Integrated Multiomics Analyses Reveal Molecular Insights into How Intermittent Fasting Ameliorates Obesity and Increases Fertility in Male Mice
Abstract
:1. Introduction
2. Methods
2.1. Animals
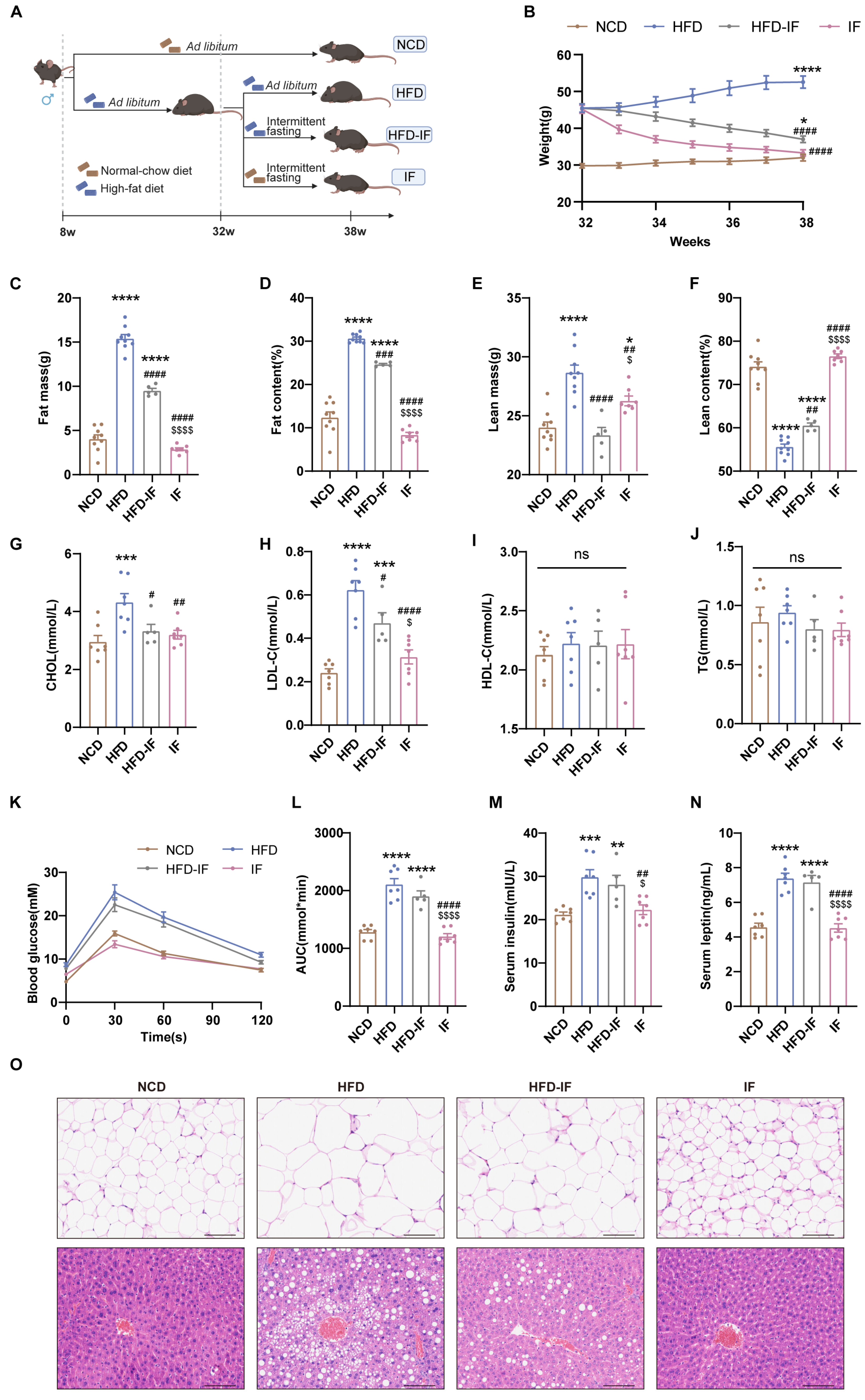
2.2. Body Composition Analysis
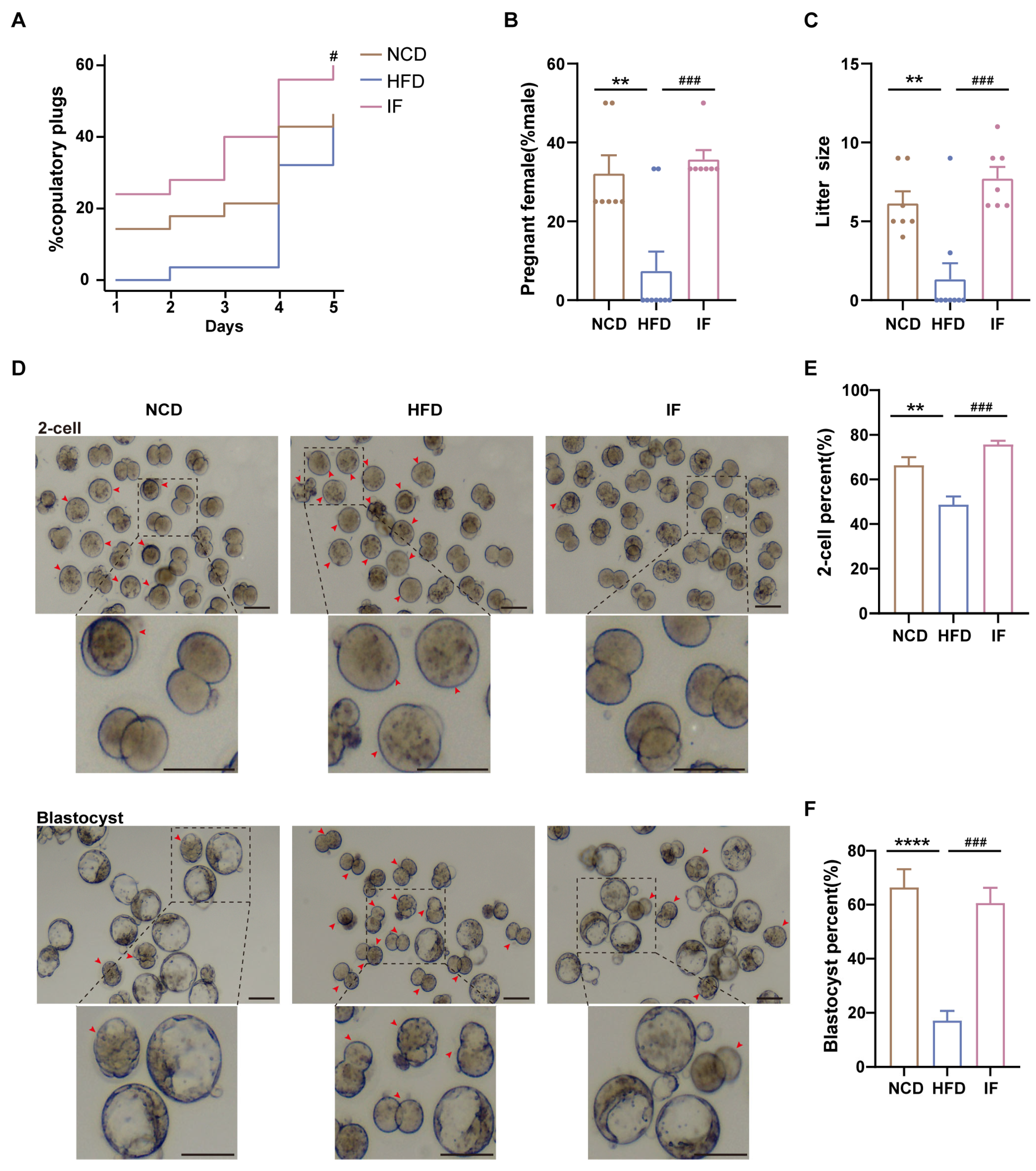
2.3. Reproductive Phenotype Assessment
2.4. Glucose Tolerance Test (GTT)
2.5. Serum and Tissue Collection
2.6. Serum Hormone and Lipid Analysis
2.7. Histological Examination
2.8. Sperm Analysis
2.9. In Vitro Fertilization and Early Embryo Development
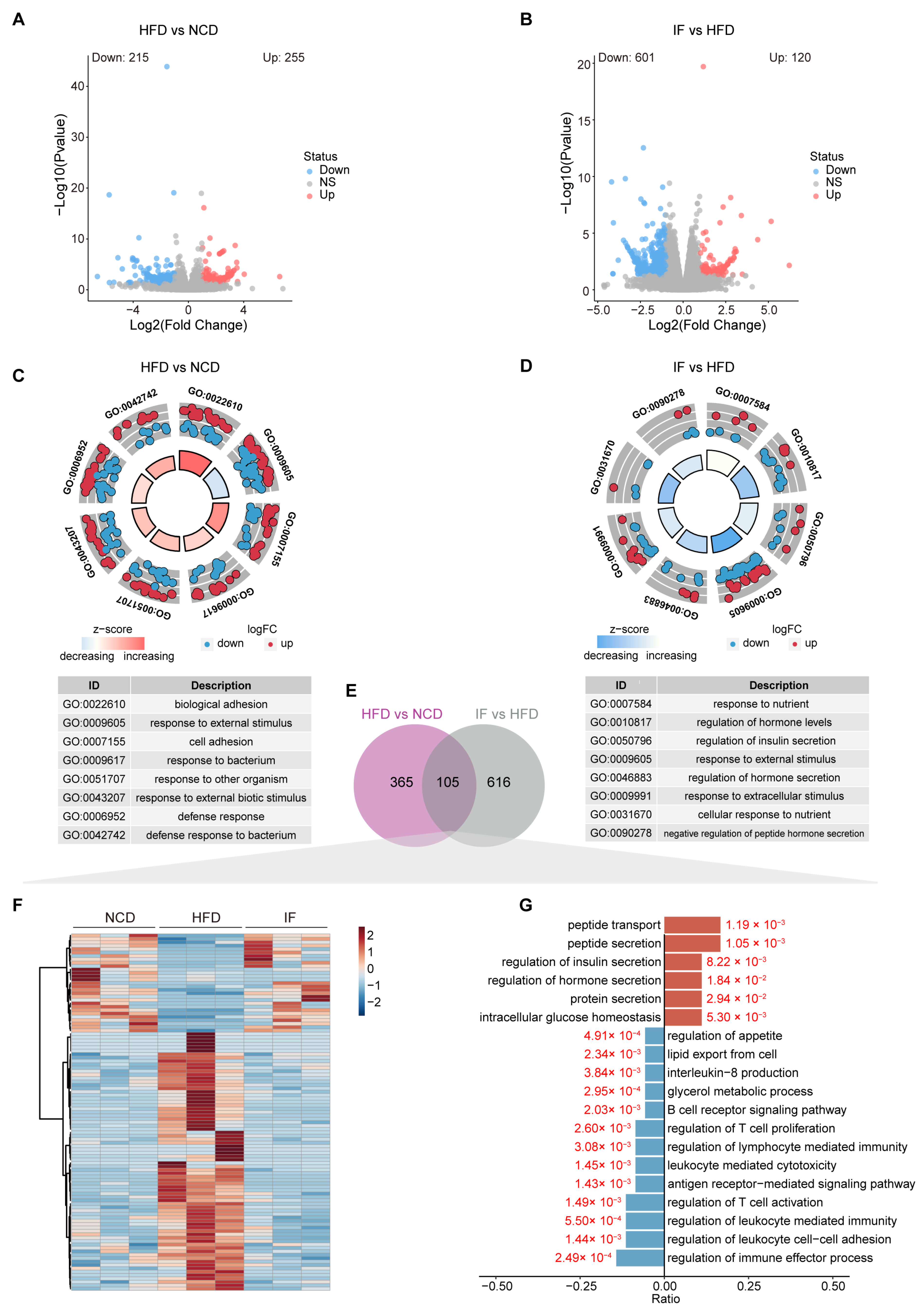
2.10. RNA Sequencing
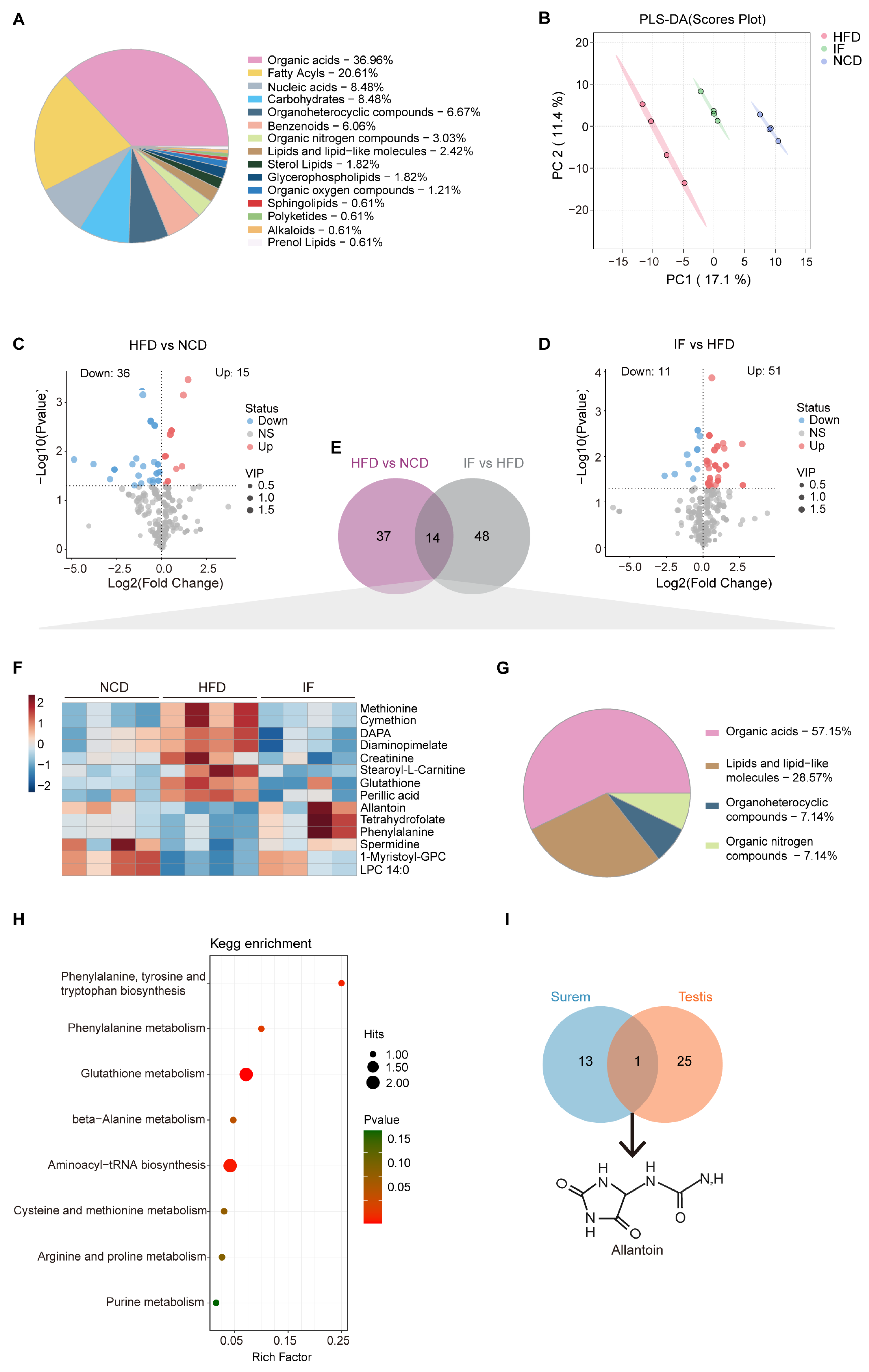
2.11. Untargeted Metabolomic Analysis by LC-MS
2.12. Real-Time Quantitative PCR
2.13. Western Blot Analysis
2.14. Immunohistochemistry (IHC) Analysis
2.15. Statistical Analysis
3. Results
3.1. IF Mitigates HFD-Induced Obesity in Mice
3.2. IF Alleviates HFD-Induced Male Infertility in Mice
3.3. IF Reverses Testis Metabolomic Changes in HFD-Fed Mice
3.4. IF Reverses Serum Metabolomic Changes in HFD-Fed Mice
3.5. IF Reverses the Testis Transcriptome in HFD-Fed Mice
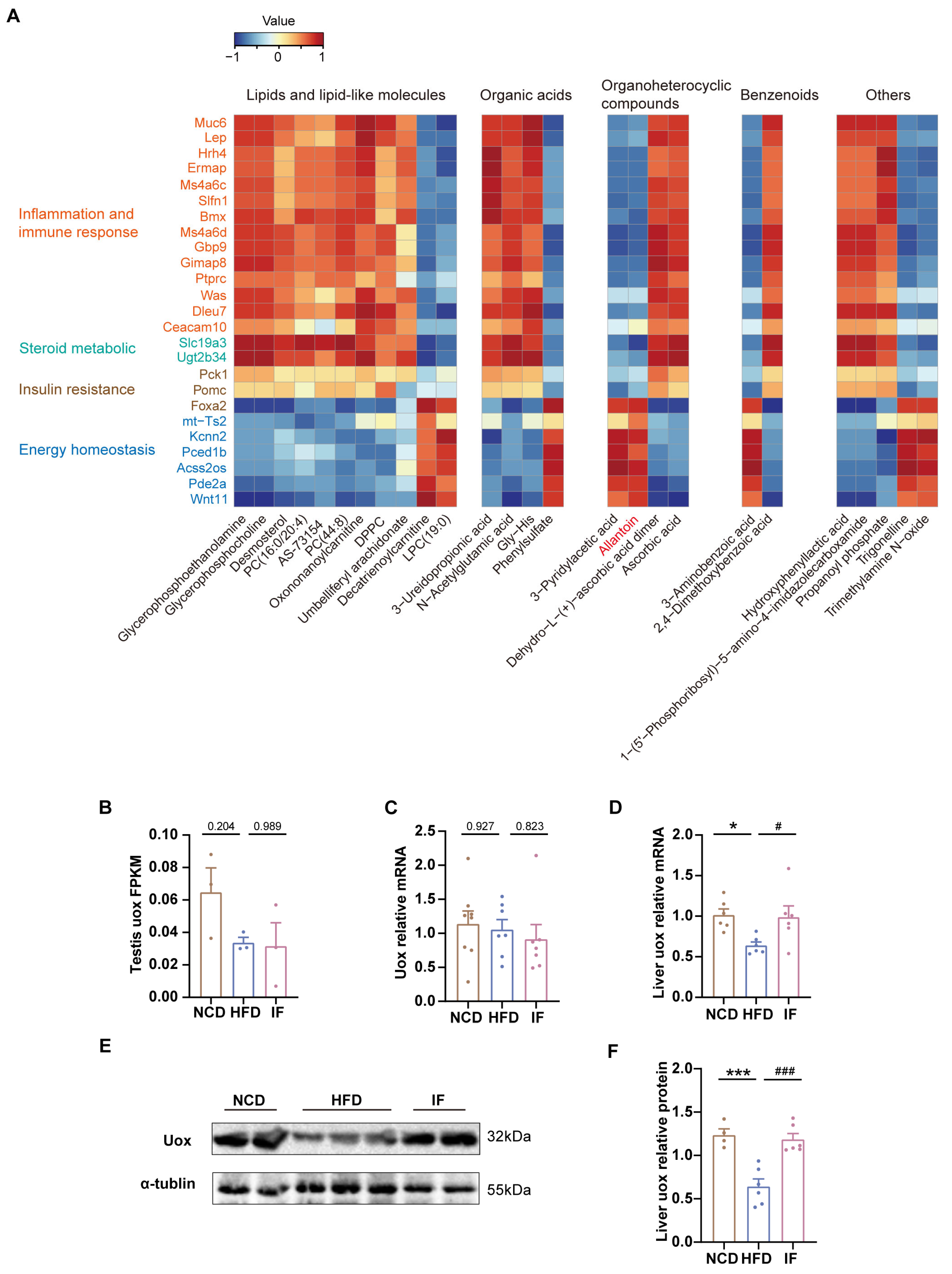
3.6. Integrated Analysis Reveals That IF Is Associated with Allantoin Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shen, Z.; Gu, W.; Lyu, Z.; Qi, X.; Mu, Y.; Ning, Y. Meinian Investigator Group: Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 2023, 25, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Lotti, F.; Marchiani, S.; Corona, G.; Maggi, M. Metabolic Syndrome and Reproduction. Int. J. Mol. Sci. 2021, 22, 1988. [Google Scholar] [CrossRef]
- Service, C.A.; Puri, D.; Al Azzawi, S.; Hsieh, T.-C.; Patel, D.P. The impact of obesity and metabolic health on male fertility: A systematic review. Fertil. Steril. 2023, 120, 1098–1111. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef]
- Teerds, K.J.; de Rooij, D.G.; Keijer, J. Functional relationship between obesity and male reproduction: From humans to animal models. Hum. Reprod. Update 2011, 17, 667–683. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, M.; Sun, M.; Luo, D.; Guan, Q.; Yu, C. Restoring Impaired Fertility Through Diet: Observations of Switching From High-Fat Diet During Puberty to Normal Diet in Adulthood Among Obese Male Mice. Front. Endocrinol. 2022, 13, 839034. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, T.; Zhang, Y.; Liu, X.; Huang, H. Effects of parental overweight and obesity on offspring’s mental health: A meta-analysis of observational studies. PLoS ONE 2022, 17, e0276469. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Poulimeneas, D.; Mamalaki, E.; Anastasiou, C.A. Dietary modifications for weight loss and weight loss maintenance. Metabolism 2019, 92, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Ryan, D.H. Evidence-based weight loss interventions: Individualized treatment options to maximize patient outcomes. Diabetes Obes. Metab. 2021, 23, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Freire, R. Scientific evidence of diets for weight loss: Different macronutrient composition, intermittent fasting, and popular diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Cardiometabolic Benefits of Intermittent Fasting. Annu. Rev. Nutr. 2021, 41, 333–361. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Honjoh, S.; Yamamoto, T.; Uno, M.; Nishida, E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 2009, 457, 726–730. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Madkour, M.I.; TEl-Serafi, A.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S.; Faris, M.A.-I.E. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019, 155, 107801. [Google Scholar] [CrossRef]
- Liu, B.; Page, A.J.; Hatzinikolas, G.; Chen, M.; Wittert, G.A.; Heilbronn, L.K. Intermittent Fasting Improves Glucose Tolerance and Promotes Adipose Tissue Remodeling in Male Mice Fed a High-Fat Diet. Endocrinology 2019, 160, 169–180. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N.; et al. Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Zhao, J.; Li, M.; Zhang, Y.; Hu, H.; Wei, L.; Zhou, K.; Qin, H.; Qu, P.; et al. Effect of Dietetic Obesity on Testicular Transcriptome in Cynomolgus Monkeys. Genes 2023, 14, 557. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Hu, L.; Wang, Y.; Liu, K.; Le, J.; Tan, Y.; Li, T.; Xue, H.; Wei, Y.; et al. Pyrroloquinoline quinone inhibits PCSK9-NLRP3 mediated pyroptosis of Leydig cells in obese mice. Cell Death Dis. 2023, 14, 723. [Google Scholar] [CrossRef]
- El-Shehawi, A.M.; El-Shazly, S.; Ahmed, M.; Alkafafy, M.; Sayed, S.; Farouk, S.; Alotaibi, S.S.; Elseehy, M.M. Transcriptome Analysis of Testis from HFD-Induced Obese Rats (Rattus norvigicus) Indicated Predisposition for Male Infertility. Int. J. Mol. Sci. 2020, 21, 6493. Available online: https://pubmed.ncbi.nlm.nih.gov/32899471/ (accessed on 5 September 2020). [CrossRef]
- Lin, T.; Zhang, S.; Zhou, Y.; Wu, L.; Liu, X.; Huang, H. Small RNA perspective of physical exercise-related improvement of male reproductive dysfunction due to obesity. Front. Endocrinol. 2022, 13, 1038449. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Abu Bakar, A.B.; Noor, M.M.; Nna, V.U.; Othman, Z.A.; Zakaria, Z.; Eleazu, C.O.; Mohamed, M. Bee bread mitigates downregulation of steroidogenic genes, decreased spermatogenesis, and epididymal oxidative stress in male rats fed with high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E351–E366. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Lin, W.; Simbulan, R.K.; Giritharan, G.; Piane, L.D.; Kolahi, K.; Ameri, K.; Maltepe, E.; et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014, 155, 1956–1969. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Li, R.; Yin, J.; Sun, H.; Zhu, C.; Kong, Q.; Mou, H.; Nie, S. Amelioration of hydrolyzed guar gum on high-fat diet-induced obesity: Integrated hepatic transcriptome and metabolome. Carbohydr. Polym. 2022, 297, 120051. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, C.; Huang, P.; Chen, L.; Ma, Y.; Ding, H. Effects of chronic exposure to a high fat diet, nutritive or non-nutritive sweeteners on hypothalamic-pituitary-adrenal (HPA) and -gonadal (HPG) axes of male Sprague-Dawley rats. Eur. J. Nutr. 2024, 63, 2209–2220. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.-S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Parmar, M.; Khosravizadeh, Z.; Kuchakulla, M.; Manoharan, M.; Arora, H. The role of leptin and obesity on male infertility. Curr. Opin. Urol. 2020, 30, 334–339. [Google Scholar] [CrossRef]
- George, B.T.; Jhancy, M.; Dube, R.; Kar, S.S.; Annamma, L.M. The Molecular Basis of Male Infertility in Obesity: A Literature Review. Int. J. Mol. Sci. 2024, 25, 179. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Molecular biology of steroid hormone synthesis. Endocr. Rev. 1988, 9, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Vance, J.E. Physiological consequences of disruption of mammalian phospholipid biosynthetic genes. J. Lipid Res. 2009, 50 Suppl, S132–S137. [Google Scholar] [CrossRef]
- Hamamah, S.; Seguin, F.; Barthelemy, C.; Akoka, S.; Pape, A.L.; Lansac, J.; Royere, D. 1H nuclear magnetic resonance studies of seminal plasma from fertile and infertile men. Reproduction 1993, 97, 51–55. [Google Scholar] [CrossRef]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef]
- Mason, S.A.; Della Gatta, P.A.; Snow, R.J.; Russell, A.P.; Wadley, G.D. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: Findings of a randomized controlled study. Free Radic Biol Med. 2016, 93, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, J.; Chen, J.; Li, W.; Xie, X. Reduced Glycolysis Contributed to Inhibition of Testis Spermatogenesis in Rats After Chronic Methamphetamine Exposure. Med. Sci. Monit. 2019, 25, 5453–5464. [Google Scholar] [CrossRef]
- Han, J.; Zhao, C.; Guo, H.; Liu, T.; Li, Y.; Qi, Y.; Deussing, J.M.; Zhang, Y.; Tan, J.; Han, H.; et al. Obesity induces male mice infertility via oxidative stress, apoptosis, and glycolysis. Reproduction 2023, 166, 27–36. [Google Scholar] [CrossRef]
- Woo, S.-L.; Yang, J.; Hsu, M.; Yang, A.; Zhang, L.; Lee, R.-P.; Gilbuena, I.; Thames, G.; Huang, J.; Rasmussen, A.; et al. Effects of branched-chain amino acids on glucose metabolism in obese, prediabetic men and women: A randomized, crossover study. Am. J. Clin. Nutr. 2019, 109, 1569–1577. [Google Scholar] [CrossRef]
- Honda, T.; Ishigami, M.; Luo, F.; Lingyun, M.; Ishizu, Y.; Kuzuya, T.; Hayashi, K.; Nakano, I.; Ishikawa, T.; Feng, G.-G.; et al. Branched-chain amino acids alleviate hepatic steatosis and liver injury in choline-deficient high-fat diet induced NASH mice. Metabolism 2017, 69, 177–187. [Google Scholar] [CrossRef]
- Orgeron, M.L.; Stone, K.P.; Wanders, D.; Cortez, C.C.; Van, N.T.; Gettys, T.W. Chapter Eleven—The Impact of Dietary Methionine Restriction on Biomarkers of Metabolic Health. In Progress in Molecular Biology and Translational Science; Tao, Y.-X., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 351–376. [Google Scholar]
- Ma, L.; Ni, Y.; Hu, L.; Zhao, Y.; Zheng, L.; Yang, S.; Ni, L.; Fu, Z. Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in preexisting obese mice. Life Sci. 2021, 265, 118739. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione system enhancement for cardiac protection: Pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 2023, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Stockman, M.-C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, L.; Su, P.; Ma, L.; Zhang, M.; Zhang, Y.Z. Autophagy: A multifaceted player in the fate of sperm. Hum. Reprod. Update 2022, 28, 200–231. [Google Scholar] [CrossRef]
- Alkafafy, M.E.; Sayed, S.M.; El-Shehawi, A.M.; El-Shazly, S.; Farouk, S.; Alotaibi, S.S.; Madkour, D.A.; Orabi, S.H.; Elbaz, H.T.; Ahmed, M.M. Moringa oleifera ethanolic extract ameliorates the testicular dysfunction resulted from HFD-induced obesity rat model. Andrologia 2021, 53, e14126. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Nna, V.U.; Zakaria, Z.; Othman, Z.A.; Eleazu, C.O.; Abu Bakar, A.B.; Ahmad, A.; Usman, U.Z.; Abdul Rahman, W.F.W.; Mohamed, M. Protective effects of bee bread on testicular oxidative stress, NF-κB-mediated inflammation, apoptosis and lactate transport decline in obese male rats. Biomed. Pharmacother. 2020, 131, 110781. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Z.; Xia, Y.; Shao, F.; Xia, W.; Wei, Y.; Li, X.; Qian, X.; Lee, J.-H.; Du, L.; et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 2020, 580, 530–535. [Google Scholar] [CrossRef]
- Malik, I.A.; Durairajanayagam, D.; Singh, H.J. Leptin and its actions on reproduction in males. Asian J. Androl. 2019, 21, 296. [Google Scholar] [CrossRef]
- Su, M.; Lin, Y.; Cui, C.; Tian, X.; Lai, L. ERMAP is a B7 family-related molecule that negatively regulates T cell and macrophage responses. Cell Mol. Immunol. 2021, 18, 1920–1933. [Google Scholar] [CrossRef]
- Rivers, E.; Thrasher, A.J. Wiskott-Aldrich syndrome protein: Emerging mechanisms in immunity. Eur. J. Immunol. 2017, 47, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Al Barashdi, M.A.; Ali, A.; McMullin, M.F.; Mills, K. Protein tyrosine phosphatase receptor type C (PTPRC or CD45). J. Clin. Pathol. 2021, 74, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.A.; Katayama, C.D.; Hedrick, S.M. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity 1998, 9, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gauthier, B.R.; Hagenfeldt-Johansson, K.A.; Iezzi, M.; Wollheim, C.B. Foxa2 (HNF3beta) controls multiple genes implicated in metabolism-secretion coupling of glucose-induced insulin release. J. Biol. Chem. 2002, 277, 17564–17570. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Teng, Y.; Mu, J.; Sriwastva, M.K.; Zhang, L.; Hood, J.L.; Yan, J.; Zhang, X.; Park, J.W.; et al. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics 2022, 12, 1388–1403. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, V.; Angeloni, C.; Giovannini, M.; Borghi, C. Purine Metabolism Dysfunctions: Experimental Methods of Detection and Diagnostic Potential. Int. J. Mol. Sci. 2023, 24, 7027. [Google Scholar] [CrossRef]
- Chung, H.-H.; Lee, K.S.; Cheng, J.-T. Decrease of Obesity by Allantoin via Imidazoline I 1 -Receptor Activation in High Fat Diet-Fed Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 589309. [Google Scholar] [CrossRef]
- Pg, Y.; Sb, C.; Mt, L. Allantoin, a Potential Metabolite That Promotes AMPK Phosphorylation and Suppresses Cholesterol Biosynthesis Via the Mevalonate Pathway and Bloch Pathway. Appl. Biochem. Biotechnol. 2020, 191, 226–244. [Google Scholar] [CrossRef]
- Florentino, I.F.; Silva, D.P.B.; Galdino, P.M.; Lino, R.C.; Martins, J.L.R.; Silva, D.M.; de Paula, J.R.; Tresvenzol, L.M.F.; Costa, E.A. Antinociceptive and anti-inflammatory effects of Memora nodosa and allantoin in mice. J. Ethnopharmacol. 2016, 186, 298–304. [Google Scholar] [CrossRef]
- Sun, R.; Huang, J.; Yang, N.; He, J.; Yu, X.; Feng, S.; Xie, Y.; Wang, G.; Ye, H.; Aa, J. Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity. Molecules 2019, 24, 4524. [Google Scholar] [CrossRef]
- Yu, L.; Xu, M.; Yan, Y.; Huang, S.; Yuan, M.; Cui, B.; Lv, C.; Zhang, Y.; Wang, H.; Jin, X.; et al. ZFYVE28 mediates insulin resistance by promoting phosphorylated insulin receptor degradation via increasing late endosomes production. Nat. Commun. 2023, 14, 6833. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hou, X.; Yuan, X.; Cui, L.; Liu, Z.; Li, X.; Ma, L.; Cheng, X.; Xin, Y.; Wang, C.; et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018, 93, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Gong, L.; Lu, S.; Zhou, Y.; Liu, L.; Duan, Z.; Xiang, R.; Gonzalez, F.J.; Li, G. Circadian transcriptional pathway atlas highlights a proteasome switch in intermittent fasting. Cell Rep. 2022, 41, 111547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jarman, J.B.; Low, Y.S.; Augustijn, H.E.; Huang, S.; Chen, H.; DeFeo, M.E.; Sekiba, K.; Hou, B.-H.; Meng, X.; et al. A widely distributed gene cluster compensates for uricase loss in hominids. Cell 2023, 186, 3400–3413.e20. [Google Scholar] [CrossRef]
- Angoorani, P.; Ejtahed, H.-S.; Hasani-Ranjbar, S.; Siadat, S.D.; Soroush, A.R.; Larijani, B. Gut microbiota modulation as a possible mediating mechanism for fasting-induced alleviation of metabolic complications: A systematic review. Nutr. Metab. 2021, 18, 105. [Google Scholar] [CrossRef]
- Özkul, C.; Yalınay, M.; Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019, 30, 1030–1035. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Lin, T.; Bao, Y.; She, J.; Liu, X.; Hu, J.; Peng, A.; Liu, X.; Huang, H. Integrated Multiomics Analyses Reveal Molecular Insights into How Intermittent Fasting Ameliorates Obesity and Increases Fertility in Male Mice. Nutrients 2025, 17, 1029. https://doi.org/10.3390/nu17061029
Zhang S, Lin T, Bao Y, She J, Liu X, Hu J, Peng A, Liu X, Huang H. Integrated Multiomics Analyses Reveal Molecular Insights into How Intermittent Fasting Ameliorates Obesity and Increases Fertility in Male Mice. Nutrients. 2025; 17(6):1029. https://doi.org/10.3390/nu17061029
Chicago/Turabian StyleZhang, Shuyu, Tingting Lin, Yucheng Bao, Junsen She, Xuanqi Liu, Jiaxue Hu, Aibing Peng, Xinmei Liu, and Hefeng Huang. 2025. "Integrated Multiomics Analyses Reveal Molecular Insights into How Intermittent Fasting Ameliorates Obesity and Increases Fertility in Male Mice" Nutrients 17, no. 6: 1029. https://doi.org/10.3390/nu17061029
APA StyleZhang, S., Lin, T., Bao, Y., She, J., Liu, X., Hu, J., Peng, A., Liu, X., & Huang, H. (2025). Integrated Multiomics Analyses Reveal Molecular Insights into How Intermittent Fasting Ameliorates Obesity and Increases Fertility in Male Mice. Nutrients, 17(6), 1029. https://doi.org/10.3390/nu17061029





