Effects of Multivitamin Supplementation on Metabolic Parameters in High- and Low-Fat Diet-Fed C57BL/6J Mice: Potential Links to Adipose Tissue Browning and Gut Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Designs
2.2. GTT and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
2.3. Indirect Calorimetry
2.4. Cold Tolerance Test
2.5. Infrared (IR) Thermography
2.6. Histological Analysis
2.7. Adipocyte Size
2.8. Serum Biochemical Analysis of Insulin and Lipid Profiles
2.9. Quantitative Real-Time PCR Analysis
2.10. Immunohistochemistry
2.11. 16S rRNA Analysis and Bioinformatics
2.12. Statistical Analysis
3. Results
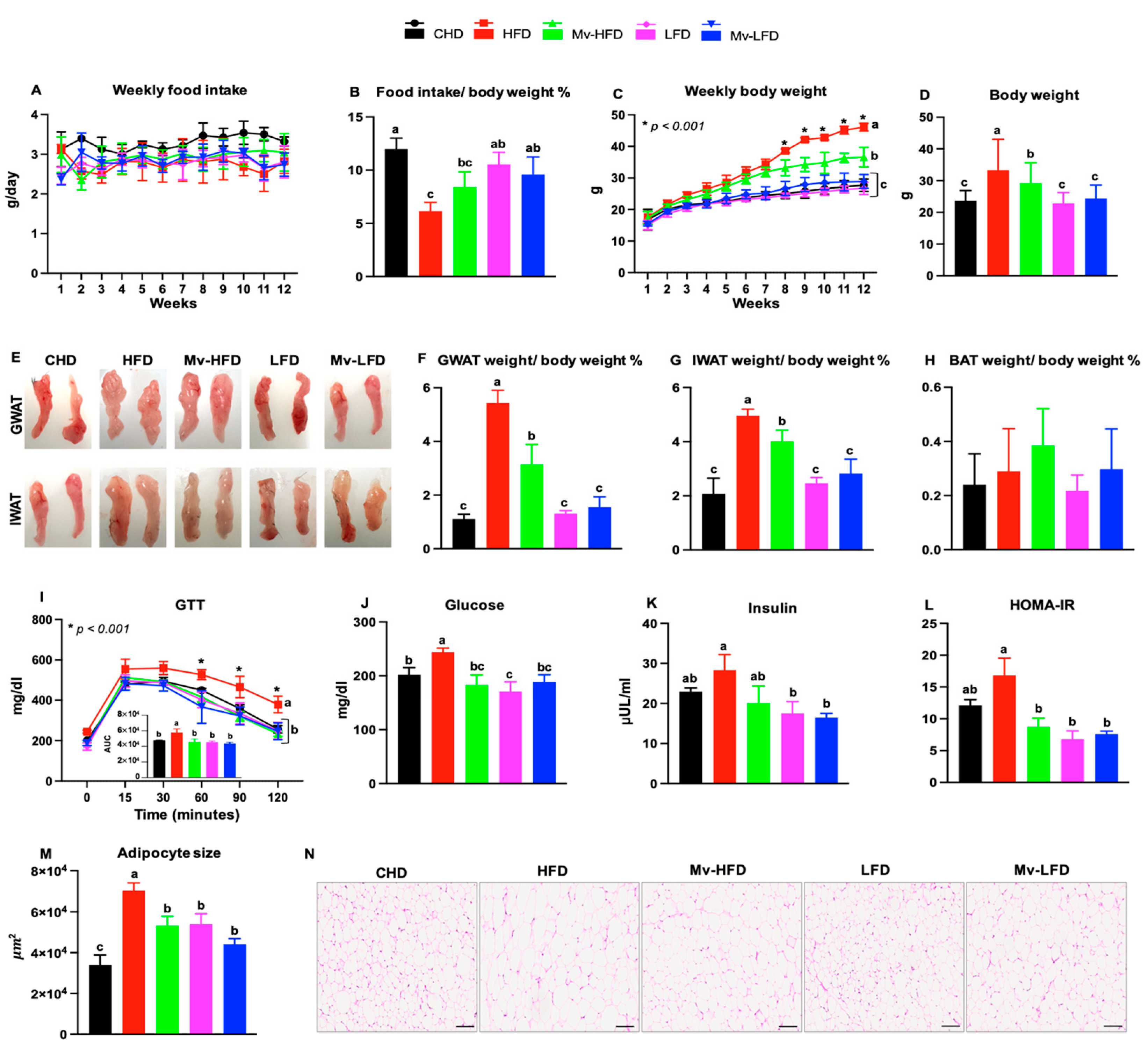
3.1. Food Intake Varied with Dietary Fat Content
3.2. Mv-HFD Decreased HFD-Induced Body Weight Gain
3.3. Mv-HFD Decreased HFD-Induced Increases in Gonadal WAT (GWAT) and IWAT Weights
3.4. Mv-HFD Decreased HFD-Induced Increases in Blood Glucose and HOMA-IR
3.5. Mv-HFD Decreased HFD-Induced Increases in Adipocyte Size in IWAT
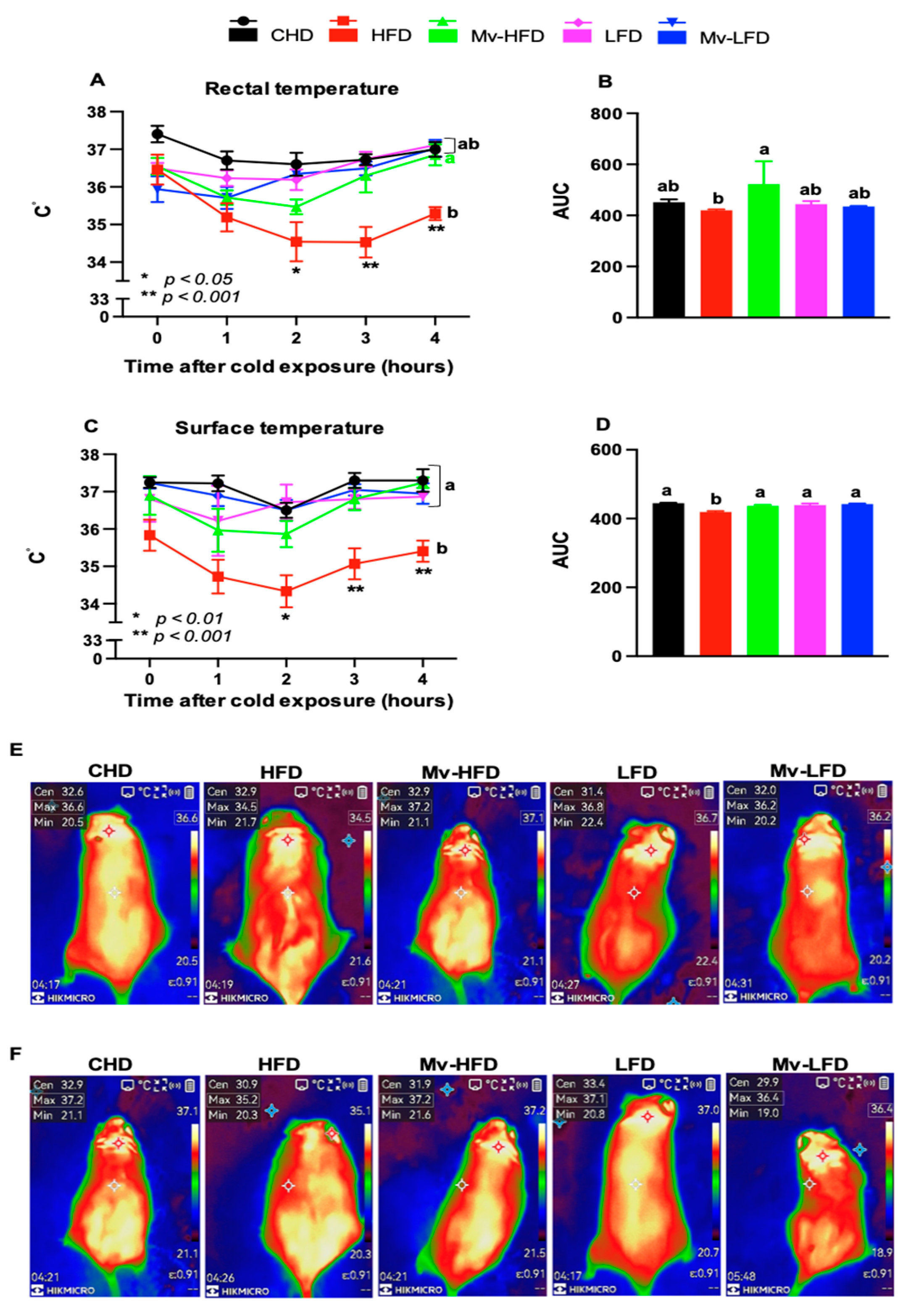
3.6. Mv-HFD Enhanced HFD-Induced Reductions in Rectal and Surface Temperatures
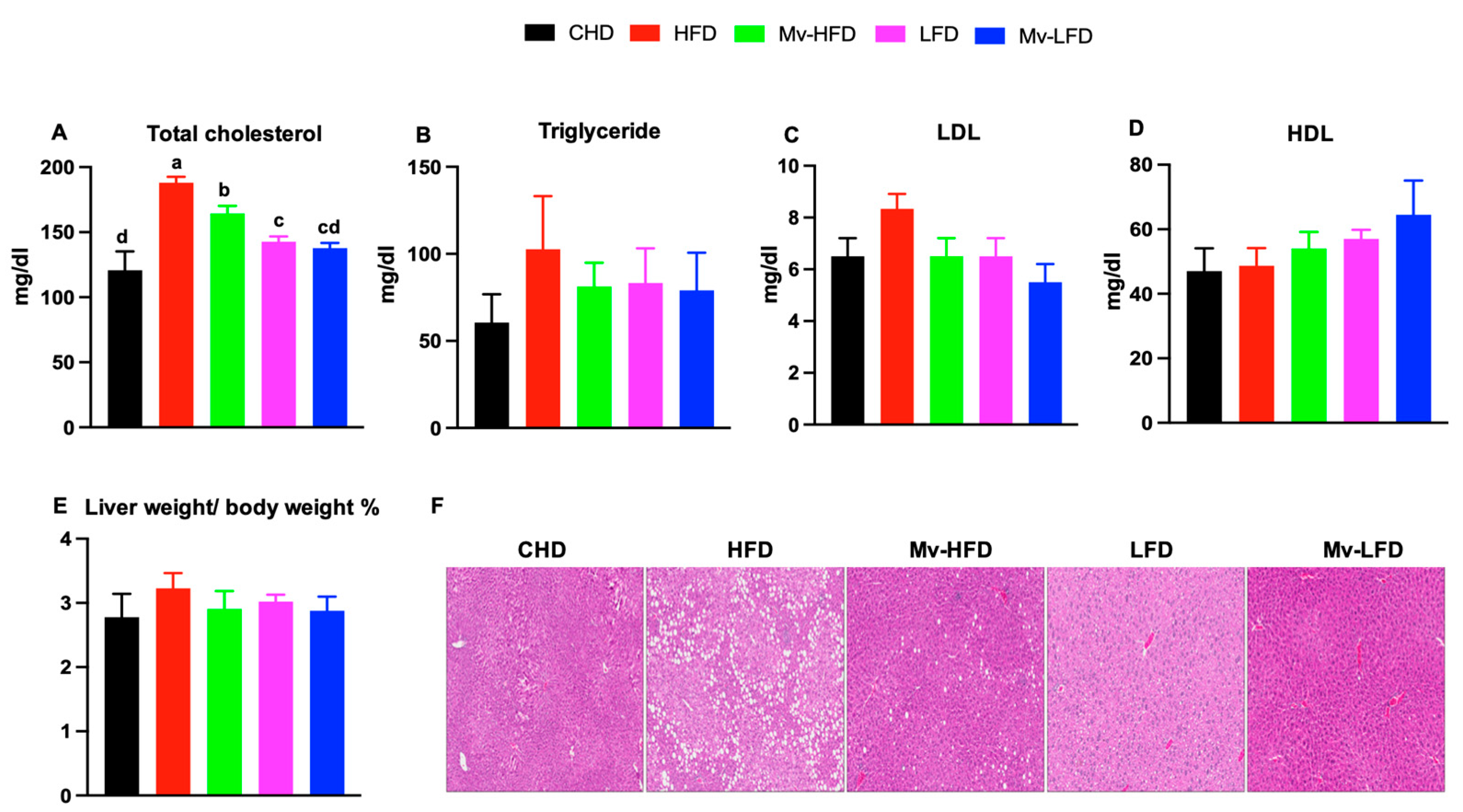
3.7. Mv-HFD Decreased HFD-Induced Increases in Total Cholesterol and Liver Lipid Accumulation
3.8. Mv-HFD Increased VO2, VCO2, and EE and Reduced RER
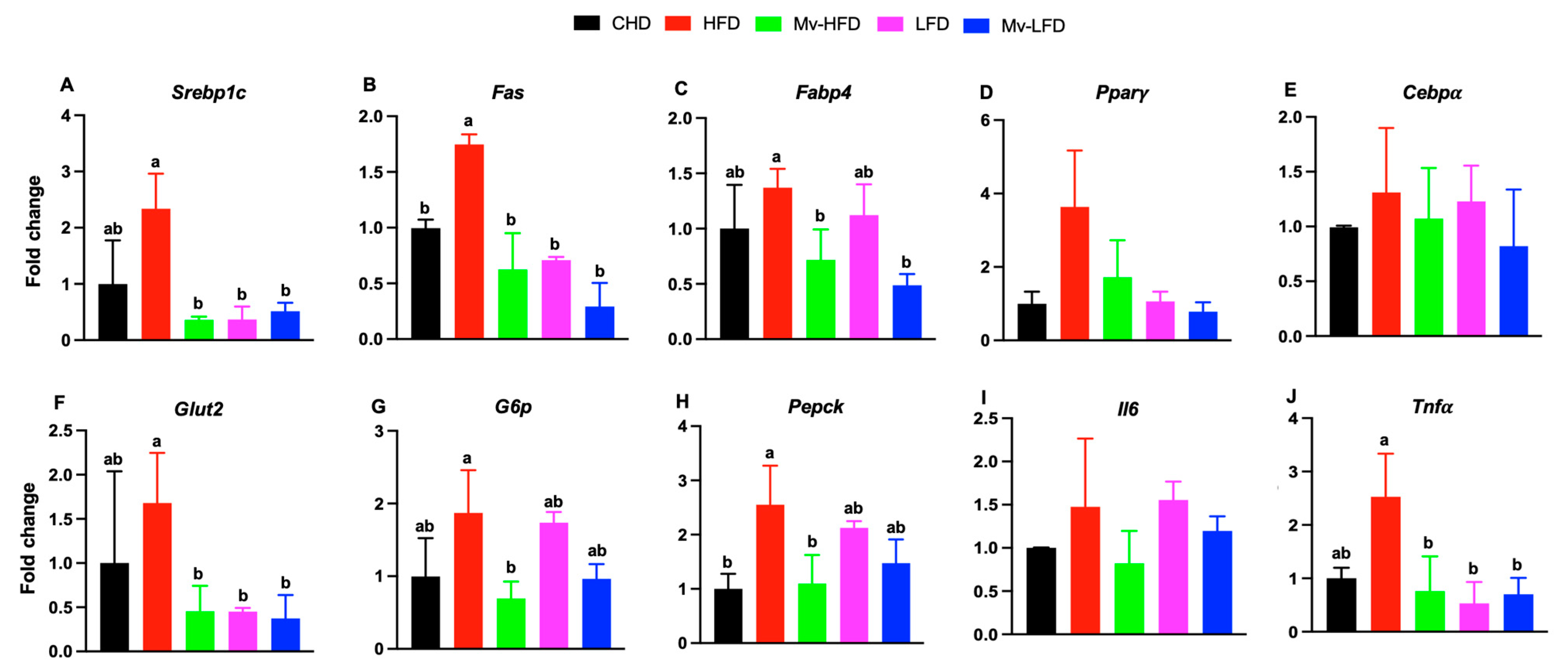
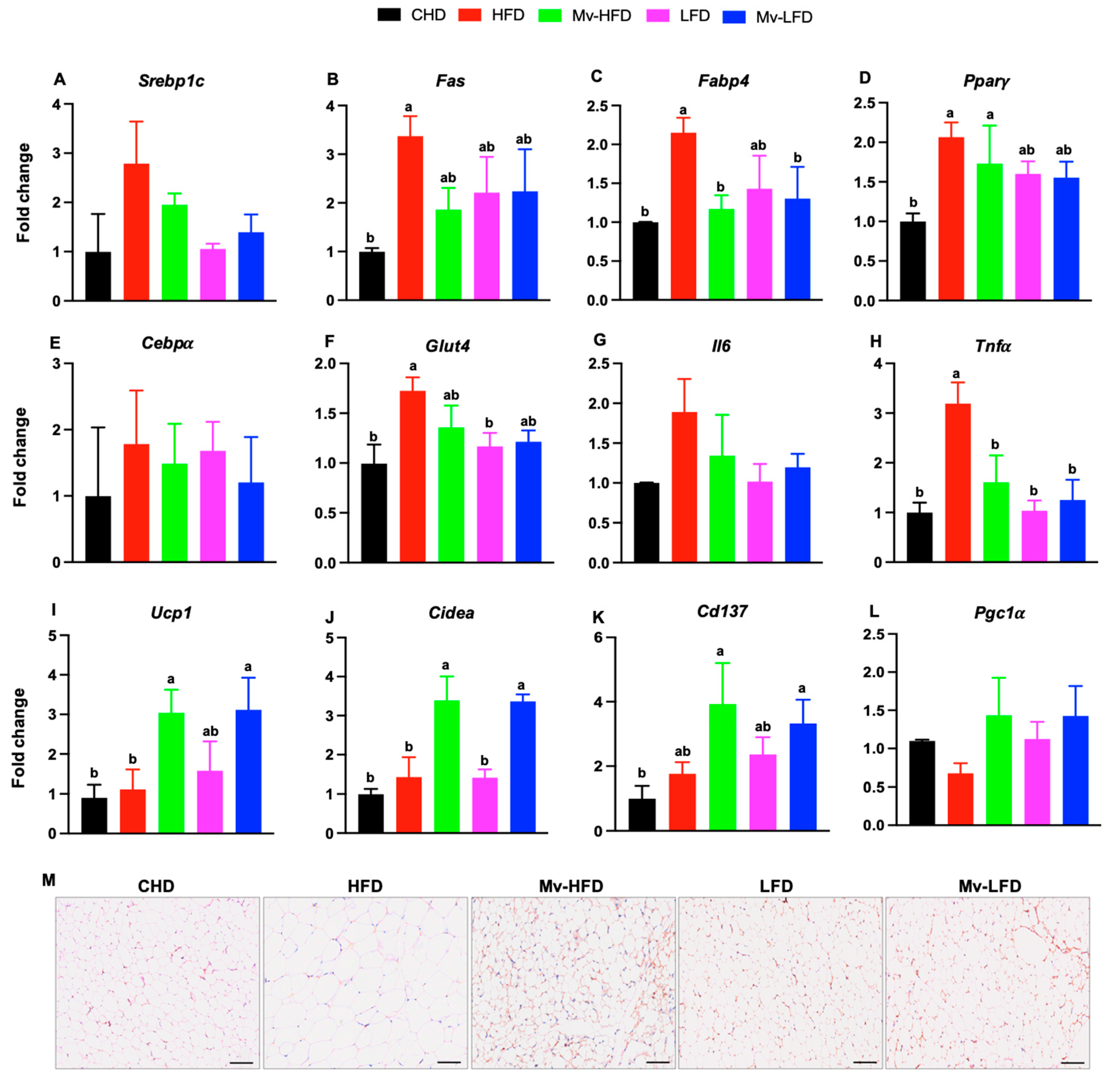
3.9. Mv-HFD, LFD, and Mv-LFD Reduced Hepatic mRNA Expression of Srebp1c, Fas, Glut2, and Tnfα
3.10. Mv-HFD and Mv-LFD Reduced IWAT mRNA Expression of Fabp4 and Tnfα
3.11. Mv-HFD and Mv-LFD Increased IWAT mRNA Expression of Ucp1, Cidea, and Cd137
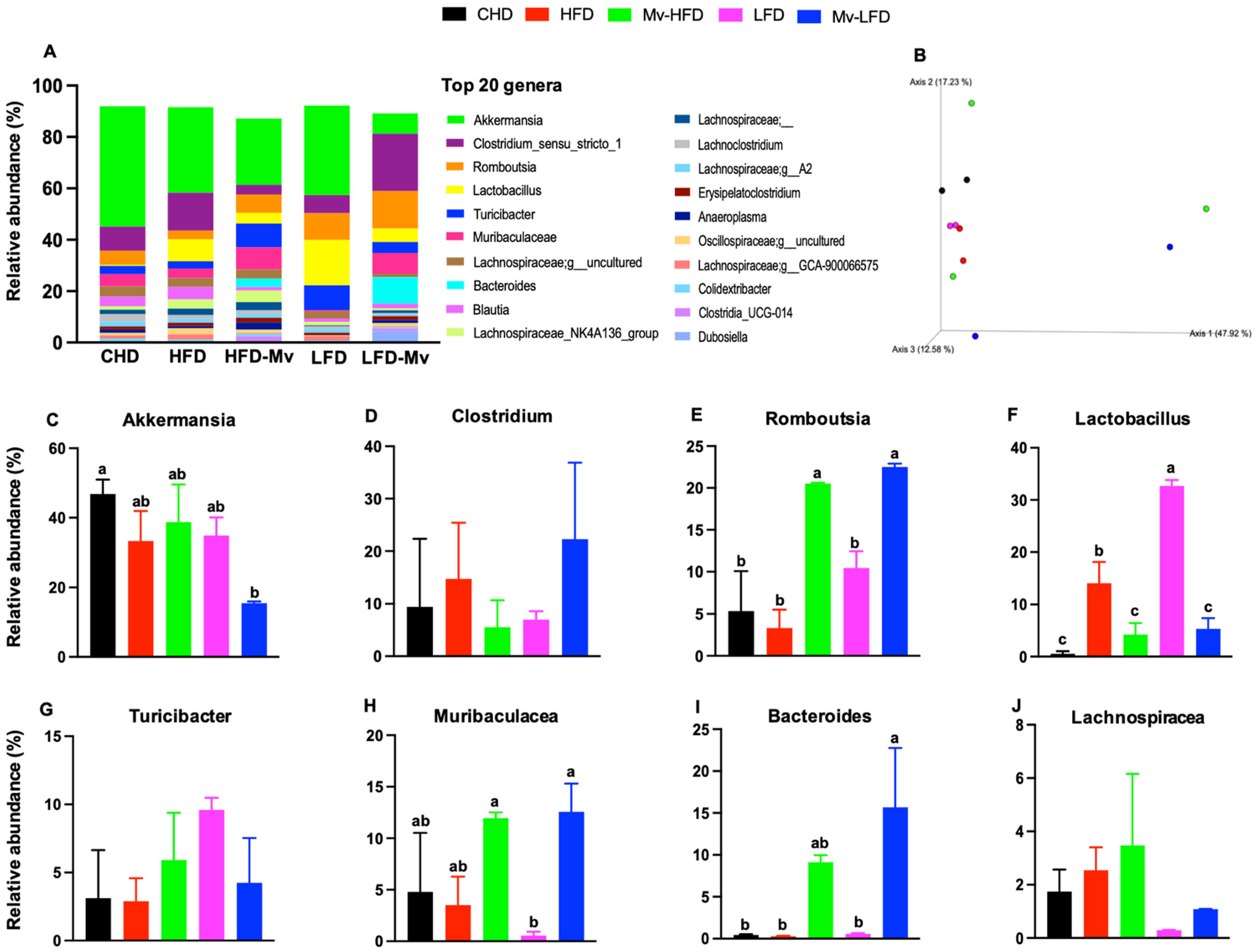
3.12. Mv-HFD and Mv-LFD Improved GM Composition at Phylum Level and α-Diversity
3.13. Mv-HFD and Mv-LFD Altered GM Composition at Genus Level and Showed Slight Trend in Improvement in -Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BAT | Brown adipose tissue |
| VCO2 | Carbon dioxide production |
| Cidea | Cell death-inducing DNA fragmentation factor-like effector A |
| Cd137 | Cluster of differentiation 137 |
| Cebpα | CCAAT/enhancer-binding protein alpha |
| CHD | Control chow diet |
| CoA | Coenzyme A |
| EE | Energy expenditure |
| Fabp4 | Fatty acid-binding protein 4 |
| Fas | Fatty acid synthase |
| GTT | Glucose tolerance test |
| Glut2 | Glucose transporter 2 |
| Glut4 | Glucose transporter 4 |
| GWAT | Gonadal white adipose tissue |
| GM | Gut microbiome |
| H&E | Hematoxylin and Eosin |
| HFD | High-fat diet |
| Il6 | Interleukin 6 |
| IWAT | Inguinal white adipose tissue |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| LFD | Low-fat diet |
| Mv-HFD | Multivitamin-supplemented HFD |
| Mv-LFD | Multivitamin-supplemented LFD |
| VO2 | Oxygen consumption |
| PA | Pantothenic acid |
| Pgc1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| Pparγ | Peroxisome proliferator-activated receptor gamma |
| Prdm16 | PR domain containing 16 |
| PCoA | Principal coordinates analysis |
| TCore | Rectal temperature |
| RER | Respiratory exchange ratio |
| RA | Retinoic acid |
| Srebp1c | Sterol regulatory element-binding protein 1c |
| TSkin | Surface temperature |
| ThTr2 | Thiamine transporter |
| Tnfα | Tumor necrosis factor alpha |
| Ucp1 | Uncoupling protein 1 |
| V4 | Variable region 4 |
| WAT | White adipose tissue |
References
- Hsu, K.-J.; Liao, C.-D.; Tsai, M.-W.; Chen, C.-N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; Hall, K.D.; Hu, F.B.; McCartney, A.L.; Roberto, C. Nutrition and the science of disease prevention: A systems approach to support metabolic health. Ann. N. Y. Acad. Sci. 2015, 1352, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Loh, Y.J.; Yang, X.; Zhang, C. The effect of calorie intake, fasting, and dietary composition on metabolic health and gut microbiota in mice. BMC Biol. 2021, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Wali, J.A.; Ni, D.; Facey, H.J.W.; Dodgson, T.; Pulpitel, T.J.; Senior, A.M.; Raubenheimer, D.; Macia, L.; Simpson, S.J. Determining the metabolic effects of dietary fat, sugars and fat-sugar interaction using nutritional geometry in a dietary challenge study with male mice. Nat. Commun. 2023, 14, 4409. [Google Scholar] [CrossRef]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms—A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef]
- Ji, T.; Fang, B.; Wu, F.; Liu, Y.; Cheng, L.; Li, Y.; Wang, R.; Zhu, L. Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice. Nutrients 2023, 15, 4978. [Google Scholar] [CrossRef]
- Lu, M.; Wan, Y.; Yang, B.; Huggins, C.E.; Li, D. Effects of low-fat compared with high-fat diet on cardiometabolic indicators in people with overweight and obesity without overt metabolic disturbance: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2018, 119, 96–108. [Google Scholar] [CrossRef]
- Lang, P.; Hasselwander, S.; Li, H.; Xia, N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci. Rep. 2019, 9, 19556. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.M.; Keijer, J.; Herreman, L.; Palm, I.; Hegeman, M.A.; Swarts, H.J.M.; van Schothorst, E.M. Adipose tissue metabolism and inflammation are differently affected by weight loss in obese mice due to either a high-fat diet restriction or change to a low-fat diet. Genes Nutr. 2014, 9, 391. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, Y.; Li, D.; Zhang, S.; Wu, Y.; Zhang, Q.; Bai, W. New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases. iMeta 2023, 2, e69. [Google Scholar] [CrossRef]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Manta, K.; Bujak, A.L.; Simone, A.C.; Fuda, M.R.; Nilsson, M.I.; Hettinga, B.P.; Hughes, M.C.; Perry, C.G.R.; Tarnopolsky, M.A. A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice. Nutrients 2021, 13, 3726. [Google Scholar] [CrossRef]
- Miao, Y.; Jiang, Z.; Song, H.; Zhang, Y.; Chen, H.; Liu, W.; Wei, X.; Li, L.; Li, W.; Li, X. Vitamin D supplementation alleviates high fat diet-induced metabolic associated fatty liver disease by inhibiting ferroptosis pathway. Eur. J. Nutr. 2024, 64, 50. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. Effects of dietary vitamins on obesity-related metabolic parameters. J. Nutr. Sci. 2023, 12, e47. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, A.; Zheng, M.; Wang, Q.; Liang, H.; Han, X.; Schouten, E.G. B Vitamins Can Reduce Body Weight Gain by Increasing Metabolism-related Enzyme Activities in Rats Fed on a High-Fat Diet. Curr. Med. Sci. 2018, 38, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Zhu, K.; Feng, R.N.; Sun, C.H. Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int. J. Obes. 2010, 34, 1070–1077. [Google Scholar] [CrossRef]
- Kalisz, M.; Chmielowska, M.; Martyńska, L.; Domańska, A.; Bik, W.; Litwiniuk, A. All-trans-retinoic acid ameliorates atherosclerosis, promotes perivascular adipose tissue browning, and increases adiponectin production in Apo-E mice. Sci. Rep. 2021, 11, 4451. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, R. Vitamin D3 affects browning of white adipocytes by regulating autophagy via PI3K/Akt/mTOR/p53 signaling in vitro and in vivo. Apoptosis 2022, 27, 992–1003. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Ye, R.; Yan, C.; Lin, J.; Huang, Y.; Jiang, X.; Yuan, S.; Chen, L.; Jiang, R.; et al. Pantothenate protects against obesity via brown adipose tissue activation. Am. J. Physiol.-Endocrinol. Metab. 2022, 323, E69–E79. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef]
- Horvath, C.; Wolfrum, C. Feeding brown fat: Dietary phytochemicals targeting non-shivering thermogenesis to control body weight. Proc. Nutr. Soc. 2020, 79, 338–356. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Yun, J.W. β-Carotene stimulates browning of 3T3-L1 white adipocytes by enhancing thermogenesis via the β3-AR/p38 MAPK/SIRT signaling pathway. Phytomedicine 2022, 96, 153857. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.A.; Greenway, F.L.; Zhang, D.; Ghosh, S.; Coulter, C.R.; James, S.L.; He, Y.; Cusimano, L.A.; Rebello, C.J. Naringenin and β-carotene convert human white adipocytes to a beige phenotype and elevate hormone-stimulated lipolysis. Front. Endocrinol. 2023, 14, 1148954. [Google Scholar] [CrossRef]
- Serra, F.; Bonet, M.L.; Puigserver, P.; Oliver, J.; Palou, A. Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int. J. Obes. 1999, 23, 650–655. [Google Scholar] [CrossRef]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Vázquez, F.; Bonet, M.L.; Picó, C.; Palou, A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem. J. 1996, 317 Pt 3, 827–833. [Google Scholar] [CrossRef]
- Mercader, J.; Ribot, J.; Murano, I.; Felipe, F.; Cinti, S.; Bonet, M.L.; Palou, A. Remodeling of White Adipose Tissue after Retinoic Acid Administration in Mice. Endocrinology 2006, 147, 5325–5332. [Google Scholar] [CrossRef]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.-J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, D.F.; Lopez-Legarrea, P.; Quintero, P.; Martinez, J.A. Vitamin C in the treatment and/or prevention of obesity. J. Nutr. Sci. Vitaminol. 2014, 60, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Du, L.; Sheng, C.; You, H.; Wang, X.; Qu, S. Vitamin C status and its change in relation to glucose-lipid metabolism in overweight and obesity patients following laparoscopic sleeve gastrectomy. Eur. J. Clin. Nutr. 2022, 76, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Djurasevic, S.F.; Cvijic, G.; Djordjevic, J.; Davidovic, V. The influence of vitamin C supplementation on the oxidative status of rat interscapular brown adipose tissue. J. Therm. Biol. 2008, 33, 238–243. [Google Scholar] [CrossRef]
- Arianti, R.; Vinnai, B.Á.; Győry, F.; Guba, A.; Csősz, É.; Kristóf, E.; Fésüs, L. Availability of abundant thiamine determines efficiency of thermogenic activation in human neck area derived adipocytes. J. Nutr. Biochem. 2023, 119, 109385. [Google Scholar] [CrossRef]
- Vinnai, B.; Arianti, R.; Győry, F.; Bacso, Z.; Fésüs, L.; Kristóf, E. Extracellular thiamine concentration influences thermogenic competency of differentiating neck area-derived human adipocytes. Front. Nutr. 2023, 10, 1207394. [Google Scholar] [CrossRef]
- Tang, W.; Zhan, W.; Wei, M.; Chen, Q. Associations Between Different Dietary Vitamins and the Risk of Obesity in Children and Adolescents: A Machine Learning Approach. Front. Endocrinol. 2021, 12, 816975. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Wallace, T.C. Multivitamins and Nutritional Adequacy in Middle-Aged to Older Americans by Obesity Status. J. Diet. Suppl. 2020, 17, 684–697. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Kang, S. The Role of the Gut Microbiome in Energy Balance with a Focus on the Gut-Adipose Tissue Axis. Front. Genet. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.S.; Golden, J.P.; Vogt, S.K.; Gereau IV, R.W. A simple and inexpensive method for determining cold sensitivity and adaptation in mice. J. Vis. Exp. 2015, 97, 52640. [Google Scholar]
- Kumar, R.; Eipers, P.; Little, R.B.; Crowley, M.; Crossman, D.K.; Lefkowitz, E.J.; Morrow, C.D. Getting started with microbiome analysis: Sample acquisition to bioinformatics. Curr. Protoc. Hum. Genet. 2014, 82, 18.8.1–18.8.29. [Google Scholar] [CrossRef]
- Melhorn, S.J.; Krause, E.G.; Scott, K.A.; Mooney, M.R.; Johnson, J.D.; Woods, S.C.; Sakai, R.R. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol. Behav. 2010, 99, 33–39. [Google Scholar] [CrossRef]
- Merino, O.; Gregorio, B.; Sampaio, F.; Sanchez, R.; Risopatrón, J. Role of Vitamin D in the Development of Obesity. Int. J. Morphol. 2017, 35, 1568–1575. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Duester, G. Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Yadav, A.S.; Isoherranen, N.; Rubinow, K.B. Vitamin A homeostasis and cardiometabolic disease in humans: Lost in translation? J. Mol. Endocrinol. 2022, 69, R95–R108. [Google Scholar] [CrossRef]
- Jeyakumar, S.M.; Vajreswari, A.; Giridharan, N.V. Vitamin A regulates obesity in WNIN/Ob obese rat; independent of stearoyl-CoA desaturase-1. Biochem. Biophys. Res. Commun. 2008, 370, 243–247. [Google Scholar] [CrossRef]
- Felipe, F.; Mercader, J.; Ribot, J.; Palou, A.; Bonet, M.L. Effects of retinoic acid administration and dietary vitamin A supplementation on leptin expression in mice: Lack of correlation with changes of adipose tissue mass and food intake. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1740, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Noy, N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol. Cell Biol. 2009, 29, 3286–3296. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.; Felipe, F.; Bonet, M.L.; Palou, A. Changes of Adiposity in Response to Vitamin A Status Correlate with Changes of PPARγ2 Expression. Obes. Res. 2001, 9, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Nan, W.; Si, H.; Yang, Q.; Shi, H.; Zhang, T.; Shi, Q.; Li, G.; Zhang, H.; Liu, H. Effect of Vitamin A Supplementation on Growth Performance, Serum Biochemical Parameters, Intestinal Immunity Response and Gut Microbiota in American Mink (Neovison vison). Animals 2021, 11, 1577. [Google Scholar] [CrossRef]
- Chen, B.-W.; Zhang, K.-W.; Chen, S.-J.; Yang, C.; Li, P.-G. Vitamin A Deficiency Exacerbates Gut Microbiota Dysbiosis and Cognitive Deficits in Amyloid Precursor Protein/Presenilin 1 Transgenic Mice. Front. Aging Neurosci. 2021, 13, 753351. [Google Scholar] [CrossRef]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Zsálig, D.; Berta, A.; Tóth, V.; Szabó, Z.; Simon, K.; Figler, M.; Pusztafalvi, H.; Polyák, É. A Review of the Relationship between Gut Microbiome and Obesity. Appl. Sci. 2023, 13, 610. [Google Scholar] [CrossRef]
- Lee, H.-K.; Kim, N.-E.; Shin, C.M.; Oh, T.J.; Yoon, H.; Park, Y.S.; Kim, N.; Won, S.; Lee, D.H. Gut microbiome signature of metabolically healthy obese individuals according to anthropometric, metabolic and inflammatory parameters. Sci. Rep. 2024, 14, 3449. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Gao, D.; Wilding, J.; Trayhurn, P.; Bing, C. Vitamin D signalling in adipose tissue. Br. J. Nutr. 2012, 108, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health1. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Lu, S.; Cao, Z.-B. Interplay between Vitamin D and Adipose Tissue: Implications for Adipogenesis and Adipose Tissue Function. Nutrients 2023, 15, 4832. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Śliwińska, A. The action of vitamin D in adipose tissue: Is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.-M.; Buring, J.E.; et al. Association of Body Weight with Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Seida, J.C.; Mitri, J.; Colmers, I.N.; Majumdar, S.R.; Davidson, M.B.; Edwards, A.L.; Hanley, D.A.; Pittas, A.G.; Tjosvold, L.; Johnson, J.A. Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.M.; Biscaia, P.B.; Brunoski, J.; Ribeiro, R.A.; Franco, G.C.N.; Scomparin, D.X. Vitamin D supplementation decreases visceral adiposity and normalizes leptinemia and circulating TNF-α levels in western diet-fed obese rats. Life Sci. 2021, 278, 119550. [Google Scholar] [CrossRef]
- Jahn, D.; Dorbath, D.; Kircher, S.; Nier, A.; Bergheim, I.; Lenaerts, K.; Hermanns, H.M.; Geier, A. Beneficial Effects of Vitamin D Treatment in an Obese Mouse Model of Non-Alcoholic Steatohepatitis. Nutrients 2019, 11, 77. [Google Scholar] [CrossRef]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Peiretti, F.; Darmon, P.; Landrier, J.-F. Vitamin D Limits Chemokine Expression in Adipocytes and Macrophage Migration In Vitro and in Male Mice. Endocrinology 2015, 156, 1782–1793. [Google Scholar] [CrossRef]
- Xiang, L.; Du, T.; Zhang, J.; Zhang, Y.; Zhou, Y.; Zhao, Y.; Zhou, Y.; Ma, L. Vitamin D(3) supplementation shapes the composition of gut microbiota and improves some obesity parameters induced by high-fat diet in mice. Eur. J. Nutr. 2024, 63, 155–172. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lee, J.; Shin, Y.; Yoon, M. Ascorbic acid reduces insulin resistance and pancreatic steatosis by regulating adipocyte hypertrophy in obese ovariectomized mice. Can. J. Physiol. Pharmacol. 2023, 101, 294–303. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.; O’Harte, F.P.; Mooney, M.H.; Barnett, C.R.; Flatt, P.R. Vitamin C supplementation decreases insulin glycation and improves glucose homeostasis in obese hyperglycemic (ob/ob) mice. Metabolism 2002, 51, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jang, J.; Lee, D.; Yoon, M. Vitamin C Inhibits Visceral Adipocyte Hypertrophy and Lowers Blood Glucose Levels in High-Fat-Diet-Induced Obese C57BL/6J Mice. Biomed. Sci. Lett. 2018, 24, 311–318. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, J.; Shin, S.S.; Yoon, M. Ascorbic acid inhibits visceral obesity and nonalcoholic fatty liver disease by activating peroxisome proliferator-activated receptor α in high-fat-diet-fed C57BL/6J mice. Int. J. Obes. 2019, 43, 1620–1630. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, L.; Mei, L.; Zhao, X.; Han, P.; Liu, J.; Meng, C.; Li, R.; Zhong, R.; Wang, K.; et al. Vitamin C and vitamin D(3) alleviate metabolic-associated fatty liver disease by regulating the gut microbiota and bile acid metabolism via the gut-liver axis. Front. Pharmacol. 2023, 14, 1163694. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome—A pilot study. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Kerns, J.C.; Arundel, C.; Chawla, L.S. Thiamin deficiency in people with obesity. Adv. Nutr. 2015, 6, 147–153. [Google Scholar] [CrossRef]
- Nath, A.; Tran, T.; Shope, T.R.; Koch, T.R. Prevalence of clinical thiamine deficiency in individuals with medically complicated obesity. Nutr. Res. 2017, 37, 29–36. [Google Scholar] [CrossRef]
- Muroyama, K.; Murosaki, S.; Yamamoto, Y.; Ishijima, A.; Toh, Y. Effects of intake of a mixture of thiamin, arginine, caffeine, and citric acid on adiposity in healthy subjects with high percent body fat. Biosci. Biotechnol. Biochem. 2003, 67, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, K.; Murosaki, S.; Yamamoto, Y.; Odaka, H.; Chung, H.C.; Miyoshi, M. Anti-obesity effects of a mixture of thiamin, arginine, caffeine, and citric acid in non-insulin dependent diabetic KK mice. J. Nutr. Sci. Vitaminol. 2003, 49, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kono, T.; Terasaki, F.; Yasui, K.; Soyama, A.; Otsuka, K.; Fujita, S.; Yamane, K.; Manabe, M.; Usui, K.; et al. Thiamine prevents obesity and obesity-associated metabolic disorders in OLETF rats. J. Nutr. Sci. Vitaminol. 2010, 56, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hosomi, K.; Kawashima, H.; Chen, Y.-A.; Mohsen, A.; Ohno, H.; Konishi, K.; Tanisawa, K.; Kifushi, M.; Kogawa, M.; et al. Dietary Vitamin B1 Intake Influences Gut Microbial Community and the Consequent Production of Short-Chain Fatty Acids. Nutrients 2022, 14, 2078. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Ortañez, J.; Leano, J.; Curras-Collazo, M.; Deol, P.; Sladek, F.; Degnan, P. Gut Microbiome Response to the Reduction of Diet Induced Obesity by Vitamin B1 Supplementation. Physiology 2024, 39, 2484. [Google Scholar] [CrossRef]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef]
- Takeda, Y.; Dai, P. Functional roles of pantothenic acid, riboflavin, thiamine, and choline in adipocyte browning in chemically induced human brown adipocytes. Sci. Rep. 2024, 14, 18252. [Google Scholar] [CrossRef] [PubMed]
- Taleban, R.; Heidari-Beni, M.; Qorbani, M.; Motlagh, M.E.; Malekshah, A.F.-T.; Moafi, M.; Zavareh, N.H.-T.; Kelishadi, R. Is dietary vitamin B intake associated with weight disorders in children and adolescents? The weight disorders survey of the CASPIAN-IV Study. Health Promot. Perspect. 2019, 9, 299–306. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Imoto, S.; Ihara, K.; Nakaji, S. Association between Nutrients and Visceral Fat in Healthy Japanese Adults: A 2-Year Longitudinal Study Brief Title: Micronutrients Associated with Visceral Fat Accumulation. Nutrients 2019, 11, 2698. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Rumberger, J.A.; Azumano, I.; Napolitano, J.J.; Citrolo, D.; Kamiya, T. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: A triple-blinded placebo and diet-controlled investigation. Vasc. Health Risk Manag. 2014, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.S.; Amarasena, S.; Mayengbam, S. B Vitamins and Their Roles in Gut Health. Microorganisms 2022, 10, 1168. [Google Scholar] [CrossRef]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal Microbial Community Structure Is Stable over Time and Related to Variation in Macronutrient and Micronutrient Intakes in Lactating Women. J. Nutr. 2015, 145, 2379–2388. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Xun, P.; Lin, H.; Zhou, C.; Huang, Z.; Yu, W.; Yang, Y.; Huang, X.; Wang, Y.; Huang, Q.; Tan, L. Effects of dietary pantothenic acid supplement on hepatic antioxidative abilities and intestinal microflora in juvenile golden pompano (Trachinotus ovatus). Isr. J. Aquac.—Bamidgeh 2019, 71, 20991. [Google Scholar] [CrossRef]
- Yao, C.; Chou, J.; Wang, T.; Zhao, H.; Zhang, B. Pantothenic Acid, Vitamin C, and Biotin Play Important Roles in the Growth of Lactobacillus helveticus. Front. Microbiol. 2018, 9, 1194. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, M.; Heath, B.; McGinness, L. Effects of Multivitamin Supplementation on Metabolic Parameters in High- and Low-Fat Diet-Fed C57BL/6J Mice: Potential Links to Adipose Tissue Browning and Gut Microbiome. Nutrients 2025, 17, 1045. https://doi.org/10.3390/nu17061045
Abbasi M, Heath B, McGinness L. Effects of Multivitamin Supplementation on Metabolic Parameters in High- and Low-Fat Diet-Fed C57BL/6J Mice: Potential Links to Adipose Tissue Browning and Gut Microbiome. Nutrients. 2025; 17(6):1045. https://doi.org/10.3390/nu17061045
Chicago/Turabian StyleAbbasi, Mehrnaz, Braeden Heath, and Lauren McGinness. 2025. "Effects of Multivitamin Supplementation on Metabolic Parameters in High- and Low-Fat Diet-Fed C57BL/6J Mice: Potential Links to Adipose Tissue Browning and Gut Microbiome" Nutrients 17, no. 6: 1045. https://doi.org/10.3390/nu17061045
APA StyleAbbasi, M., Heath, B., & McGinness, L. (2025). Effects of Multivitamin Supplementation on Metabolic Parameters in High- and Low-Fat Diet-Fed C57BL/6J Mice: Potential Links to Adipose Tissue Browning and Gut Microbiome. Nutrients, 17(6), 1045. https://doi.org/10.3390/nu17061045





