A Narrative Review on Higenamine: Pharmacological Properties and Clinical Applications
Abstract
:1. Introduction
2. Methods
- (1)
- Study design: Only randomized controlled trials (RCTs) or mechanistic pathway studies will be included. RCTs must have a clear randomization process, while mechanistic studies should focus on elucidating the biological pathways or mechanisms of action related to higenamine;
- (2)
- Intervention: Studies must explicitly use higenamine as the primary drug or dietary supplement. This includes research investigating its pharmacological effects, therapeutic potential, or physiological impacts;
- (3)
- Language: Studies published in English or Chinese will be considered to ensure accessibility and relevance to the target audience;
- (4)
- Study subjects: Both animal and human studies are eligible for inclusion, provided they meet the other criteria. This allows for a comprehensive evaluation of higenamine’s effects across different biological systems.
- (1)
- Study type: Studies focused solely on drug testing (e.g., doping testing methods and testing model establishment) will be excluded;
- (2)
- Language: Studies published in languages other than English or Chinese will be excluded to maintain consistency and avoid potential translation errors;
- (3)
- Intervention: Studies that treat higenamine as a minor component of classical drug formulation or compound mixture will be excluded. The focus is research on where higenamine is the primary agent of interest.
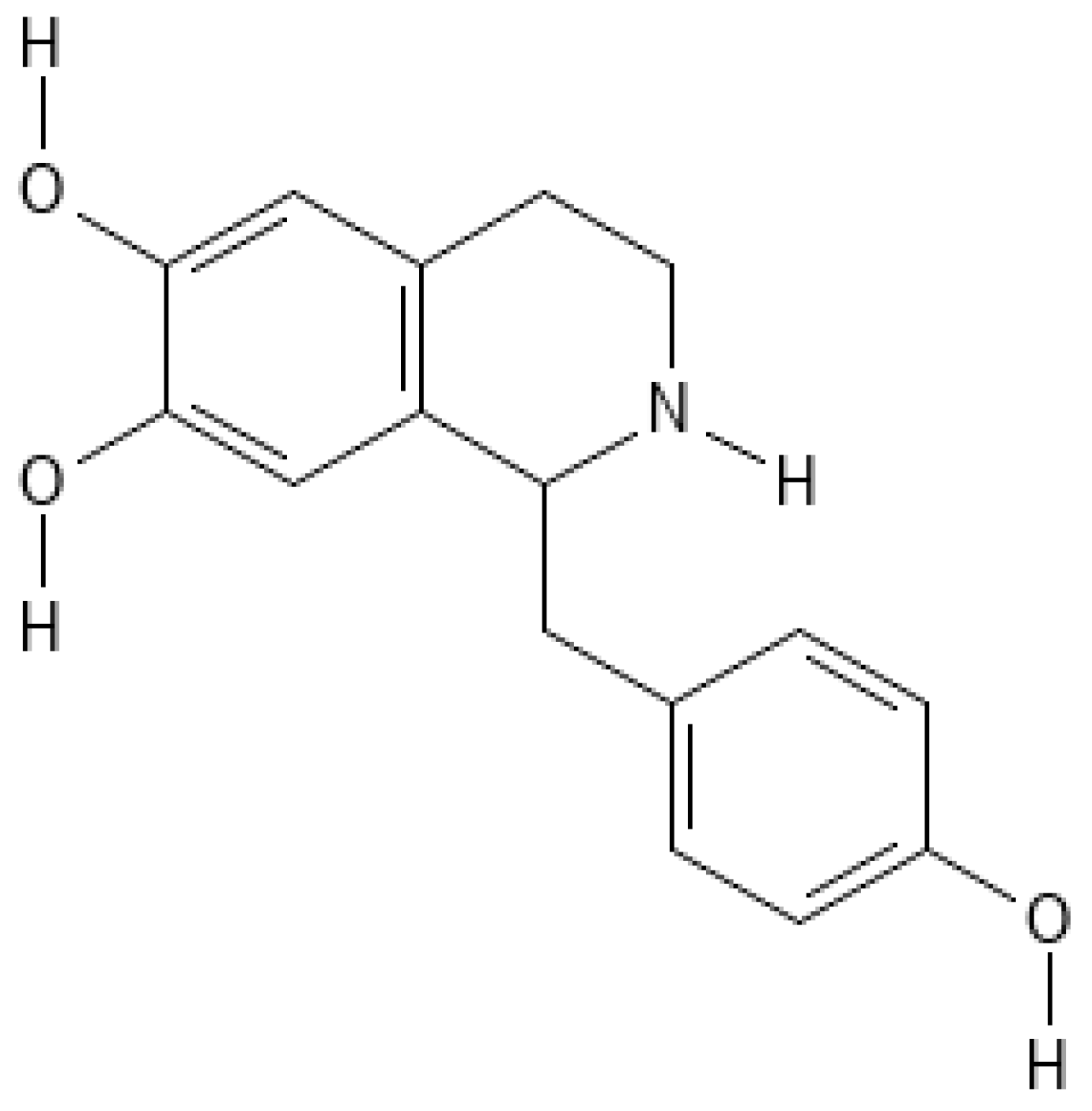
3. Chemical Structure and Properties
3.1. Structural Insights and Receptor Interactions
3.2. Chemical Properties
4. Pharmacological Properties
4.1. Pharmacokinetic Profile
4.2. Cardiovascular Effects
4.3. Weight Loss and Lipolysis
4.4. Anti-Inflammatory and Antioxidant Effects and Mechanisms
4.4.1. Ischemia and Reperfusion Injury Prevention
4.4.2. Neuroprotective Effect
4.4.3. Anti-Inflammatory Effects
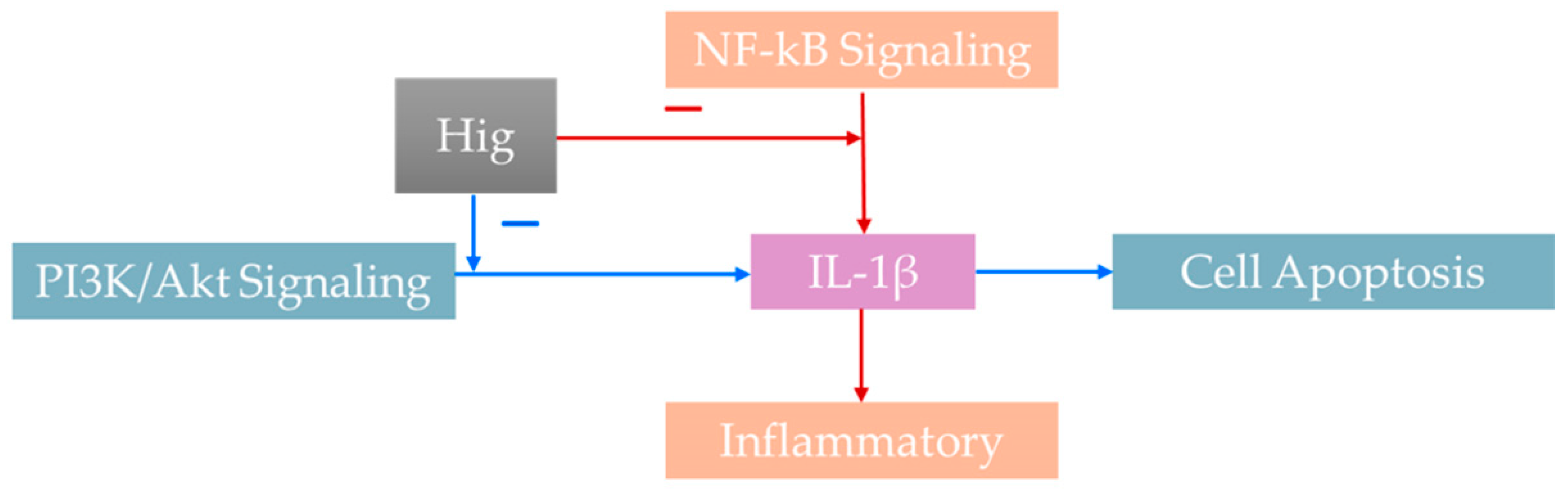
4.5. PI3K/Akt Signaling Pathway
4.5.1. Cell Apoptosis
4.5.2. Role in Diabetic Gastroparesis
4.5.3. Treatment of Rheumatoid Arthritis
4.6. Treatment of Osteoporosis
4.7. Potential Health Risks
5. Clinical Applications and Anti-Doping
5.1. Respiratory Disorders
5.2. Anti-Doping
6. Discussion
6.1. The Main Findings of the Review
6.1.1. Clinical Applications of Higenamine
6.1.2. Mechanism of Action of Higenamine
6.2. Strength and Limitations of the Review
6.3. The Clinical Implication of Findings
6.4. The New Direction for Future Research
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, J.; Li, M.; Zhang, W.; Wang, H.; Bai, Y.; Hao, J.; Liu, C.; Deng, K.; Zhao, Y. Role of Higenamine in Heart Diseases: A Mini-Review. Front. Pharmacol. 2021, 12, 798495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, Y.; Wei, H.; Fei, Q.; Luan, Z.; Wang, X.; Xu, Y.; Lu, J. Identification and characterization of higenamine metabolites in human urine by quadrupole-orbitrap LC-MS/MS for doping control. J. Pharm. Biomed. Anal. 2022, 214, 114732. [Google Scholar] [CrossRef] [PubMed]
- Kam, S.C.; Do, J.M.; Choi, J.H.; Jeon, B.T.; Roh, G.S.; Chang, K.C.; Hyun, J.S. The relaxation effect and mechanism of action of higenamine in the rat corpus cavernosum. Int. J. Impot. Res. 2012, 24, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Santos, R.; Avezum, J.; Abidão-Neto, B.; Cameron, L.C. Dietary higenamine from Annonaceae family fruits as a possible source of unintentional doping. Forensic Sci. Int. 2023, 342, 111539. [Google Scholar] [CrossRef]
- Wellstein, A.; Belz, G.G.; Palm, D. Beta adrenoceptor subtype binding activity in plasma and beta blockade by propranolol and beta-1 selective bisoprolol in humans. Evaluation with Schild-plots. J. Pharmacol. Exp. Ther. 1988, 246, 328–337. [Google Scholar] [CrossRef]
- Bai, G.; Yang, Y.; Shi, Q.; Liu, Z.; Zhang, Q.; Zhu, Y.-Y. Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist1. Acta Pharmacol. Sin. 2008, 29, 1187–1194. [Google Scholar] [CrossRef]
- Kimura, I.; Makino, M.; Takamura, Y.; Islam, M.A.; Kimura, M. Positive chronotropic and inotropic effects of higenamine and its enhancing action on the aconitine-induced tachyarrhythmia in isolated murine atria. Jpn. J. Pharmacol. 1994, 66, 75–80. [Google Scholar] [CrossRef]
- Toogood, P.L.; Clauw, D.J.; Phadke, S.; Hoffman, D. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Where will the drugs come from? Pharmacol. Res. 2021, 165, 105465. [Google Scholar] [CrossRef]
- Dolores, H.M.; Villaseñor, A.; Piña, O.S.; Mercado Márquez, C.; Bejarano, B.V.; Bonaparte, M.E.G.; López-Arellano, R. Evaluation of R- (−) and S- (+) Clenbuterol enantiomers during a doping cycle or continuous ingestion of contaminated meat using chiral liquid chromatography by LC-TQ-MS. Drug Test. Anal. 2019, 11, 1238–1247. [Google Scholar] [CrossRef]
- Pellegrini, M.; Itani, L.; Rossi, A.P.; Kreidieh, D.; El Masri, D.; Tannir, H.; El Ghoch, M. Approaching Sarcopenic Obesity in Young and Middle-Aged Female Adults in Weight Management Settings: A Narrative Review. Healthcare 2022, 10, 2042. [Google Scholar] [CrossRef]
- Bencivenga, L.; Liccardo, D.; Napolitano, C.; Visaggi, L.; Rengo, G.; Leosco, D. β-Adrenergic Receptor Signaling and Heart Failure: From Bench to Bedside. Heart Fail. Clin. 2019, 15, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.K.; Tao, Y.X. Physiology and pathophysiology of the β(3)-adrenergic receptor. Prog. Mol. Biol. Transl. Sci. 2019, 161, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.M.; Ahmadian, M.; Keinan, O.; Abu-Odeh, M.; Zhao, P.; Zhou, X.; Keller, M.P.; Gao, H.; Yu, R.T.; Liddle, C.; et al. β3-Adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J. Clin. Investig. 2022, 132, e153357. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.A.; Hsu, M.C. Determination of doping higenamine in Chinese herbal medicines and their concentrated preparations by LC-MS/MS. J. Pharm. Biomed. Anal. 2024, 246, 116188. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, B.; Zhang, H.; Hu, L.; Wang, J. Higenamine, a Dual Agonist for β 1- and β 2-Adrenergic Receptors Identified by Screening a Traditional Chinese Medicine Library. Planta Med. 2019, 85, 738–744. [Google Scholar] [CrossRef]
- Kato, E.; Kimura, S.; Kawabata, J. Ability of higenamine and related compounds to enhance glucose uptake in L6 cells. Bioorg Med. Chem. 2017, 25, 6412–6416. [Google Scholar] [CrossRef]
- Chen, D.T.; Rao, W.; Shen, X.; Chen, L.; Wan, Z.J.; Sheng, X.P.; Fan, T.Y. Pharmacological effects of higenamine based on signalling pathways and mechanism of action. Front. Pharmacol. 2022, 13, 981048. [Google Scholar] [CrossRef]
- Graham, R.M. Adrenergic receptors: Structure and function. Cleve Clin. J. Med. 1990, 57, 481–491. [Google Scholar] [CrossRef]
- Wong, K.K.; Lo, C.F.; Chen, C.M. Endothelium-dependent higenamine-induced aortic relaxation in isolated rat aorta. Planta Medica 1997, 63, 130–132. [Google Scholar] [CrossRef]
- Lee, S.R.; Schriefer, J.M.; Gunnels, T.A.; Harvey, I.C.; Bloomer, R.J. Acute oral intake of a higenamine-based dietary supplement increases circulating free fatty acids and energy expenditure in human subjects. Lipids Health Dis. 2013, 12, 148. [Google Scholar] [CrossRef]
- Zhang, N.; Lian, Z.; Peng, X.; Li, Z.; Zhu, H. Applications of Higenamine in pharmacology and medicine. J. Ethnopharmacol. 2017, 196, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Rangelov Kozhuharov, V.; Ivanov, K.; Ivanova, S. Higenamine in Plants as a Source of Unintentional Doping. Plants 2022, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.K.; Kim, J.M.; Jin, J.L.; Chang, K.C.; Lee, D.H.; Yun-Choi, H.S. Effects of higenamine and its 1-naphthyl analogs, YS-49 and YS-51, on platelet TXA2 synthesis and aggregation. Thromb. Res. 2007, 120, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Nojima, H.; Okazaki, M.; Kimura, I. Counter Effects of Higenamine and Coryneine, Components of Aconite Root, on Acetylcholine Release from Motor Nerve Terminal in Mice. J. Asian Nat. Prod. Res. 2000, 2, 195–203. [Google Scholar] [CrossRef]
- Kim, D.; Yun, J.; Roh, E.; Shin, H.S.; Kim, J.E. Higenamine Reduces Fine-Dust-Induced Matrix Metalloproteinase (MMP)-1 in Human Keratinocytes. Plants 2023, 12, 2479. [Google Scholar] [CrossRef]
- Romeo, I.; Parise, A.; Galano, A.; Russo, N.; Alvarez-Idaboy, J.R.; Marino, T. The Antioxidant Capability of Higenamine: Insights from Theory. Antioxidants 2020, 9, 358. [Google Scholar] [CrossRef]
- Bai, X.; Ding, W.; Yang, S.; Guo, X. Higenamine inhibits IL-1β-induced inflammation in human nucleus pulposus cells. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Lo, C.F.; Chen, C.M. Pharmacokinetics of higenamine in rabbits. Biopharm. Drug Dispos. 1996, 17, 791–803. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, J.; Hu, P.; Zhang, J.Y.; Liu, T.; Zhao, Q.; Li, B.L. A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy Chinese subjects. Acta Pharmacol. Sin. 2012, 33, 1353–1358. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, L.; Wang, J.; Wang, J.; Wang, L.; Wang, R.; Li, R.; Liu, H.; Wei, S.; Li, H.; et al. Therapeutic effects of higenamine combined with [6]-gingerol on chronic heart failure induced by doxorubicin via ameliorating mitochondrial function. J. Cell Mol. Med. 2020, 24, 4036–4050. [Google Scholar] [CrossRef]
- Jin, C.; Chai, Y.; Hu, Z.; Tian, W.; Ling, W.; Li, J.; Wu, M. Higenamine Attenuates Doxorubicin-Induced Cardiac Remodeling and Myocyte Apoptosis by Suppressing AMPK Activation. Front. Cell Dev. Biol. 2022, 10, 809996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-X.; Ling, W.; Xue, C.; Zhou, Z.; Zhang, Y.; Yan, C.; Wu, M.-P. Higenamine Attenuates Cardiac Fibroblast Activation and Fibrosis via Inhibition of TGF-β1/Smad Signaling. Eur. J. Pharmacol. 2021, 900, 174013. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.H.; Zhou, Y.P.; Liu, W.H.; Fan, L.L.; Tseng, K.Y. Comparison of cardiovascular effects of aconite root and higenamine in dogs (author’s transl). Zhongguo Yao Li Xue Bao 1980, 1, 34–39. [Google Scholar]
- Yu, F.; Kong, L.; Wang, S. Influence of racemic higenamine on the sinus node. Exp. Ther. Med. 2013, 5, 591–595. [Google Scholar] [CrossRef]
- Zhang, N.; Qu, K.; Wang, M.; Yin, Q.; Wang, W.; Xue, L.; Fu, H.; Zhu, H.; Zijian, l. Identification of higenamine as a novel α1-adrenergic receptor antagonist: Higenamine, a novel α1-adrenergic receptor antagonist. Phytother. Res. 2019, 33, 708–717. [Google Scholar] [CrossRef]
- Yun-Choi, H.S.; Pyo, M.K.; Park, K.M.; Chang, K.C.; Lee, D.H. Anti-thrombotic effects of higenamine. Planta Med. 2001, 67, 619–622. [Google Scholar] [CrossRef]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Natural compounds against cytotoxic drug-induced cardiotoxicity: A review on the involvement of PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22683. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, Y.J.; Kim, H.J.; Park, M.K.; Seo, H.G.; Lee, J.H.; Yun-Choi, H.S.; Chang, K.C. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemia-reperfusion injury. Apoptosis 2006, 11, 1091–1100. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhuang, X.D.; Xu, Z.W.; Lu, L.H.; Guo, H.L.; Wu, W.K.; Liao, X.X. Higenamine Combined with [6]-Gingerol Suppresses Doxorubicin-Triggered Oxidative Stress and Apoptosis in Cardiomyocytes via Upregulation of PI3K/Akt Pathway. Evid. Based Complement. Alternat Med. 2013, 2013, 970490. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Keizers, P.H.J.; Boyer, F.E.; Venhuis, B.J. The stimulant higenamine in weight loss and sports supplements. Clin. Toxicol. 2019, 57, 125–130. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Vanhee, C.; Ohana, D.; Venhuis, B.J. Nine prohibited stimulants found in sports and weight loss supplements: Deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine. Clin. Toxicol. 2021, 59, 975–981. [Google Scholar] [CrossRef]
- Jędrejko, K.; Catlin, O.; Stewart, T.; Anderson, A.; Muszyńska, B.; Catlin, D.H. Unauthorized ingredients in “nootropic” dietary supplements: A review of the history, pharmacology, prevalence, international regulations, and potential as doping agents. Drug Test. Anal. 2023, 15, 803–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Santillo, M.F. Cytochrome P450 2D6 and 3A4 enzyme inhibition by amine stimulants in dietary supplements. Drug Test. Anal. 2016, 8, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Stajić, A.; Anđelković, M.; Dikić, N.; Rašić, J.; Vukašinović-Vesić, M.; Ivanović, D.; Jančić-Stojanović, B. Determination of higenamine in dietary supplements by UHPLC/MS/MS method. J. Pharm. Biomed. Anal. 2017, 146, 48–52. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Jingfen, W.; Fei, D.; Mei, P. Higenamine alleviates cerebral ischemia-reperfusion injury in rats. Front. Biosci. (Landmark Ed.) 2019, 24, 859–869. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wu, C.; Guo, S.; Su, J.; Zhao, W.; Xing, H. Higenamine protects neuronal cells from oxygen-glucose deprivation/reoxygenation-induced injury. J. Cell Biochem. 2019, 120, 3757–3764. [Google Scholar] [CrossRef]
- Yang, B.; Ma, S.; Zhang, C.; Sun, J.; Zhang, D.; Chang, S.; Lin, Y.; Zhao, G. Higenamine Attenuates Neuropathic Pain by Inhibition of NOX2/ROS/TRP/P38 Mitogen-Activated Protein Kinase/NF-ĸB Signaling Pathway. Front. Pharmacol. 2021, 12, 716684. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Chen, J.; Deng, Y.; Lu, W.; Chen, D. pH Effect and Chemical Mechanisms of Antioxidant Higenamine. Molecules 2018, 23, 2176. [Google Scholar] [CrossRef]
- Wu, M.P.; Zhang, Y.S.; Zhou, Q.M.; Xiong, J.; Dong, Y.R.; Yan, C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol. Res. 2016, 104, 115–123. [Google Scholar] [CrossRef]
- Ha, Y.M.; Kim, M.Y.; Park, M.K.; Lee, Y.S.; Kim, Y.M.; Kim, H.J.; Lee, J.H.; Chang, K.C. Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. Apoptosis 2012, 17, 463–474. [Google Scholar] [CrossRef]
- Yang, S.; Chu, S.-F.; Ai, Q.; Zhang, Z.; Gao, Y.; Lin, M.; Liu, Y.; Hu, Y.; Li, X.; Peng, Y.; et al. Anti-inflammatory effects of higenamine (Hig) on LPS-activated mouse microglia (BV2) through NF-κB and Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2020, 85, 106629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Wang, Y.; Wu, J.; Li, J. Higenamine promotes M2 macrophage activation and reduces Hmgb1 production through HO-1 induction in a murine model of spinal cord injury. Int. Immunopharmacol. 2014, 23, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, W.; Qi, X.; Zhou, J.; Liu, Z.; Cheng, Y. Natural alkaloids from Lotus Plumule Ameliorate Lipopolysaccharide-induced Depression-like Behavior: Integrating Network Pharmacology and Molecular Mechanism Evaluation. Food Funct. 2019, 10, 6062–6073. [Google Scholar] [CrossRef]
- Yao, J.; Chen, C.; Sun, Y.; Lin, Y.; Tian, Z.; Liu, X.; Wang, H.; Long, J.; Yan, Q.; Lin, M.; et al. Higenamine exerts antidepressant effect by improving the astrocytic gap junctions and inflammatory response. J. Affect. Disord. 2024, 348, 107–115. [Google Scholar] [CrossRef]
- Yang, X.; Du, W.; Zhang, Y.; Wang, H.; He, M. Neuroprotective Effects of Higenamine Against the Alzheimer’s Disease Via Amelioration of Cognitive Impairment, A Burden, Apoptosis and Regulation of Akt/GSK3β Signaling Pathway. Dose-Response 2020, 18, 1559325820972205. [Google Scholar] [CrossRef]
- Du, J.; Mehrabi Nasab, E.; Athari, S.S.; Qian, L. Bronchodilatory effect of higenamine as antiallergic asthma treatment. J. Investig. Med. 2022, 70, 1753–1758. [Google Scholar] [CrossRef]
- Shao, X.X.; Xu, Y.; Xiao, H.Y.; Hu, Y.; Jiang, Y. Higenamine improves DSS-induced ulcerative colitis in mice through the Galectin-3/TLR4/NF-κB pathway. Tissue Cell 2023, 82, 102111. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, B.; Liang, X.; Liu, C.; Xia, T.; Xie, Y.; Deng, X.; Tan, X. Higenamine alleviates allergic rhinitis by activating AKT1 and suppressing the EGFR/JAK2/c-JUN signaling. Phytomedicine 2021, 86, 153565. [Google Scholar] [CrossRef]
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, S.; Cao, Z.; Yang, L.; Lu, F.; Li, Y.; Hu, L.; Bai, X. Higenamine mitigates interleukin-1β-induced human nucleus pulposus cell apoptosis by ROS-mediated PI3K/Akt signaling. Mol. Cell Biochem. 2021, 476, 3889–3897. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e116. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, C.; Teng, D.; Su, S.; Luo, X.; Liu, Z.; Liao, G. Discovery of higenamine as a potent, selective and cellular active natural LSD1 inhibitor for MLL-rearranged leukemia therapy. Bioorg Chem. 2021, 109, 104723. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.Q.; Hao, J.; Yang, X.; He, J.H.; Liang, J.; Yuan, J.W.; Mao, Y.; Liu, D.; Cao, R.; Wu, X.Z.; et al. Higenamine enhances the antitumor effects of cucurbitacin B in breast cancer by inhibiting the interaction of AKT and CDK2. Oncol. Rep. 2018, 40, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Long, C.; Deng, X.; Tang, A.; Xie, J.; Chen, L.; Wang, Z. Higenamine inhibits apoptosis and maintains survival of gastric smooth muscle cells in diabetic gastroparesis rat model via activating the β2-AR/PI3K/AKT pathway. Biomed. Pharmacother. 2017, 95, 1710–1717. [Google Scholar] [CrossRef]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef]
- Duan, W.; Chen, J.; Wu, Y.; Zhang, Y.; Xu, Y. Protective effect of higenamine ameliorates collagen-induced arthritis through heme oxygenase-1 and PI3K/Akt/Nrf-2 signaling pathways. Exp. Ther. Med. 2016, 12, 3107–3112. [Google Scholar] [CrossRef]
- Kang, Y.J.; Lee, G.W.; Ku, E.B.; Lee, H.; Chang, K.-C. Inhibition by higenamine of lipopolysaccharide-induced iNOS mRNA expression and NO production in rat aorta. Korean J. Physiol. Pharmacol. 1997, 1, 297–302. [Google Scholar]
- Dong, H.; Liu, R.; Zou, K.; Jin, Z.; Kang, J.; Zhang, Y.; Zhang, X.; Sun, Z.; Yu, G.; Huang, N.; et al. Higenamine Promotes Osteogenesis Via IQGAP1/SMAD4 Signaling Pathway and Prevents Age- and Estrogen-Dependent Bone Loss in Mice. J. Bone Miner. Res. 2023, 38, 775–791. [Google Scholar] [CrossRef]
- Lo, C.F.; Chen, C.M. Acute toxicity of higenamine in mice. Planta Med. 1997, 63, 95–96. [Google Scholar] [CrossRef]
- Biesterbos, J.W.H.; Sijm, D.; van Dam, R.; Mol, H.G.J. A health risk for consumers: The presence of adulterated food supplements in the Netherlands. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1273–1288. [Google Scholar] [CrossRef]
- Lim, H.S.; Jang, Y.; Park, G. Effects of the Higenamine, a Potent Compound from Aconitum, on UVB-Induced Photoaging in Hairless Mice. Evid. Based Complement. Alternat Med. 2022, 2022, 9116642. [Google Scholar] [CrossRef] [PubMed]
- Buels, K.S.; Fryer, A.D. Muscarinic receptor antagonists: Effects on pulmonary function. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 317–341. [Google Scholar] [CrossRef]
- Rasic, J.S.; Ivanovic, N.D.; Andjelkovic, M.S.; Nedeljkovic, I.P.; Nikolic, I.R.; Stojanovic, S.D.; Ristic-Medic, D.K.; Takic, M.M.; Djordjevic, B.I.; Dikic, N.V. Influence of Higenamine on Exercise Performance of Recreational Female Athletes: A Randomized Double-Blinded Placebo-Controlled Trial. Front. Psychol. 2021, 12, 633110. [Google Scholar] [CrossRef] [PubMed]
- Hudzik, T.J.; Patel, M.; Brown, A. β(2) -Adrenoceptor agonist activity of higenamine. Drug Test. Anal. 2021, 13, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Leaney, A.E.; Heath, J.; Midforth, E.; Beck, P.; Brown, P.; Mawson, D.H. Presence of higenamine in beetroot containing ‘foodstuffs’ and the implication for WADA-relevant anti-doping testing. Drug Test. Anal. 2023, 15, 173–180. [Google Scholar] [CrossRef]
- Rubin, R. Dietary Supplement Sellers Receive Warnings. Jama 2022, 327, 2281. [Google Scholar] [CrossRef]

| First Author | Experimental Subjects | Outcomes | Results | Treating Disease/Unhealthy Symptoms |
|---|---|---|---|---|
| Wen, J. [30] | Rats | Effects on cardiac functions, energy metabolism and related pathways, mitochondrial respiratory function | Higenamine combined with 6-gingerol can improve cardiac function, reduce serum indexes, and reduce histological damage to the heart. Combining with 6-gingerol prevents DOX-induced cardiotoxicity through cardiotonic effects and promotes myocardial mitochondrial metabolism through the LKB1/AMPK/Sirt1 pathway to prevent CHF. | DOX-induced chronic heart failure |
| Jin, C. [31] | Rats | Assessment of reactive oxygen species (ROS) levels, superoxide dismutase levels | Higenamine alleviates DOX-induced chronic myocardial injury by inhibiting AMPK activation and ROS production. | Cardiotoxicity is caused by DOX |
| Zhu, J. [32] | Rats | Heart size, cardiac fibrosis, heart function | Higenamine has a beneficial effect on myocardial death during acute ischemia. It may act through TGF-β/Smad signaling and activation of CFs which ameliorate pathological cardiac fibrosis and dysfunction. | Effect of HG on chronic cardiac remodeling |
| Huang, N. [33] | Dogs | Blood pressure, heart rate | The heart rate increased and blood pressure decreased after the injection of 5 μg/kg of higenamine. | Effects on cardiovascular system, higenamine acts matched group |
| Yu, F. [34] | Rabbits | Sinus node recovery time, corrected sinus node recovery time, total sinoatrial conduction time, sinus cycle length | Higenamine can treat arrhythmia caused by sinus node damage, enhance the autonomy of the sinus node, and improve the sinus and atrioventricular conduction functions. | The mechanism of racemic higenamine in treating sick sinus syndrome |
| Zhang, N. [35] | Rats | Effects on arterial pressure, higenamine binding for α1-adrenergic receptor, and so on | Higenamine can lower blood pressure in normotension, spontaneous hypertension, and induced hypertension models. | Whether higenamine can act as an α1-adrenoceptor antagonist in affecting blood pressure |
| Yun-Choi, H.S. [36] | Rats | Anti-thrombotic activities of higenamine, protection of mice from thrombotic challenge | The results showed that higenamine can resist the formation of thrombotic effects. | Higenamine’s anti-platelet and anti-thrombotic effects |
| Yarmohammadi, F. [37] | - | - | Higenamine can protect the heart by regulating the PI3K/Akt signaling pathway. | Higenamine’s role in regulating PI3K/Akt pathway to protect cardiotoxicity caused by drugs |
| Lee, Y.S. [38] | Rats | Caspase-3 activity, heme oxygenase (HO) enzyme activity, and so on | Higenamine can trigger anti-apoptosis induced by zinc protoporphyrin IX (ZnPP IX), and HO protects beneficial effects produced by higenamine. | Higenamine’s effects on myocardial I/R-induced injury |
| Chen, Y.L. [39] | Rat cardiomyocytes (newborn) | Cytochrome c releasement from mitochondria, cell viability assay in H9c2 cell and neonatal rat cardiomyocytes | Combined treatment of higenamine and 6-gingerol can activate the PI3K/Akt signaling pathway and have a protective effect on DOX-induced cardiotoxicity. | Heart failure caused by DOX |
| First Author | Experimental Subjects | Outcomes | Results | Treating Disease/Unhealthy Symptoms |
|---|---|---|---|---|
| Wang, X. [45] | Rats | Neurological function, TNF-α, interleukins (ILs) | Higenamine can improve neurological function and inhibit I/R-induced serum (TNF-α) and ILs (such as IL-1 and IL-6). | In the I/R model |
| Zhang, Y. [46] | Rats | Oxygen–glucose deprivation/reperfusion (OGD/R)-induced cell injury in neuronal cells, OGD/R-induced oxidative stress, Akt and Nrf2/HO-1 signaling pathways | Higenamine can prevent and treat brain I/R-induced damage. | Test higenamine’s acts in OGD/R-induced neuronal cells injury |
| Yang, B. [47] | Rats | The level of ROS, glutathione (GSH), superoxide dismutase (SOD), THF-α, IL-6 | Higenamine may play a role in chronic diseases related to inflammatory and oxidative stress. | The possible mechanism of higenamine in treating neuropathic pain |
| Xie, Y.L. [48] | - | Antioxidant value | Higenamine is a powerful antioxidant. | Antioxidant activity study of higenamine |
| Wu, M.P. [49] | Rats | Level of caspase-3, Akt, and pAkt | Higenamine’s anti-apoptosis and myocardium-protecting function is related to the β2-AR/PI3K/Akt pathway. | The molecular target and mechanism responsible for the effect of higenamine in cardioprotection |
| Ha, Y.M. [50] | The experiment was based on C6 cells | Nrf-2 transfection, signal pathway of induction of HO-1 by higenamine | Higenamine can increase Nrf-2 luciferase activity and transfer Nrf-2 to the nucleus. | Test higenamine’s benefits in hypoxic injuries such as stroke |
| Yang, S. [51] | Rats | The level of TNF-α, IL-6, ROS, NO mediated by iNOS, PGE2 mediated by COX2 | Higenamine achieves its anti-inflammatory and antioxidant effects by inhibiting NF-κB and activating Nrf2/HO-1 signaling pathway expression. | Explore the neuroprotection effects of higenamine in neuronal inflammation |
| Zhang, Z. [52] | Rats | Adoptive transfer of HG-treated macrophages, factors of immunohistochemistry | Higenamine increases the expression of IL-4 and IL-10, promoting the rise in HO-1. | The effects of higenamine on spinal cord injury |
| Chen, S. [53] | Rats | NO production, immunohistochemistry | Higenamine attenuates LPS-induced depressive-like behavior by modulating brain-derived neurotrophic factor (BDNF)-mediated ER stress and autophagy. | Explore the molecular mechanism of depression-like behaviors |
| Yao, J. [54] | Rats | Changes in astrocyte GJs function, expression and phosphorylation of connexin 43 | It is possible that higenamine may ameliorate and treat depression by improving astrocytes. | The potential of higenamine in treating neurodisease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Cheng, L.; Li, H.; Yu, L.; You, T.; Xu, Z.; Zhou, Z.; Zhao, H.; Liu, C.; Shu, S. A Narrative Review on Higenamine: Pharmacological Properties and Clinical Applications. Nutrients 2025, 17, 1030. https://doi.org/10.3390/nu17061030
Shi H, Cheng L, Li H, Yu L, You T, Xu Z, Zhou Z, Zhao H, Liu C, Shu S. A Narrative Review on Higenamine: Pharmacological Properties and Clinical Applications. Nutrients. 2025; 17(6):1030. https://doi.org/10.3390/nu17061030
Chicago/Turabian StyleShi, Hanghao, Long Cheng, Huixin Li, Longqi Yu, Ting You, Zhiqin Xu, Zixiang Zhou, Haotian Zhao, Chang Liu, and Shengfang Shu. 2025. "A Narrative Review on Higenamine: Pharmacological Properties and Clinical Applications" Nutrients 17, no. 6: 1030. https://doi.org/10.3390/nu17061030
APA StyleShi, H., Cheng, L., Li, H., Yu, L., You, T., Xu, Z., Zhou, Z., Zhao, H., Liu, C., & Shu, S. (2025). A Narrative Review on Higenamine: Pharmacological Properties and Clinical Applications. Nutrients, 17(6), 1030. https://doi.org/10.3390/nu17061030





