Serotonin Transporter Gene Polymorphisms Predict Adherence to Weight Loss Programs Independently of Obesity-Related Genes
Abstract
:1. Introduction
1.1. Adherence and Serotonin Transporter Gene
1.2. Ethnic Differences in Genes and Obesity
1.3. Serotonin Transporter and Energy Metabolism
1.4. Objective of This Study
2. Materials and Methods
2.1. Participants
2.2. Dietary and Exercise Advice
2.3. Genotyping
2.4. Anthropometry
- Initial Weight: the starting weight before the intervention.
- Final Weight: the weight after the intervention.
2.5. Blood Biochemistry
2.6. Eating Behavior Questionnaire
2.7. Statistical Analysis
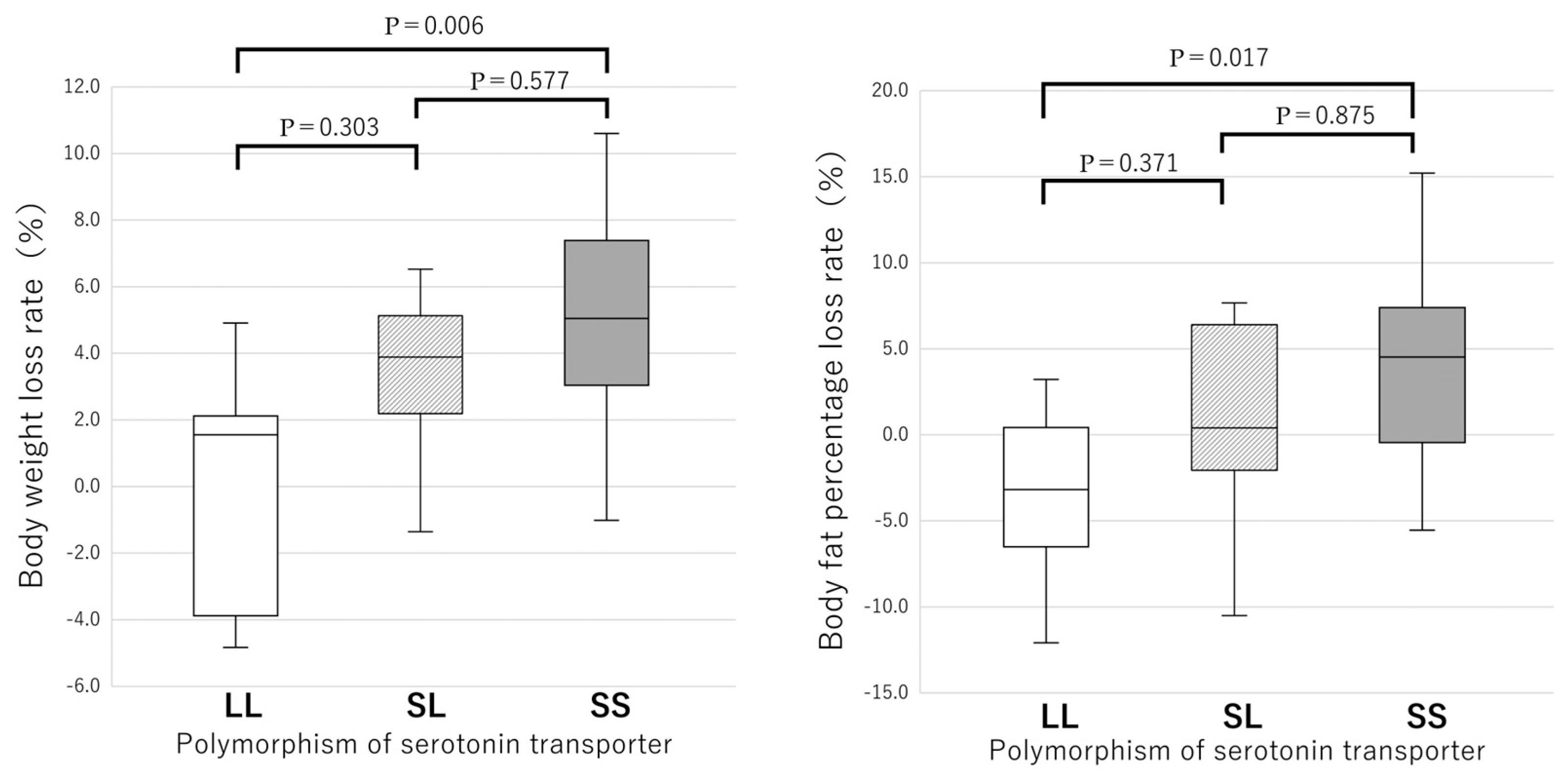
3. Results
3.1. Genotypes
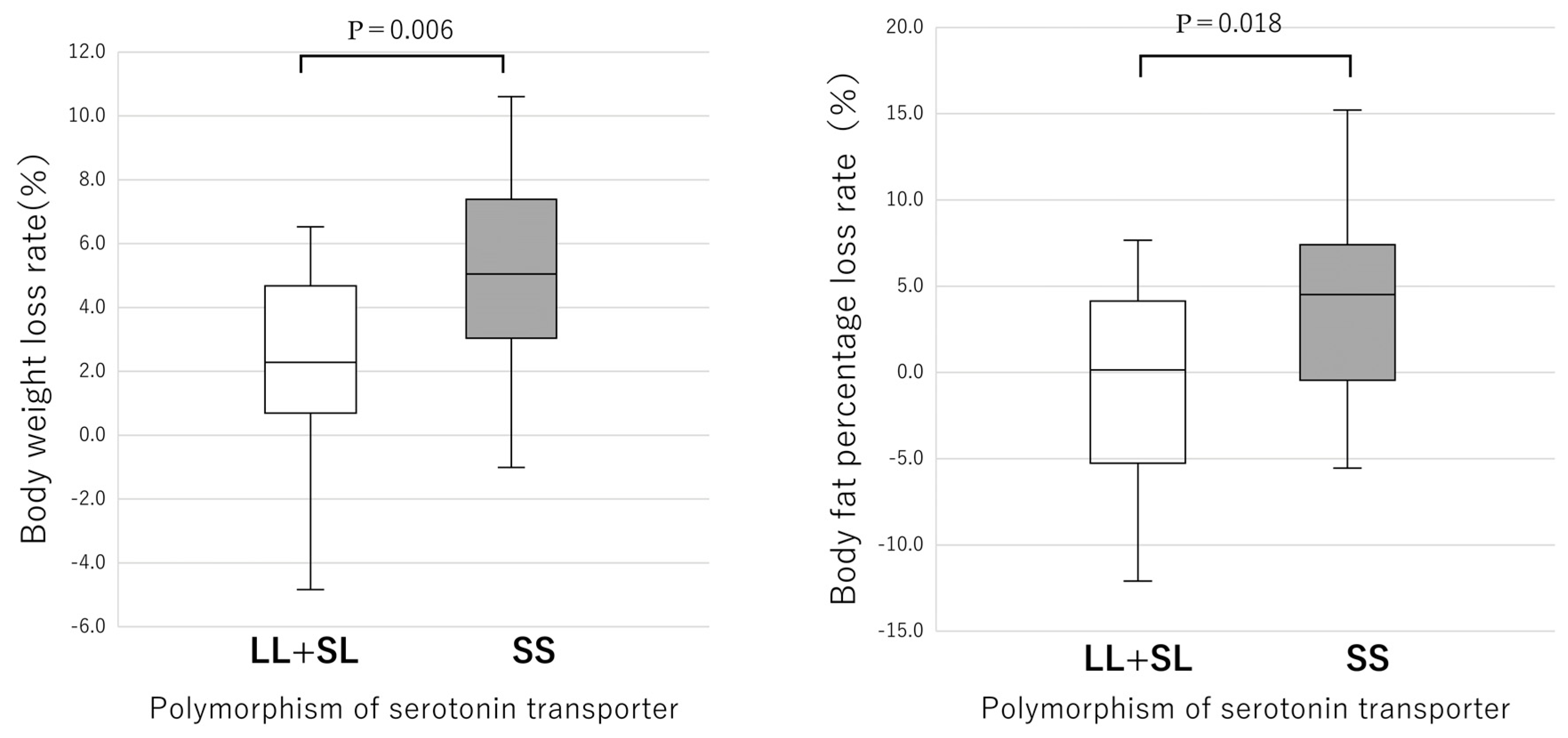
3.2. Anthropometry and Blood Biochemistry
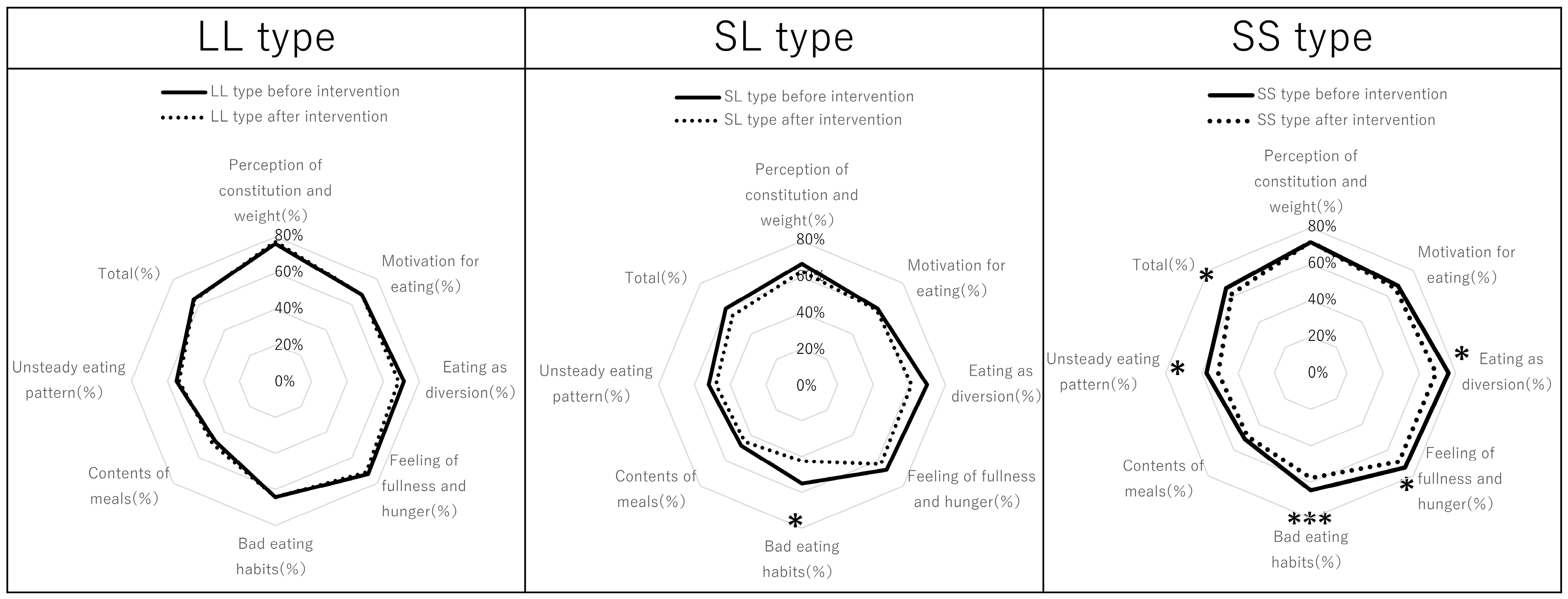
3.3. Eating Behavior Scores
4. Discussion
Strength and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| β3AR | Beta 3 adrenergic receptor |
| BMI | Body mass index |
| DBP | Diastolic blood pressure |
| FBG | Fasting blood glucose |
| HbA1c | Hemoglobin A1c |
| 5-HT | 5-hydroxytryptamine or serotonin |
| 5-HTT | Serotonin transporter |
| 5-HTTLPR | 5-HTT-linked polymorphic region |
| L | Long allele of 5-HTTLPR |
| LDL-C | Low-density cholesterol |
| LL | Homozygote of long allele of 5-HTTLPR |
| SL | Heterozygote of L and S alleles of 5-HTTLPR |
| S | Short allele of 5-HTTLPR |
| SBP | Systolic blood pressure |
| SS | Homozygote of S alleles of 5-HTTLPR |
| TC | Total cholesterol |
| UCP1 | Uncoupling protein 1 |
References
- Desroches, S.; Lapointe, A.; Ratté, S.; Gravel, K.; Légaré, F.; Turcotte, S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst. Rev. 2013, 2, CD008722. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.; Akter, S.; Kashino, I.; Goto, A.; Mizoue, T.; Noda, M.; Sasazuki, S.; Sawada, N.; Tsugane, S.; Japan Public Health Center based Prospective Study Group. Quality of diet and mortality among Japanese men and women: Japan Public Health Center based Prospective Study. BMJ 2016, 352, i1209. [Google Scholar] [CrossRef]
- Burleson, J.; Stephens, D.E.; Rimal, R.N. Adherence Definitions, Measurement Modalities, and Psychometric Properties in HIV, Diabetes, and Nutritional Supplementation Studies: A Scoping Review. Patient Prefer. Adherence 2025, 19, 319–344. [Google Scholar] [CrossRef]
- Carrasco-Marín, F.; Parra-Soto, S.; Bonpoor, J.; Phillips, N.; Talebi, A.; Petermann-Rocha, F.; Pell, J.; Ho, F.; Martínez-Maturana, N.; Celis-Morales, C.; et al. Adherence to dietary recommendations by socioeconomic status in the United Kingdom biobank cohort study. Front. Nutr. 2024, 11, 1349538. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Calati, R.; Mandelli, L.; De Ronchi, D. Serotonin transporter gene variants and behavior: A comprehensive review. Curr. Drug Targets 2006, 7, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Fukushima, A.; Sakuma, K.; Yanagisawa, Y.; Kagawa, Y. Serotonin transporter polymorphisms affect human blood glucose control. Biochem. Biophys. Res. Commun. 2005, 334, 1165–1171. [Google Scholar] [CrossRef]
- Lesch, K.P.; Balling, U.; Gross, J.; Strauss, K.; Wolozin, B.L.; Murphy, D.L.; Riederer, P. Organization of the human serotonin transporter gene. J. Neural Transm. Gen. Sect. 1994, 95, 157–162. [Google Scholar] [CrossRef]
- Meng, Y.; Groth, S.W.; Hodgkinson, C.A.; Mariani, T.J. Serotonin system genes contribute to the susceptibility to obesity in Black adolescents. Obes. Sci. Pract. 2021, 7, 441–449. [Google Scholar] [CrossRef]
- Schinka, J.A.; Busch, R.M.; Robichaux-Keene, N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol. Psychiatry 2004, 9, 197–202. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Svrakic, D.M.; Przybeck, T.R. A psychobiological model of temperament and character. Arch. Gen. Psychiat. 1993, 50, 975–990. [Google Scholar] [CrossRef]
- Gillespiea, N.A.; Cloninger, C.R.; Heathc, A.C.; Martina, N.G. The genetic and environmental relationship between Cloninger’s dimensions of temperament and character. Pers. Individ. Dif. 2003, 35, 1931–1946. [Google Scholar]
- Goldberg, L.R. The structure of phenotypic personality traits. Am. Psychol. 1993, 48, 26–34. [Google Scholar] [PubMed]
- Park, C.S.; Choi, J.; Kwak, S.; Lee, S.-P.; Kim, H.-K.; Kim, Y.-J.; Kwak, S.H.; Park, J.-B. Association between personality, lifestyle behaviors, and cardiovascular diseases in type 2 diabetes mellitus: A population-based cohort study of UK Biobank data. BMJ Open Diabetes Res. Care. 2024, 12, e004244. [Google Scholar] [PubMed]
- Lo, M.-T.; Hinds, D.A.; Tung, J.Y.; Franz, C.; Chun-Chieh Fan, C.-C.; Wang, Y.C.; Smeland, O.B.; Schork, A.; Holland, D.; Kauppi, K.; et al. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat. Genet. 2017, 49, 152–156. [Google Scholar] [CrossRef]
- Gondo, Y.; Hirose, N.; Arai, Y.; Yamamura, K.; Shimizu, K.; Michiyo Takayama, M.; Yoshinori Ebihara, Y.; Nakazawa, S.; Inagaki, H.; Masui, Y.; et al. Contribution of an affect-associated gene to human longevity: Prevalence of the long-allele genotype of the serotonin transporter-linked gene in Japanese centenarians. Mech. Ageing Dev. 2005, 126, 1178–1184. [Google Scholar]
- Kim, J.-H.; Kim, H.-K.; Sang-Wha Lee, S.-W.; Son, Y.-D.; Kim, J.-H. The relationship between character traits and in vivo cerebral serotonin transporter availability in healthy subjects: A high-resolution PET study with C-11 DASB. Pharmaceuticals 2023, 16, 759. [Google Scholar] [CrossRef]
- Hariri, A.R.; Weinberger, D.R. Functional neuroimaging of genetic variation in serotonergic neurotransmission. Genes Brain Behav. 2003, 2, 341–349. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueno, S.; Sano, A.; Tanabe, H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol. Psychiatry 2000, 5, 32–38. [Google Scholar]
- Kagawa, Y. Influence of nutritional intakes in Japan and the United States on COVID-19 infection. Nutrients 2022, 14, 633. [Google Scholar] [CrossRef]
- Tecott, L.H. Serotonin and the orchestration of energy balance. Cell Metab. 2007, 6, 352–361. [Google Scholar]
- McGlashon, J.M.; Gorecki, M.C.; Kozlowski, A.E.; Thirnbeck, C.K.; Markan, K.R.; Leslie, K.L.; Kotas, M.E.; Potthoff, M.J.; Richerson, G.B.; Gillum, M.P. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab. 2015, 21, 692–705. [Google Scholar] [CrossRef]
- Sookoian, S.; Gianotti, T.F.; Gemma, C.; Burgueño, A.; Pirola, C.J. Contribution of the functional 5-HTTLPR variant of the SLC6A4 gene to obesity risk in male adults. Obesity 2008, 16, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Ikegami, H.; Kawaguchi, Y.; Ogihara, T.J. Meta-analysis of the association of Trp64Arg polymorphism of beta 3-adrenergic receptor gene with body mass index. Clin. Endocrinol. Metabol. 1998, 83, 2441–2444. [Google Scholar]
- Zhu, L.Y.; Hu, L.Y.; Li, X.L.; Wang, G.Y.; Shan, W.; Ma, L.C.; Wang, X.H. Relationship between Trp64Arg mutation in the beta3-adrenergic receptor gene and metabolic syndrome: A seven-year follow-up study. Chin. Med. J. 2010, 123, 2375–2378. [Google Scholar]
- Jia, J.J.; Tian, Y.B.; Cao, Z.H.; Tao, L.L.; Zhang, X.; Gao, S.Z.; Ge, C.R.; Lin, Q.Y.; Jois, M. The polymorphisms of UCP1 genes associated with fat metabolism, obesity and diabetes. Mol. Biol. Rep. 2010, 37, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Chathoth, S.; Ismai, M.H.; Vatte, C.; Cyrus, C.; Ali, Z.A.; Ahmed, K.H.; Sadananda Acharya, S.; Barqi, A.M.A.; Ali, A.A. Association of Uncoupling Protein 1 (UCP1) gene polymorphism with obesity: A case-control study. BMC Med. Genet. 2018, 19, 203. [Google Scholar]
- Carey, G.B. Mechanisms regulating adipocyte lipolysis. Adv. Exp. Med. Biol. 1998, 441, 157–170. [Google Scholar] [PubMed]
- Kim, Y.; Yeung, S.L.A.; Sharp, S.J.; Wang, M.; Jang, H.; Luo, S.; Brage, S.; Wijndaele, K. Genetic susceptibility, screen-based sedentary activities and incidence of coronary heart disease. BMC Med. 2022, 20, 188. [Google Scholar]
- Cassidy, S.; Chau, J.Y.; Catt, M.; Bauman, A.; Michael, I.; Trenell, M.I. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioral phenotype of cardiovascular disease and type 2 diabetes. BMJ Open 2016, 6, e010038. [Google Scholar]
- Miller, T.A. Health literacy and adherence to medical treatment in chronic and acute illness: A meta-analysis. Patient Educ. Couns. 2016, 99, 1079–1086. [Google Scholar]
- Leibowitz, S.F.; Alexander, J.T. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatry 1998, 44, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, Y.; Kagawa, A. Secondary prevention of cardiovascular diseases of outpatients of the nutrition clinic. In Nutritional Prevention of Cardiovascular Diseases; Lovenberg, W., Yamori, Y., Eds.; Academic Press: Cambridge, MA, USA, 1984; pp. 339–348. [Google Scholar]
- Hirai, C.; Kagawa, Y. The concentrations of blood sugar and HbA1c are significantly higher in g/g homozygotes of adiponectin t45g polymorphism than in heterozygotes and wild types. Asia Pac. J. Public Health 2008, 20, 80–86. [Google Scholar]
- Obata, K.; Segawa, O.; Yakabe, M.; Ishida, Y.; Kuroita, T.; Ikeda, K.; Kawakami, B.; Kawamura, Y.; Yohda, M.; Matsunaga, T.; et al. Development of a novel method for operating magnetic particles, Magtration Technology, and its use for automating nucleic acid purification. J. Biosci. Bioeng. 2001, 91, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, G.A.; Vollger, M.R.; Eichler, E.E. Long-read human genome sequencing and its applications. Nat. Rev. Genet. 2020, 21, 597–614. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, M.; Busscher, L.; Menafra, R.; Zhai, Q.; van den Berg, R.R.; Kingan, S.B.; Gonzaludo, N.; Hon, T.; Han, T.; Arbiza, L.; et al. Design and performance of a long-read sequencing panel for pharmacogenomics. bioRxiv 2022. Available online: https://www.biorxiv.org/content/10.1101/2022.10.25.513646v1.abstract (accessed on 1 January 2020).
- Kagawa, Y.; Hiraoka, M.; Miyashita-Hatano, Y.; Shishido-Oki, M.; Yoshida, M.; Kondou, S.; Sugiura, M.; Sawakami-Kobayashi, K.; Takahashi, M.; Tajima, H.; et al. Automated single nucleotide polymorphism typing using bead array in capillary tube. J. Biosci. Bioeng. 2010, 110, 505–508. [Google Scholar] [CrossRef]
- Japan Society for the Study of Obesity. Guidelines for the Management of Obesity Disease 2016 (In Japanese); Life Science Publisher: Tokyo, Japan, 2016; pp. 40–42. [Google Scholar]
- Miwa, T.; Tajirika, S.; Hanai, T.; Imamura, N.; Adachi, M.; Horita, R.; Fukao, T.; Shimizu, M.; Yamamoto, M. Usefulness of a questionnaire for assessing the relationship between eating behavior and steatotic liver disease among Japanese male young adults. Sci. Rep. 2024, 14, 2194. [Google Scholar] [CrossRef]
- Chapman, B.P.; Franks, P.; Duberstein, P.R.; Jerant, A. Differences between individual and societal health state valuations: Any link with personality? Med. Care 2009, 47, 902–907. [Google Scholar] [CrossRef]
- Bonnet, G.; Gómez-Abellán, P.; Vera, B.; Juan Francisco Sánchez-Romera, J.F.; Hernández-Martínez, A.M.; Sookoian, S.; Pirola, C.J.; Garaulet, M. Serotonin-transporter promoter polymorphism modulates the ability to control food intake: Effect on total weight loss. Mol. Nutr. Food Res. 2017, 61, 1700494. [Google Scholar] [CrossRef]
- Mõttus, R.; McNeill, G.; Jia, X.; Craig, L.C.A.; Starr, J.M.; Deary, I.J. The associations between personality, diet and body mass index in older people. Health Psychol. 2013, 32, 353–560. [Google Scholar] [CrossRef]
- Yeh, Y.-W.; Ho, P.-S.; Kuo, S.-C.; Chen, C.-Y.; Liang, C.-S.; Yen, C.-H.; Huang, C.-C.; Ma, K.-H.; Shiue, C.-Y.; Huang, W.-S.; et al. Disproportionate reduction of serotonin transporter may predict the response and adherence to antidepressants in patients with major depressive disorder: A positron emission tomography study with 4-[18F]-ADAM. Int. J. Neuropsychopharmacol. 2015, 18, pyu120. [Google Scholar] [PubMed]
- The 1000 Genome Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Kagawa, Y.; Yanagisawa, Y.; Hasegawa, K.; Suzuki, H.; Yasuda, K.; Kudo, H.; Abe, M.; Matsuda, S.; Ishikawa, Y.; Tsuchiya, N.; et al. Single nucleotide polymorphisms of thrifty genes for energy metabolism: Evolutionary origins and prospects for intervention to prevent obesity-related diseases. Biochem. Biophys. Res. Commun. 2002, 295, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015, 8, 1–31. [Google Scholar] [PubMed]
- Du, I.; Bakish, D.; Hrdina, P.D. Gender differences in association between serotonin transporter gene polymorphism and personality traits. Psychiatr. Genet. 2000, 10, 159–164. [Google Scholar]
- Philibert, R.A.; Sandhu, H.; Hollenbeck, N.; Gunter, T.; Adams, W.; Madan, A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008, 147, 543–549. [Google Scholar] [CrossRef]




| Mean ± SD | |
|---|---|
| Body weight (kg) | 66.3 ± 13.8 |
| BMI (kg/m2) | 27.2 ± 5.6 |
| Body fat percentage (%) | 35.3 ± 6.1 |
| Waist circumference (cm) | 93.4 ± 13.9 |
| Systolic blood pressure (mmHg) | 133 ± 18 |
| Diastolic blood pressure (mmHg) | 79 ± 12 |
| Triglycerides (mg/dL) | 108 ± 65 |
| Total cholesterol (mg/dL) | 219 ± 38 |
| LDL cholesterol (mg/dL) | 129 ± 34 |
| Fasting blood glucose (mg/dL) | 94 ± 22 |
| HbA1c (%) | 5.8 ± 0.6 |
| Before Intervention | After Intervention | p Value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Body weight (kg) | 66.3 | ±13.8 | 63.6 | ±13.6 | <0.001 |
| BMI (kg/m2) | 27.2 | ±5.6 | 26.1 | ±5.6 | <0.001 |
| Body fat percentage (%) | 35.3 | ±6.1 | 34.5 | ±5.8 | 0.004 |
| Waist circumference (cm) | 93.4 | ±13.9 | 90.3 | ±13.6 | <0.001 |
| Systolic blood pressure (mmHg) | 133 | ±18 | 122 | ±16 | <0.001 |
| Diastolic blood pressure (mmHg) | 79 | ±12 | 72 | ±11 | <0.001 |
| Triglyceride (mg/dL) | 108 | ±65 | 103 | ±45 | 0.456 |
| Total cholesterol (mg/dL) | 219 | ±38 | 219 | ±31 | 0.845 |
| LDL cholesterol (mg/dL) | 129 | ±34 | 129 | ±27 | 0.884 |
| Fasting blood glucose (mg/dL) | 94 | ±22 | 91 | ±18 | 0.115 |
| HbA1c (%) | 5.84 | ±0.59 | 5.75 | ±0.63 | 0.003 |
| LL Type (n = 7) | SL Type (n = 10) | SS Type (n = 39) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||
| Mean ± SD | Mean ± SD | p Value | Mean ± SD | Mean ± SD | p Value | Mean ± SD | Mean ± SD | p Value | |
| Body weight (kg) | 69.7 ± 19.8 | 70.1 ± 22.3 | 1.000 | 62.1 ± 12.7 | 57.2 ± 8.3 | 0.007 | 67.5 ± 13.4 | 64.1 ± 12.5 | <0.001 |
| BMI (kg/m2) | 28.6 ± 7.6 | 28.9 ± 8.8 | 0.917 | 25.4 ± 4.1 | 24.5 ± 3.8 | 0.009 | 27.4 ± 5.6 | 26.0 ± 5.2 | <0.001 |
| Body fat percentage (%) | 33.9 ± 5.6 | 35.0 ± 5.5 | 0.910 | 34.8 ± 6.9 | 34.2 ± 6.2 | 0.333 | 35.7 ± 6.0 | 34.4 ± 5.9 | <0.001 |
| Waist circumference (cm) | 98.0 ± 15.8 | 97.0 ± 18.3 | 0.672 | 88.3 ± 11.4 | 87.0 ± 9.3 | 0.306 | 93.9 ± 14.0 | 90.0 ± 13.5 | <0.001 |
| Systolic blood pressure (mmHg) | 131 ± 22 | 123 ± 23 | 0.116 | 134 ± 21 | 123 ± 17 | 0.058 | 133 ± 17 | 122 ± 15 | <0.001 |
| Diastolic blood pressure (mmHg) | 76 ± 11 | 72 ± 16 | 0.128 | 76 ± 11 | 66 ± 11 | 0.038 | 80 ± 12 | 73 ± 9 | <0.001 |
| Triglycerides (mg/dL) | 99 ± 43 | 93 ± 32 | 0.612 | 93 ± 54 | 103 ± 66 | 0.919 | 113 ± 70 | 104 ± 48 | 0.433 |
| Total cholesterol (mg/dL) | 191 ± 25 | 192 ± 16 | 0.866 | 222 ± 33 | 231 ± 38 | 0.906 | 223 ± 40 | 221 ± 29 | 0.759 |
| LDL cholesterol (mg/dL) | 107 ± 23 | 111 ± 15 | 0.446 | 133 ± 30 | 137 ± 31 | 0.415 | 132 ± 36 | 131 ± 27 | 0.794 |
| Fasting blood glucose (mg/dL) | 90 ± 9 | 86 ± 3 | 0.207 | 114 ± 30 | 101 ± 29 | 0.014 | 89 ± 16 | 89 ± 15 | 0.816 |
| HbA1c (%) | 5.6 ± 0.2 | 5.5 ± 0.3 | 0.026 | 6.2 ± 0.9 | 6.1 ± 1.1 | 0.356 | 5.8 ± 0.5 | 5.7 ± 0.5 | 0.045 |
| LL + SL Type (n = 17) | SS Type (n = 39) | |||||
|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |
| Mean | Mean | Mean | Mean | |||
| Perception of constitution and weight (%) | 71.1 | 69.1 | 0.599 | 72.0 | 72.2 | 0.859 |
| Motivation for eating (%) | 63.2 | 62.7 | 0.975 | 67.9 | 66.0 | 0.355 |
| Eating as diversion (%) | 70.6 | 64.2 | 0.078 | 76.1 | 69.1 | 0.040 |
| Feeling of fullness and hunger (%) | 69.4 | 66.2 | 0.180 | 73.6 | 69.0 | 0.010 |
| Bad eating habits (%) | 58.8 | 51.5 | 0.115 | 64.5 | 57.7 | <0.001 |
| Contents of meals (%) | 47.6 | 46.8 | 0.788 | 51.5 | 49.4 | 0.319 |
| Unsteady eating pattern (%) | 53.2 | 50.3 | 0.248 | 57.6 | 51.0 | 0.001 |
| Total (%) | 61.8 | 58.4 | 0.169 | 66.1 | 61.6 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsuda, M.; Furou, M.; Kamachi, K.; Sakamoto, K.; Shoji, K.; Ishihara, O.; Kagawa, Y. Serotonin Transporter Gene Polymorphisms Predict Adherence to Weight Loss Programs Independently of Obesity-Related Genes. Nutrients 2025, 17, 1094. https://doi.org/10.3390/nu17061094
Yatsuda M, Furou M, Kamachi K, Sakamoto K, Shoji K, Ishihara O, Kagawa Y. Serotonin Transporter Gene Polymorphisms Predict Adherence to Weight Loss Programs Independently of Obesity-Related Genes. Nutrients. 2025; 17(6):1094. https://doi.org/10.3390/nu17061094
Chicago/Turabian StyleYatsuda, Mana, Miyako Furou, Keiko Kamachi, Kaori Sakamoto, Kumiko Shoji, Osamu Ishihara, and Yasuo Kagawa. 2025. "Serotonin Transporter Gene Polymorphisms Predict Adherence to Weight Loss Programs Independently of Obesity-Related Genes" Nutrients 17, no. 6: 1094. https://doi.org/10.3390/nu17061094
APA StyleYatsuda, M., Furou, M., Kamachi, K., Sakamoto, K., Shoji, K., Ishihara, O., & Kagawa, Y. (2025). Serotonin Transporter Gene Polymorphisms Predict Adherence to Weight Loss Programs Independently of Obesity-Related Genes. Nutrients, 17(6), 1094. https://doi.org/10.3390/nu17061094




