Evaluation of Inulin and Polyphenol Content and the Cytotoxicity of Cichorium intybus L. var. foliosum Root Extracts Obtained by Pectinase- and Pressure-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Chicory Root Extracts
2.3. Determination of Inulin Content by Lane and Eynon Method
2.4. Determination of Total Phenolic Content by the Folin–Ciocalteu Method
2.5. UHPLC-PDA Quantitation of Caffeic Acid Derivatives
2.6. In Vitro MTT Cytotoxicity Test of Chicory Extracts
2.7. Statistics
3. Results and Discussion
3.1. Inulin and Polyphenol Content
3.2. MTT Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid.-Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef]
- Bais, H.P.; Ravishankar, G.A. Cichorium intybus L—Cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agr. 2001, 81, 467–484. [Google Scholar] [CrossRef]
- Imam, K.; Xie, Y.; Liu, Y.; Wang, F.; Xin, F. Cytotoxicity of Cichorium intybus L. metabolites (Review). Oncol. Rep. 2019, 42, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, T.A.; Kelley, C.J.; Karchesy, Y.; Laurantos, M.; Nguyen-Dinh, P.; Arefi, A.G. Antimalarial activity of lactucin and lactucopicrin: Sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 2004, 95, 455–457. [Google Scholar] [CrossRef]
- Twarogowska, A.; Van Poucke, C.; Van Droogenbroeck, B. Upcycling of Belgian endive (Cichorium intybus var. foliosum) by-products. Chemical composition and functional properties of dietary fibre root powders. Food Chem. 2020, 332, 127444. [Google Scholar] [CrossRef] [PubMed]
- van Arkel, J.; Vergauwen, R.; Sevenier, R.; Hakkert, J.C.; van Laere, A.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Sink filling, inulin metabolizing enzymes and carbohydrate status in field grown chicory (Cichorium intybus L.). J. Plant Physiol. 2012, 169, 1520–1529. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Inulin-type fructans: Functional food ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef]
- Stökle, K.; Jung, D.; Kruse, A. Acid-assisted extraction and hydrolysis of inulin from chicory roots to obtain fructose-enriched extracts. Biomass Convers. Biorefinery 2023, 13, 159–170. [Google Scholar] [CrossRef]
- van Arkel, J.; Twarogowska, A.; Cornelis, Y.; De Marez, T.; Engel, J.; Maenhout, P.; de Vos, R.C.H.; Beekwilder, J.; Van Droogenbroeck, B.; Cankar, K. Effect of Root Storage and Forcing on the Carbohydrate and Secondary Metabolite Composition of Belgian Endive (Cichorium intybus L. Var. foliosum). ACS Food Sci. Technol. 2022, 2, 1546–1557. [Google Scholar] [CrossRef]
- Duda, L.; Klosinski, K.K.; Budryn, G.; Jaskiewicz, A.; Kolat, D.; Kaluzinska-Kolat, Z.; Pasieka, Z.W. Medicinal Use of Chicory (Cichorium intybus L.). Sci. Pharm. 2024, 92, 31. [Google Scholar] [CrossRef]
- Twarogowska, A.; Van Droogenbroeck, B. Influence of cultivar and growing location on composition and functionality of dietary fibre concentrates produced from forced roots of Belgian endive (Cichorium intybus var. foliosum). J. Food Compos. Anal. 2022, 106, 104281. [Google Scholar] [CrossRef]
- Piccolella, S.; Fiorentino, M.; Cimmino, G.; Esposito, A.; Pacifico, S. Cilentan Cichorium intybus L. organs: UHPLC-QqTOF-MS/MS analysis for new antioxidant scenario, exploitable locally and beyond. Future Foods 2024, 9, 100379. [Google Scholar] [CrossRef]
- Tardugno, R.; Pozzebon, M.; Beggio, M.; Del Turco, P.; Pojana, G. Polyphenolic profile of Cichorium intybus L. endemic varieties from the Veneto region of Italy. Food Chem. 2018, 266, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Perovic, J.; Saponjac, V.T.; Kojic, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroza-Solarov, M.; Ilic, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Mulabagal, V.; Wang, H.B.; Ngouajio, M.; Nair, M.G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur. Food Res. Technol. 2009, 230, 47–53. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023; World Health Organization: Geneva, Switzerland, 2013; 76p. [Google Scholar]
- Löhmar, K.; Theurillat, V. Chicory Beverages. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 1144–1149. ISBN 9780122270550. [Google Scholar] [CrossRef]
- Pouille, C.L.; Jegou, D.; Dugardin, C.; Cudennec, B.; Ravallec, R.; Hance, P.; Rambaud, C.; Hilbert, J.L.; Lucau-Danila, A. Chicory root flour—A functional food with potential multiple health benefits evaluated in a mice model. J. Funct. Foods 2020, 74, 104174. [Google Scholar] [CrossRef]
- Puhlmann, M.L.; de Vos, W.M. Back to the Roots: Revisiting the Use of the Fiber-Rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–889. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Kanayama, T.; Kitagawa, K.; Nishimura, H.; Nishihira, J. Effects of the extract from roasted chicory (Cichorium intybus L.) root containing inulin-type fructans on blood glucose, lipid metabolism, and fecal properties. J. Tradit. Complement. Med. 2015, 5, 161–167. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Pouille, C.L.; Dugardin, C.; Behra, J.; Tourret, M.; Molinie, R.; Fontaine, J.X.; Mathiron, D.; Palaric, C.; Gagneul, D.; Ravallec, R.; et al. Metabolomic monitoring of chicory during in vitro gastrointestinal digestion and correlation with bioactive properties. Food Chem. 2025, 467, 142344. [Google Scholar] [CrossRef]
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef]
- Pouille, C.L.; Ouaza, S.; Roels, E.; Behra, J.; Tourret, M.; Molinie, R.; Fontaine, J.X.; Mathiron, D.; Gagneul, D.; Taminiau, B.; et al. Chicory: Understanding the Effects and Effectors of This Functional Food. Nutrients 2022, 14, 957. [Google Scholar] [CrossRef]
- Lepczynski, A.; Herosimczyk, A.; Ozgo, M.; Marynowska, M.; Pawlikowska, M.; Barszcz, M.; Taciak, M.; Skomial, J. Dietary chicory root and chicory inulin trigger changes in energetic metabolism, stress prevention and cytoskeletal proteins in the liver of growing pigs—A proteomic study. J. Anim. Physiol. Anim. Nutr. 2017, 101, e225–e236. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak, K.; Król, B.; Juskiewicz, J.; Zdunczyk, Z. Composition and properties of chicory extracts rich in fructans and polyphenols. Pol. J. Food Nutr. Sci. 2009, 59, 35–43. [Google Scholar]
- Iovlev, D.P.; Farakhov, M.I.; Akberov, R.R.; Stekolshchikov, I.R.; Akhmerov, A.V. Modernization of operating chicory factories using continuous pulsating extractors. New Technol. 2022, 18, 40–52. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Aamer, R.A.; Ali, A.N.A. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020, 65, 59–67. [Google Scholar] [CrossRef]
- Kardamanidis, A.; Misailidis, N.; Da Gama Ferreira, R.; Petrides, D. Inulin Production from Chicory Roots—Process Modeling and Techno-Economic Assessment (TEA) Using SuperPro Designer. 2024. Available online: https://www.researchgate.net/publication/378141057_Inulin_Production_from_Chicory_Roots_-_Process_Modeling_and_Techno-Economic_Assessment_TEA_using_SuperPro_Designer?channel=doi&linkId=65c9431334bbff5ba7fe2e66&showFulltext=true (accessed on 15 January 2025).
- Jaskiewicz, A.; Budryn, G.; Nowak, A.; Efenberger-Szmechtyk, M. Novel Biodegradable Starch Film for Food Packaging with Antimicrobial Chicory Root Extract and Phytic Acid as a Cross-Linking Agent. Foods 2020, 9, 1696. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman: London, UK, 1989. [Google Scholar]
- Ibrahim, R.M. Utilization of Chickpea Split (Cicer arietinum L.) in Preparing Some Gluten-Free Casein-Free Food Products for Autism Children. Food Nutr. Sci. 2022, 13, 284–315. [Google Scholar] [CrossRef]
- Sinkovic, L.; Jamnik, P.; Korosec, M.; Vidrih, R.; Meglic, V. In-vitro and in-vivo antioxidant assays of chicory plants (Cichorium intybus L.) as influenced by organic and conventional fertilisers. BMC Plant Biol. 2020, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Skala, E.; Kicel, A.; Olszewska, M.A.; Kiss, A.K.; Wysokinska, H. Establishment of hairy root cultures of Rhaponticum carthamoides (Willd.) Iljin for the production of biomass and caffeic acid derivatives. Biomed. Res. Int. 2015, 2015, 181098. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: London, UK, 2009.
- Girek, M.; Klosinski, K.; Grobelski, B.; Pizzimenti, S.; Cucci, M.A.; Daga, M.; Barrera, G.; Pasieka, Z.; Czarnecka, K.; Szymanski, P. Novel tetrahydroacridine derivatives with iodobenzoic moieties induce G0/G1 cell cycle arrest and apoptosis in A549 non-small lung cancer and HT-29 colorectal cancer cells. Mol. Cell Biochem. 2019, 460, 123–150. [Google Scholar] [CrossRef]
- Klosinski, K.; Girek, M.; Czarnecka, K.; Pasieka, Z.; Skibinski, R.; Szymanski, P. Biological assessment of new tetrahydroacridine derivatives with fluorobenzoic moiety in vitro on A549 and HT-29 cell lines and in vivo on animal model. Hum. Cell 2020, 33, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin extraction from common inulin-containing plant sources. Ind. Crop Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.; Yu, K.W.; Imm, J.Y.; Suh, H.J. Enzyme-assisted extraction of cactus bioactive molecules under high hydrostatic pressure. J. Sci. Food Agric. 2014, 94, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Iwahashi, H. Properties of Polysaccharides Extracted from Using High Hydrostatic Pressure Processing and Hot Water Treatment. J. Food Process Eng. 2015, 38, 197–206. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Carrillo, C.; Zhu, Z.; Brncic, M.; Berrada, H.; Barba, F.J. Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina. Foods 2021, 10, 2153. [Google Scholar] [CrossRef]
- Sikodia, N.; Battan, B.; Chahal, S.; Sharma, J. Enhanced bio-extraction of medicinally important plants using xylano-pectinolytic enzymes produced concurrently by Bacillus pumilus AJK. South Asian J. Exp. Biol. 2024, 13, 407–415. [Google Scholar] [CrossRef]
- Hakkinen, S.T.; Cankar, K.; Nohynek, L.; Suomalainen, M.; van Arkel, J.; Siika-Aho, M.; Twarogowska, A.; Van Droogenbroeck, B.; Oksman-Caldentey, K.M. Enzyme-treated chicory for cosmetics: Application assessment and techno-economic analysis. AMB Express 2022, 12, 152. [Google Scholar] [CrossRef]
- Baiano, A.; Fiore, A. Enzymatic-Assisted Recovery of Antioxidants from Chicory and Fennel by-Products. Waste Biomass Valorizat. 2024, 16, 957–969. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kaur, N.; Kaur, N. Preparation of inulin from chicory roots. J. Sci. Ind. Res. India 2003, 62, 916–920. [Google Scholar]
- Sagcan, N.; Sagcan, H.; Bozkurt, F.; Gunes, A.N.B.; Fakir, H.; Dertli, E.; Sagdic, O. Optimization of Inulin Extraction from Chicory Roots and an Ultrafiltration Application to Obtain Purified Inulin and Hydrolyzed Fructooligosaccharides. J. Agr. Sci.-Tarim. Bili 2024, 30, 166–178. [Google Scholar] [CrossRef]
- Jayarathna, G.N.; Jayasena, D.D.; Mudannayake, D.C. Garlic Inulin as a Fat Replacer in Vegetable Fat Incorporated Low-Fat Chicken Sausages. Food Sci. Anim. Resour. 2022, 42, 295–312. [Google Scholar] [CrossRef]
- Brkljača, J.; Bodroža-Solarov, M.; Krulj, J.; Terzić, S.; Mikić, A.; Jeromela, A.M. Quantification of Inulin Content in Selected Accessions of Jerusalem Artichoke (Helianthus tuberosus L.). Helia 2014, 37, 105–112. [Google Scholar] [CrossRef]
- Aykul, S.; Martinez-Hackert, E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal. Biochem. 2016, 508, 97–103. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, J.B.; McAlpine, J.B.; Napoitano, J.G. Importance of Purity Evaluation and the Potential of Quantitative <SUP>1</SUP>H NMR as a Purity Assay. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef]
- Eray, N.; Kartal, D.İ.; Çelik, İ. Antioxidant Properties of Cichorium intybus L. (Chicory) Extracts and Their Cytotoxic Effects on HepG2 Cells. Yuz. Yıl Univ. J. Agric. Sci. 2020, 30, 444–453. [Google Scholar] [CrossRef]
- Firozian, F.; Emadi, M.A.; Chehardoli, G.; Ghafari, F. Inulin Stearate Self-assembly Micro-rod Containing Paclitaxel: Synthesis and In Vitro Cytotoxicity MTT Assay in HeLa Cell Line. J. Pharm. Innov. 2022, 17, 1215–1220. [Google Scholar] [CrossRef]
- Peymaeei, F.; Sadeghi, F.; Safari, E.; Khorrami, S.; Falahati, M.; Roudbar Mohammadi, S.; Roudbary, M. Candida albicans Beta-Glucan Induce Anti- Cancer Activity of Mesenchymal Stem Cells against Lung Cancer Cell Line: An In-Vitro Experimental Study. Asian Pac. J. Cancer Prev. 2020, 21, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Spampatti, M.; Vlotides, G.; Spottl, G.; Maurer, J.; Goke, B.; Auernhammer, C.J. Aspirin inhibits cell viability and mTOR downstream signaling in gastroenteropancreatic and bronchopulmonary neuroendocrine tumor cells. World J. Gastroenterol. 2014, 20, 10038–10049. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar] [PubMed]


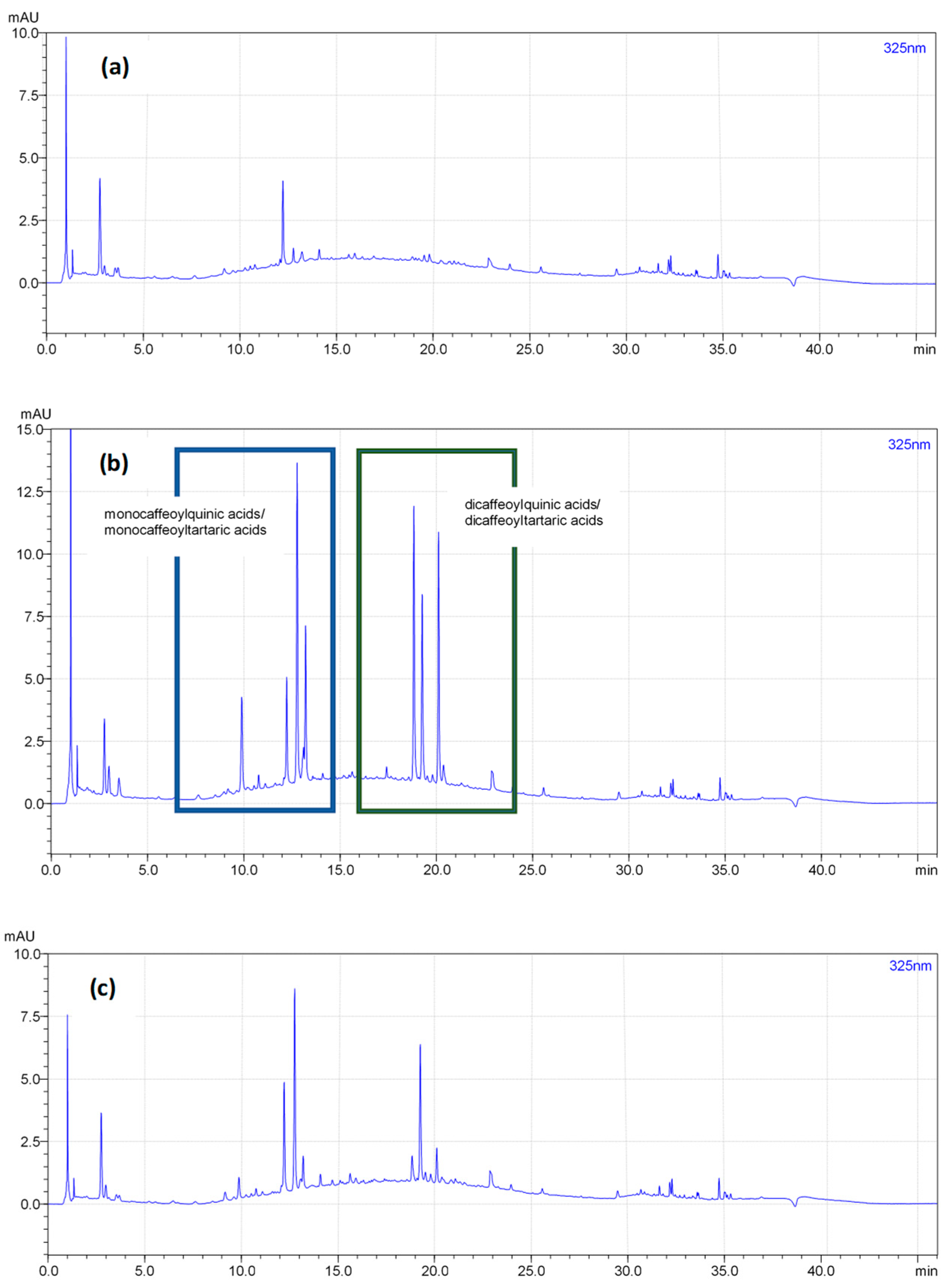

| Constituent | Enzyme-Assisted Extraction (Method 1) | Pressure-Assisted Extraction (Method 2) | Enzyme + Pressure-Assisted Extraction (Method 3) |
| Inulin (g/100 g dm) | 43.88 ± 0.98 c | 47.34 ± 1.47 b | 50.95 ± 0.69 a |
| TPC (mg/100 g dm) | 816.7 ± 56.4 b | 881.2 ± 49.6 b | 906.4 ± 14.1 b |
| MCA (mg/100 g dm) | 11.46 ± 0.08 c | 94.08 ± 2.12 a | 54.57 ± 2.67 b |
| DCA (mg/100 g dm) | <LOQ | 92.99 ± 0.88 a | 38.49 ± 1.57 b |
| TCA (mg/100 g dm) | 11.46 ± 0.08 c | 187.1 ± 3.01 a | 93.07 ± 4.23 b |
| Enzyme-Assisted Extraction (Method 1) | Pressure-Assisted Extraction (Method 2) | Enzyme + Pressure-Assisted Extraction (Method 3) | |||
|---|---|---|---|---|---|
| IC50 (mg/mL) | IC50 (mg/mL) | IC50 (mg/mL) | |||
| mean | 7.31 | mean | 5.76 | mean | 4.72 |
| SD | 1.00 | SD | 0.32 | SD | 0.58 |
| RSD (%) | 13.67 | RSD | 5.51 | RSD | 12.22 |
| Paracetamol | Inulin | ||
|---|---|---|---|
| IC50 (mg/mL) | IC50 (mg/mL) | ||
| mean | 3.02 | mean | 19.77 |
| SD | 0.22 | SD | 2.40 |
| RSD (%) | 7.19 | RSD | 12.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda, Ł.; Budryn, G.; Olszewska, M.A.; Rutkowska, M.; Kruczkowska, W.; Grabowska, K.; Kołat, D.; Jaśkiewicz, A.; Pasieka, Z.W.; Kłosiński, K.K. Evaluation of Inulin and Polyphenol Content and the Cytotoxicity of Cichorium intybus L. var. foliosum Root Extracts Obtained by Pectinase- and Pressure-Assisted Extraction. Nutrients 2025, 17, 1040. https://doi.org/10.3390/nu17061040
Duda Ł, Budryn G, Olszewska MA, Rutkowska M, Kruczkowska W, Grabowska K, Kołat D, Jaśkiewicz A, Pasieka ZW, Kłosiński KK. Evaluation of Inulin and Polyphenol Content and the Cytotoxicity of Cichorium intybus L. var. foliosum Root Extracts Obtained by Pectinase- and Pressure-Assisted Extraction. Nutrients. 2025; 17(6):1040. https://doi.org/10.3390/nu17061040
Chicago/Turabian StyleDuda, Łukasz, Grażyna Budryn, Monika Anna Olszewska, Magdalena Rutkowska, Weronika Kruczkowska, Katarzyna Grabowska, Damian Kołat, Andrzej Jaśkiewicz, Zbigniew Włodzimierz Pasieka, and Karol Kamil Kłosiński. 2025. "Evaluation of Inulin and Polyphenol Content and the Cytotoxicity of Cichorium intybus L. var. foliosum Root Extracts Obtained by Pectinase- and Pressure-Assisted Extraction" Nutrients 17, no. 6: 1040. https://doi.org/10.3390/nu17061040
APA StyleDuda, Ł., Budryn, G., Olszewska, M. A., Rutkowska, M., Kruczkowska, W., Grabowska, K., Kołat, D., Jaśkiewicz, A., Pasieka, Z. W., & Kłosiński, K. K. (2025). Evaluation of Inulin and Polyphenol Content and the Cytotoxicity of Cichorium intybus L. var. foliosum Root Extracts Obtained by Pectinase- and Pressure-Assisted Extraction. Nutrients, 17(6), 1040. https://doi.org/10.3390/nu17061040






