Protocatechuic Acid Attenuates Inflammation in Macrophage-like Vascular Smooth Muscle Cells in ApoE−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Primary VSMCs
2.2. Transdifferentiation of VSMCs into MLCs
2.3. Cell Treatments
2.4. Dosage Information
2.5. Mouse Study
2.6. Cell Viability Assay
2.7. RNA Preparation and Quantitative Real-Time PCR
2.8. Protein Extraction and Western Blot
2.9. Inflammatory Cytokines Analysis
2.10. DNA-Binding Activity of Nuclear p65
2.11. Immunocytochemistry
2.12. Small Interfering RNA (siRNA)-Dependent Knockdown of Exportin-1
2.13. Statistical Methods
3. Results
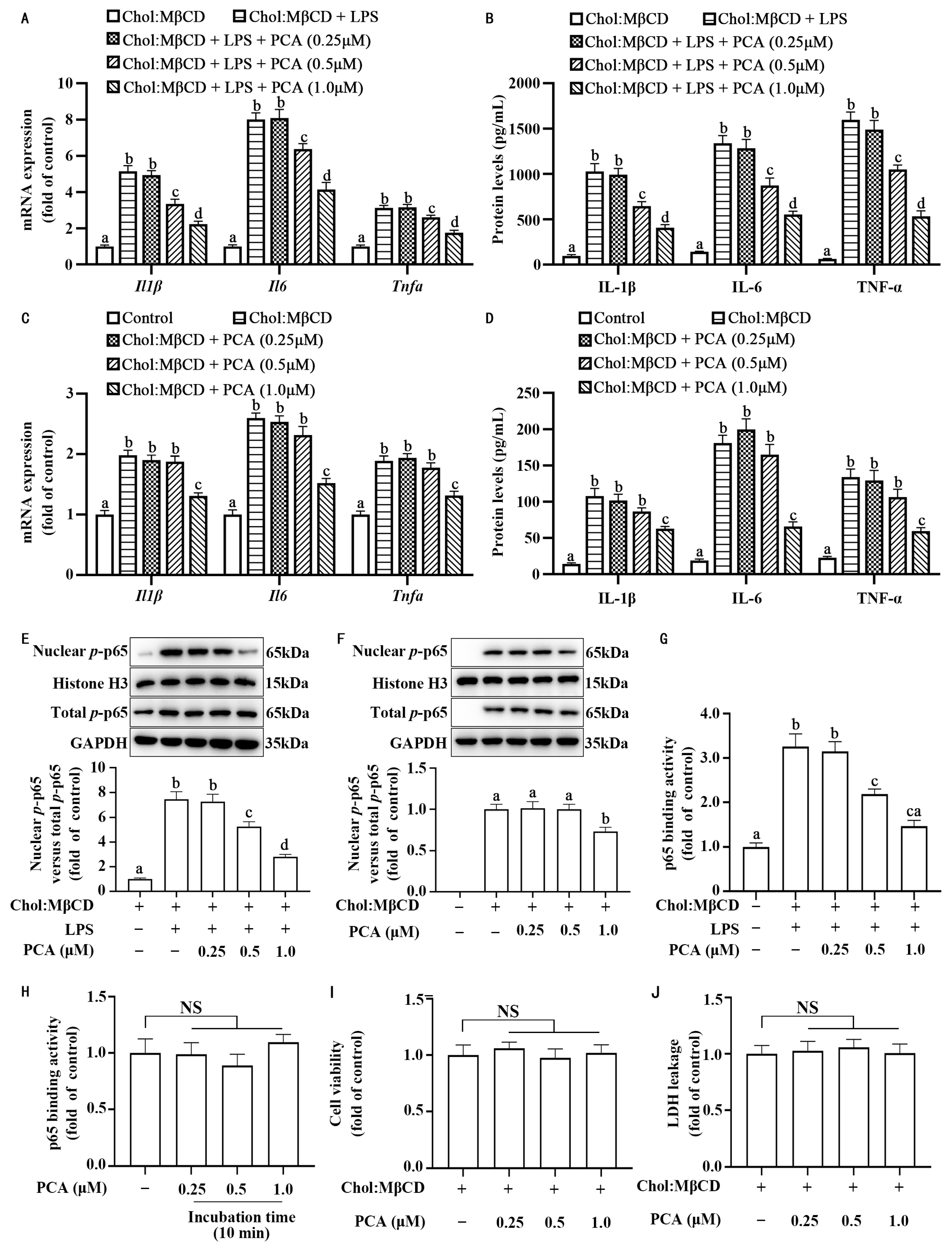
3.1. Protocatechuic Acid Attenuates Inflammatory Response in MLCs
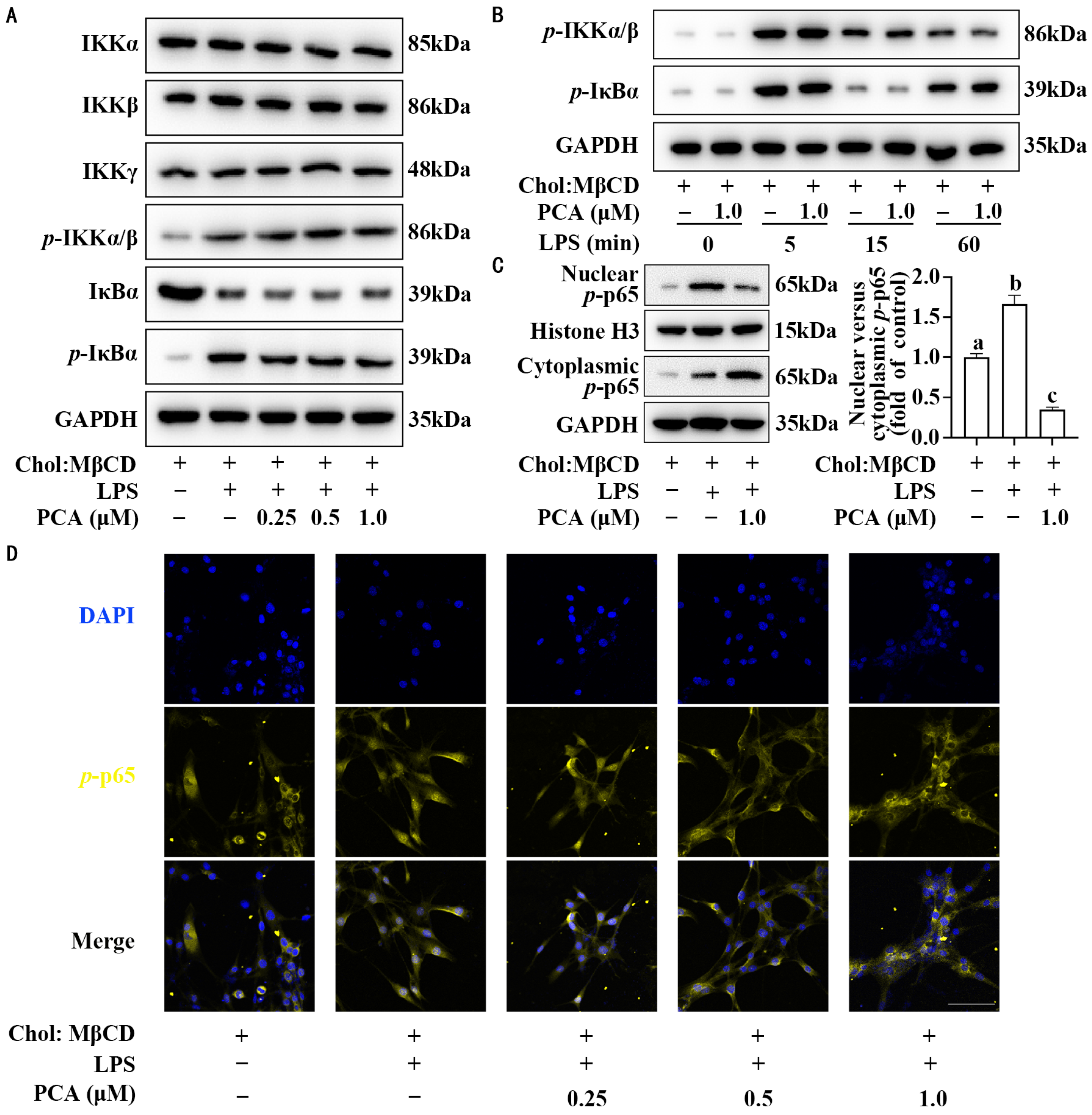
3.2. Protocatechuic Acid Reduces the Ratio of Nuclear to Cytoplasmic p-p65 Without Affecting Its Expression in MLCs
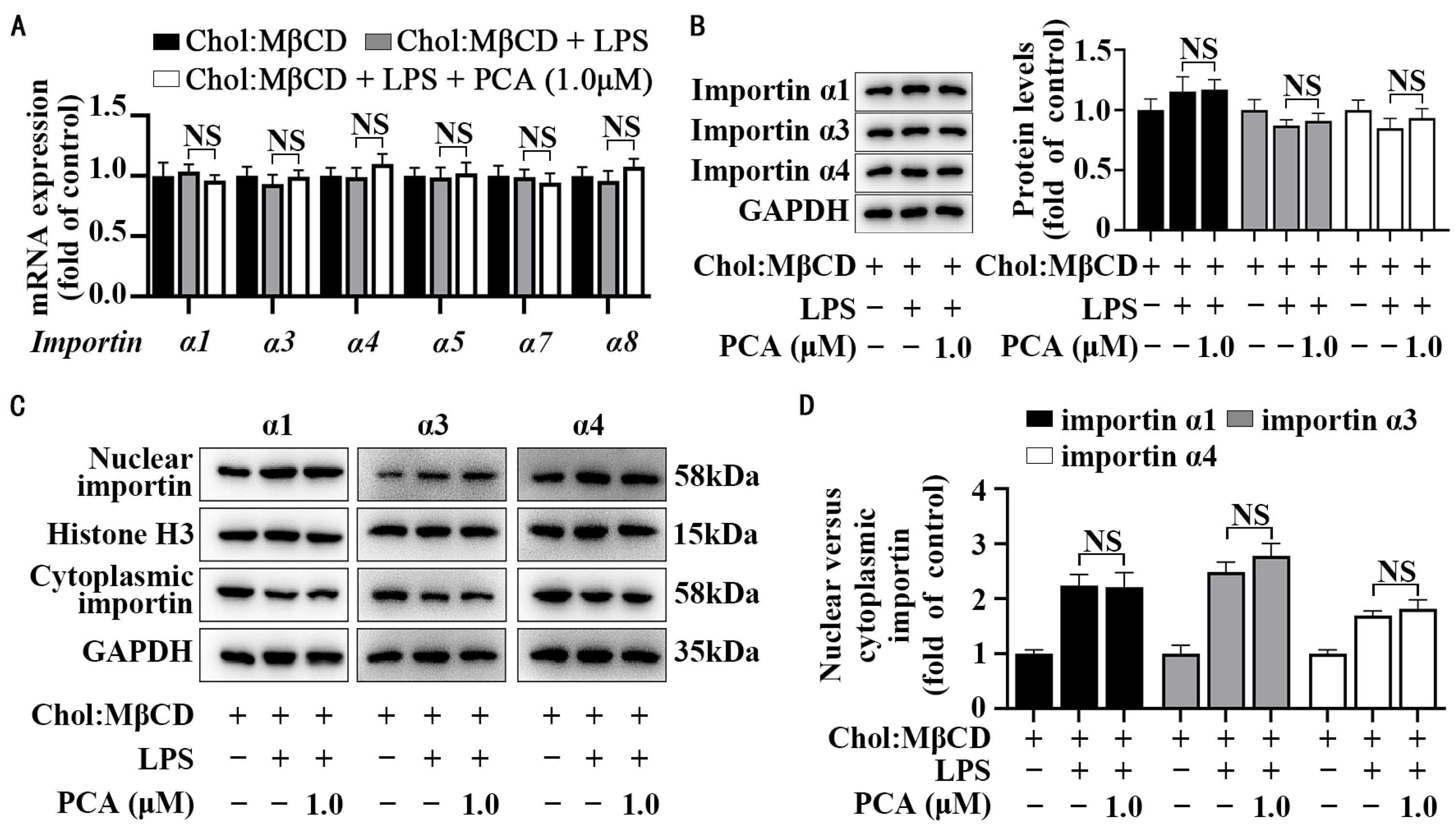
3.3. Protocatechuic Acid Does Not Affect Importin Abundance and Cellular Distribution in MLCs
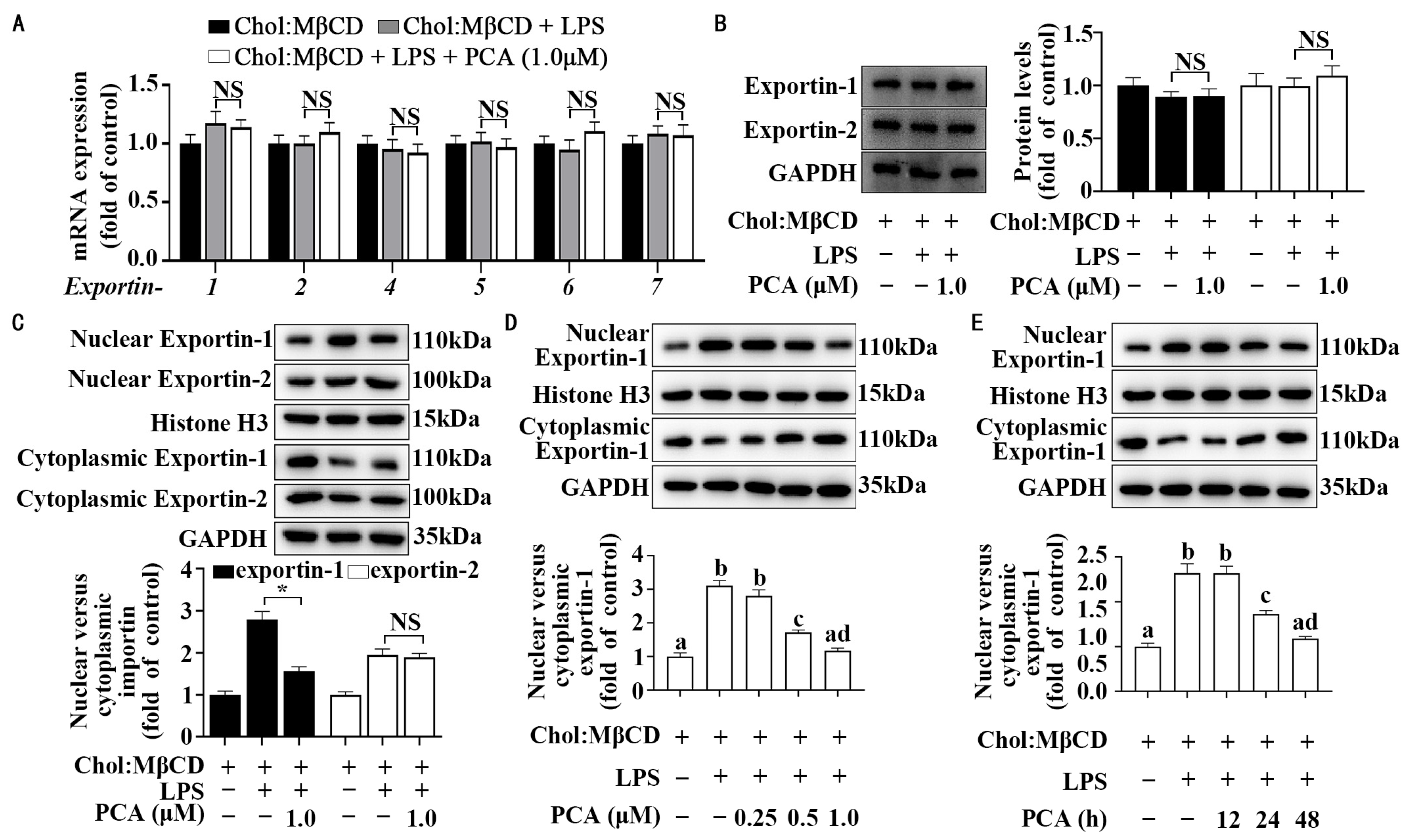
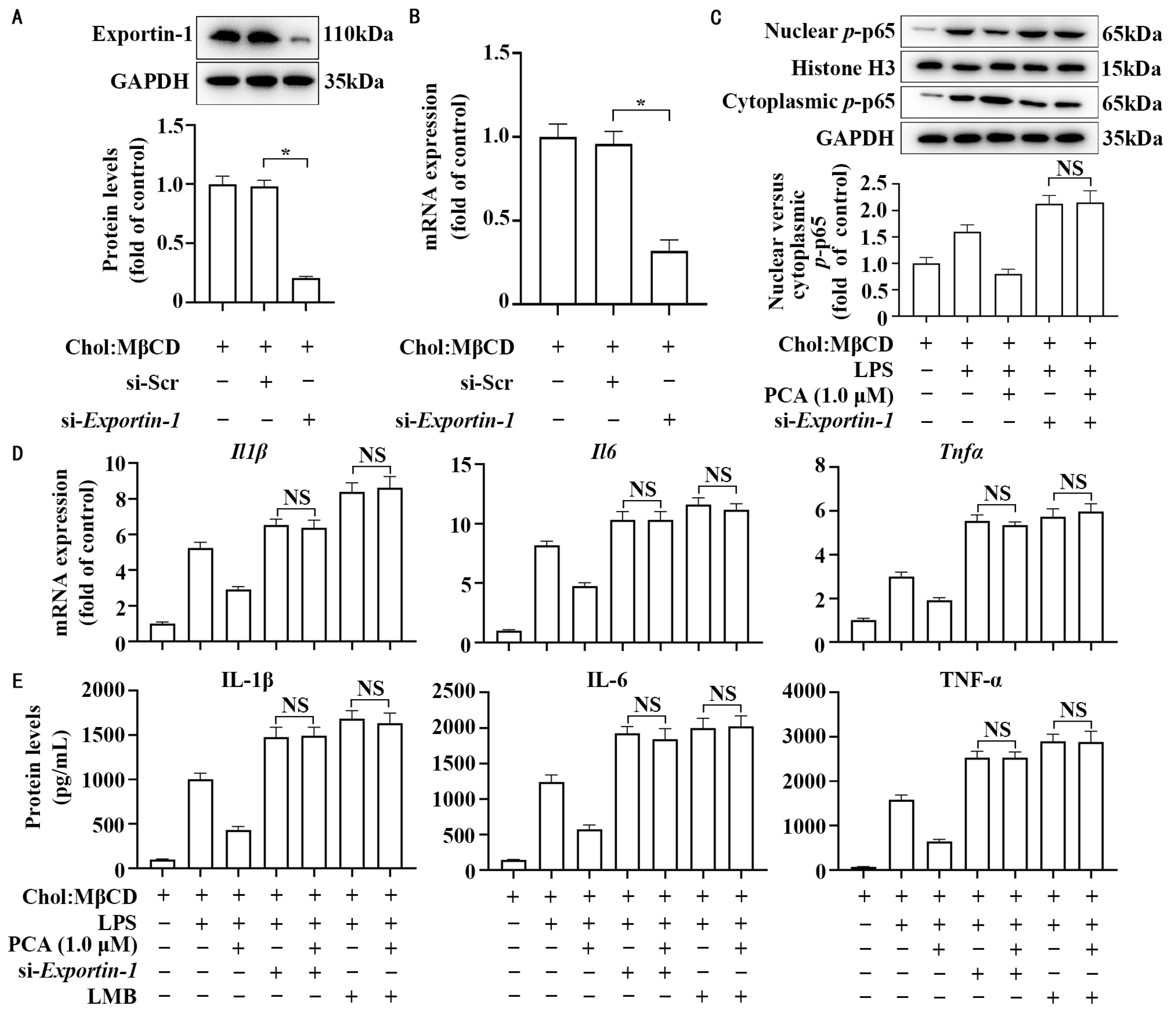
3.4. Protocatechuic Acid Regulates Cellular Distribution of Exportin-1 Without Affecting Its Expression in MLCs
3.5. Exportin-1 Mediates the Anti-Inflammatory Property of Protocatechuic Acid in MLCs
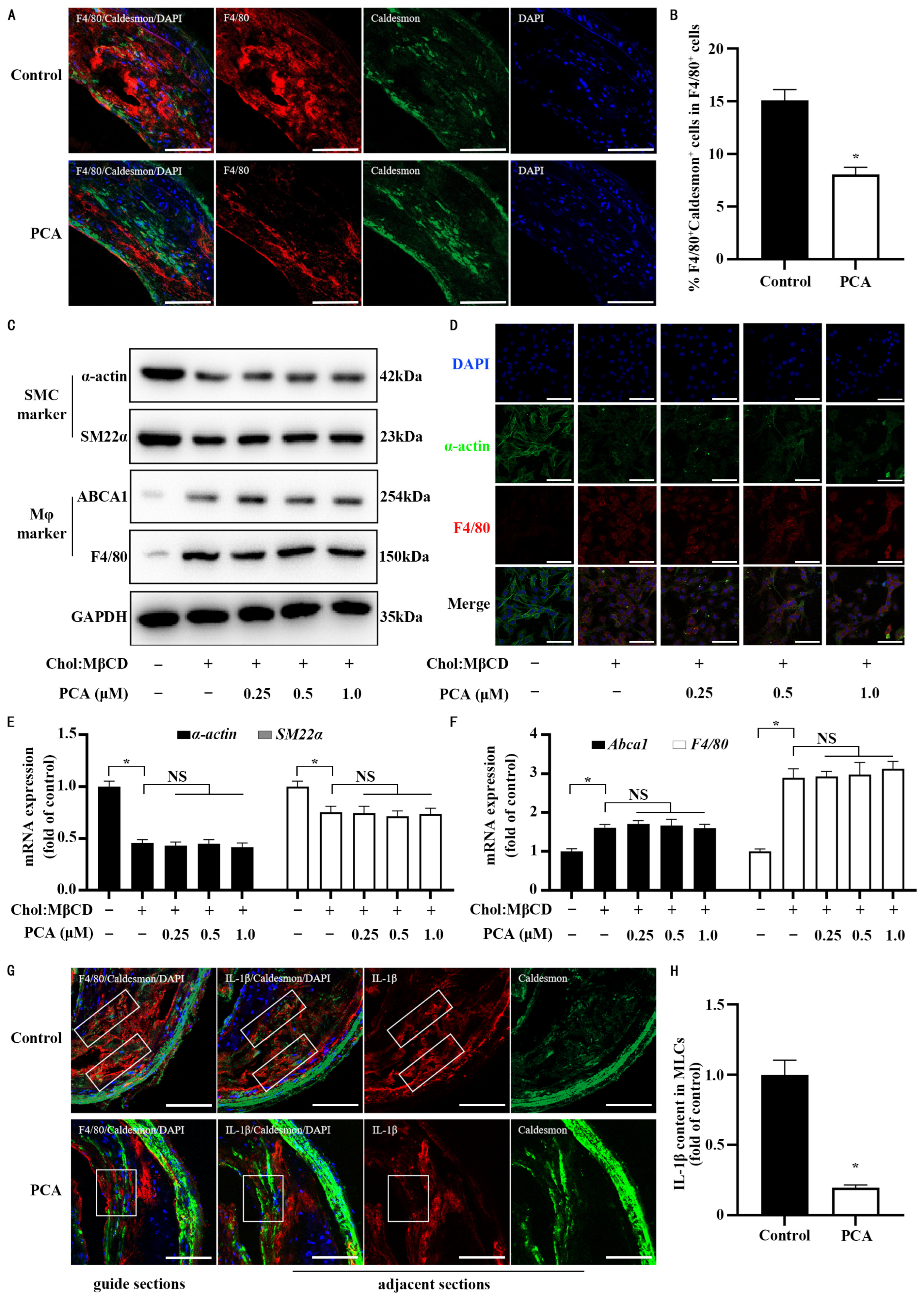
3.6. Dietary Protocatechuic Acid Reduces Inflammation Burden in MLCs Within Atherosclerotic Plaques and Inhibits the Transdifferentiation Progress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| Chol:MβCD | cholesterol and methyl-β-cyclodextrin complexes |
| DAPI | 4′,6-diamidino-2-phenylindole |
| IL | interleukin |
| LDH | lactate dehydrogenase |
| LMB | leptomycin B |
| LPS | lipopolysaccharide |
| Mφ | macrophage |
| MLCs | macrophage-like cells |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear factor kappa B |
| NS | nonsignificant |
| ox-LDL | oxidized LDL |
| PCA | protocatechuic acid |
| POVPC | 1-palmitoyl 2-(5-oxovaleroyl) phosphatidylcholine |
| p-IκBα | phosphorylated NF-κB inhibitor α |
| p-IKK | phosphorylated NF-κB kinase inhibitor |
| siRNA | small interfering RNA |
| si-Exportin-1 | Exportin-1 small interfering RNA |
| si-Scr | scrambled small interfering RNA |
| VSMCs | vascular smooth muscle cells |
| SM22α | smooth muscle 22α |
References
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ho, S.E.; Xue, C.; Cui, J.; Johanson, Q.S.; Sachs, N.; Ross, L.S.; Li, F.; Solomon, R.A.; Connolly, E.S.; et al. Atherosclerosis Is a Smooth Muscle Cell–Driven Tumor-like Disease. Circulation 2024, 149, 1885–1898. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Harman, J.L.; Jorgensen, H.F. The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br. J. Pharmacol. 2019, 176, 3741–3753. [Google Scholar] [CrossRef] [PubMed]
- Swiatlowska, P.; Tipping, W.; Marhuenda, E.; Severi, P.; Fomin, V.; Yang, Z.; Xiao, Q.; Graham, D.; Shanahan, C.; Iskratsch, T. Hypertensive Pressure Mechanosensing Alone Triggers Lipid Droplet Accumulation and Transdifferentiation of Vascular Smooth Muscle Cells to Foam Cells. Adv. Sci. 2024, 11, e2308686. [Google Scholar] [CrossRef]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar] [CrossRef]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef]
- Vengrenyuk, Y.; Nishi, H.; Long, X.; Ouimet, M.; Savji, N.; Martinez, F.O.; Cassella, C.P.; Moore, K.J.; Ramsey, S.A.; Miano, J.M.; et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 535–546. [Google Scholar] [CrossRef]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Dubland, J.A.; Allahverdian, S.; Besler, K.J.; Ortega, C.; Wang, Y.; Pryma, C.S.; Boukais, K.; Chan, T.; Seidman, M.A.; Francis, G.A. Low LAL (Lysosomal Acid Lipase) Expression by Smooth Muscle Cells Relative to Macrophages as a Mechanism for Arterial Foam Cell Formation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e354–e368. [Google Scholar] [CrossRef] [PubMed]
- Higashimori, M.; Tatro, J.B.; Moore, K.J.; Mendelsohn, M.E.; Galper, J.B.; Beasley, D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Dwivedi, N.; Bechtel, T.J.; Paulsen, J.L.; Muth, A.; Bawadekar, M.; Li, G.; Thompson, P.R.; Shelef, M.A.; Schiffer, C.A.; et al. Citrullination of NF-kappaB p65 promotes its nuclear localization and TLR-induced expression of IL-1beta and TNFalpha. Sci. Immunol. 2017, 2, eaal3062. [Google Scholar] [CrossRef]
- Fagerlund, R.; Kinnunen, L.; Kohler, M.; Julkunen, I.; Melen, K. NF-kappaB is transported into the nucleus by importin alpha3 and importin alpha4. J. Biol. Chem. 2005, 280, 15942–15951. [Google Scholar] [CrossRef]
- Lin, H.C.; Li, J.; Cheng, D.D.; Zhang, X.; Yu, T.; Zhao, F.Y.; Geng, Q.; Zhu, M.X.; Kong, H.W.; Li, H.; et al. Nuclear export protein CSE1L interacts with P65 and promotes NSCLC growth via NF-kappaB/MAPK pathway. Mol. Ther. Oncolytics 2021, 21, 23–36. [Google Scholar] [CrossRef]
- Azmi, A.S.; Uddin, M.H.; Mohammad, R.M. The nuclear export protein XPO1—From biology to targeted therapy. Nat. Rev. Clin. Oncol. 2021, 18, 152–169. [Google Scholar] [CrossRef]
- Boughanem, H.; Torres-Pena, J.D.; Arenas-de Larriva, A.P.; Romero-Cabrera, J.L.; Gomez-Luna, P.; Martin-Piedra, L.; Rodriguez-Cantalejo, F.; Tinahones, F.J.; Yubero Serrano, E.M.; Soehnlein, O.; et al. Mediterranean diet, neutrophil count, and carotid intima-media thickness in secondary prevention: The CORDIOPREV study. Eur. Heart J. 2025, 46, 719–729. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 2011, 124, 841–851.E2. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, W. Health Benefits and Future Research of Phytochemicals: A Literature Review. J. Nutr. 2025, 155, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Wang, D.; Zou, T.; Yang, Y.; Yan, X.; Ling, W. Cyanidin-3-O-beta-glucoside with the aid of its metabolite protocatechuic acid, reduces monocyte infiltration in apolipoprotein E-deficient mice. Biochem. Pharmacol. 2011, 82, 713–719. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Cheng, C.K.; He, L.; Pu, Y.; Zhang, Y.; Lin, X.; Xu, A.; Lau, C.W.; Tian, X.Y.; Ma, R.C.W.; et al. Endothelial UCP2 Is a Mechanosensitive Suppressor of Atherosclerosis. Circ. Res. 2022, 131, 424–441. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Ijaz, M.; Buabeid, M.; Kharaba, Z.J.; Yaseen, H.S.; Murtaza, G. Therapeutic Effects and Safe Uses of Plant-Derived Polyphenolic Compounds in Cardiovascular Diseases: A Review. Drug Des. Devel Ther. 2021, 15, 4713–4732. [Google Scholar] [CrossRef] [PubMed]
- Satheesh Babu, A.K.; Srinivasan, H.; Anandh Babu, P.V. Breaking bugs: Gut microbes metabolize dietary components and modulate vascular health. Crit. Rev. Food Sci. Nutr. 2024, 64, 12411–12419. [Google Scholar] [CrossRef]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Jing, J.; Guo, J.; Dai, R.; Zhu, C.; Zhang, Z. Targeting gut microbiota and immune crosstalk: Potential mechanisms of natural products in the treatment of atherosclerosis. Front. Pharmacol. 2023, 14, 1252907. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Yang, H.; Wang, D.; Ling, W. Influence of Intestinal Microbiota on the Catabolism of Flavonoids in Mice. J. Food Sci. 2016, 81, H3026–H3034. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Chen, F.; Zhang, X.; Clinton, S.K.; Tang, X.; Sun, Z.; Chen, T. Suppression of Oxidative Stress and NFkappaB/MAPK Signaling by Lyophilized Black Raspberries for Esophageal Cancer Prevention in Rats. Nutrients 2017, 9, 413. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Wang, L.S.; Zimmerman, N.P.; Ransom, B.W.; Carmella, S.G.; Kuo, C.T.; Chen, J.H.; Oshima, K.; Huang, Y.W.; Hecht, S.S.; et al. Dietary Consumption of Black Raspberries or Their Anthocyanin Constituents Alters Innate Immune Cell Trafficking in Esophageal Cancer. Cancer Immunol. Res. 2016, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, Q.; He, L.; Weng, H.; Su, D.; Liu, X.; Ling, W.; Wang, D. Protocatechuic Acid Inhibits Vulnerable Atherosclerotic Lesion Progression in Older Apoe−/− Mice. J. Nutr. 2020, 150, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, J.; Chen, Z.; Huang, S.; Yan, C.; Kwek, E.; He, Z.; Zhu, H.; Chen, Z.Y. Protocatechuic acid alleviates TMAO-aggravated atherosclerosis via mitigating inflammation, regulating lipid metabolism, and reshaping gut microbiota. Food Funct. 2024, 15, 881–893. [Google Scholar] [CrossRef]
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2010, 58, 12722–12728. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Du, Y.; Zhang, X.; Xiang, P.; Chen, G.; Ling, W.; Wang, D. Protocatechuic acid boosts continual efferocytosis in macrophages by derepressing KLF4 to transcriptionally activate MerTK. Sci. Signal 2023, 16, eabn1372. [Google Scholar] [CrossRef]
- Amini, A.M.; Spencer, J.P.E.; Yaqoob, P. Effects of pelargonidin-3-O-glucoside and its metabolites on lipopolysaccharide-stimulated cytokine production by THP-1 monocytes and macrophages. Cytokine 2018, 103, 29–33. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Pang, J.; Zhang, H.; Luo, J.; Qian, X.; Chen, Q.; Ling, W. Attenuation of Atherosclerosis by Protocatechuic Acid via Inhibition of M1 and Promotion of M2 Macrophage Polarization. J. Agric. Food Chem. 2019, 67, 807–818. [Google Scholar] [CrossRef]
- Vari, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiao, Y.; Song, F.; Yang, Y.; Xia, M.; Ling, W. Increased plasma S-adenosyl-homocysteine levels induce the proliferation and migration of VSMCs through an oxidative stress-ERK1/2 pathway in apoE−/− mice. Cardiovasc. Res. 2012, 95, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA 2003, 100, 13531–13536. [Google Scholar] [CrossRef]
- Archbold, H.C.; Jackson, K.L.; Arora, A.; Weskamp, K.; Tank, E.M.; Li, X.; Miguez, R.; Dayton, R.D.; Tamir, S.; Klein, R.L.; et al. TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Sci. Rep. 2018, 8, 4606. [Google Scholar] [CrossRef]
- Zheng, J.; Xiong, H.; Li, Q.; He, L.; Weng, H.; Ling, W.; Wang, D. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Sci. Nutr. 2019, 7, 3071–3080. [Google Scholar] [CrossRef]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Trogan, E.; Fisher, E.A. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol. Biol. 2005, 293, 221–231. [Google Scholar] [CrossRef]
- Maitra, U.; Deng, H.; Glaros, T.; Baker, B.; Capelluto, D.G.; Li, Z.; Li, L. Molecular mechanisms responsible for the selective and low-grade induction of proinflammatory mediators in murine macrophages by lipopolysaccharide. J. Immunol. 2012, 189, 1014–1023. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Chapman, M.J.; Zamorano, J.L.; Parhofer, K.G. Reducing residual cardiovascular risk in Europe: Therapeutic implications of European medicines agency approval of icosapent ethyl/eicosapentaenoic acid. Pharmacol. Ther. 2022, 237, 108172. [Google Scholar] [CrossRef]
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Wong, N.D.; Zhao, Y.; Quek, R.G.W.; Blumenthal, R.S.; Budoff, M.J.; Cushman, M.; Garg, P.; Sandfort, V.; Tsai, M.; Lopez, J.A.G. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2017, 11, 1223–1233. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J.; Group, C.T. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2018, 391, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Hong, K.; Wang, J.; Kang, X.; Xue, H.; Gao, Y.; Liang, H.; Huang, W.; Zhan, J.; You, Y. Ferulic acid and protocatechuic acid alleviate atherosclerosis by promoting UCP1 expression to inhibit the NLRP3-IL-1beta signaling pathway. Food Funct. 2025, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Dejani, N.N.; Elshabrawy, H.A.; Bezerra Filho, C.; de Sousa, D.P. Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives. Biomolecules 2021, 11, 1254. [Google Scholar] [CrossRef]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef]
- Han, Z.; Hu, H.; Yin, M.; Lin, Y.; Yan, Y.; Han, P.; Liu, B.; Jing, B. HOXA1 participates in VSMC-to-macrophage-like cell transformation via regulation of NF-kappaB p65 and KLF4: A potential mechanism of atherosclerosis pathogenesis. Mol. Med. 2023, 29, 104. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Bergmark, C.; Laurila, A.; Horkko, S.; Han, K.H.; Friedman, P.; Dennis, E.A.; Witztum, J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: Evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA 1999, 96, 6353–6358. [Google Scholar] [CrossRef]
- Bao, Z.; Li, L.; Geng, Y.; Yan, J.; Dai, Z.; Shao, C.; Sun, Z.; Jing, L.; Pang, Q.; Zhang, L.; et al. Advanced Glycation End Products Induce Vascular Smooth Muscle Cell-Derived Foam Cell Formation and Transdifferentiate to a Macrophage-Like State. Mediat. Inflamm. 2020, 2020, 6850187. [Google Scholar] [CrossRef] [PubMed]
- Pidkovka, N.A.; Cherepanova, O.A.; Yoshida, T.; Alexander, M.R.; Deaton, R.A.; Thomas, J.A.; Leitinger, N.; Owens, G.K. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ. Res. 2007, 101, 792–801. [Google Scholar] [CrossRef]
- Masella, R.; Vari, R.; D’Archivio, M.; Di Benedetto, R.; Matarrese, P.; Malorni, W.; Scazzocchio, B.; Giovannini, C. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J. Nutr. 2004, 134, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, R.; Liu, H.; Xue, H.; Zhang, R.; Han, S.; Ji, L.; Huang, W.; Zhan, J.; You, Y. Research progress on intervention effect and mechanism of protocatechuic acid on nonalcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2022, 62, 9053–9075. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Wang, L.S.; Bei, D.; Wang, J.; See, W.A.; Mallery, S.R.; Stoner, G.D.; Liu, Z. Pharmacokinetics of protocatechuic acid in mouse and its quantification in human plasma using LC-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 908, 39–44. [Google Scholar] [CrossRef]
- Liang, S.; Zhao, Z.; Liu, L.; Zhang, Y.; Liu, X. Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment. Molecules 2024, 29, 4724. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Esposito, A.; Choi, H.; Matarese, M.; Benedetti, V.; Di Malta, C.; Monfregola, J.; Medina, D.L.; Lippincott-Schwartz, J.; Ballabio, A. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun. 2018, 9, 3312. [Google Scholar] [CrossRef]
- Mitchell, L.; Hobbs, G.A.; Aghajanian, A.; Campbell, S.L. Redox regulation of Ras and Rho GTPases: Mechanism and function. Antioxid. Redox Signal 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, J.H.; Chou, F.P.; Wang, C.J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-kappaB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef]

| Antibody | Cat# | Source |
|---|---|---|
| GAPDH | K200057m | Solarbio, Beijing, China |
| Histone H3 | ab1791 | Abcam, Cambridge, UK |
| IKKα 1 | AF6012 | Affinity Biosciences, Cincinnati, OH |
| IKKβ | AF6009 | Affinity Biosciences, Cincinnati, OH |
| IKKγ | AF6495 | Affinity Biosciences, Cincinnati, OH |
| phospho-IKKα/β | 2697S | Cell Signaling Technology, Danvers, MA, USA |
| IκBα 2 | 4814S | Cell Signaling Technology, Danvers, MA, USA |
| phospho-IκBα | AP0707 | Abclonal, Woburn, MA, USA |
| Caldesmon | 85702 | NOVUS, Oakland, NE, USA |
| α-actin | A2547/ab5694 | Sigma-Adrich, St. Louis, MO, USA/Abcam, Cambridge, UK |
| SM22α 3 | PA5-29767 | Invitrogen, Waltham, MA, USA |
| F4/80 | Ab16911 | Abcam, Cambridge, UK |
| ABCA1 4 | ab18180 | Abcam, Cambridge, UK |
| NF-κB/phospho-p65 5 | 11014 | SAB, Johannesburg, South Africa |
| IL-1β 6 | AF-401-NA | R&D, Minneapolis, MN, USA |
| Importin α1 | 10819-1-AP | Proteintech, Rosemont, IL, USA |
| Importin α3 | 12463-1-AP | Proteintech, Rosemont, IL, USA |
| Importin α4 | 67892-1-IG | Proteintech, Rosemont, IL, USA |
| Exportin-1 | 66763-1-IG | Proteintech, Rosemont, IL, USA |
| Exportin-2 | 67306-1-IG | Proteintech, Rosemont, IL, USA |
| Alexa Fluor® 647 anti-rabbit lgG | A21244 | Invitrogen, Waltham, MA, USA |
| Alexa Fluor® 488 anti-mouse lgG | A10680 | Invitrogen, Waltham, MA, USA |
| Alexa Fluor® 546 anti-goat lgG | A11056 | Invitrogen, Waltham, MA, USA |
| Genes | Forward (5′−3′) | Reverse (5′−3′) |
|---|---|---|
| Il6 1 | GTCCTTCAGAGAGATACAGAAACT | AGCTTATCTGTTAGGAGACCATTG |
| Il1b 2 | CCAGCTTCAAATCTCACAGCAG | CTTCTTTGGGTATTGCTTGGGATC |
| Tnfα 3 | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| Importin α1 | AAACGTCAGCTCCTTTCCTGAT | GGGATAGCACCTCCATCCAC |
| Importin α3 | TCGGGAACTTCTGCACAGAC | ACACCGCTTGTTCACAAACATT |
| Importin α4 | CCAGTGATCGAAATCCACCAA | CGTTTGTTCAGACGTTCCAGAT |
| Importin α5 | TGGAGTTCCTCAAACGAAAAGAA | TTTGTCAGGACCCAAGCTGAT |
| Importin α7 | AAGAACAATGCCTTAAACCCTGA | AGCAGACTATCAAACATGGCAG |
| Importin α8 | CTACCTCAAAGGCTCCCAAAG | CGGAGTTGTAGACTGGAAGCAA |
| Exportin-1 | CTGCTTGATTTCAGCCAAAAACT | GTATTTCGTGTTCATGTTCTGCG |
| Exportin-2 | ATGGAGTCCTTCGTACAGCG | TCATTTGCATGGGTACTGCAC |
| Exportin-4 | CGGTAACTGCAAGCGAGTCTT | CTCAGGACTTGGTTGGCGA |
| Exportin-5 | TGTGCGAGGAGCTAGTGAAAG | TCTGACGATGGCAATTTGTGTT |
| Exportin-6 | ATAAGATGGAAATCCGTAGCTGC | GGCGTCCAATATCAACGATAACT |
| Exportin-7 | CGTGTCTCGGACAAACAACC | GCTTGAGTCACGAAAGTTGCC |
| α-actin | GCTTCGCTGGTGATGATGCTC | AGTTGGTGATGATGCCGTGTTC |
| SM22α 4 | CCAACAAGGGTCCATCCTACG | ATCTGGGCGGCCTACATCA |
| F4/80 | CCTGGACGAATCCTGTGAAG | GGTGGGACCACAGAGAGTTG |
| Abca1 5 | GCTTCGCTGGTGATGATGCTC | AGTTGGTGATGATGCCGTGTTC |
| Gapdh 6 | AACGACCCCTTCATTGAC | TCCACGACATACTCAGCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Du, Y.; Chen, G.; Mao, Y.; Zhang, W.; Kang, M.; Zhu, S.; Wang, D. Protocatechuic Acid Attenuates Inflammation in Macrophage-like Vascular Smooth Muscle Cells in ApoE−/− Mice. Nutrients 2025, 17, 1090. https://doi.org/10.3390/nu17061090
Li S, Du Y, Chen G, Mao Y, Zhang W, Kang M, Zhu S, Wang D. Protocatechuic Acid Attenuates Inflammation in Macrophage-like Vascular Smooth Muscle Cells in ApoE−/− Mice. Nutrients. 2025; 17(6):1090. https://doi.org/10.3390/nu17061090
Chicago/Turabian StyleLi, Shuangshuang, Yushi Du, Guanyu Chen, Yihui Mao, Wenyu Zhang, Mengxi Kang, Shasha Zhu, and Dongliang Wang. 2025. "Protocatechuic Acid Attenuates Inflammation in Macrophage-like Vascular Smooth Muscle Cells in ApoE−/− Mice" Nutrients 17, no. 6: 1090. https://doi.org/10.3390/nu17061090
APA StyleLi, S., Du, Y., Chen, G., Mao, Y., Zhang, W., Kang, M., Zhu, S., & Wang, D. (2025). Protocatechuic Acid Attenuates Inflammation in Macrophage-like Vascular Smooth Muscle Cells in ApoE−/− Mice. Nutrients, 17(6), 1090. https://doi.org/10.3390/nu17061090








