T.; Malafarina, V.; Welch, A.; Cruz-Jentoft, A. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef]
- Mikkelsen, S.; Geisler, L.; Holst, M. Malnutrition measured by unintended weight loss among patients in general practice. Nutrition 2022, 96, 111554. [Google Scholar] [CrossRef]
- Kirkland, L.L.; Kashiwagi, D.T.; Brantley, S.; Scheurer, D.; Varkey, P. Nutrition in the hospitalized patient. J. Hosp. Med. 2013, 8, 52–58. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H.E. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Kanno, Y.; Watanabe, S.; Yokokawa, T.; Abe, S.; Miyata, M.; Sato, T.; Suzuki, S.; Oikawa, M.; Kobayashi, A. Impact of nutritional indices on mortality in patients with heart failure. Open Heart 2018, 5, e000730. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.; Forssell, H.; Fagerström, C. Malnutrition, functional ability and mortality among older people aged≥ 60 years: A 7-year longitudinal study. Eur. J. Clin. Nutr. 2016, 70, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, M.; Lauck, S.; Webb, J.G.; Asgar, A.W.; Perrault, L.P.; Piazza, N.; Martucci, G.; Lachapelle, K.; Noiseux, N.; Kim, D.H. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation 2018, 138, 2202–2211. [Google Scholar] [CrossRef]
- de Boer, A.; Ter Horst, G.J.; Lorist, M.M. Physiological and psychosocial age-related changes associated with reduced food intake in older persons. Ageing Res. Rev. 2013, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.d.P.; Figueiredo, N.M.; Chociay, S.; Silva, T.A.; Seixas, R.A.M.; Luchesi, B.M. Factors associated with nutritional risk and appetite loss in long-aged older people. Rev. Nutr. 2021, 34, e200308. [Google Scholar] [CrossRef]
- Barg, F.K.; Huss-Ashmore, R.; Wittink, M.N.; Murray, G.F.; Bogner, H.R.; Gallo, J.J. A mixed-methods approach to understanding loneliness and depression in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2006, 61, S329–S339. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Vellas, B.; Garry, P. Mini Nutritional Assessment: A Practical Assessment Tool for Grading the Nutritional State of Elderly Patients; Serdi Publishing Company: Paris, France, 1997; Volume 1. [Google Scholar]
- Basolo, A.; Ando, T.; Chang, D.C.; Hollstein, T.; Krakoff, J.; Piaggi, P.; Votruba, S. Reduced Albumin Concentration Predicts Weight Gain and Higher Ad Libitum Energy Intake in Humans. Front. Endocrinol. 2021, 12, 642568. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.Y.; Wu, T.H.; Liu, C.S.; Lin, C.H.; Lin, C.C.; Lai, M.M.; Lin, W.Y. Body mass index and albumin levels are prognostic factors for long-term survival in elders with limited performance status. Aging 2020, 12, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Kuikka, L.K.; Salminen, S.; Ouwehand, A.; Gueimonde, M.; Strandberg, T.E.; Finne-Soveri, U.H.; Sintonen, H.; Pitkälä, K.H. Inflammation markers and malnutrition as risk factors for infections and impaired health-related quality of life among older nursing home residents. J. Am. Med. Dir. Assoc. 2009, 10, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Fatyga, P.; Pac, A.; Fedyk-Łukasik, M.; Grodzicki, T.; Skalska, A. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur. Geriatr. Med. 2020, 11, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Akpınar, Ş.; Tek, N.A. Age-Related Changes in Circadian Rhythm and Association with Nutrition. Curr. Nutr. Rep. 2023, 12, 376–382. [Google Scholar] [CrossRef]
- Leitão, C.; Mignano, A.; Estrela, M.; Fardilha, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The effect of nutrition on aging—A systematic review focusing on aging-related biomarkers. Nutrients 2022, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K.; Voulgaridou, G.; Kondyli, F.S.; Drakaki, M.; Sianidou, K.; Andrianopoulou, R.; Rodopaios, N.; Pritsa, A. Nutritional and Nutrition-Related Biomarkers as Prognostic Factors of Sarcopenia, and Their Role in Disease Progression. Diseases 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Tomasiewicz, A.; Polański, J.; Tański, W. Advancing the Understanding of Malnutrition in the Elderly Population: Current Insights and Future Directions. Nutrients 2024, 16, 2502. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.E.; Kolbe-Alexander, T.L.; Nel, J.H. Development of a novel nutrition screening tool for use in elderly South Africans. Public Health Nutr. 2005, 8, 468–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guarente, L. Sirtuins as potential targets for metabolic syndrome. Nature 2006, 444, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S. A review of Sirt1 and Sirt1 modulators in cardiovascular and metabolic diseases. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 156–164. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Oberdoerffer, P.; Michan, S.; McVay, M.; Mostoslavsky, R.; Vann, J.; Park, S.-K.; Hartlerode, A.; Stegmuller, J.; Hafner, A.; Loerch, P. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008, 135, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Kujawowicz, K.; Mirończuk-Chodakowska, I.; Witkowska, A.M. Sirtuin 1 as a potential biomarker of undernutrition in the elderly: A narrative review. Crit. Rev. Food Sci. Nutr. 2024, 64, 9532–9553. [Google Scholar] [CrossRef]
- Goswami, N.; Abulafia, C.; Vigo, D.; Moser, M.; Cornelissen, G.; Cardinali, D. Falls risk, circadian rhythms and melatonin: Current perspectives. Clin. Interv. Aging 2020, 15, 2165–2174. [Google Scholar] [CrossRef]
- Korkmaz, A.; Topal, T.; Tan, D.-X.; Reiter, R.J. Role of melatonin in metabolic regulation. Rev. Endocr. Metab. Disord. 2009, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mc Carthy, C.E. Sleep disturbance, sleep disorders and co-morbidities in the care of the older person. Med. Sci. 2021, 9, 31. [Google Scholar] [CrossRef]
- Miner, B.; Kryger, M.H. Sleep in the aging population. Sleep Med. Clin. 2020, 15, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cong, B.; Shan, B.; Zhang, J.; Chen, H.; Wang, T.; Ma, C.; Qin, J.; Wen, D.; Yu, F. Cholecystokinin octapeptide exerts its therapeutic effects on collagen-induced arthritis by suppressing both inflammatory and Th17 responses. Rheumatol. Int. 2011, 31, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Li, S.-J.; Yao, Y.-X.; Zhu, G.-J.; Ling, Y.-L. Effect of cholecystokinin octapeptide on tumor necrosis factor α transcription and nuclear factor-κB activity induced by lipopolysaccharide in rat pulmonary interstitial macrophages. World J. Gastroenterol. 2002, 8, 718. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ni, Z.; Cong, B.; Gao, W.; Xu, S.; Wang, C.; Yao, Y.; Ma, C.; Ling, Y. CCK-8 inhibits LPS-induced IL-1β production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-κB pathway. Shock 2007, 27, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.B. Chapter 10—Synaptic transmission in the central nervous system. In Introduction to Neuropharmacology; Bradley, P.B., Ed.; Butterworth-Heinemann: Oxford, UK, 1989; pp. 127–174. [Google Scholar]
- Donini, L.M.; Savina, C.; Cannella, C. Eating Habits and Appetite Control in the Elderly: The Anorexia of Aging. Int. Psychogeriatr. 2003, 15, 73–87. [Google Scholar] [CrossRef]
- Bogacka, A.; Sobczak-Czynsz, A.; Balejko, E.; Heberlej, A.; Ciechanowski, K. Effect of Diet and Supplementation on Serum Vitamin C Concentration and Antioxidant Activity in Dialysis Patients. Nutrients 2023, 15, 78. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.H.; Lim, S.L. Ferric Reducing Capacity Versus Ferric Reducing Antioxidant Power for Measuring Total Antioxidant Capacity. Lab. Med. 2013, 44, 51–55. [Google Scholar] [CrossRef]
- Gorni, D.; Finco, A. Oxidative stress in elderly population: A prevention screening study. Aging Med. 2020, 3, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mądra-Gackowska, K.; Szewczyk-Golec, K.; Gackowski, M.; Woźniak, A.; Kędziora-Kornatowska, K. Evaluation of Selected Parameters of Oxidative Stress and Adipokine Levels in Hospitalized Older Patients with Diverse Nutritional Status. Antioxidants 2023, 12, 569. [Google Scholar] [CrossRef]
- Moshtagh, M.; Moodi, M.; Moezi, S.A.; Sharifi, F.; Khazdair, M.R. Inflammatory and Oxidative Stress Biomarkers in the Elderly, the Birjand Longitudinal Aging Study. BioMed Res. Int. 2023, 2023, 4683542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Garrido, M.; Rodríguez, A.; Terrón, M. Tryptophan and Melatonin-Enriched Foodstuffs to Improve Antioxidant Status in Aging. In Aging; Elsevier: Amsterdam, The Netherlands, 2014; pp. 129–136. [Google Scholar]
- Chrustek, A.; Olszewska-Słonina, D. Melatonin as a powerful antioxidant. Acta Pharm. 2021, 71, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.E.; Steketee, J.D.; Saphier, D. Antioxidant properties of melatonin—An emerging mystery. Biochem. Pharmacol. 1998, 56, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, H.; Li, L.; Li, X.; Ge, J.; Reiter, R.J.; Wang, Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT 3-SOD 2-dependent pathway. J. Pineal Res. 2017, 63, e12431. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Zhou, X.; Lu, X.; Liu, X.; Li, G.; Long, J. Melatonin attenuates sepsis-induced acute lung injury through improvement of epithelial sodium channel-mediated alveolar fluid clearance via activation of SIRT1/SGK1/Nedd4-2 signaling pathway. Front. Pharmacol. 2020, 11, 590652. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin Functions in Health and Disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Steinberg, G.R. SIRT1 Takes a Backseat to AMPK in the Regulation of Insulin Sensitivity by Resveratrol. Diabetes 2010, 59, 551–553. [Google Scholar] [CrossRef]
- Chakrabarti, P.; English, T.; Karki, S.; Qiang, L.; Tao, R.; Kim, J.; Luo, Z.; Farmer, S.R.; Kandror, K.V. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 2011, 52, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jiang, X.; Ma, H.; Wang, Y.; Xue, P.; Liu, Y. SIRT1 and insulin resistance. J. Diabetes Complicat. 2016, 30, 178–183. [Google Scholar] [CrossRef]
- Schug, T.T.; Li, X. Sirtuin 1 in lipid metabolism and obesity. Ann. Med. 2011, 43, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin Stimulates the SIRT1/Nrf2 Signaling Pathway Counteracting Lipopolysaccharide (LPS)-Induced Oxidative Stress to Rescue Postnatal Rat Brain. CNS Neurosci. Ther. 2017, 23, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kilic, U.; Kilic, E.; Tuzcu, Z.; Tuzcu, M.; Ozercan, I.H.; Yilmaz, O.; Sahin, F.; Sahin, K. Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutr. Metab. 2013, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Z.; Jiang, A.; Wu, D.; Li, S.; Liu, Z.; Wei, Z.; Yang, Z.; Guo, C. Protective effects of pterostilbene on lipopolysaccharide-induced acute lung injury in mice by inhibiting NF-κB and activating Nrf2/HO-1 signaling pathways. Front. Pharmacol. 2021, 11, 591836. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chen, Y.; Zheng, M.; Ryu, J.; Cho, G.J.; Surh, Y.-J.; Sato, D.; Hamada, H.; Ryter, S.W.; Kim, U.-H. Pterostilbene 4′-β-Glucoside Attenuates LPS-Induced Acute Lung Injury via Induction of Heme Oxygenase-1. Oxid. Med. Cell. Longev. 2018, 2018, 2747018. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Rajashekaraiah, V. Ferric reducing ability of plasma: A potential oxidative stress marker in stored plasma. Acta Haematol. Pol. 2021, 52, 61–67. [Google Scholar] [CrossRef]
- Banks, A.S.; Kon, N.; Knight, C.; Matsumoto, M.; Gutiérrez-Juárez, R.; Rossetti, L.; Gu, W.; Accili, D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008, 8, 333–341. [Google Scholar] [CrossRef]
- Luen Tang, B. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Chen, H.; Zaky, A.; Pollock, C.; Saad, S. SIRT1 overexpression attenuates offspring metabolic and liver disorders as a result of maternal high-fat feeding. J. Physiol. 2019, 597, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yan, L.; Tan, Y.; Chen, S.; Zhang, K.; Gong, Z.; Liu, W.; Zou, H.; Song, R.; Zhu, J.; et al. Melatonin improves mitochondrial function by preventing mitochondrial fission in cadmium-induced rat proximal tubular cell injury via SIRT1–PGC-1α pathway activation. Ecotoxicol. Environ. Saf. 2022, 242, 113879. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Naaz, S.; Mishra, S.; Pal, P.K.; Chattopadhyay, A.; Das, A.R.; Bandyopadhyay, D. Activation of SIRT1/PGC 1α/SIRT3 pathway by melatonin provides protection against mitochondrial dysfunction in isoproterenol induced myocardial injury. Heliyon 2020, 6, e05159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, N.; Qi, J.; Wang, S.; Hao, J.; Wu, Y.; Chen, H.; Tian, Z.; Wang, B.; Chen, D.; et al. CCK reduces the food intake mainly through CCK1R in Siberian sturgeon (Acipenser baerii Brandt). Sci. Rep. 2017, 7, 12413. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Cholecystokinin—From Local Gut Hormone to Ubiquitous Messenger. Front. Endocrinol. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.; Turner, M.; Naumovski, N.; Somerset, S. Role of cholecystokinin in satiation: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 2182–2190. [Google Scholar] [CrossRef]
- Smith, G. The Peripheral Control of Appetite. Lancet 1983, 322, 88–90. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, S.; Wei, X.; Zhang, M.; Chen, Y.; Mao, X.; Chen, G.; Liu, C. Short-term moderate caloric restriction in a high-fat diet alleviates obesity via AMPK/SIRT1 signaling in white adipocytes and liver. Food Nutr. Res. 2022, 66, 7909. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Nutraceutical activation of Sirt1: A review. Open Heart 2022, 9, e002171. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Wang, J.; Liu, Q. The controversial links among calorie restriction, SIRT1, and resveratrol. Free Radic. Biol. Med. 2011, 51, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Francini, F.; Schinella, G.R.; Ríos, J.-L. Activation of AMPK by medicinal plants and natural products: Its role in type 2 diabetes mellitus. Mini-Rev. Med. Chem. 2019, 19, 880–901. [Google Scholar] [CrossRef]
- Angin, Y.; Beauloye, C.; Horman, S.; Bertrand, L. Regulation of carbohydrate metabolism, lipid metabolism, and protein metabolism by AMPK. In AMP-Activated Protein Kinase; Springer: Cham, Switzerland, 2016; pp. 23–43. [Google Scholar]
- Kumar, G.S.; Kulkarni, A.; Khurana, A.; Kaur, J.; Tikoo, K. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem.-Biol. Interact. 2014, 223, 125–133. [Google Scholar] [CrossRef]
- Shengyu, C.; Yinhua, L.; Yuanhong, L.; Jinbo, Z.; Can, F.; Hao, X.; Changjiang, Z. Selenium alleviates heart remodeling through Sirt1/AKT/GSK-3β pathway. Int. Immunopharmacol. 2022, 111, 109158. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.M.; Huber, F.M.; Hoelz, A. Structural and Functional Analysis of Human SIRT1. J. Mol. Biol. 2014, 426, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, Y.; Zhang, F.; Xia, Y.; Liu, J.; Huang, R.; Wang, Y.; Hu, Y.; Wu, J.; Dai, C. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 2014, 59, 2196–2206. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Kanasaki, K.; Takeda-Watanabe, A.; Koya, D. Sirtuins as possible drug targets in type 2 diabetes. Curr. Drug Targets 2013, 14, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, L.; Zhang, Y.; Geriletu; Yang, J.; Zhang, Y.; Xing, Y. Vitamin C Protected Human Retinal Pigmented Epithelium from Oxidant Injury Depending on Regulating SIRT1. Sci. World J. 2014, 2014, 750634. [Google Scholar] [CrossRef]
- Zillikens, M.C.; van Meurs, J.B.; Rivadeneira, F.; Hofman, A.; Oostra, B.A.; Sijbrands, E.J.; Witteman, J.C.; Pols, H.A.; van Duijn, C.M.; Uitterlinden, A.G. Interactions between dietary vitamin E intake and SIRT1 genetic variation influence body mass index. Am. J. Clin. Nutr. 2010, 91, 1387–1393. [Google Scholar] [CrossRef]
- Saboori, S.; Koohdani, F.; Nematipour, E.; Yousefi Rad, E.; Saboor-Yaraghi, A.A.; Javanbakht, M.H.; Eshraghian, M.R.; Ramezani, A.; Djalali, M. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1α and serum antioxidant enzymes in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 489–494. [Google Scholar] [CrossRef]
- Naqvi, A.; Hoffman, T.A.; DeRicco, J.; Kumar, A.; Kim, C.-S.; Jung, S.-B.; Yamamori, T.; Kim, Y.-R.; Mehdi, F.; Kumar, S.; et al. A single-nucleotide variation in a p53-binding site affects nutrient-sensitive human SIRT1 expression. Hum. Mol. Genet. 2010, 19, 4123–4133. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Zhou, Y.; Zhang, D. Reduced serum SIRT1 levels in patients with Parkinson’s disease: A cross-sectional study in China. Neurol. Sci. 2021, 42, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kinnula, V.L.; Gorbunova, V.; Yao, H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev. Med. 2012, 54, S20–S28. [Google Scholar] [CrossRef]
- Yang, T.; Zhan, Z.; Zhang, L.; Zhu, J.; Liu, Y.; Zhang, L.; Ge, J.; Zhao, Y.; Zhang, L.; Dong, J. Prevalence and risk factors for malnutrition in patients with Parkinson’s disease. Front. Neurol. 2020, 11, 533731. [Google Scholar] [CrossRef] [PubMed]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Duran, X.; Meza-Valderrama, D.; Rodríguez, D.A.; Muñoz, E.; Tejero-Sánchez, M.; Muns, M.D.; Guillén-Solà, A.; et al. Malnutrition According to GLIM Criteria Is Associated with Mortality and Hospitalizations in Rehabilitation Patients with Stable Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Tiwari, P.; Kaur, A.; Singh, T.G. Sirtuin acetylation and deacetylation: A complex paradigm in neurodegenerative disease. Mol. Neurobiol. 2021, 58, 3903–3917. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 activity control in neurodegenerative diseases. Front. Pharmacol. 2021, 11, 585821. [Google Scholar] [CrossRef]
- Min, S.-W.; Sohn, P.D.; Cho, S.-H.; Swanson, R.A.; Gan, L. Sirtuins in neurodegenerative diseases: An update on potential mechanisms. Front. Aging Neurosci. 2013, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Pizzorni, N.; Ciammola, A.; Casazza, G.; Ginocchio, D.; Bianchi, F.; Feroldi, S.; Poletti, B.; Nanetti, L.; Mariotti, C.; Mora, G. Predictors of malnutrition risk in neurodegenerative diseases: The role of swallowing function. Eur. J. Neurol. 2022, 29, 2493–2498. [Google Scholar] [CrossRef]
- Sheard, J.M. Malnutrition and neurodegenerative diseases. Curr. Nutr. Rep. 2014, 3, 102–109. [Google Scholar] [CrossRef]
- Almeida, M.; Porter, R.M. Sirtuins and FoxOs in osteoporosis and osteoarthritis. Bone 2019, 121, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, J.; Villarroya, F.; Puri, P.L.; Vinciguerra, M. SIRT1 signaling as potential modulator of skeletal muscle diseases. Curr. Opin. Pharmacol. 2012, 12, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Russell, D.; Whitwell, J.; Jeejeebhoy, K.N. Skeletal muscle function in malnutrition. Am. J. Clin. Nutr. 1982, 36, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Romeo, S.; Ferro, Y.; Migliaccio, V.; Gazzaruso, C.; Pujia, A. Osteoporosis in chronic inflammatory disease: The role of malnutrition. Endocrine 2013, 43, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.J. Apelin and Sirtuin 1 dysregulation induce endocrine and metabolic disorders in chronic disease. Glob. J. Endocrinol. Met. 2017, 1, 1–2. [Google Scholar] [CrossRef]
- Andreux, P.A.; Van Diemen, M.P.; Heezen, M.R.; Auwerx, J.; Rinsch, C.; Groeneveld, G.J.; Singh, A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci. Rep. 2018, 8, 8548. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Jiang, S.; Li, H.; Chen, L.; Wu, Y.; Essien, A.E.; Opoku, M.; Naranmandakh, S.; Liu, S. SIRT1 signaling pathways in sarcopenia: Novel mechanisms and potential therapeutic targets. Biomed. Pharmacother. 2024, 177, 116917. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Wagner, R.; Rzucidlo, E.M. Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H533–H541. [Google Scholar] [CrossRef]
- Breitenstein, A.; Wyss, C.A.; Spescha, R.D.; Franzeck, F.C.; Hof, D.; Riwanto, M.; Hasun, M.; Akhmedov, A.; von Eckardstein, A.; Maier, W. Peripheral blood monocyte Sirt1 expression is reduced in patients with coronary artery disease. PLoS ONE 2013, 8, e53106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Narisawa, M.; Jin, X.; Murohara, T.; Kuzuya, M. Sirtuin 1 as a potential therapeutic target in pulmonary artery hypertension. J. Hypertens. 2018, 36, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Guclu, A.; Erdur, F.; Turkmen, K. The emerging role of sirtuin 1 in cellular metabolism, diabetes mellitus, diabetic kidney disease and hypertension. Exp. Clin. Endocrinol. Diabetes 2016, 124, 131–139. [Google Scholar] [CrossRef]
- Choi, M.-J.; Seo, J.-W.; Yoon, J.-W.; Lee, S.-K.; Kim, S.-J.; Lee, Y.-K.; Noh, J.-W.; Koo, J.-R. The malnutrition-inflammation-depression-arteriosclerosis complex is associated with an increased risk of cardiovascular disease and all-cause death in chronic hemodialysis patients. Nephron Clin. Pract. 2013, 122, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Anzaki, K.; Kanda, D.; Ikeda, Y.; Takumi, T.; Tokushige, A.; Ohmure, K.; Sonoda, T.; Arikawa, R.; Ohishi, M. Impact of malnutrition on prognosis and coronary artery calcification in patients with stable coronary artery disease. Curr. Probl. Cardiol. 2023, 48, 101185. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Huang, H.; Xu, W.; Cui, K.; Ruan, Y.; Guo, Y.; Wang, J.; Bin, J.; Wang, Y.; Chen, Y. Prognostic impact of malnutrition in patients with coronary artery disease: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, S.; Soysal, P.; Bulut, E.A.; Isik, A. Malnutrition and malnutrition risk can be associated with systolic orthostatic hypotension in older adults. J. Nutr. Health Aging 2018, 22, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Bitla, A.R.; Kumari, N.M.; Reddy, N.S.; Nagaraju, K.V.; Sachan, A.; Kumar, V.P.; Suchitra, M.M.; Srinivasa Rao, P.V.L.N. Antioxidant status in patients with metabolic syndrome as measured by ferric reducing ability of plasma (FRAP) assay. J. Clin. Res. 2012, 1, 114–120. [Google Scholar] [CrossRef]
- Chapple, I. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, K.; Deng, L.; Chen, Y.; Nice, E.C.; Huang, C. Redox regulation of inflammation: Old elements, a new story. Med. Res. Rev. 2015, 35, 306–340. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Hirabayashi, T.; Nakanishi, R.; Tanaka, M.; Nisa, B.u.; Maeshige, N.; Kondo, H.; Fujino, H. Reduced metabolic capacity in fast and slow skeletal muscle via oxidative stress and the energy-sensing of AMPK/SIRT1 in malnutrition. Physiol. Rep. 2021, 9, e14763. [Google Scholar] [CrossRef] [PubMed]
- Kujawowicz, K.; Mirończuk-Chodakowska, I.; Cyuńczyk, M.; Witkowska, A.M. Identifying Malnutrition Risk in the Elderly: A Single- and Multi-Parameter Approach. Nutrients 2024, 16, 2537. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Toward a conceptual definition of frail community dwelling older people. Nurs. Outlook 2010, 58, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Brennan, I.M.; Otto, B.; Feltrin, K.L.; Meyer, J.H.; Horowitz, M.; Feinle-Bisset, C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides 2007, 28, 607–611. [Google Scholar] [CrossRef]
- Nahata, M.; Fujitsuka, N.; Sekine, H.; Shimobori, C.; Ohbuchi, K.; Iizuka, S.; Mogami, S.; Ohnishi, S.; Takeda, H. Decline in liver mitochondria metabolic function is restored by hochuekkito through sirtuin 1 in aged mice with malnutrition. Front. Physiol. 2022, 13, 848960. [Google Scholar] [CrossRef]
- Coto-Montes, A.; Boga, J.A.; Tan, D.X.; Reiter, R.J. Melatonin as a potential agent in the treatment of sarcopenia. Int. J. Mol. Sci. 2016, 17, 1771. [Google Scholar] [CrossRef]
- Besora-Moreno, M.; Llauradó, E.; Valls, R.M.; Tarro, L.; Pedret, A.; Solà, R. Antioxidant-rich foods, antioxidant supplements, and sarcopenia in old-young adults ≥55 years old: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin. Nutr. 2022, 41, 2308–2324. [Google Scholar] [CrossRef]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S.; Diets, T.-g.S.o.W.o.; Groups, H.S. Inverse association between dietary habits with high total antioxidant capacity and prevalence of frailty among elderly Japanese women: A multicenter cross-sectional study. J. Nutr. Health Aging 2014, 18, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Niu, H.; Sha, G.; Zhang, Y.; Liu, P.; Li, Y. Serum SIRT1 Is Associated with Frailty and Adipokines in Older Adults. J. Nutr. Health Aging 2019, 23, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mohan, N.; Upadhyay, A.D.; Singh, A.P.; Sahu, V.; Dwivedi, S.; Dey, A.B.; Dey, S. Identification of serum sirtuins as novel noninvasive protein markers for frailty. Aging Cell 2014, 13, 975–980. [Google Scholar] [CrossRef]
- Sasaki, T.; Kim, H.-J.; Kobayashi, M.; Kitamura, Y.-I.; Yokota-Hashimoto, H.; Shiuchi, T.; Minokoshi, Y.; Kitamura, T. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology 2010, 151, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Lee, C.E.; Bookout, A.L.; Lee, S.; Williams, K.W.; Anderson, J.; Elmquist, J.K.; Coppari, R. Brain SIRT1: Anatomical distribution and regulation by energy availability. J. Neurosci. 2008, 28, 9989–9996. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Ben-Josef, G.; West, T.; Wozniak, D.F.; Holtzman, D.M.; Herzog, E.D.; Imai, S.-I. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 2010, 30, 10220–10232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Wolden-Hanson, T.; Mitton, D.; McCants, R.; Yellon, S.; Wilkinson, C.; Matsumoto, A.; Rasmussen, D. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology 2000, 141, 487–497. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcón, M.; Ruiz, R.; Abuhamadah, S.; El-Mir, M.Y.; Vázquez, G.F. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Prunet-Marcassus, B.; Desbazeille, M.; Bros, A.; Louche, K.; Delagrange, P.; Renard, P.; Casteilla, L.; Pénicaud, L. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 2003, 144, 5347–5352. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.; Fuentes-Broto, L.; Paredes, S.; Reiter, R. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; A-Serrano, M.M.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef] [PubMed]
- Gawron-Skarbek, A.; Chrzczanowicz, J.; Kostka, J.; Nowak, D.; Drygas, W.; Jegier, A.; Kostka, T. Cardiovascular Risk Factors and Total Serum Antioxidant Capacity in Healthy Men and in Men with Coronary Heart Disease. BioMed Res. Int. 2014, 2014, 216964. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Morua, W.; Villafan-Bernal, J.R.; Ramírez-Moreno, E.; García-Ortiz, H.; Martínez-Portilla, R.J.; Contreras-Cubas, C.; Martínez-Hernández, A.; Centeno-Cruz, F.; Pedroza-Montoya, F.E.; Orozco, L.; et al. Total Antioxidant Capacity in Obese and Non-Obese Subjects and Its Association with Anthropo-Metabolic Markers: Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Libuy, M.; Feliú, F.; Hasson, D. Oxidative Stress-Related Biomarkers in Essential Hypertension and Ischemia-Reperfusion Myocardial Damage. Dis. Markers 2013, 35, 974358. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative stress and hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Yavuzer, H.; Yavuzer, S.; Cengiz, M.; Erman, H.; Doventas, A.; Balci, H.; Erdincler, D.S.; Uzun, H. Biomarkers of lipid peroxidation related to hypertension in aging. Hypertens. Res. 2016, 39, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Birch-Machin, M.A. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Lehucher-Michel, M.P.; Lesgards, J.F.; Delubac, O.; Stocker, P.; Durand, P.; Prost, M. Oxidative stress and human disease. Current knowledge and perspectives for prevention. Presse Medicale 2001, 30, 1076–1081. [Google Scholar] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.; Selcoki, Y.; Nazlı, Y.; Çolak, N.; Yalçın, K.S.; Canbal, M.; Demirçelik, B.; Yiğitoğlu, R.; Eryonucu, B. Relationship between total antioxidant capacity and the severity of coronary artery. J. Clin. Exp. Investig. 2012, 3, 22–28. [Google Scholar]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid hormones, oxidative stress, and inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [PubMed]
- Bhimte, B.; Agrawal, B.; Sharma, V.; Chauhan, S.S. Oxidative stress status in hypothyroid patients. Biomed. Res. 2012, 23, 286–288. [Google Scholar]
- Mancini, A.; Festa, R.; Donna, V.d.; Leone, E.; Littarru, G.P.; Silvestrini, A.; Meucci, E.; Pontecorvi, A. Hormones and antioxidant systems: Role of pituitary and pituitary-dependent axes. J. Endocrinol. Investig. 2010, 33, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Abilés, J.; de la Cruz, A.P.; Castaño, J.; Rodríguez-Elvira, M.; Aguayo, E.; Moreno-Torres, R.; Llopis, J.; Aranda, P.; Argüelles, S.; Ayala, A. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: A cohort study. Crit. Care 2006, 10, R146. [Google Scholar] [CrossRef]
- Parhofer, K.G. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Merino, B.; Cano, V.; Domínguez, G.; Pérez-Castells, J.; Fernández-Alfonso, M.S.; Sengenès, C.; Chowen, J.A.; Ruiz-Gayo, M. Cholecystokinin is involved in triglyceride fatty acid uptake by rat adipose tissue. J. Endocrinol. 2018, 236, 137–150. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Lin, X.; Okoro, E.U.; Guo, Z. Cholecystokinin elevates mouse plasma lipids. PLoS ONE 2012, 7, e51011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burman, M.; Hörnsten, C.; Öhlin, J.; Olofsson, B.; Nordström, P.; Gustafson, Y. Prevalence of obesity and malnutrition in four cohorts of very old adults, 2000–2017. J. Nutr. Health Aging 2022, 26, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, C.; Mogensen, K.M.; Robinson, M.K. Malnutrition in patients with obesity: An overview perspective. Nutr. Clin. Pract. 2024, 39, 1300–1316. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G. Double burden of malnutrition in persons with obesity. Rev. Endocr. Metab. Disord. 2020, 21, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Barrón-Pavón, V.; González-Stager, M.A.; Rodríguez-Fernández, A. Relationship between body composition and the risk of non-communicable chronic diseases in active older women from Chillán (Chile). Rev. Esp. Salud Publica 2023, 97, e202306045. [Google Scholar] [PubMed]
- Alemán-Mateo, H.; Esparza-Romero, J.; Romero, R.U.; García, H.A.; Pérez Flores, F.A.; Ochoa Chacón, B.V.; Valencia, M.E. Prevalence of malnutrition and associated metabolic risk factors for cardiovascular disease in older adults from Northwest Mexico. Arch. Gerontol. Geriatr. 2008, 46, 375–385. [Google Scholar] [CrossRef]
- Kanda, D.; Ohishi, M. Malnutrition is one of new risk factors in patients with hypertension: The message form Fukushima Cohort Study. Hypertens. Res. 2024, 47, 2589–2591. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-S.; Yen, C.-H.; Huang, Y.-Y.; Chiu, C.-J.; Lin, P.-T. Associations between Coenzyme Q10 Status, Oxidative Stress, and Muscle Strength and Endurance in Patients with Osteoarthritis. Antioxidants 2020, 9, 1275. [Google Scholar] [CrossRef]
- Rospleszcz, S.; Dermyshi, D.; Müller-Peltzer, K.; Strauch, K.; Bamberg, F.; Peters, A. Association of serum uric acid with visceral, subcutaneous and hepatic fat quantified by magnetic resonance imaging. Sci. Rep. 2020, 10, 442. [Google Scholar] [CrossRef]
- de Freitas Lima, L.; de Faria Ghetti, F.; Hermsdorff, H.; de Oliveira, D.; Teixeira, G.; de Castro Ferreira, L.; Moreira, A. Dietary total antioxidant capacity is positively associated with muscular strength in cirrhotic outpatients: A cross-sectional study. J. Hum. Nutr. Diet. 2020, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Schilp, J.; Wijnhoven, H.A.; Deeg, D.J.; Visser, M. Early determinants for the development of undernutrition in an older general population: Longitudinal Aging Study Amsterdam. Br. J. Nutr. 2011, 106, 708–717. [Google Scholar] [CrossRef]
- Ritchie, C.S.; Joshipura, K.; Silliman, R.A.; Miller, B.; Douglas, C.W. Oral health problems and significant weight loss among community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M366–M371. [Google Scholar] [CrossRef] [PubMed]
- Nazri, N.S.; Vanoh, D.; Leng, S.K. Malnutrition, low diet quality and its risk factors among older adults with low socio-economic status: A scoping review. Nutr. Res. Rev. 2021, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M. Effect of drugs on nutritional status and drug–nutrition interactions in older patients. Geriatr. Gerontol. Int. 2023, 23, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Landi, F.; Smoyer, K.E.; Tarasenko, L.; Groarke, J. Association of anorexia/appetite loss with malnutrition and mortality in older populations: A systematic literature review. J. Cachexia Sarcopenia Muscle 2023, 14, 706–729. [Google Scholar] [CrossRef]
- Chang, S.F. Frailty is a major related factor for at risk of malnutrition in community-dwelling older adults. J. Nurs. Scholarsh. 2017, 49, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin. Nutr. 2016, 35, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Ng, T.-P. Frailty and malnutrition: Related and distinct syndrome prevalence and association among community-dwelling older adults: Singapore longitudinal ageing studies. J. Am. Med. Dir. Assoc. 2017, 18, 1019–1028. [Google Scholar] [CrossRef]
- Kubo, Y.; Nakashima, D.; Tomiyama, N.; Noritake, K.; Yorozuya, K.; Tsubouchi, Y.; Iitsuka, T.; Fujii, K. Association between muscle quality and nutritional status among community-dwelling older adults: A cross-sectional study. Nutr. Health 2024. [Google Scholar] [CrossRef]
- Tey, S.L.; Chew, S.T.H.; How, C.H.; Yalawar, M.; Baggs, G.; Chow, W.L.; Cheong, M.; Ong, R.H.S.; Husain, F.S.; Kwan, S.C. Factors associated with muscle mass in community-dwelling older people in Singapore: Findings from the SHIELD study. PLoS ONE 2019, 14, e0223222. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Walter, S.; Carnicero, J.A.; García-García, F.J.; Sánchez-Puelles, J.-M.; Sánchez-Puelles, C.; Rodríguez-Mañas, L. Better Nutritional Status Is Positively Associated with mRNA Expression of SIRT1 in Community-Dwelling Older Adults in the Toledo Study for Healthy Aging. J. Nutr. 2018, 148, 1408–1414. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Swaab, D.F. Melatonin rhythmicity: Effect of age and Alzheimer’s disease. Exp. Gerontol. 2003, 38, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Munmun, F.; Witt-Enderby, P.A. Melatonin effects on bone: Implications for use as a therapy for managing bone loss. J. Pineal Res. 2021, 71, e12749. [Google Scholar] [CrossRef] [PubMed]
- Kotlarczyk, M.P.; Lassila, H.C.; O’Neil, C.K.; D’Amico, F.; Enderby, L.T.; Witt-Enderby, P.A.; Balk, J.L. Melatonin osteoporosis prevention study (MOPS): A randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J. Pineal Res. 2012, 52, 414–426. [Google Scholar] [CrossRef]
- Tuft, C.; Matar, E.; Menczel Schrire, Z.; Grunstein, R.R.; Yee, B.J.; Hoyos, C.M. Current insights into the risks of using melatonin as a treatment for sleep disorders in older adults. Clin. Interv. Aging 2023, 18, 49–59. [Google Scholar] [CrossRef]
- MacIntosh, C.G.; Morley, J.E.; Wishart, J.; Morris, H.; Jansen, J.B.M.J.; Horowitz, M.; Chapman, I.M. Effect of Exogenous Cholecystokinin (CCK)-8 on Food Intake and Plasma CCK, Leptin, and Insulin Concentrations in Older and Young Adults: Evidence for Increased CCK Activity as a Cause of the Anorexia of Aging. J. Clin. Endocrinol. Metab. 2001, 86, 5830–5837. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Lu, Q.C.; Cai, Y. Effect of cholecystokinin on experimental neuronal aging. World J. Gastroenterol. 2005, 11, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Little, T.; Horowitz, M.; Feinle-Bisset, C. Role of cholecystokinin in appetite control and body weight regulation. Obes. Rev. 2005, 6, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.O.; Shannon, O.M.; Matu, J.; Holliday, A.; Ispoglou, T.; Deighton, K. Differences in circulating appetite-related hormone concentrations between younger and older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2020, 32, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of aging: Risk factors, consequences, and potential treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Onder, G.; Vetrano, D.L.; Ortolani, E.; Tosato, M.; Marzetti, E.; Landi, F. Anorexia of aging: A modifiable risk factor for frailty. Nutrients 2013, 5, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Uriz-Otano, F.; Gil-Guerrero, L.; Iniesta, R. The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013, 74, 293–302. [Google Scholar] [CrossRef]
- Berthélemy, P.; Bouisson, M.; Vellas, B.; Moreau, J.; Nicole-Vaysse; Albarede, J.L.; Ribet, A. Postprandial Cholecystokinin Secretion in Elderly with Protein-Energy Undernutrition. J. Am. Geriatr. Soc. 1992, 40, 365–369. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N | Overall Sample | Risk of Malnutrition | p ** | |
|---|---|---|---|---|---|
| Yes (n = 58) * | No (n = 95) * | ||||
| SIRT-1 (ng/mL) | 153 | 0.92 (0.53, 2.22) | 1.11 (0.58, 2.54) | 0.84 (0.49, 2.06) | 0.265 |
| CCK-8 (pg/mL) | 153 | 160.53 (121.02, 217.24) | 175.84 (126.96, 225.41) | 156.64 (118.24, 209.97) | 0.114 |
| Melatonin (MT) (pg/mL) | 153 | 431.02 (338.12, 494.25) | 416.25 (340.14, 471.40) | 441.62 (343.43, 499.35) | 0.362 |
| TAC (µmol/L) | 153 | 1165.50 (1017.17, 1305.50) | 1099.67 (992.17, 1255.92) | 1197.17 (1023.00, 1320.50) | 0.090 |
| Parameter | Sex * | Optimal Cut-Off Point | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|
| SIRT-1 | Female | ≥0.56 ng/mL | 0.54 | 0.77 | 0.39 | 0.57 |
| SIRT-1 | Male | ≥1.09 ng/mL | 0.54 | 0.80 | 0.44 | 0.57 |
| CCK-8 | Female | ≥168.55 pg/mL | 0.61 | 0.50 | 0.69 | 0.58 |
| CCK-8 | Male | ≥179.44 pg/mL | 0.57 | 0.90 | 0.44 | 0.64 |
| Melatonin | Female | ≥214.93 pg/mL | 0.45 | 0.96 | 0.10 | 0.46 |
| Melatonin | Male | ≥382.91 pg/mL | 0.43 | 1.00 | 0.20 | 0.51 |
| TAC | Female | ≥1028.83 µmol/L | 0.47 | 0.67 | 0.34 | 0.42 |
| TAC | Male | ≥1682.17 µmol/L | 0.77 | 0.30 | 0.96 | 0.54 |
| Characteristic n = 153 | SIRT-1 ng/mL | CCK-8, pg/mL | Melatonin, pg/mL | TAC, µmol/L |
|---|---|---|---|---|
| Smoking status | ||||
| Yes (n = 14) * | 1.43 (0.64, 2.59) | 163.35 (124.40, 214.06) | 449.64 (357.44, 471.51) | 1217.17 (989.67, 1327.17) |
| No (n = 139) * | 0.87 (0.51, 2.09) | 160.53 (120.18, 216.53) | 430.77 (341.96, 497.75) | 1165.50 (1020.50, 1298.00) |
| p ** | 0.308 | 0.967 | 0.759 | 0.960 |
| Hypertension | ||||
| Yes (n = 83) * | 0.67 (0.44, 1.96) | 175.85 (137.68, 220.65) | 443.17 (380.71, 497.75) | 1202.17 (1060.50, 1331.33) |
| No (n = 70) * | 1.03 (0.58, 2.62) | 151.32 (114.80, 203.44) | 416.78 (331.52, 477.79) | 1082.17 (952.17, 1263.42) |
| p ** | 0.119 | 0.060 | 0.282 | 0.004 |
| Diabetes | ||||
| Yes (n = 22) * | 0.77 (0.40, 2.45) | 181.52 (149.67, 247.72) | 440.90 (328.47, 541.48) | 1157.17 (1017.58, 1343.00) |
| No (n = 131) * | 0.97 (0.54, 2.13) | 158.60 (119.06, 212.82) | 431.01 (349.26, 493.73) | 1165.50 (1016.33, 1303.00) |
| p ** | 0.720 | 0.110 | 0.956 | 0.753 |
| Cardiovascular disease | ||||
| Yes (n = 40) * | 0.65 (0.53, 1.78) | 181.52 (134.78, 230.37) | 433.89 (377.42, 565.13) | 1228.00 (1109.25, 1352.58) |
| No (n = 113) * | 0.97 (0.53, 2.40) | 156.84 (116.19, 204.86) | 431.01 (334.51, 484.89) | 1102.17 (1007.17, 1278.83) |
| p ** | 0.416 | 0.067 | 0.680 | 0.030 |
| Hypothyroidism | ||||
| Yes (n = 26) * | 0.66 (0.41, 2.40) | 156.74 (108.34, 210.03) | 428.42 (382.97, 462.52) | 1098.83 (968.00, 1203.00) |
| No (n = 127) * | 0.99 (0.55, 2.13) | 162.93 (125.01, 217.70) | 431.02 (327.95, 499.35) | 1183.83 (1020.50, 1325.50) |
| p ** | 0.503 | 0.300 | 0.784 | 0.047 |
| Hyperlipidemia | ||||
| Yes (n = 54) * | 0.85 (0.45, 2.32) | 187.95 (136.00, 230.47) | 420.39 (375.94, 495.78) | 1170.50 (1026.33, 1318.00) |
| No (n = 99) * | 0.97 (0.56, 2.20) | 156.72 (117.48, 197.00) | 437.73 (332.52, 493.73) | 1145.50 (988.83, 1299.67) |
| p ** | 0.585 | 0.019 | 0.756 | 0.549 |
| Obesity | ||||
| Yes (n = 69) * | 0.88 (0.52, 2.06) | 166.26 (129.92, 218.42) | 439.56 (367.73, 498.84) | 1198.83 (1083.83, 1365.50) |
| No (n = 84) * | 0.94 (0.54, 2.42) | 157.94 (118.29, 214.58) | 425.76 (329.23, 480.62) | 1085.50 (953.42, 1255.92) |
| p ** | 0.822 | 0.499 | 0.423 | 0.005 |
| Parameter | SIRT-1 | CCK-8 | Melatonin | TAC | ||||
|---|---|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | Rho | p | |
| GS, kg | 0.11 | 0.194 | 0.16 | 0.044 | 0.13 | 0.114 | 0.22 | 0.006 |
| FS | 0.11 | 0.191 | 0.02 | 0.768 | −0.08 | 0.324 | −0.13 | 0.105 |
| RD | 0.04 | 0.649 | 0.06 | 0.488 | −0.12 | 0.155 | −0.11 | 0.192 |
| LA | 0.14 | 0.080 | 0.15 | 0.059 | −0.01 | 0.916 | −0.02 | 0.790 |
| PS | −0.01 | 0.222 | 0.03 | 0.737 | −0.12 | 0.138 | −0.03 | 0.753 |
| ASMI, kg/m2 | 0.06 | 0.444 | 0.12 | 0.148 | 0.05 | 0.564 | 0.38 | <0.001 |
| TBW, L | 0.05 | 0.564 | 0.13 | 0.096 | 0.10 | 0.221 | 0.35 | <0.001 |
| TEE | 0.02 | 0.778 | 0.11 | 0.157 | 0.14 | 0.092 | 0.36 | <0.001 |
| REE | 0.03 | 0.702 | 0.15 | 0.071 | 0.14 | 0.075 | 0.39 | <0.001 |
| FFMI kg/m2 | 0.07 | 0.413 | 0.14 | 0.088 | 0.03 | 0.696 | 0.35 | <0.001 |
| FMI kg/m2 | −0.16 | 0.054 | −0.14 | 0.077 | 0.02 | 0.797 | 0.09 | 0.277 |
| PA | 0.10 | 0.235 | 0.06 | 0.439 | 0.2 | 0.795 | 0.16 | 0.055 |
| VAT, L | 0.11 | 0.185 | 0.22 | 0.007 | 0.13 | 0.115 | 0.41 | <0.001 |
| Predictors | Risk of Malnutrition | ||
|---|---|---|---|
| OR | CI 95% | p | |
| (Intercept) | 0.12 | 0.05–0.24 | <0.001 |
| Smoking | |||
| No | Reference level | ||
| Yes | 4.54 | 1.14–19.08 | 0.033 |
| SSRI and MAOI medication | |||
| No | Reference level | ||
| Yes | 3.01 | 0.86–11.55 | 0.092 |
| FS | |||
| No | Reference level | ||
| Yes | 6.60 | 2.82–16.24 | <0.001 |
| LA | |||
| No | Reference level | ||
| Yes | 2.75 | 1.20–6.48 | 0.018 |
| ASMI (centered by Mdn = 7.70 kg/m2) | 0.61 | 0.39–0.88 | 0.015 |
| VAT (centered by Mdn = 1.86 L | 1.37 | 0.90–2.22 | 0.163 |
| CCK-8 (centered by Mdn = 160.60 pg/mL) | 1.01 | 1.00–1.01 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawowicz, K.; Mirończuk-Chodakowska, I.; Cyuńczyk, M.; Witkowska, A.M. Malnutrition Risk in Older Adults: Evaluating the Diagnostic Relevance of Serum Biomarkers: SIRT-1, CCK-8, Melatonin, and Total Antioxidant Capacity (TAC). Nutrients 2025, 17, 726. https://doi.org/10.3390/nu17040726
Kujawowicz K, Mirończuk-Chodakowska I, Cyuńczyk M, Witkowska AM. Malnutrition Risk in Older Adults: Evaluating the Diagnostic Relevance of Serum Biomarkers: SIRT-1, CCK-8, Melatonin, and Total Antioxidant Capacity (TAC). Nutrients. 2025; 17(4):726. https://doi.org/10.3390/nu17040726
Chicago/Turabian StyleKujawowicz, Karolina, Iwona Mirończuk-Chodakowska, Monika Cyuńczyk, and Anna Maria Witkowska. 2025. "Malnutrition Risk in Older Adults: Evaluating the Diagnostic Relevance of Serum Biomarkers: SIRT-1, CCK-8, Melatonin, and Total Antioxidant Capacity (TAC)" Nutrients 17, no. 4: 726. https://doi.org/10.3390/nu17040726
APA StyleKujawowicz, K., Mirończuk-Chodakowska, I., Cyuńczyk, M., & Witkowska, A. M. (2025). Malnutrition Risk in Older Adults: Evaluating the Diagnostic Relevance of Serum Biomarkers: SIRT-1, CCK-8, Melatonin, and Total Antioxidant Capacity (TAC). Nutrients, 17(4), 726. https://doi.org/10.3390/nu17040726







