Abstract
Intensive Care Unit-Acquired Weakness (ICUAW) is a very common condition in patients admitted to intensive care units (ICUs), even after relatively short stays. This weakness can develop with a pre-existing background of sarcopenia or cachexia, although these conditions are not always the direct cause. Over the years, much of the literature has focused on the nutritional aspect of the issue, leading to the development of widely accepted guidelines recommending the initiation of early nutrition, with the goal of achieving caloric and protein targets within the first five days of ICU admission. Despite adherence to these guidelines, several studies have shown a significant loss of muscle mass in critically ill patients, which directly impacts their ability to generate strength. However, it has become increasingly evident that nutrition alone is not sufficient to counteract this muscle loss, which is often closely linked to the prolonged immobility experienced by ICU patients due to a variety of clinical and logistical factors. In particular, there is growing evidence suggesting that even the introduction of early and minimal rehabilitation—including passive mobilization—when combined with appropriate nutritional support, can be a valuable strategy to help reduce the incidence of ICUAW. In this narrative review, we aim to summarize the current scientific knowledge on this topic, emphasizing the importance of an integrated approach that combines nutrition and early mobilization. Such a combined strategy not only holds the potential to reduce the acute incidence of ICUAW but also contributes to better recovery outcomes and, eventually, improved quality of life for these patients.
1. Introduction
Intensive Care Unit-Acquired Weakness (ICUAW) is a complex syndrome characterized by severe muscle weakness which develops during an ICU patient’s stay and is primarily not attributable to pre-existing conditions [1]. It is closely associated with many conditions, such as prolonged immobilization, systemic inflammation, and the metabolic and neuromuscular disorders associated with critical illness [2]. ICUAW not only complicates functional recovery but also extends ICU and hospital stays, often leading to long-term disability in survivors [3,4]. Even if the loss of muscle mass is a sign of different conditions such as malnutrition, sarcopenia, and cachexia [5,6], recent guidelines emphasize that muscle strength may be a more reliable indicator of adverse outcomes than muscle mass alone [7]. In fact, sarcopenia is characterized not only by muscle loss but also by a decline in muscle quality, with structural alterations at both the microscopic and macroscopic levels [8]. Furthermore, malnutrition accelerates muscle loss and functional decline, which can evolve into physical frailty, leading to impaired mobility and disability. Thus, understanding these interconnected processes help in developing effective interventions aimed to preserve muscle function and improve patient outcomes [9].
In critically ill patients, muscle loss is not simply a consequence of reduced nutrient intake but depends on profound disruptions in metabolic pathways which include dysregulated proteostasis, impaired muscle protein synthesis and breakdown during fasting, disturbances in glucose and insulin homeostasis, inflammation, neuromuscular function, and microvascular function [10]. Moreover, prolonged hospital stays and pharmacological treatments exacerbate long-term complications [11]. Lastly, ICUAW is often a part of Post-Intensive Care Syndrome (PICS), a complex condition that includes physical, cognitive, and psychological impairments that can persist long after ICU discharge [12].
This review explores how early mobilization and nutritional support work synergically to mitigate ICUAW, highlighting their combined role in improving patient outcomes.
2. Literature Selection
In this narrative review, we selected relevant literature through a structured search of six major databases: PubMed (1996–present), Embase (1974–present), Scopus (2004–present), SpringerLink (1950–present), Ovid Emcare (1995–present), and Google Scholar (2004–present). We included studies that provided original data or comprehensive analyses on nutritional and rehabilitation support, focusing primarily on randomized controlled trials, observational studies, and meta-analyses. The search strategy involved using keywords such as “early nutrition”, “early rehabilitation”, “muscular assessment”, and “ICU” to identify relevant studies. Two authors, AM and PF, were responsible for retrieving and reviewing the full texts of articles that met the search criteria. They carefully examined titles and abstracts to determine relevance and obtained full-text versions for detailed evaluation. The quality of the selected articles was assessed by evaluating their method, sample size, study design, and relevance to the topic of combined nutrition and rehabilitation in the ICU. Given the nature of a narrative review, we did not perform a formal quality assessment of the included studies, and selection was based on relevance and scientific merit. While this approach allows for a broader discussion of the topic, it also presents limitations compared to systematic reviews, as it may be subject to selection bias and does not provide a quantitative synthesis of the evidence.
3. Assessment of Nutritional and Physical Status in ICU
Despite remarkable medical innovations, significant challenges remain in accurately assessing both muscle quantity and quality, making the accurate diagnosis of sarcopenia particularly complex [5]. Determining the nutritional needs of ICU patients adds another difficulty. The traditional measures—such as body mass index, predictive ideal body weight, or adjustments according to body mass index—fail to accurately reflect cellular mass or account for the substantial fluid shifts commonly seen in critically ill patients, leading to potential misinterpretations of nutritional status [13]. Furthermore, serum biomarkers such as albumin, transthyretin, and transferrin, although routinely employed to assess nutrition, also have limitations in critical care [14]. For instance, their levels change due to acute infections, inflammation, and renal or liver dysfunction, making them often unreliable indicators in these settings [15]. Indirect calorimetry has been introduced to overcome these limitations, with the aim of targeting the nutritional needs of each individual patient [16]. The major limitation is that it is not a readily available device in the majority of ICUs [17].
Given these limitations, there is a need for more precise and advanced tools specifically tailored to the physiological complexities of critically ill patients [18]. Nutrition screening should be the first step in this process, integrated into routine patient care. Several validated instruments, including the Malnutrition Universal Screening Tool (MUST) [19], the Nutrition Risk Screening 2002 (NRS-2002) [20], and the NUTRIC score [21,22,23], are commonly used to assess malnutrition risk in ICU patients. Recent efforts have focused on developing numerical screening tools to better identify patients at risk of malnutrition early on. The SCREENIC score was created for this purpose, specifically for intensive care patients [24]. It includes six questions based on patient factors like comorbidities, age, and muscle mass loss, and it was found to have good accuracy in predicting prolonged ICU and hospital stays. One of the most challenging aspects in literature is the lack of a standardized approach to diagnosing ICUAW, without a uniform consensus on the primary diagnostic criteria. Over the years, different studies have progressively integrated complementary diagnostic tools, combining muscle strength assessment with imaging techniques such as ultrasound [25]. In this regard, recently, muscle ultrasound has emerged as a valuable tool for a non-invasive and dynamic method for evaluating both muscle mass and quality. Unlike “static” measurements such as BMI or biochemical markers, ultrasound allows a real-time tracking of muscle changes, providing detailed insights into tissue composition and structure [26]. Parameters such as muscle thickness, echogenicity (which reflects alterations in muscle tissue), and overall architecture can be directly visualized [27]. Despite these highly useful features, it is important to highlight that the ultrasound technique has inherent limitations. These are primarily due to operator-dependent variability in performing the measurement and the specific setting used for muscle assessment, which largely depends on the available ultrasound machine [28]. More recent devices offer dedicated presets that can influence a study’s execution.
Assessing muscle strength is equally important, with various methods available, each with specific applications and limitations. While grip strength correlates moderately with overall limb strength, its utility in ICU patients is often restricted, as critically ill patients may not be able to actively participate in testing during the acute phase of illness [29]. Another widely used approach is the Medical Research Council (MRC) scale, which assesses motor performance on a graded scale from 5 (normal strength) to 0 (no visible muscle contraction) [30]. A prospective cohort study demonstrated agreement between handgrip dynamometry and the MRC score for ICUAW diagnosis [31]. Thus, once patients stabilize and become more cooperative, these assessments offer valuable insights into their functional recovery, guiding rehabilitation strategies with a more personalized approach to patients at a high risk of ICUAW.
4. Benefits of Early Mobilization in ICU
Early mobilization in ICU refers to initiating physical activity—including passive movements, active exercises, sitting, or standing—as soon as it is clinically safe for the patient [32]. This practice has gained recognition as a cornerstone of modern critical care due to its substantial impact on the recovery of critically ill patients. Its benefits extend beyond merely preventing muscle loss since early mobilization counteract the physical, the functional, and the psychological consequences of critical illness [33].
4.1. Preservation of Muscle Mass and Function
One of the most significant advantages of early mobilization is its role in preserving muscle mass and function [34]. Critically ill patients, especially those undergoing prolonged mechanical ventilation, deep sedation, or extended bed rest, experience rapid muscle atrophy. Research indicates that patients can lose up to 20% of their muscle mass within the first week of immobility [35]. This weakness impacts both peripheral muscles and respiratory function, often leading to difficulties in weaning from mechanical ventilation and prolonged ICU stays [4]. Early mobilization provides a sort of protective strategy against these complications. With this prospective, even apparently minimal movements, such as passive range-of-motion exercises or assisted limb mobilization, play an important role in maintaining neuromuscular integrity [11].
Emerging research is beginning to reveal the molecular mechanisms that explain the positive effects of physical activity on muscle preservation [36]. These processes include molecules like Bassoon, neuregulin-1, and Insulin-like growth factor-1 [37,38].
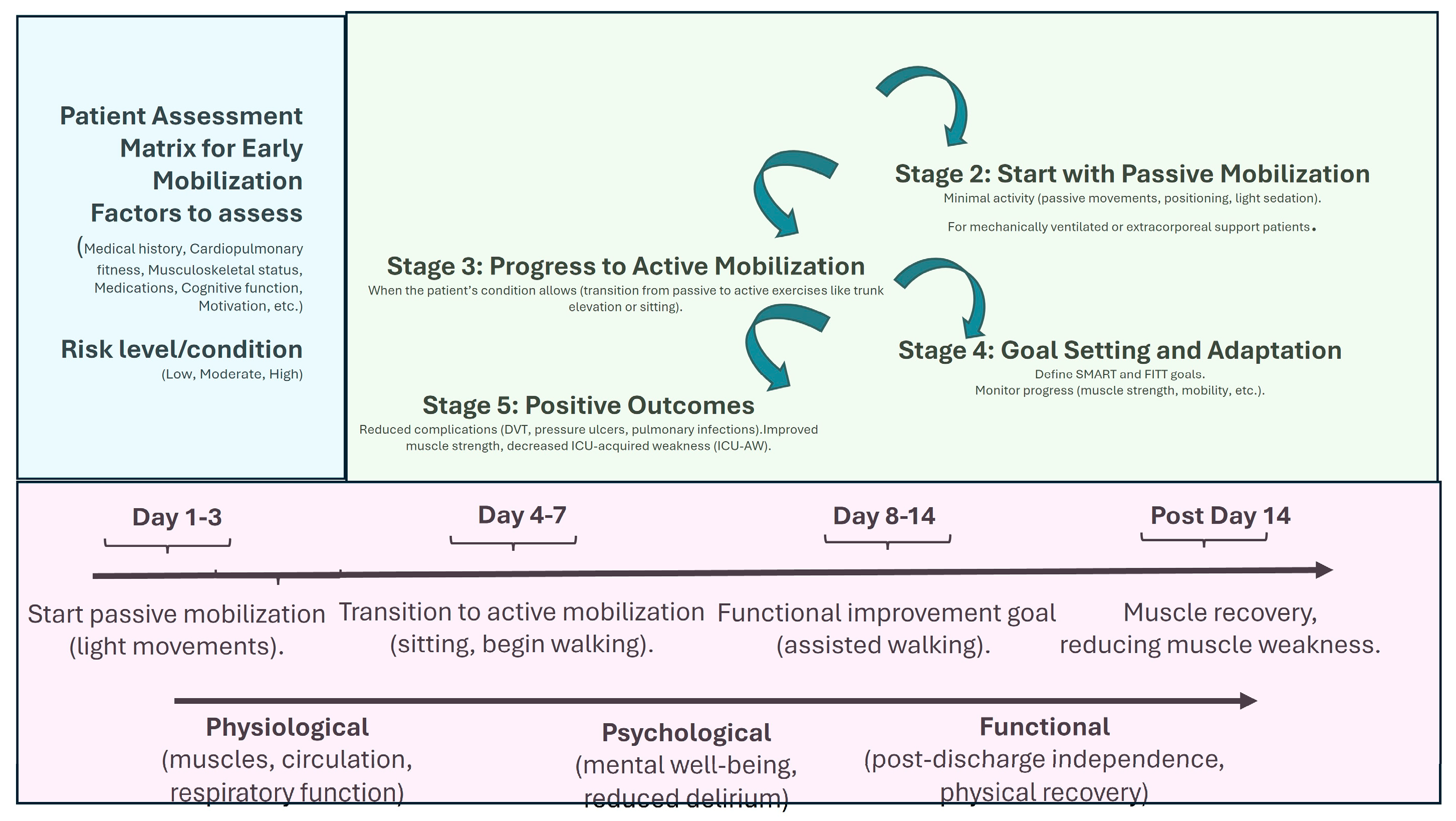
In terms of rehabilitation, a systematic review by Wang et al. [39], analyzing 60 randomized controlled trials with over 5000 participants, demonstrated that initiating physical rehabilitation in the ICU significantly improved patients’ functional status at hospital discharge and contributed to shorter ICU and hospital stays, even though it did not alter other clinical outcomes. Additionally, Biolo et al. [40] highlighted that exercise improves the muscle’s response to exogenous amino acids, suggesting that movement itself actively contributes to optimizing muscle protein synthesis. Figure 1 shows the main strategies and implications of mobilization programs.
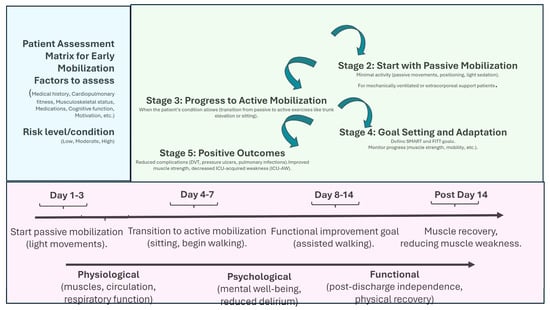
Figure 1.
Early mobilization benefits and temporal managing. This figure outlines the step-by-step process for implementing early mobilization in ICU patients. SMART = Specific, Measurable, Achievable, Realistic, and Time-bound; FITT = Frequency, Intensity, Time, and Type.
4.2. Improved Functional Outcomes
Patients who participate in early mobilization during their ICU stay consistently show better functional recovery than those who remain inactive, and these benefits are particularly evident in post-discharge mobility and self-sufficiency. Research suggests that early mobilization shortens the rehabilitation period post-ICU, accelerating the return to daily activities [41,42]. On the other hand, prolonged immobility in critically ill patients is associated with several complications, including deep vein thrombosis, pulmonary infections, pressure ulcers, and joint stiffness [43]. Early mobilization plays a key role in mitigating these risks since simply sitting up or standing can help expand lung capacity and improve secretion clearance, significantly lowering the likelihood of ventilator-associated pneumonia [44]. A secondary analysis of the PREVENT trial examined how different levels of early mobility during the first three days in the ICU impacted patient outcomes [45]. The findings suggested that patients who were involved in higher levels of mobility had a lower risk of death within 90 days. In addition to these benefits, it alleviated prolonged pressure on vulnerable areas of the body, such as bony prominences, reducing the risk of skin pressure-ulcers and their associated complications [46]. Several studies have consistently reported a direct correlation between early mobilization and improved prognosis in ICUAW. While this evidence strongly supports the beneficial role of early mobilization, some authors have rightly pointed out that multiple factors interact to enhance its effectiveness [47]. Among these are resource staffing, equipment, education, financial support, and the engagement of both staff and family in early mobilization, as well as therapeutic strategies that, in addition to nutrition, include the careful management of sedation and vasoactive drugs, which are directly dependent on the type of underlying pathology.
The ICU environment, often characterized by sensory deprivation, sleep disruption, and frequent sedation, predisposes patients to psychological distress, delirium, and PICS [48]. It has been suggested that mobilization may be able to decrease the incidence of all these detrimental factors [49]. In a randomized controlled trial involving hundreds of ICU patients who had been sedated and on mechanical ventilation for less than 72 h, those who engaged in early mobilization during daily sedation discontinuities were significantly more likely to recover independent functional status by the time of discharge [50].
Despite all these observations, early mobilization also presents potential risks that must be carefully managed. These include the possibility of hemodynamic instability, as physical exertion may lead to variable blood pressure level or heart rate [51]. Furthermore, early mobilization may worsen respiratory function in some patients, particularly those with severe pulmonary conditions [52].
4.3. Long-Term Outcomes and Follow-Up Care After ICU Discharge
Many patients who survive a critical illness face long-lasting challenges that can significantly affect their lives. One of the most prevalent concerns is PICS, which encompasses a range of physical, cognitive, and psychological issues that persist well after leaving the ICU [53]. A recent metanalysis comparing early active mobilization with usual care showed that early mobilization was associated with improved physical function in survivors at 6 months [54].
In addition to physical recovery, addressing emotional and cognitive challenges is just as important as physical rehabilitation. In a randomized controlled trial involving 200 mechanically ventilated ICU patients, early physical and occupational therapy (early mobilization) was compared to usual care [55]. The primary outcome, cognitive impairment one year after hospital discharge, was significantly lower in the early mobilization group (24%) compared to the usual care group (43%). The intervention group also showed fewer ICU-acquired weaknesses and better physical quality of life scores. These findings suggest that early mobilization may reduce long-term cognitive impairment but warrant further investigation due to the increased risk of adverse events.
It is also important to provide patients and families with the information and resources they need to understand what to expect in the recovery process and how to manage potential complications [56].
4.4. Challenges in Implementation
Despite the well-documented benefits, early mobilization is not without challenges. The most significant limitation is the clinical stability of patients [57]. Mobilization can only proceed when vital signs, hemodynamic parameters, and respiratory function are within safe limits. In the acute phase of critical illness, where mechanical ventilation and sedation are often necessary, active mobilization may not be feasible.
Passive mobilization strategies, such as manual limb movements or passive cycling, can help overcome this challenge, allowing for early intervention while maintaining patient safety [58]. Genc et al. [59] highlighted the positive impact of passive movements in critically ill patients, using a regimen of 10 repetitions for each joint movement. For patients with impaired bowel function, Morisawa et al. [60] showed that passive lower limb and trunk movements, including 10 repetitions of joint movements and an additional 10 min of trunk rotation, were effective. However, other studies have reported mixed outcomes. In a randomized controlled trial including 48 ICU patients, Stiller et al. [61] showed that passive mobilizations, as applied to a cohort of medium- to long-term ICU patients, did not reduced joint stiffness. Therefore, since the evidence on this issue remains inconclusive, it is reasonable to carefully evaluate the appropriateness and timing of initiating passive mobilization on an individual basis, particularly in patients experiencing hemodynamic and respiratory instability.
Another challenge is the need for sufficient staffing and training. Successful mobilization programs require close coordination among intensivists, nurses, physiotherapists, and occupational therapists. This largely depends on the type of organization of the various integrated units [62]. The optimal setting, as suggested in few studies, could involve the implementation of individualized care plans based on principles like SMART (Specific, Measurable, Achievable, Realistic, and Time-bound) [63] or FITT (Frequency, Intensity, Time, and Type). However, implementing individualized care plans is not always straightforward. Some authors have suggested simple key points that could help improve this critical aspect, facilitating a more effective and tailored approach to patient rehabilitation [64,65], in part already discussed above. Regarding mobilization, there are still significant uncertainties about the optimal approach to exercise, particularly in terms of timing, modality, and dosage of interventions. Some evidence suggests that initiating rehabilitation within the first 2–3 days of ICU admission may yield better outcomes compared to later initiation [66]. Various techniques can be employed, including active mobilization, in-bed cycling, neuromuscular electrical stimulation (either alone or combined with passive or active exercises), tilt tables, and different rehabilitation devices. Moreover, factors such as the appropriate intensity, duration, and frequency of these interventions play a crucial role in optimizing their effectiveness [67].
Lastly, a relatively recent development is the introduction of virtual reality in the ICU. Some authors have started to propose the use of these devices in combination with upper- and lower-limb rehabilitation activities. In a proof-of-concept study, virtual reality was shown to be a feasible, safe, and well-received rehabilitation tool [68]. Twenty patients participated in 79 virtual reality sessions using a dedicated app designed for bedridden patients in the supine position. Each session lasted around 14 min, with 10 min dedicated to active training. Importantly, physiotherapists reported no significant barriers, and no adverse events were recorded. Similarly, another author showed a significant impact on mobility scales. A study involving ten ICU patients, all mechanically ventilated for at least 48 h, participated in virtual reality sessions three times a week for 20 min, completing progressively challenging puzzles to improve arm function [69]. Patients completed three weekly sessions, with 13 min of active training per session, and the mobility significantly improved from baseline to the end of the training period [70].
5. Benefits of Early Nutrition in ICU
Early initiation of nutrition—ideally within 24–48 h of ICU admission—is recommended in ICU patients [13]. Although guidelines suggest starting nutrition within the first 48 h and reaching the predicted or calculated targets within the first 5 days, there is still debate over whether early initiation should be considered within the first 24 or 48 h, or even beyond this timeframe [71]. In a prospective, randomized trial with 100 ICU patients, those who started enteral nutrition at admission showed significantly higher serum albumin and prealbumin levels, shorter ICU stays and ventilator time, along with fewer complications compared to those who started enteral nutrition at 24–48 h [72]. A meta-analysis including 16 randomized controlled trials found that starting enteral nutrition within 24 h of ICU admission did not reduce mortality compared to other types of nutrition support [73]. However, early enteral nutrition reduced mortality compared to delayed enteral intake.
In any case, the benefits of enteral nutrition have been demonstrated in several studies. Rehal et al. [74] showed how full dosing of enteral nutrition significantly improved whole-body protein balance, which is crucial for improving protein metabolism in ICU patients. However, the optimal timing and approach to nutritional support remain the subject of ongoing debate. For instance, a randomized multi-center trial by Caesar et al. [75] found that delaying parenteral nutrition led to faster recovery and fewer complications, suggesting that the benefits of early full nutritional support may not always be universally applicable and might even cause harm in some cases, as pointed out by Gunst et al. [76]. Moreover, EN was described as being associated with improved mucosal trophism, leading to a reduction in the formation of neutrophil extracellular traps (NETs) and the expression of NET-associated proteins [77]. This effect is linked to the regulation of immune pathways, as early EN attenuated the activation of TLR4, NFκB, and MAPK signaling [78]. On the other hand, providing early nutrition may have some potential risks that need to be carefully considered. Overfeeding can strain metabolic balance, leading to issues like hyperglycemia or liver stress [79], while refeeding syndrome—especially in malnourished patients—can cause dangerous electrolyte shifts and cardiac complications [80]. Digestive issues, such as delayed gastric emptying or diarrhea, may also impact tolerance.
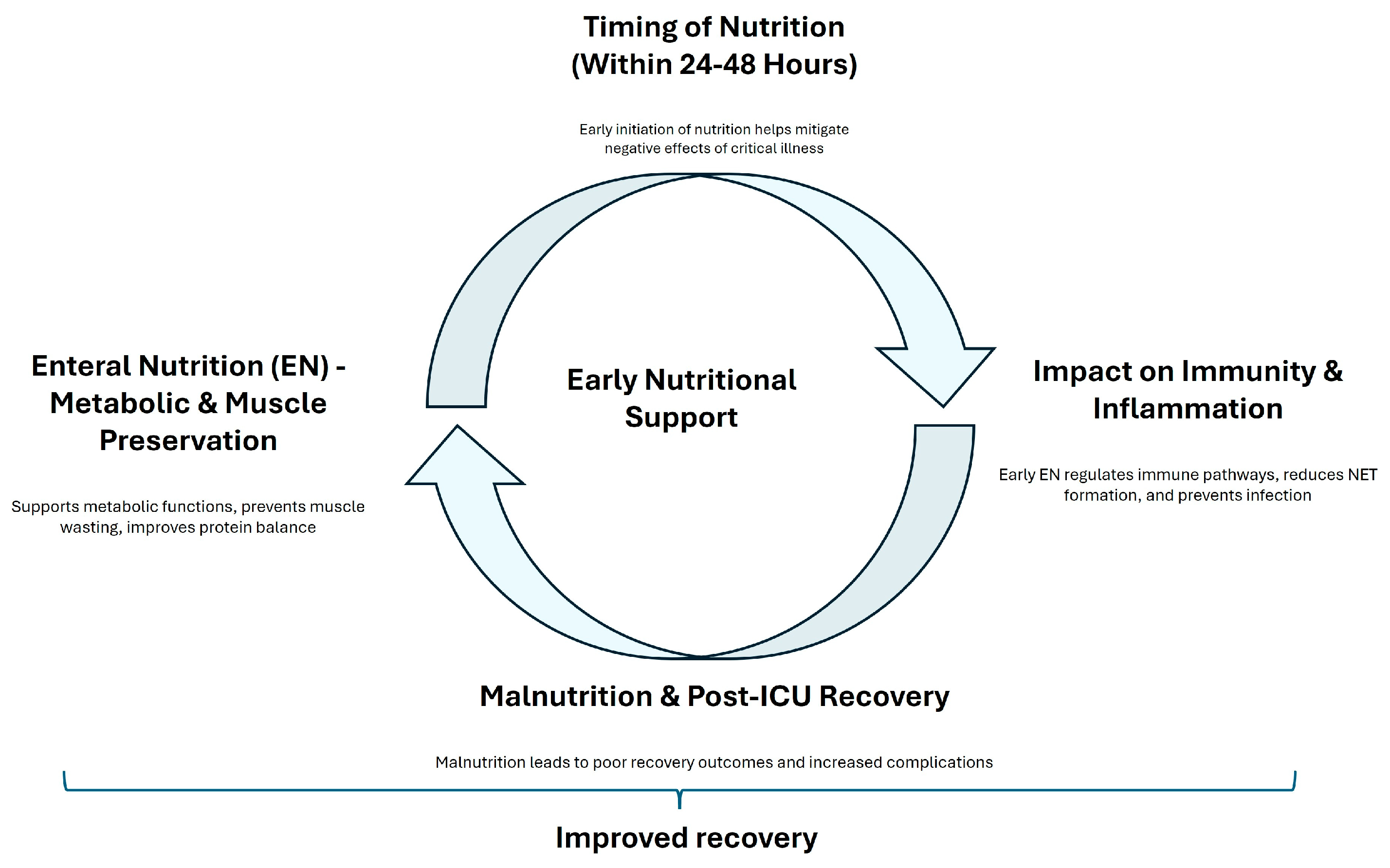
Despite these mixed findings, there is a consensus that nutritional interventions should be personalized based on the individual patients’ needs. Chapple et al. [81] noted that although protein intake often meets international guidelines, the actual delivery of protein to ICU patients frequently falls short. Additionally, Heyland et al. [82] reported that high-dose protein supplementation did not significantly improve hospital discharge times and might even have adverse effects on patients with acute kidney injury. Vallet also demonstrated that negative energy balance is associated with increased complications [83]. The prevalence of malnutrition among ICU survivors, as highlighted by Moisey et al. [84], further supports the importance of ongoing nutritional rehabilitation after discharge. A dual-center randomized controlled trial by Zhou et al. [85] demonstrated that early mobilization combined with guideline-based nutrition significantly reduced the incidence of ICUAW and improved muscle strength compared to standard care. Figure 2 summarized the principal effects of early nutritional support. The first branch emphasizes how EN supports metabolic functions, prevents muscle wasting, and improves protein balance, leading to enhanced recovery. The second branch stresses the importance of early nutritional intervention in mitigating the negative effects of critical illness. A contrasting branch shows the potential benefits of delayed parenteral nutrition, suggesting that postponing parenteral nutrition might reduce complications and accelerate recovery. The third branch focuses on how early EN regulates immune pathways, reduces infection risk, and promotes faster recovery by modulating key signaling pathways. The final branch points out the adverse effects of malnutrition on recovery and the need for ongoing nutritional support after ICU discharge to prevent complications and ensure long-term recovery.
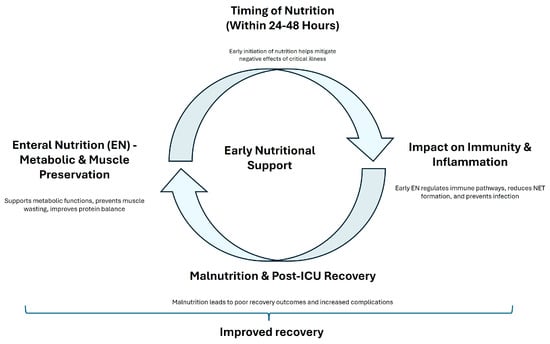
Figure 2.
A concept map of early nutritional support. This figure illustrates the concept of ’early nutritional support’ and its impact on patient recovery.
6. The Combined Effect of Early Mobilization and Nutrition on ICUAW
The combination of early mobilization and early nutritional support may influence recovery, in essence through muscle preservation and metabolic optimization. Table 1 summarizes the main published observations.

Table 1.
Principal investigation on combined effects of nutrition and rehabilitation.
Mobilization stimulates muscle activity, promoting a series of responses that stimulates protein synthesis and improve neuromuscular function [90]. Nakamura et al. [86] showed that high-protein delivery, when paired with active rehabilitation, led to better preservation of muscle volume. Similarly, de Azevedo [87] demonstrated how high-protein intake, alongside resistance exercise, improved physical quality of life and survival rates. Recent studies have highlighted the distinct metabolic effects of exercise and amino acids in regulating anabolic intracellular signaling, muscle protein synthesis, and muscle mass [91]. Mechano-sensors, such as intracellular calcium concentrations and the accumulation of specific molecules, produced by phospholipase D, activate a protein complex involved in muscle synthesis in response to mechanical stress [92]. Nutrient sensing mechanisms, including certain proteins involved in cell signaling, modulate the localization and activation of this protein complex when amino acid concentrations increase [93]. Jones et al. [88] suggested how the combined effects of a 6-week physiotherapy program and an essential amino acid supplement improved patients’ outcomes. They showed that their combination led to improved walking distance, as well as reduced anxiety and depression [71].
Additionally, mobilization activates metabolic pathways related to glucose metabolism and insulin sensitivity. Patel et al. [89] conducted a secondary analysis of 104 mechanically ventilated patients from a randomized controlled trial, comparing early occupational and physical therapy with conventional therapy, focusing on the impact of insulin dose and early mobilization on the incidence ICUAW. Their logistic regression analysis revealed that both early mobilization and higher insulin doses significantly reduced the incidence of ICUAW. A dual-center randomized controlled trial by Zhou et al. [85] demonstrated that early mobilization combined with guideline-based nutrition significantly reduced the incidence of ICUAW and improved muscle strength compared to standard care.
Future studies should focus on adequately powered randomized controlled trials to validate these approaches and further explore the long-term effects of prolonged nutritional interventions on critical patient-centered outcomes such as quality of life and functional recovery [72].
Practical Implications
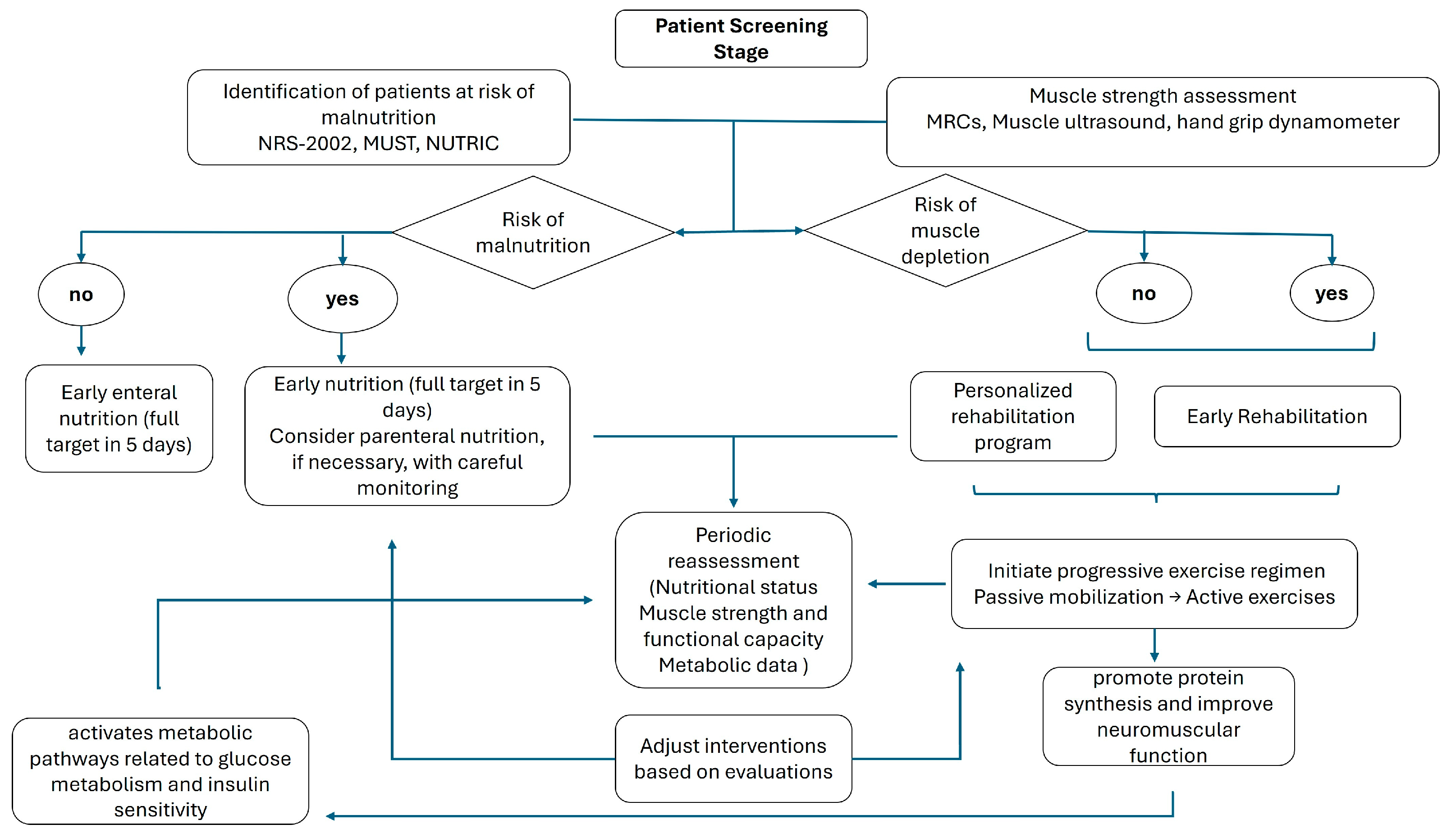
A detailed flow diagram accompanies a summary of previous observations, delineating the sequential steps—from initial screening and assessment to the implementation and ongoing evaluation of integrated nutrition and rehabilitation protocols (Figure 3).
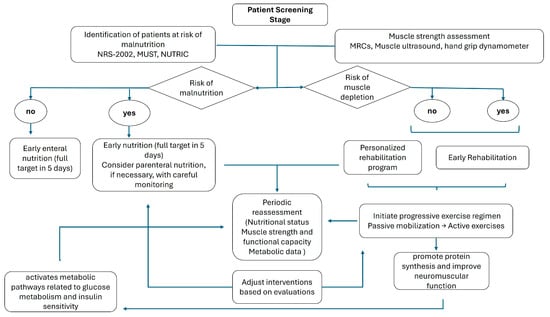
Figure 3.
Flow diagram of practical implications.
Initially, rigorous screening procedures must be employed to identify individuals at heightened risk for malnutrition and physical deconditioning. This screening should utilize validated instruments to establish a baseline nutritional profile and determine the need for early intervention. This assessment is particularly important in patients anticipated to have an ICU stay exceeding 72 h, especially in medical patients and those admitted with critical conditions [94]. Conversely, this assessment holds relatively less significance in postoperative patients and those with conditions that are likely to result in a shorter ICU stay.
Following the identification of at-risk patients, a detailed evaluation of muscle strength and function is important. Quantitative assessments, including muscle ultrasound and validated force scales, are recommended to ascertain the degree of deconditioning and to guide the intensity of subsequent rehabilitative measures. Concurrently, it is critical to monitor nutritional intake and energy expenditure accurately. Indirect calorimetry provides a measure of metabolic requirements [76], while serial ultrasound evaluations can yield precise data regarding muscle mass and adipose tissue distribution, thus supplying a comprehensive assessment of the patient’s nutritional and functional status [95].
After these assessments, a patient-specific nutritional plan should be formulated, addressing individualized energy, protein, and micronutrient requirements. Early initiation of enteral nutrition mitigates the risk of further nutritional deficits and supports anabolic processes. In scenarios where enteral feeding is contraindicated or not feasible, parenteral nutrition should be administered with vigilant monitoring of caloric delivery and metabolic parameters [13].
Simultaneously, early mobilization and physical rehabilitation must be planned to counteract the deleterious effects of immobility [96]. A progressive exercise regimen—ranging from passive mobilization to active resistance training—should be tailored to the patient’s current functional capacity. This rehabilitation strategy aims to preserve skeletal muscle integrity, enhance neuromuscular function, and expedite functional recovery.
Periodic re-evaluation of muscle strength and structure with objective measures is essential to refine and adapt the rehabilitation protocol in response to the patient’s evolving clinical status. Similarly, outcome measures should include serial evaluations of nutritional status, muscle strength, functional capacity, and metabolic indices.
7. Conclusions
In conclusion, the integrated use of early mobilization and early nutritional support is a fundamental aspect of ICU care that goes beyond addressing muscle weakness or malnutrition. By targeting both the physical and metabolic systems, this combined approach promotes an optimal proposal for recovery, helping critically ill patients regain strength, independence, and overall quality of life after their ICU stay. This synergy between mobilization and nutrition is essential not only for short-term recovery but also for long-term health outcomes, highlighting the importance of integrating these interventions into routine ICU care protocols. Since we did not conduct a formal analysis, as would be carried out in a systematic review, even if the aspects discussed are recognized in other systematic reviews and some guidelines, they should be considered in their specific context.
Author Contributions
Conceptualization, P.F., A.M., and M.U.; methodology, P.F. and M.U.; software, A.M.; validation, A.G., M.G., and G.S.; formal analysis, P.F. and A.M.; data curation, P.F. and A.M.; writing—original draft preparation, P.F. and A.M.; writing—review and editing, M.U. and G.B.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge that AI tools were used in the editing process of this manuscript. We thank John J Marini for English editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ICU | Intensive Care Unit |
| ICUAW | Intensive care unit-acquired weakness |
| PICS | Post-intensive care syndrome |
| NETs | neutrophil extracellular traps |
References
- Lad, H.; Saumur, T.M.; Herridge, M.S.; Dos Santos, C.C.; Mathur, S.; Batt, J.; Gilbert, P.M. Intensive Care Unit-Acquired Weakness: Not Just Another Muscle Atrophying Condition. Int. J. Mol. Sci. 2020, 21, 7840. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, Z.; Jiang, L.; Wang, Y.; Xi, X. Risk Factors for Intensive Care Unit-Acquired Weakness: A Systematic Review and Meta-Analysis. Acta Neurol. Scand. 2018, 138, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van Mechelen, H.; Clerckx, B.; Vanhullebusch, T.; Mesotten, D.; Wilmer, A.; Casaer, M.P.; Meersseman, P.; Debaveye, Y.; Van Cromphaut, S.; et al. Acute Outcomes and 1-Year Mortality of Intensive Care Unit-Acquired Weakness. A Cohort Study and Propensity-Matched Analysis. Am. J. Respir. Crit. Care Med. 2014, 190, 410–420. [Google Scholar] [CrossRef]
- Thille, A.W.; Boissier, F.; Muller, M.; Levrat, A.; Bourdin, G.; Rosselli, S.; Frat, J.-P.; Coudroy, R.; Vivier, E. Role of ICU-Acquired Weakness on Extubation Outcome among Patients at High Risk of Reintubation. Crit. Care 2020, 24, 86. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. Frailty, Sarcopenia, and Malnutrition Frequently (Co-)Occur in Hospitalized Older Adults: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef]
- Prado, C.M.; Landi, F.; Chew, S.T.H.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in Muscle Health and Nutrition: A Toolkit for Healthcare Professionals. Clin. Nutr. 2022, 41, 2244–2263. [Google Scholar] [CrossRef]
- Ambrosino, N.; Venturelli, E.; Vagheggini, G.; Clini, E. Rehabilitation, Weaning and Physical Therapy Strategies in Chronic Critically Ill Patients. Eur. Respir. J. 2012, 39, 487–492. [Google Scholar] [CrossRef]
- Rawal, G.; Yadav, S.; Kumar, R. Post-Intensive Care Syndrome: An Overview. J. Transl. Int. Med. 2017, 5, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.-C.; et al. ESPEN Practical and Partially Revised Guideline: Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- De Waele, E.; Jonckheer, J.; Wischmeyer, P. Indirect Calorimetry In Critical Illness: A New Standard of Care? Curr. Opin. Crit. Care 2021, 27, 334–343. [Google Scholar] [CrossRef] [PubMed]
- De Waele, E.; van Zanten, A.R.H. Routine Use of Indirect Calorimetry in Critically Ill Patients: Pros and Cons. Crit. Care 2022, 26, 123. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Díaz Chavarro, B.C.; Molina-Recio, G.; Assis Reveiz, J.K.; Romero-Saldaña, M. Factors Associated with Nutritional Risk Assessment in Critically Ill Patients Using the Malnutrition Universal Screening Tool (MUST). J. Clin. Med. 2024, 13, 1236. [Google Scholar] [CrossRef]
- Coruja, M.K.; Cobalchini, Y.; Wentzel, C.; Fink, J.D.S. Nutrition Risk Screening in Intensive Care Units: Agreement Between NUTRIC and NRS 2002 Tools. Nutr. Clin. Pract. 2020, 35, 567–571. [Google Scholar] [CrossRef]
- Yildirim, M.; Yildirim, Z.S.; Deniz, M. Effect of the Modified NUTRIC Score in Predicting the Prognosis of Patients Admitted to Intensive Care Units. BMC Anesthesiol. 2024, 24, 473. [Google Scholar] [CrossRef] [PubMed]
- Wełna, M.; Adamik, B.; Kübler, A.; Goździk, W. The NUTRIC Score as a Tool to Predict Mortality and Increased Resource Utilization in Intensive Care Patients with Sepsis. Nutrients 2023, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Sanaie, S.; Sarfaraz, T.; Shadvar, K.; Fattahi, V.; Hamishekar, H.; Vahedian-Azimi, A.; Samim, A.; Rahimi-Bashar, F. Prognostic Values of Modified NUTRIC Score to Assess Outcomes in Critically Ill Patients Admitted to the Intensive Care Units: Prospective Observational Study. BMC Anesthesiol. 2023, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Razzera, E.L.; Milanez, D.S.J.; Silva, F.M. Derivation of the Screening of Nutritional Risk in Intensive Care Risk Prediction Score: A Secondary Analysis of a Prospective Cohort Study. JPEN J. Parenter. Enteral Nutr. 2024, 48, 82–92. [Google Scholar] [CrossRef]
- Rodriguez, B.; Schefold, J.C.; Z’Graggen, W.J. Diagnosis of “Intensive Care Unit-Acquired Weakness” and “Critical Illness Myopathy”: Do the Diagnostic Criteria Need to Be Revised? Clin. Neurophysiol. Pract. 2024, 9, 236–241. [Google Scholar] [CrossRef]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The Current Use of Ultrasound to Measure Skeletal Muscle and Its Ability to Predict Clinical Outcomes: A Systematic Review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef]
- Formenti, P.; Umbrello, M.; Coppola, S.; Froio, S.; Chiumello, D. Clinical Review: Peripheral Muscular Ultrasound in the ICU. Ann. Intensive Care 2019, 9, 57. [Google Scholar] [CrossRef]
- Venco, R.; Artale, A.; Formenti, P.; Deana, C.; Mistraletti, G.; Umbrello, M. Methodologies and Clinical Applications of Lower Limb Muscle Ultrasound in Critically Ill Patients: A Systematic Review and Meta-Analysis. Ann. Intensive Care 2024, 14, 163. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic Value of Grip Strength: Findings from the Prospective Urban Rural Epidemiology (PURE) Study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Connolly, B.A.; Jones, G.D.; Curtis, A.A.; Murphy, P.B.; Douiri, A.; Hopkinson, N.S.; Polkey, M.I.; Moxham, J.; Hart, N. Clinical Predictive Value of Manual Muscle Strength Testing during Critical Illness: An Observational Cohort Study. Crit. Care 2013, 17, R229. [Google Scholar] [CrossRef]
- Bragança, R.D.; Ravetti, C.G.; Barreto, L.; Ataíde, T.B.L.S.; Carneiro, R.M.; Teixeira, A.L.; Nobre, V. Use of Handgrip Dynamometry for Diagnosis and Prognosis Assessment of Intensive Care Unit Acquired Weakness: A Prospective Study. Heart Lung 2019, 48, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Singam, A. Mobilizing Progress: A Comprehensive Review of the Efficacy of Early Mobilization Therapy in the Intensive Care Unit. Cureus 2024, 16, e57595. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tronstad, O.; Flaws, D.; Churchill, L.; Jones, A.Y.M.; Nakamura, K.; Fraser, J.F. From Bedside to Recovery: Exercise Therapy for Prevention of Post-Intensive Care Syndrome. J. Intensive Care 2024, 12, 11. [Google Scholar] [CrossRef]
- Hodgson, C.L.; Berney, S.; Harrold, M.; Saxena, M.; Bellomo, R. Clinical Review: Early Patient Mobilization in the ICU. Critical Care 2013, 17, 207. [Google Scholar] [CrossRef]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The Rate and Assessment of Muscle Wasting during Critical Illness: A Systematic Review and Meta-Analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, H.; Stanford, J.A.; Mori, Y. Role of Exercise in Maintaining the Integrity of the Neuromuscular Junction. Muscle Nerve 2014, 49, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, H.; Numata, T.; Chen, J.; Aoki, Y.; Wang, Y.; Starr, M.P.; Mori, Y.; Stanford, J.A. Active Zone Protein Bassoon Co-Localizes with Presynaptic Calcium Channel, Modifies Channel Function, and Recovers from Aging Related Loss by Exercise. PLoS ONE 2012, 7, e38029. [Google Scholar] [CrossRef]
- Lebrasseur, N.K.; Coté, G.M.; Miller, T.A.; Fielding, R.A.; Sawyer, D.B. Regulation of Neuregulin/ErbB Signaling by Contractile Activity in Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2003, 284, C1149–C1155. [Google Scholar] [CrossRef]
- Wang, Y.T.; Lang, J.K.; Haines, K.J.; Skinner, E.H.; Haines, T.P. Physical Rehabilitation in the ICU: A Systematic Review and Meta-Analysis. Crit. Care Med. 2022, 50, 375–388. [Google Scholar] [CrossRef]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am. J. Physiol. 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Zang, K.; Chen, B.; Wang, M.; Chen, D.; Hui, L.; Guo, S.; Ji, T.; Shang, F. The Effect of Early Mobilization in Critically Ill Patients: A Meta-Analysis. Nurs. Crit. Care 2020, 25, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Mi, J.; Zhang, Z.; Luo, X.; Gan, R.; Mu, S. Effects of the High-Intensity Early Mobilization on Long-Term Functional Status of Patients with Mechanical Ventilation in the Intensive Care Unit. Crit. Care Res. Pract. 2024, 2024, 4118896. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Cao, J.; Jiao, J.; Wang, Y.; Liu, G.; Liu, Y.; Li, F.; Song, B.; Jin, J.; et al. The Association between Major Complications of Immobility during Hospitalization and Quality of Life among Bedridden Patients: A 3 Month Prospective Multi-Center Study. PLoS ONE 2018, 13, e0205729. [Google Scholar] [CrossRef] [PubMed]
- Mezidi, M.; Guérin, C. Effects of Patient Positioning on Respiratory Mechanics in Mechanically Ventilated ICU Patients. Ann. Transl. Med. 2018, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Al-Dorzi, H.M.; AlQahtani, S.; Al-Dawood, A.; Al-Hameed, F.M.; Burns, K.E.A.; Mehta, S.; Jose, J.; Alsolamy, S.J.; Abdukahil, S.A.I.; Afesh, L.Y.; et al. Association of Early Mobility with the Incidence of Deep-Vein Thrombosis and Mortality among Critically Ill Patients: A Post Hoc Analysis of PREVENT Trial. Crit. Care 2023, 27, 83. [Google Scholar] [CrossRef]
- Regan, M.A.; Teasell, R.W.; Wolfe, D.L.; Keast, D.; Mortenson, W.B.; Aubut, J.-A. A Systematic Review of Therapeutic Interventions for Pressure Ulcers Following Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2009, 90, 213–231. [Google Scholar] [CrossRef]
- Potter, K.; Miller, S.; Newman, S. Environmental Factors Affecting Early Mobilization and Physical Disability Post-Intensive Care: An Integrative Review Through the Lens of the World Health Organization International Classification of Functioning, Disability, and Health. Dimens. Crit. Care Nurs. 2021, 40, 92–117. [Google Scholar] [CrossRef]
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-Intensive Care Syndrome: Its Pathophysiology, Prevention, and Future Directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef]
- Mart, M.F.; Williams Roberson, S.; Salas, B.; Pandharipande, P.P.; Ely, E.W. Prevention and Management of Delirium in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 112–126. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early Physical and Occupational Therapy in Mechanically Ventilated, Critically Ill Patients: A Randomised Controlled Trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Nydahl, P.; Ewers, A.; Brodda, D. Complications Related to Early Mobilization of Mechanically Ventilated Patients on Intensive Care Units. Nurs. Crit. Care 2014, 21, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, H.; Esmaeili, M.; Maroufizadeh, S.; Rahimi, B. The Effect of Early Mobilization on Respiratory Parameters of Mechanically Ventilated Patients with Respiratory Failure. Crit. Care Nurs. Q. 2022, 45, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hiser, S.L.; Fatima, A.; Ali, M.; Needham, D.M. Post-Intensive Care Syndrome (PICS): Recent Updates. J. Intensive Care 2023, 11, 23. [Google Scholar] [CrossRef]
- Paton, M.; Chan, S.; Tipping, C.J.; Stratton, A.; Serpa Neto, A.; Lane, R.; Young, P.J.; Romero, L.; Broadley, T.; Hodgson, C.L. The Effect of Mobilization at 6 Months after Critical Illness—Meta-Analysis. NEJM Evid. 2023, 2, EVIDoa2200234. [Google Scholar] [CrossRef]
- Patel, B.K.; Wolfe, K.S.; Patel, S.B.; Dugan, K.C.; Esbrook, C.L.; Pawlik, A.J.; Stulberg, M.; Kemple, C.; Teele, M.; Zeleny, E.; et al. Effect of Early Mobilisation on Long-Term Cognitive Impairment in Critical Illness in the USA: A Randomised Controlled Trial. Lancet Respir. Med. 2023, 11, 563–572. [Google Scholar] [CrossRef]
- Avgeri, K.; Zakynthinos, E.; Tsolaki, V.; Sgantzos, M.; Fotakopoulos, G.; Makris, D. Quality of Life and Family Support in Critically Ill Patients Following ICU Discharge. Healthcare 2023, 11, 1106. [Google Scholar] [CrossRef]
- Alaparthi, G.K.; Gatty, A.; Samuel, S.R.; Amaravadi, S.K. Effectiveness, Safety, and Barriers to Early Mobilization in the Intensive Care Unit. Crit. Care Res. Pract. 2020, 2020, 7840743. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, T.; Cao, L.; Ye, L.; Song, W. Early Mobilization for Critically Ill Patients. Respir. Care 2023, 68, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Genc, A.; Koca, U.; Gunerli, A. What Are the Hemodynamic and Respiratory Effects of Passive Limb Exercise for Mechanically Ventilated Patients Receiving Low-Dose Vasopressor/Inotropic Support? Crit. Care Nurs. Q. 2014, 37, 152–158. [Google Scholar] [CrossRef]
- Morisawa, T.; Takahashi, T.; Sasanuma, N.; Mabuchi, S.; Takeda, K.; Hori, N.; Ohashi, N.; Ide, T.; Domen, K.; Nishi, S. Passive Exercise of the Lower Limbs and Trunk Alleviates Decreased Intestinal Motility in Patients in the Intensive Care Unit after Cardiovascular Surgery. J. Phys. Ther. Sci. 2017, 29, 312–316. [Google Scholar] [CrossRef]
- Stiller, K.R.; Dafoe, S.; Jesudason, C.S.; McDonald, T.M.; Callisto, R.J. Passive Movements Do Not Appear to Prevent or Reduce Joint Stiffness in Medium to Long-Stay ICU Patients: A Randomized, Controlled, Within-Participant Trial. Crit. Care Explor. 2023, 5, e1006. [Google Scholar] [CrossRef]
- Legg, L.A.; Lewis, S.R.; Schofield-Robinson, O.J.; Drummond, A.; Langhorne, P. Occupational Therapy for Adults with Problems in Activities of Daily Living after Stroke. Cochrane Database Syst. Rev. 2017, 2017, CD003585. [Google Scholar] [CrossRef]
- Bovend’Eerdt, T.J.H.; Botell, R.E.; Wade, D.T. Writing SMART Rehabilitation Goals and Achieving Goal Attainment Scaling: A Practical Guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.L.; Schaller, S.J.; Nydahl, P.; Timenetsky, K.T.; Needham, D.M. Ten Strategies to Optimize Early Mobilization and Rehabilitation in Intensive Care. Crit. Care 2021, 25, 324. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Bear, D.E.; Berger, M.M.; De Waele, E.; Gunst, J.; McClave, S.A.; Prado, C.M.; Puthucheary, Z.; Ridley, E.J.; Van den Berghe, G.; et al. Personalized Nutrition Therapy in Critical Care: 10 Expert Recommendations. Crit. Care 2023, 27, 261. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.K.; Paykel, M.S.; Haines, K.J.; Hodgson, C.L. Clinical Practice Guidelines for Early Mobilization in the ICU: A Systematic Review. Crit. Care Med. 2020, 48, e1121–e1128. [Google Scholar] [CrossRef]
- Paton, M.; Lane, R.; Paul, E.; Cuthburtson, G.A.; Hodgson, C.L. Mobilization During Critical Illness: A Higher Level of Mobilization Improves Health Status at 6 Months, a Secondary Analysis of a Prospective Cohort Study. Crit. Care Med. 2021, 49, e860–e869. [Google Scholar] [CrossRef]
- Haghedooren, E.; Haghedooren, R.; Langer, D.; Gosselink, R. Feasibility and Safety of Interactive Virtual Reality Upper Limb Rehabilitation in Patients with Prolonged Critical Illness. Aust. Crit. Care 2024, 37, 949–956. [Google Scholar] [CrossRef]
- de Vries, M.; Beumeler, L.F.E.; van der Meulen, J.; Bethlehem, C.; den Otter, R.; Boerma, E.C. The Feasibility of Virtual Reality Therapy for Upper Extremity Mobilization during and after Intensive Care Unit Admission. PeerJ 2025, 13, e18461. [Google Scholar] [CrossRef]
- Burnet, K.; Kelsch, E.; Zieff, G.; Moore, J.B.; Stoner, L. How Fitting Is F.I.T.T.?: A Perspective on a Transition from the Sole Use of Frequency, Intensity, Time, and Type in Exercise Prescription. Physiol. Behav. 2019, 199, 33–34. [Google Scholar] [CrossRef]
- Fremont, R.D.; Rice, T.W. How Soon Should We Start Interventional Feeding in the ICU? Curr. Opin. Gastroenterol. 2014, 30, 178–181. [Google Scholar] [CrossRef]
- Yu, A.; Xie, Y.; Zhong, M.; Wang, F.; Huang, H.; Nie, L.; Liu, X.; Xiao, M.; Zhu, H. Comparison of the Initiation Time of Enteral Nutrition for Critically Ill Patients: At Admission vs. 24 to 48 Hours after Admission. Emerg. Med. Int. 2021, 2021, 3047732. [Google Scholar] [CrossRef]
- Tian, F.; Heighes, P.T.; Allingstrup, M.J.; Doig, G.S. Early Enteral Nutrition Provided Within 24 Hours of ICU Admission: A Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2018, 46, 1049–1056. [Google Scholar] [CrossRef]
- Rea, J.; Walters, K.; Avgerinou, C. How Effective Is Nutrition Education Aiming to Prevent or Treat Malnutrition in Community-Dwelling Older Adults? A Systematic Review. Eur. Geriatr. Med. 2019, 10, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.; Casaer, M.P.; Preiser, J.-C.; Reignier, J.; Van den Berghe, G. Toward Nutrition Improving Outcome of Critically Ill Patients: How to Interpret Recent Feeding RCTs? Crit. Care 2023, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Winter, T.A.; O’Keefe, S.J.; Callanan, M.; Marks, T. Effect of Severe Undernutrition and Subsequent Refeeding on Gut Mucosal Protein Fractional Synthesis in Human Subjects. Nutrition 2007, 23, 29–35. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, H.; Hong, Z.; Wang, C.; Zheng, T.; Ren, Y.; Chen, K.; Liu, S.; Wang, G.; Gu, G.; et al. Early Enteral Nutrition Preserves Intestinal Barrier Function through Reducing the Formation of Neutrophil Extracellular Traps (NETs) in Critically Ill Surgical Patients. Oxid. Med. Cell Longev. 2020, 2020, 8815655. [Google Scholar] [CrossRef]
- Tanaka, A.; Hamilton, K.; Eastwood, G.M.; Jones, D.; Bellomo, R. The Epidemiology of Overfeeding in Mechanically Ventilated Intensive Care Patients. Clin. Nutr. ESPEN 2020, 36, 139–145. [Google Scholar] [CrossRef]
- Papathanakos, G.; Blot, S.; Koulenti, D. Refeeding Syndrome in the ICU: A Serious Problem Still Lacking an Evidence-Based Approach. Intensive Crit. Care Nurs. 2024, 85, 103771. [Google Scholar] [CrossRef]
- Chapple, L.-A.S.; Kouw, I.W.K.; Summers, M.J.; Weinel, L.M.; Gluck, S.; Raith, E.; Slobodian, P.; Soenen, S.; Deane, A.M.; van Loon, L.J.C.; et al. Muscle Protein Synthesis after Protein Administration in Critical Illness. Am. J. Respir. Crit. Care Med. 2022, 206, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.-Y.; González, V.C.; O’Reilly, K.; Regala, R.; Wedemire, C.; et al. The Effect of Higher Protein Dosing in Critically Ill Patients with High Nutritional Risk (EFFORT Protein): An International, Multicentre, Pragmatic, Registry-Based Randomised Trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Villet, S.; Chiolero, R.; Bollmann, M.; Revelly, J.-P.; Cayeux R N, M.C.; Delarue, J.; Berger, M. Negative Impact of Hypocaloric Feeding and Energy Balance on Clinical Outcome in ICU Patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef]
- Moisey, L.L.; Merriweather, J.L.; Drover, J.W. The Role of Nutrition Rehabilitation in the Recovery of Survivors of Critical Illness: Underrecognized and Underappreciated. Crit. Care 2022, 26, 270. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, L.; Fan, Y.; Shi, B.; Wang, X.; Chen, T.; Yu, H.; Liu, J.; Wang, X.; Liu, C.; et al. Effect of Early Mobilization Combined with Early Nutrition on Acquired Weakness in Critically Ill Patients (EMAS): A Dual-Center, Randomized Controlled Trial. PLoS ONE 2022, 17, e0268599. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. High Protein versus Medium Protein Delivery under Equal Total Energy Delivery in Critical Care: A Randomized Controlled Trial. Clin. Nutr. 2021, 40, 796–803. [Google Scholar] [CrossRef]
- de Azevedo, J.R.A.; Lima, H.C.M.; Frota, P.H.D.B.; Nogueira, I.R.O.M.; de Souza, S.C.; Fernandes, E.A.A.; Cruz, A.M. High-Protein Intake and Early Exercise in Adult Intensive Care Patients: A Prospective, Randomized Controlled Trial to Evaluate the Impact on Functional Outcomes. BMC Anesthesiol. 2021, 21, 283. [Google Scholar] [CrossRef]
- Jones, C.; Eddleston, J.; McCairn, A.; Dowling, S.; McWilliams, D.; Coughlan, E.; Griffiths, R.D. Improving Rehabilitation after Critical Illness through Outpatient Physiotherapy Classes and Essential Amino Acid Supplement: A Randomized Controlled Trial. J. Crit. Care 2015, 30, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Pohlman, A.S.; Hall, J.B.; Kress, J.P. Impact of Early Mobilization on Glycemic Control and ICU-Acquired Weakness in Critically Ill Patients Who Are Mechanically Ventilated. Chest 2014, 146, 583–589. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K. Muscle Protein Synthesis in Response to Nutrition and Exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef]
- Pasiakos, S.M. Exercise and Amino Acid Anabolic Cell Signaling and the Regulation of Skeletal Muscle Mass. Nutrients 2012, 4, 740–758. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Compr. Physiol. 2019, 9, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhou, C.; Yan, Q.; Tan, Z.; Kang, J.; Tang, S. Elucidating the Underlying Mechanism of Amino Acids to Regulate Muscle Protein Synthesis: Effect on Human Health. Nutrition 2022, 103–104, 111797. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Baumgartner, A.; Bounoure, L.; Bally, M.; Deutz, N.E.; Greenwald, J.L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Nutritional Support with Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e1915138. [Google Scholar] [CrossRef]
- Rodrigues, C.N.; Ribeiro Henrique, J.; Ferreira, Á.R.S.; Correia, M.I.T.D. Ultrasonography and Other Nutrition Assessment Methods to Monitor the Nutrition Status of Critically Ill Patients. JPEN J. Parenter. Enteral Nutr. 2021, 45, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, K.; Lin, F.; Mitchell, M.L.; White, H. Early Rehabilitation in the Intensive Care Unit: An Integrative Literature Review. Aust. Crit. Care 2015, 28, 216–225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).