Impact of Prenatal Dietary Soy on Cerebellar Neurodevelopment and Function in Experimental Fetal Alcohol Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design Overview
2.2. Materials
2.3. Experimental Model
2.4. Rotarod Testing
2.5. Stereology
2.6. Sample Processing for Molecular and Biochemical Studies
2.7. Multiplex Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Duplex Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis
2.9. Statistical Analysis
3. Results
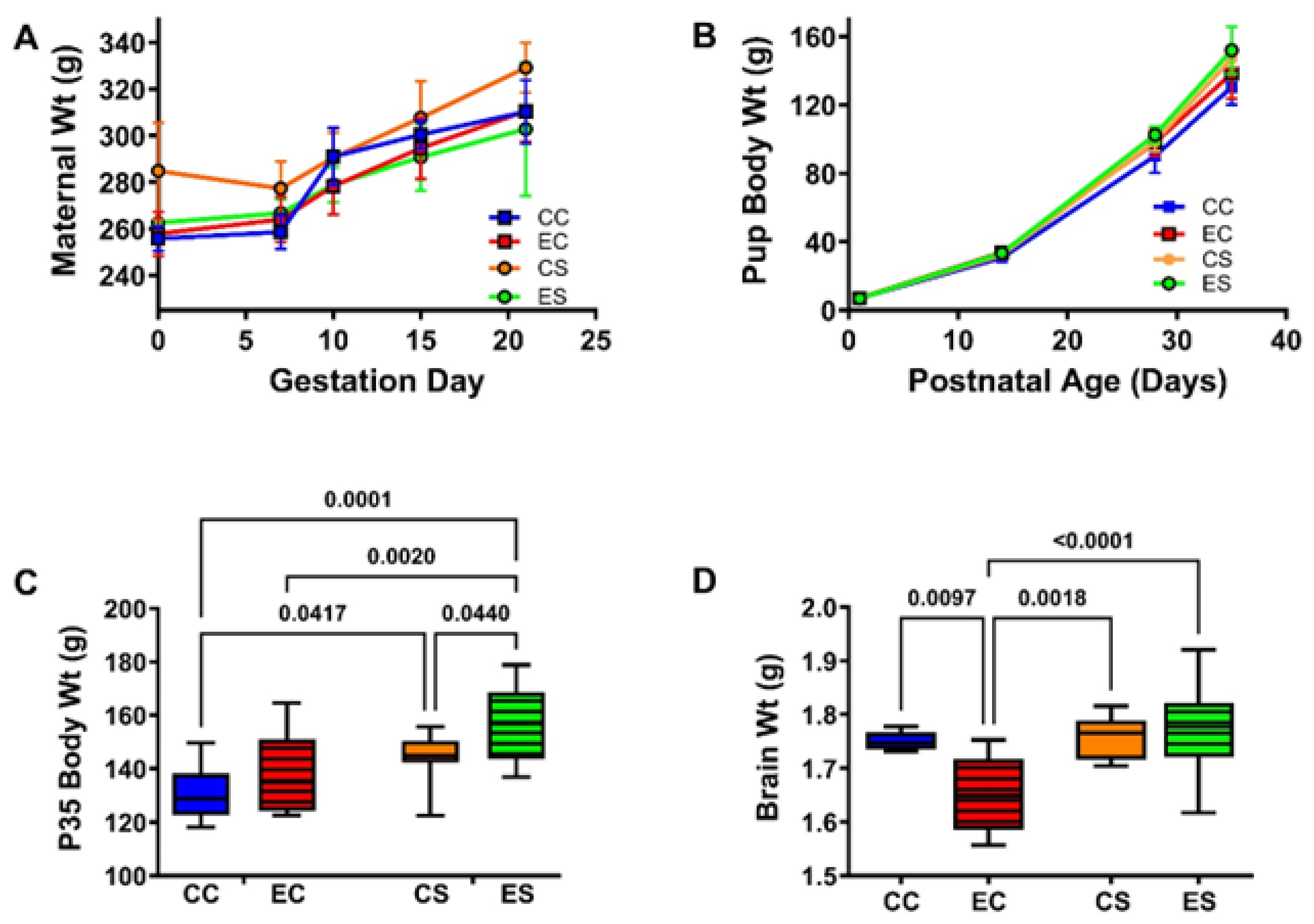
3.1. Growth and Brain Weight
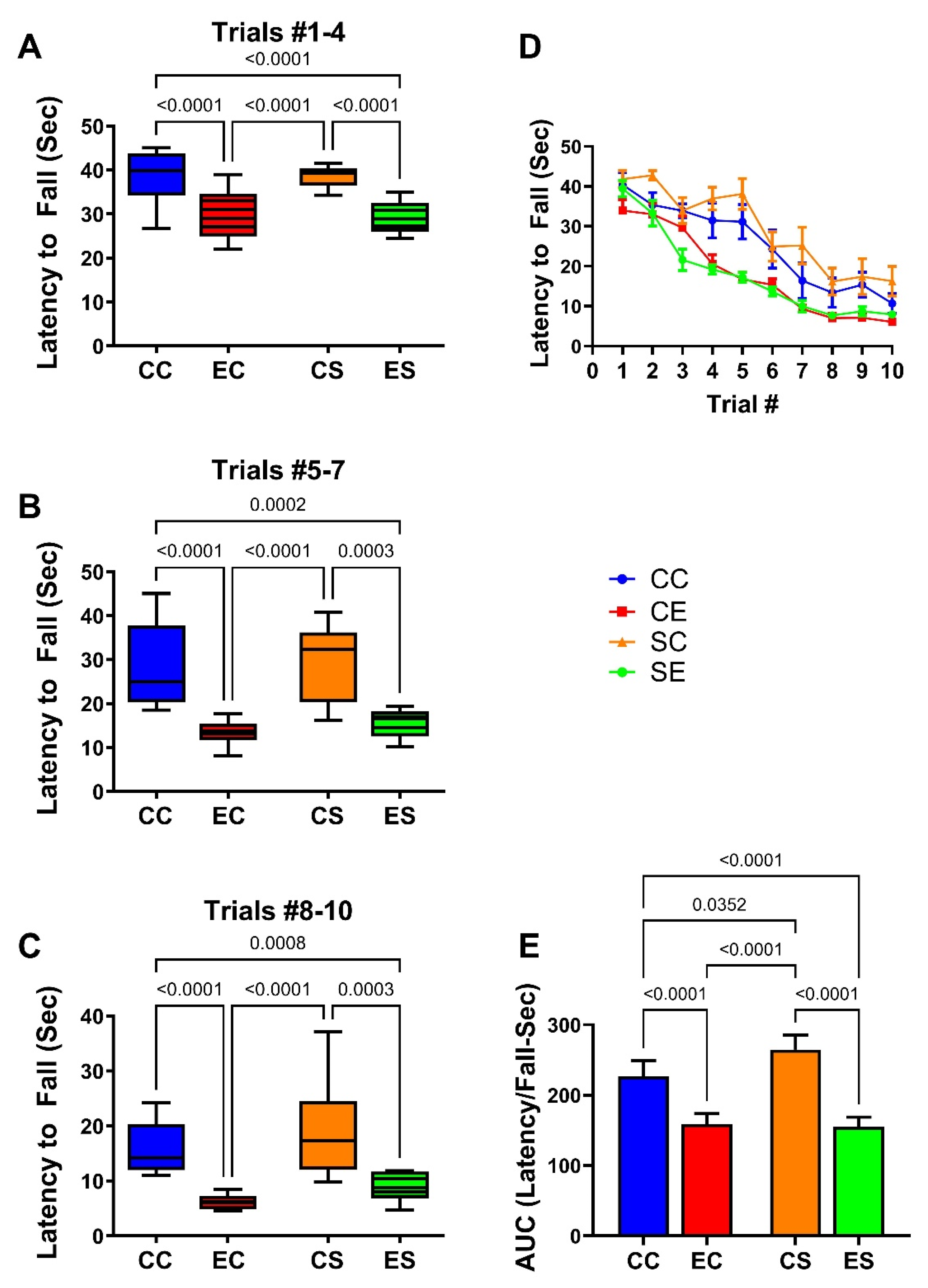
3.2. Neurobehavioral Testing
3.3. Cerebellar Pathology
3.4. Cerebellar Image Analysis
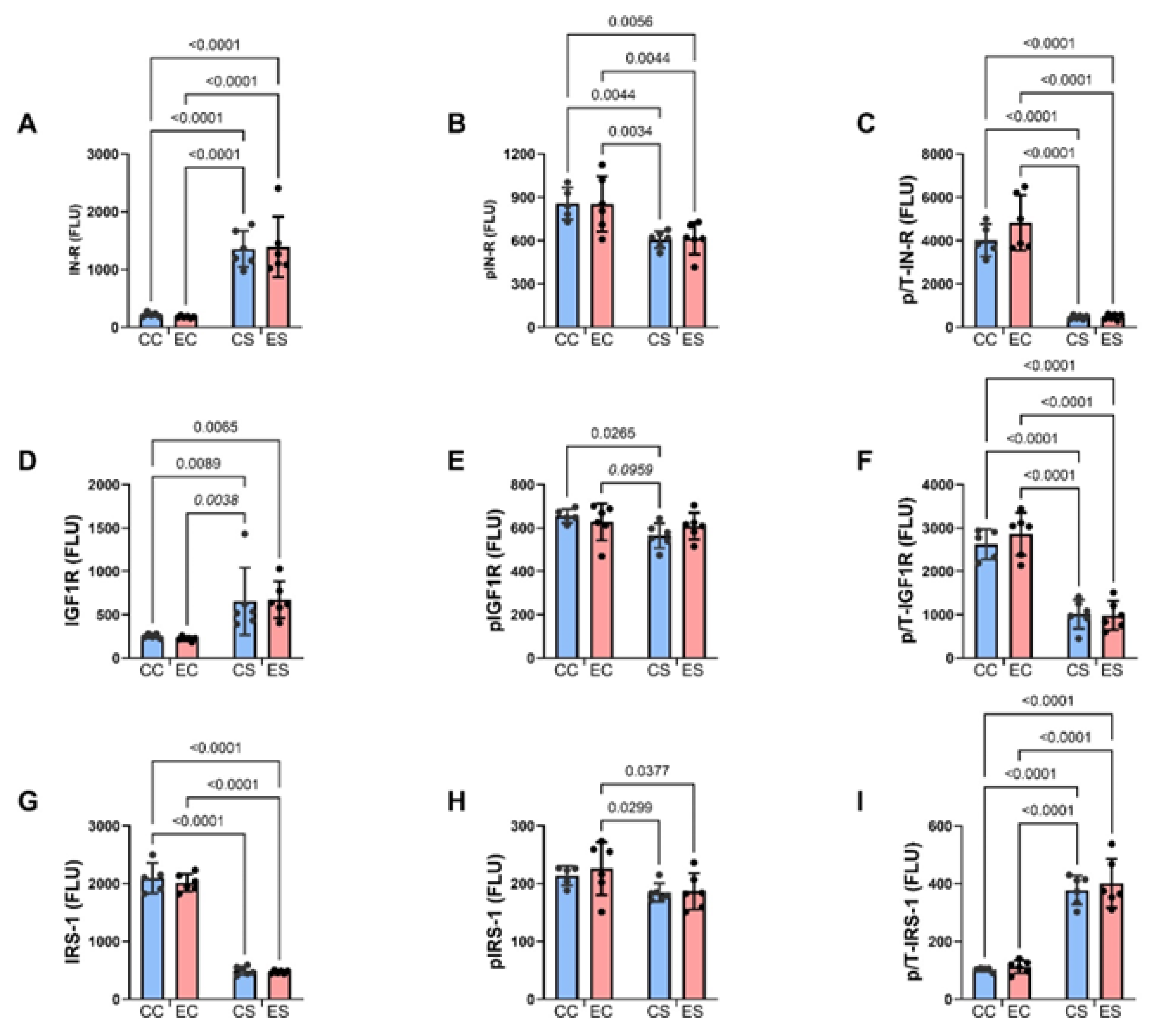
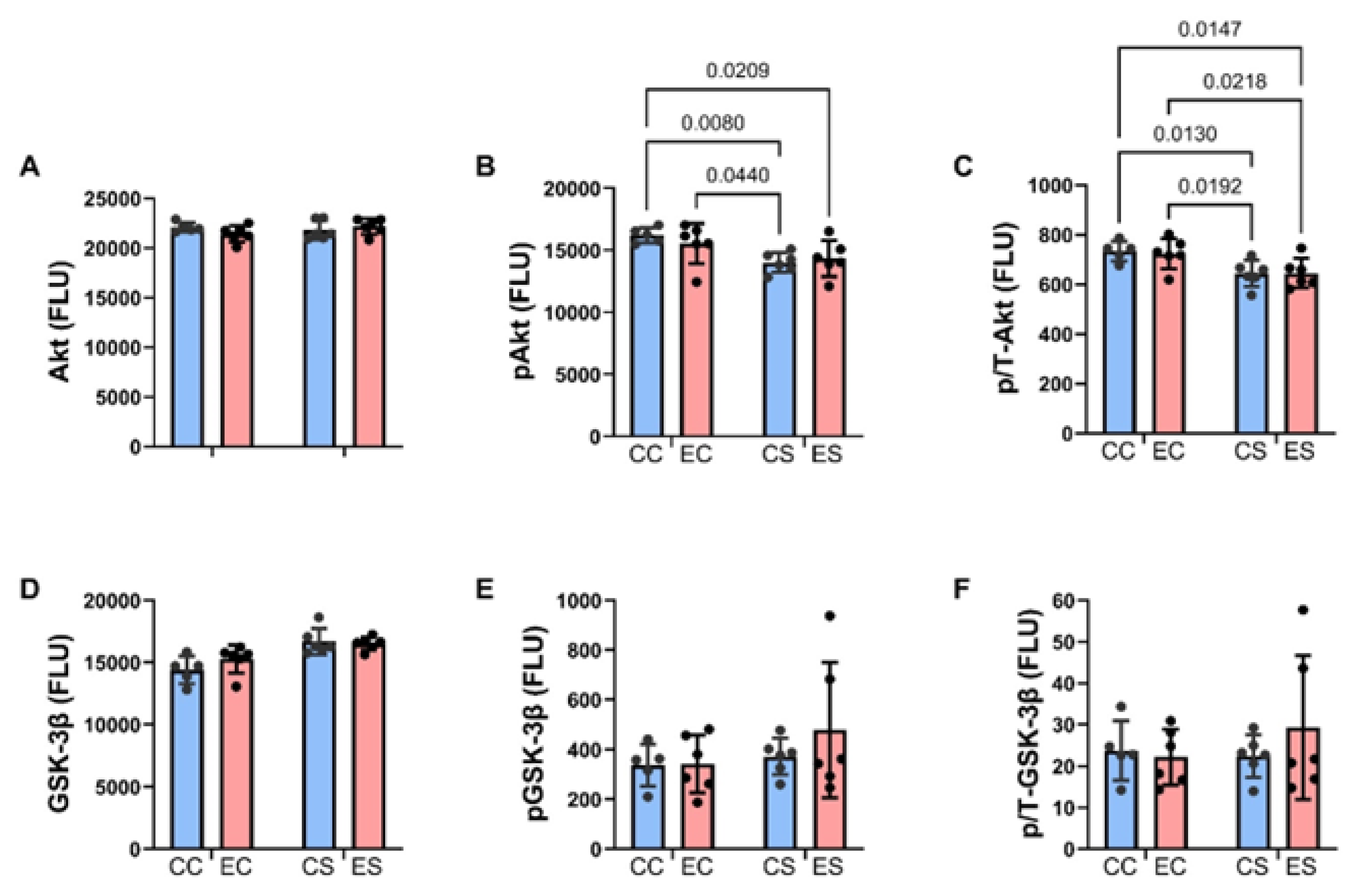
3.5. Multiplex Immunoassays of Insulin Receptor/IGF-1 Receptor/IRS-1 Pathway
| Molecule | Diet F-Ratio | p-Value | Ethanol F-Ratio | p-Value | Interaction F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| Insulin R | 79.95 | <0.0001 | 0.0002 | 0.989 | 0.078 | 0.784 |
| IGF-1R | 20.10 | 0.0003 | 0.003 | 0.954 | 0.0689 | 0.796 |
| IRS-1 | 642.9 | <0.0001 | 0.751 | 0.397 | 0.193 | 0.665 |
| Akt | 0.538 | 0.472 | 0.188 | 0.670 | 2.535 | 0.128 |
| GSK-3β | 18.61 | 0.0004 | 0.716 | 0.408 | 1.65 | 0.214 |
| pY-Insulin R | 20.86 | 0.0002 | 0.002 | 0.961 | 0.011 | 0.919 |
| pY-IGF-1R | 4.417 | 0.049 | 0.0998 | 0.756 | 1.902 | 0.184 |
| pS-IRS-1 | 7.140 | 0.015 | 0.322 | 0.577 | 0.169 | 0.686 |
| pS-Akt | 10.97 | 0.0037 | 0.119 | 0.742 | 0.947 | 0.343 |
| pS-GSK-3β | 1.574 | 0.225 | 0.672 | 0.423 | 0.565 | 0.462 |
| p/T-Insulin R | 160.5 | <0.0001 | 1.731 | 0.204 | 1.659 | 0.213 |
| p/T-IGF-1 R | 118.1 | <0.0001 | 0.395 | 0.537 | 0.674 | 0.422. |
| p/T-IRS-1 | 170.3 | <0.0001 | 0.584 | 0.454 | 0.096 | 0.761 |
| p/T-Akt | 13.75 | 0.0015 | 0.031 | 0.862 | 0.068 | 0.798 |
| p/T-GSK-3β | 0.363 | 0.554 | 0.455 | 0.515 | 0.922 | 0.349 |
| Molecule | Diet F-Ratio | p-Value | Ethanol F-Ratio | p-Value | Interaction F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| ASPH | 0.792 | 0.384 | 2.199 | 0.161 | 0.959 | 0.339 |
| NOTCH 1 | 2.044 | 0.168 | 0.041 | 0.842 | 0.000 | 0.980 |
| JAGGED 1 | 5.931 | 0.024 | 1.428 | 0.246 | 5.218 | 0.033 |
| HES1 | 1.100 | 0.307 | 1.251 | 0.277 | 3.603 | 0.072 |
| HIF-1α | 2.611 | 0.122 | 4.660 | 0.043 | 6.404 | 0.0199 |
| FIH | 4.374 | 0.049 | 0.249 | 0.623 | 9.646 | 0.0056 |

| Molecule | Diet F-Ratio | p-Value | Ethanol F-Ratio | p-Value | Interaction F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| Wnt 5a | 5.263 | 0.0327 | 7.269 | 0.0139 | 8.424 | 0.0088 |
| Wnt 5b | 4.05 | 0.0571 | 2.685 | 0.1169 | 5.063 | 0.0359 |
| Fzd 4 | 5.418 | 0.0305 | 0.99 | 0.33 | 0.006 | 0.937 |
| Fzd 6 | 5.702 | 0.0269 | 8.044 | 0.0102 | 7.776 | 0.0113 |
| EP300 | 0.060 | 0.808 | 5.788 | 0.0259 | 0.449 | 0.510 |
| Dixdc1 | 1.194 | 0.287 | 3.242 | 0.0869 | 11.59 | 0.0028 |
| Axin2 | 2.563 | 0.125 | 6.065 | 0.023 | 5.024 | 0.0365 |
3.6. Notch Pathway mRNA Studies
3.7. Wnt Pathway mRNA Studies

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayak, R.B.; Murthy, P. Fetal alcohol spectrum disorder. Indian Pediatr. 2008, 45, 977–983. [Google Scholar] [PubMed]
- Osborn, J.A.; Harris, S.R.; Weinberg, J. Fetal alcohol syndrome: Review of the literature with implications for physical therapists. Phys. Ther. 1993, 73, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Xia, Y. Neurodevelopmental Trajectories Following Prenatal Alcohol Exposure. Front. Hum. Neurosci. 2021, 15, 695855. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.P.; Infante, M.A.; Warren, K.R. Fetal alcohol spectrum disorders: An overview. Neuropsychol. Rev. 2011, 21, 73–80. [Google Scholar] [CrossRef]
- Riley, E.P.; McGee, C.L. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Exp. Biol. Med. 2005, 230, 357–365. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Moore, E.M.; Lane, B.; Pohl, K.M.; Riley, E.P.; Pfefferbaum, A. Graded Cerebellar Lobular Volume Deficits in Adolescents and Young Adults with Fetal Alcohol Spectrum Disorders (FASD). Cereb. Cortex 2020, 30, 4729–4746. [Google Scholar] [CrossRef]
- Chanraud, S.; Martelli, C.; Delain, F.; Kostogianni, N.; Douaud, G.; Aubin, H.J.; Reynaud, M.; Martinot, J.L. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 429–438. [Google Scholar] [CrossRef]
- Bookstein, F.L.; Streissguth, A.P.; Connor, P.D.; Sampson, P.D. Damage to the human cerebellum from prenatal alcohol exposure: The anatomy of a simple biometrical explanation. Anat. Rec. B New Anat. 2006, 289, 195–209. [Google Scholar] [CrossRef]
- Norman, A.L.; Crocker, N.; Mattson, S.N.; Riley, E.P. Neuroimaging and fetal alcohol spectrum disorders. Dev. Disabil. Res. Rev. 2009, 15, 209–217. [Google Scholar] [CrossRef]
- Dorrie, N.; Focker, M.; Freunscht, I.; Hebebrand, J. Fetal alcohol spectrum disorders. Eur. Child Adolesc. Psychiatry 2014, 23, 863–875. [Google Scholar] [CrossRef]
- Sawant, O.B.; Lunde, E.R.; Washburn, S.E.; Chen, W.J.; Goodlett, C.R.; Cudd, T.A. Different patterns of regional Purkinje cell loss in the cerebellar vermis as a function of the timing of prenatal ethanol exposure in an ovine model. Neurotoxicol. Teratol. 2013, 35, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cealie, M.Y.; Douglas, J.C.; Swan, H.K.; Vonkaenel, E.D.; McCall, M.N.; Drew, P.D.; Majewska, A.K. Developmental Ethanol Exposure Impacts Purkinje Cells but Not Microglia in the Young Adult Cerebellum. Cells 2024, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, I.; Alejandre-Gomez, M. Cerebellar granule cell and Bergmann glial cell maturation in the rat is disrupted by pre- and post-natal exposure to moderate levels of ethanol. Int. J. Dev. Neurosci. 2005, 23, 383–388. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Carlson, R.I.; Carter, J.J.; Longato, L.; Silbermann, E.; Wands, J.R. Ethanol inhibition of aspartyl-asparaginyl-beta-hydroxylase in fetal alcohol spectrum disorder: Potential link to the impairments in central nervous system neuronal migration. Alcohol 2009, 43, 225–240. [Google Scholar] [CrossRef]
- Niedzwiedz-Massey, V.M.; Douglas, J.C.; Rafferty, T.; Kane, C.J.M.; Drew, P.D. Ethanol effects on cerebellar myelination in a postnatal mouse model of fetal alcohol spectrum disorders. Alcohol 2021, 96, 43–53. [Google Scholar] [CrossRef]
- Adamo, M.; Raizada, M.K.; LeRoith, D. Insulin and insulin-like growth factor receptors in the nervous system. Mol. Neurobiol. 1989, 3, 71–100. [Google Scholar] [CrossRef]
- Torres-Aleman, I.; Pons, S.; Arevalo, M.A. The insulin-like growth factor I system in the rat cerebellum: Developmental regulation and role in neuronal survival and differentiation. J. Neurosci. Res. 1994, 39, 117–126. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Bowling, N.; Moskal, P. si-RNA inhibition of brain insulin or insulin-like growth factor receptors causes developmental cerebellar abnormalities: Relevance to fetal alcohol spectrum disorder. Mol. Brain 2011, 4, 13. [Google Scholar] [CrossRef]
- Qi, W.; Gundogan, F.; Gilligan, J.; Monte, S. Dietary soy prevents fetal demise, intrauterine growth restriction, craniofacial dysmorphic features, and impairments in placentation linked to gestational alcohol exposure: Pivotal role of insulin and insulin-like growth factor signaling networks. Alcohol 2023, 110, 65–81. [Google Scholar] [CrossRef]
- Lawton, M.; Tong, M.; Gundogan, F.; Wands, J.R.; de la Monte, S.M. Aspartyl-(asparaginyl) beta-hydroxylase, hypoxia-inducible factor-alpha and Notch cross-talk in regulating neuronal motility. Oxid. Med. Cell Longev. 2010, 3, 347–356. [Google Scholar] [CrossRef]
- Gundogan, F.; Elwood, G.; Greco, D.; Rubin, L.P.; Pinar, H.; Carlson, R.I.; Wands, J.R.; de la Monte, S.M. Role of aspartyl-(asparaginyl) beta-hydroxylase in placental implantation: Relevance to early pregnancy loss. Hum. Pathol. 2007, 38, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Cantarini, M.C.; de la Monte, S.M.; Pang, M.; Tong, M.; D’Errico, A.; Trevisani, F.; Wands, J.R. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology 2006, 44, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Gao, J.S.; Borgas, D.; de la Monte, S.M. Phosphorylation Modulates Aspartyl-(Asparaginyl)-beta Hydroxylase Protein Expression, Catalytic Activity and Migration in Human Immature Neuronal Cerebellar Cells. Cell Biol. 2013, 6, 133. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knofler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Mehta, S.; Hingole, S.; Chaudhary, V. The Emerging Mechanisms of Wnt Secretion and Signaling in Development. Front. Cell Dev. Biol. 2021, 9, 714746. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.V.; Jayusman, P.A.; Ibrahim, N. A Review on the Crosstalk between Insulin and Wnt/beta-Catenin Signalling for Bone Health. Int. J. Mol. Sci. 2023, 24, 12441. [Google Scholar] [CrossRef]
- Palsgaard, J.; Emanuelli, B.; Winnay, J.N.; Sumara, G.; Karsenty, G.; Kahn, C.R. Cross-talk between insulin and Wnt signaling in preadipocytes: Role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5). J. Biol. Chem. 2012, 287, 12016–12026. [Google Scholar] [CrossRef]
- Collu, G.M.; Hidalgo-Sastre, A.; Brennan, K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol. Life Sci. 2014, 71, 3553–3567. [Google Scholar] [CrossRef]
- Acar, A.; Hidalgo-Sastre, A.; Leverentz, M.K.; Mills, C.G.; Woodcock, S.; Baron, M.; Collu, G.M.; Brennan, K. Inhibition of Wnt signalling by Notch via two distinct mechanisms. Sci. Rep. 2021, 11, 9096. [Google Scholar] [CrossRef]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef]
- Tong, M.; Dominguez, C.; Didsbury, J.; de la Monte, S.M. Targeting Alzheimer’s Disease Neuro-Metabolic Dysfunction with a Small Molecule Nuclear Receptor Agonist (T3D-959) Reverses Disease Pathologies. J. Alzheimers Dis. Park. 2016, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xing, A.; Li, S. The forgotten type 2 diabetes mellitus medicine: Rosiglitazone. Diabetol. Int. 2022, 13, 49–65. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Xie, X.B.; Cheng, X.C.; Wang, R.L. Multitargeted bioactive ligands for PPARs discovered in the last decade. Chem. Biol. Drug Des. 2016, 88, 635–663. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M. Soybean, Nutrition and Health. In Soybean-Bio-Active Compounds; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Tong, M.; Ziplow, J.L.; Mark, P.; de la Monte, S.M. Dietary Soy Prevents Alcohol-Mediated Neurocognitive Dysfunction and Associated Impairments in Brain Insulin Pathway Signaling in an Adolescent Rat Model. Biomolecules 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.W.; Dudink, J.; Wijnands, B.; De Zeeuw, C.I.; Canto, C.B. Impact of Intrauterine Insults on Fetal and Postnatal Cerebellar Development in Humans and Rodents. Cells 2024, 13, 1911. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.; Tong, M.; Monte, S.M.d.l. Association between dietary soy prevention of fetal alcohol spectrum disorder and normalization of placental insulin and insulin-like growth factor signaling networks and downstream effector molecule expression. Gene Protein Dis. 2024, 3, 3113. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.; Elwood, G.; Longato, L.; Tong, M.; Feijoo, A.; Carlson, R.I.; Wands, J.R.; de la Monte, S.M. Impaired placentation in fetal alcohol syndrome. Placenta 2008, 29, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.I.; Barbosa, A.C.; Pinto Mda, S.; Lajolo, F.M. Commercial soy protein ingredients as isoflavone sources for functional foods. Plant Foods Hum. Nutr. 2007, 62, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Eberini, I.; Arnoldi, A.; Wait, R.; Sirtori, C.R. A proteomic investigation of isolated soy proteins with variable effects in experimental and clinical studies. J. Nutr. 2003, 133, 9–14. [Google Scholar] [CrossRef]
- Jimenez, J.A.; Zylka, M.J. Controlling litter effects to enhance rigor and reproducibility with rodent models of neurodevelopmental disorders. J. Neurodev. Disord. 2021, 13, 2. [Google Scholar] [CrossRef]
- Lazic, S.E.; Essioux, L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Ewenczyk, A.; Ziplow, J.; Tong, M.; Le, T.; de la Monte, S.M. Sustained Impairments in Brain Insulin/IGF Signaling in Adolescent Rats Subjected to Binge Alcohol Exposures during Development. J. Clin. Exp. Pathol. 2012, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.R.; Bae, S.H.; Wales, T.E.; Engen, J.R.; Lee, J.; Jang, H.; Park, S. The serine phosphorylations in the IRS-1 PIR domain abrogate IRS-1 and IR interaction. Proc. Natl. Acad. Sci. USA 2024, 121, e2401716121. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Ziplow, J.; Chen, W.C.; Nguyen, Q.G.; Kim, C.; de la Monte, S.M. Motor Function Deficits Following Chronic Prenatal Ethanol Exposure are Linked to Impairments in Insulin/IGF, Notch and Wnt Signaling in the Cerebellum. J. Diabetes Metab. 2013, 4, 238. [Google Scholar] [CrossRef]
- Gundogan, F.; Gilligan, J.; Qi, W.; Chen, E.; Naram, R.; de la Monte, S.M. Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta 2015, 36, 523–530. [Google Scholar] [CrossRef]
- Gundogan, F.; Qi, W.; Gilligan, J.; de la Monte, S. Effects of dietary soy on ethanol-impaired placentation and fetal growth. Placenta 2013, 34, A37. [Google Scholar] [CrossRef]
- Yalcin, E.B.; Tong, M.; de la Monte, S.M. Altered Oligodendroglial and Neuroglial Gene Expression in Adult Rat Cerebral White Matter Following Short- and Long-Term Ethanol Exposures and Brief Abstinence. J. Drug Alc. Res. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Crews, F.T.; Nixon, K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009, 44, 115–127. [Google Scholar] [CrossRef]
- Meyerhoff, D.J.; Bloomer, C.; Schuff, N.; Ezekiel, F.; Norman, D.; Clark, W.; Weiner, M.W.; Fein, G. Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: Proton magnetic resonance spectroscopic imaging. Addict. Biol. 1999, 4, 405–419. [Google Scholar] [CrossRef]
- Monnig, M.A.; Tonigan, J.S.; Yeo, R.A.; Thoma, R.J.; McCrady, B.S. White matter volume in alcohol use disorders: A meta-analysis. Addict. Biol. 2013, 18, 581–592. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Adalsteinsson, E.; Sullivan, E.V. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol. Aging 2006, 27, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Ostrea, E.M., Jr.; Cheng, C.T.; Kisseih, E.; Maddipati, K.R.; Thomas, R.L. Effect of docosahexaenoic acid and olive oil supplementation on pup weight in alcohol-exposed pregnant rats. Front. Pediatr. 2024, 12, 1334285. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Kobus, S.M.; Ji, Y.; Bennett, B.M.; Reynolds, J.N.; Brien, J.F. Chronic prenatal ethanol exposure increases apoptosis in the hippocampus of the term fetal guinea pig. Neurotoxicol. Teratol. 2005, 27, 871–881. [Google Scholar] [CrossRef]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef]
- Forbes, K.; Westwood, M. Maternal growth factor regulation of human placental development and fetal growth. J. Endocrinol. 2010, 207, 1–16. [Google Scholar] [CrossRef]
- McDonald, T.J.; Nijland, M.J.; Nathanielsz, P.W. The insulin-like growth factor system and the fetal brain: Effects of poor maternal nutrition. Rev. Endocr. Metab. Disord. 2007, 8, 71–84. [Google Scholar] [CrossRef]
- Khalil, D.A.; Lucas, E.A.; Juma, S.; Smith, B.J.; Payton, M.E.; Arjmandi, B.H. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J. Nutr. 2002, 132, 2605–2608. [Google Scholar] [CrossRef]
- Gao, Q.G.; Xie, J.X.; Wong, M.S.; Chen, W.F. IGF-I receptor signaling pathway is involved in the neuroprotective effect of genistein in the neuroblastoma SK-N-SH cells. Eur. J. Pharmacol. 2012, 677, 39–46. [Google Scholar] [CrossRef]
- Rocamora, N.; Garcia-Ladona, F.J.; Palacios, J.M.; Mengod, G. Differential expression of brain-derived neurotrophic factor, neurotrophin-3, and low-affinity nerve growth factor receptor during the postnatal development of the rat cerebellar system. Brain Res. Mol. Brain Res. 1993, 17, 1–8. [Google Scholar] [CrossRef]
- Vinters, H.V.; Gatti, R.A.; Rakic, P. Sequence of cellular events in cerebellar ontogeny relevant to expression of neuronal abnormalities in ataxia-telangiectasia. Kroc Found. Ser. 1985, 19, 233–255. [Google Scholar]
- West, J.R.; Parnell, S.E.; Chen, W.J.; Cudd, T.A. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcohol. Clin. Exp. Res. 2001, 25, 1051–1057. [Google Scholar] [PubMed]
- de La Monte, S.M.; Sutherland, G.T. Dual Stages of Alcohol-Related Cerebral White Matter Degeneration Reviewed: Early-Stage Stress/Neuroinflammation Versus Late-Stage Impaired Insulin/IGF Signaling Through Akt-mTOR—Review. ASN Neuro, 2025, in press.
- Kim, M.; Jun, S.; Park, H.; Tanaka-Yamamoto, K.; Yamamoto, Y. Regulation of cerebellar network development by granule cells and their molecules. Front. Mol. Neurosci. 2023, 16, 1236015. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Yeon, J.E.; Tong, M.; Longato, L.; Chaudhry, R.; Pang, M.Y.; Duan, K.; Wands, J.R. Insulin resistance in experimental alcohol-induced liver disease. J. Gastroenterol. Hepatol. 2008, 23, e477–e486. [Google Scholar] [CrossRef]
- Lasky, J.L.; Wu, H. Notch signaling, brain development, and human disease. Pediatr. Res. 2005, 57, 104R–109R. [Google Scholar] [CrossRef]
- Ables, J.L.; Breunig, J.J.; Eisch, A.J.; Rakic, P. Not(ch) just development: Notch signalling in the adult brain. Nat. Rev. Neurosci. 2011, 12, 269–283. [Google Scholar] [CrossRef]
- Dinchuk, J.E.; Henderson, N.L.; Burn, T.C.; Huber, R.; Ho, S.P.; Link, J.; O’Neil, K.T.; Focht, R.J.; Scully, M.S.; Hollis, J.M.; et al. Aspartyl beta -hydroxylase (Asph) and an evolutionarily conserved isoform of Asph missing the catalytic domain share exons with junctin. J. Biol. Chem. 2000, 275, 39543–39554. [Google Scholar] [CrossRef]
- Ince, N.; de la Monte, S.M.; Wands, J.R. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is associated with malignant transformation. Cancer Res. 2000, 60, 1261–1266. [Google Scholar]
- Jia, S.; VanDusen, W.J.; Diehl, R.E.; Kohl, N.E.; Dixon, R.A.; Elliston, K.O.; Stern, A.M.; Friedman, P.A. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J. Biol. Chem. 1992, 267, 14322–14327. [Google Scholar] [CrossRef]
- McGinnis, K.; Ku, G.M.; VanDusen, W.J.; Fu, J.; Garsky, V.; Stern, A.M.; Friedman, P.A. Site-directed mutagenesis of residues in a conserved region of bovine aspartyl (asparaginyl) beta-hydroxylase: Evidence that histidine 675 has a role in binding Fe2+. Biochemistry 1996, 35, 3957–3962. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Park, J.S.; Manzanero, S.; Choi, Y.; Baik, S.H.; Okun, E.; Gelderblom, M.; Fann, D.Y.; Magnus, T.; Launikonis, B.S.; et al. Evidence that collaboration between HIF-1alpha and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol. Dis. 2014, 62, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, L.; Yu, M.; Yang, F.; Wu, B.; Lu, S.; Tu, M.; Xu, H. HIF-1alpha is Critical for the Activation of Notch Signaling in Neurogenesis During Acute Epilepsy. Neuroscience 2018, 394, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Linke, S.; Dias, J.M.; Zheng, X.; Gradin, K.; Wallis, T.P.; Hamilton, B.R.; Gustafsson, M.; Ruas, J.L.; Wilkins, S.; et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 3368–3373. [Google Scholar] [CrossRef]
- Coleman, M.L.; McDonough, M.A.; Hewitson, K.S.; Coles, C.; Mecinovic, J.; Edelmann, M.; Cook, K.M.; Cockman, M.E.; Lancaster, D.E.; Kessler, B.M.; et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J. Biol. Chem. 2007, 282, 24027–24038. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1alpha. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.; Bedoya, A.; Gilligan, J.; Lau, E.; Mark, P.; De Paepe, M.E.; de la Monte, S.M. siRNA inhibition of aspartyl-asparaginyl beta-hydroxylase expression impairs cell motility, Notch signaling, and fetal growth. Pathol. Res. Pr. 2011, 207, 545–553. [Google Scholar] [CrossRef]
- Aihara, A.; Huang, C.K.; Olsen, M.J.; Lin, Q.; Chung, W.; Tang, Q.; Dong, X.; Wands, J.R. A cell-surface beta-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology 2014, 60, 1302–1313. [Google Scholar] [CrossRef]
- Silbermann, E.; Moskal, P.; Bowling, N.; Tong, M.; de la Monte, S.M. Role of aspartyl-(asparaginyl)-beta-hydroxylase mediated notch signaling in cerebellar development and function. Behav. Brain Funct. 2010, 6, 68. [Google Scholar] [CrossRef]
- Boopathy, A.V.; Pendergrass, K.D.; Che, P.L.; Yoon, Y.S.; Davis, M.E. Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res. Ther. 2013, 4, 43. [Google Scholar] [CrossRef]
- Clark, J.L.; Taylor, C.G.; Zahradka, P. Rebelling against the (Insulin) Resistance: A Review of the Proposed Insulin-Sensitizing Actions of Soybeans, Chickpeas, and Their Bioactive Compounds. Nutrients 2018, 10, 434. [Google Scholar] [CrossRef]
- Tovar, A.R.; Torre-Villalvazo, I.; Ochoa, M.; Elias, A.L.; Ortiz, V.; Aguilar-Salinas, C.A.; Torres, N. Soy protein reduces hepatic lipotoxicity in hyperinsulinemic obese Zucker fa/fa rats. J. Lipid Res. 2005, 46, 1823–1832. [Google Scholar] [CrossRef]
- Wagner, J.D.; Zhang, L.; Shadoan, M.K.; Kavanagh, K.; Chen, H.; Tresnasari, K.; Kaplan, J.R.; Adams, M.R. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism 2008, 57, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Halterman, M.W.; Miller, C.C.; Federoff, H.J. Hypoxia-Inducible Factor-1α Mediates Hypoxia-Induced Delayed Neuronal Death That Involves p53. J. Neurosci. 1999, 19, 6818–6824. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Wang, D.C.; Wang, Y.Q.; Huang, A.F.; Xu, W.D. Emerging role of hypoxia-inducible factor-1alpha in inflammatory autoimmune diseases: A comprehensive review. Front. Immunol. 2022, 13, 1073971. [Google Scholar] [CrossRef]
- Mialet-Perez, J.; Belaidi, E. Interplay between hypoxia inducible Factor-1 and mitochondria in cardiac diseases. Free Radic. Biol. Med. 2024, 221, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Halleskog, C.; Mulder, J.; Dahlstrom, J.; Mackie, K.; Hortobagyi, T.; Tanila, H.; Kumar Puli, L.; Farber, K.; Harkany, T.; Schulte, G. WNT signaling in activated microglia is proinflammatory. Glia 2011, 59, 119–131. [Google Scholar] [CrossRef]
- Chen, C.M.; Orefice, L.L.; Chiu, S.L.; LeGates, T.A.; Hattar, S.; Huganir, R.L.; Zhao, H.; Xu, B.; Kuruvilla, R. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc. Natl. Acad. Sci. USA 2017, 114, E619–E628. [Google Scholar] [CrossRef]
- Subashini, C.; Dhanesh, S.B.; Chen, C.M.; Riya, P.A.; Meera, V.; Divya, T.S.; Kuruvilla, R.; Buttler, K.; James, J. Wnt5a is a crucial regulator of neurogenesis during cerebellum development. Sci. Rep. 2017, 7, 42523. [Google Scholar] [CrossRef]
- Suthon, S.; Perkins, R.S.; Bryja, V.; Miranda-Carboni, G.A.; Krum, S.A. WNT5B in Physiology and Disease. Front. Cell Dev. Biol. 2021, 9, 667581. [Google Scholar] [CrossRef]
- Pascual-Vargas, P.; Salinas, P.C. A Role for Frizzled and Their Post-Translational Modifications in the Mammalian Central Nervous System. Front. Cell Dev. Biol. 2021, 9, 692888. [Google Scholar] [CrossRef]
- Corda, G.; Sala, A. Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis 2017, 6, e364. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Martens, H.; Hassouna, I.; Oliveira, B.; Erck, C.; Zafeiriou, M.P.; Peteri, U.K.; Hesse, D.; Gerhart, S.; Altas, B.; et al. Widespread Expression of Erythropoietin Receptor in Brain and Its Induction by Injury. Mol. Med. 2015, 21, 803–815. [Google Scholar] [CrossRef]
- Rouhi, L.; Fan, S.; Cheedipudi, S.M.; Braza-Boils, A.; Molina, M.S.; Yao, Y.; Robertson, M.J.; Coarfa, C.; Gimeno, J.R.; Molina, P.; et al. The EP300/TP53 pathway, a suppressor of the Hippo and canonical WNT pathways, is activated in human hearts with arrhythmogenic cardiomyopathy in the absence of overt heart failure. Cardiovasc. Res. 2022, 118, 1466–1478. [Google Scholar] [CrossRef]
- Singh, K.K.; Ge, X.; Mao, Y.; Drane, L.; Meletis, K.; Samuels, B.A.; Tsai, L.H. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 2010, 67, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.D.; Idrus, N.M.; Monk, B.R.; Dominguez, H.D. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 827–837. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J. Neurodev. Disord. 2020, 12, 9. [Google Scholar] [CrossRef]


| Variable | Diet F-Ratio | p-Value | Ethanol F-Ratio | p-Value | Interaction F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| Dam’s Weight GD0 | 3.363 | 0.175 | 2.496 | 0.153 | 1.698 | 0.229 |
| Dam’s Weight-GD21/P0 | 0.075 | 0.791 | 1.775 | 0.327 | 1.088 | 0.328 |
| Offspring P0 Birth Weight | 0.129 | 0.722 | 1.075 | 0.302 | 0.676 | 0.417 |
| Offspring P35 Body Weight | 14.41 | 0.0006 | 6.119 | 0.0185 | 0.358 | 0.554 |
| Offspring P35 Brain Weight | 7.491 | 0.0096 | 3.229 | 0.08 | 5.413 | 0.0257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Monte, S.M.; Tong, M.; Ziplow, J.; Mark, P.; Van, S.; Nguyen, V.A. Impact of Prenatal Dietary Soy on Cerebellar Neurodevelopment and Function in Experimental Fetal Alcohol Spectrum Disorder. Nutrients 2025, 17, 812. https://doi.org/10.3390/nu17050812
de la Monte SM, Tong M, Ziplow J, Mark P, Van S, Nguyen VA. Impact of Prenatal Dietary Soy on Cerebellar Neurodevelopment and Function in Experimental Fetal Alcohol Spectrum Disorder. Nutrients. 2025; 17(5):812. https://doi.org/10.3390/nu17050812
Chicago/Turabian Stylede la Monte, Suzanne M., Ming Tong, Jason Ziplow, Princess Mark, Stephanie Van, and Van Ahn Nguyen. 2025. "Impact of Prenatal Dietary Soy on Cerebellar Neurodevelopment and Function in Experimental Fetal Alcohol Spectrum Disorder" Nutrients 17, no. 5: 812. https://doi.org/10.3390/nu17050812
APA Stylede la Monte, S. M., Tong, M., Ziplow, J., Mark, P., Van, S., & Nguyen, V. A. (2025). Impact of Prenatal Dietary Soy on Cerebellar Neurodevelopment and Function in Experimental Fetal Alcohol Spectrum Disorder. Nutrients, 17(5), 812. https://doi.org/10.3390/nu17050812






