Dark Chocolate Mitigates Premenstrual Performance Impairments and Muscle Soreness in Female CrossFit® Athletes: Evidence from a Menstrual-Phase-Specific Trial
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Sample Size Calculation and Study Design
2.3. Supplementation Procedures
2.4. CINDY WOD
2.5. Examination of Delayed-Onset Muscle Soreness by the VAS
2.6. Handgrip Strength Test (HGS)
2.7. Stroop Test (ST)
2.8. Data Analysis
3. Results
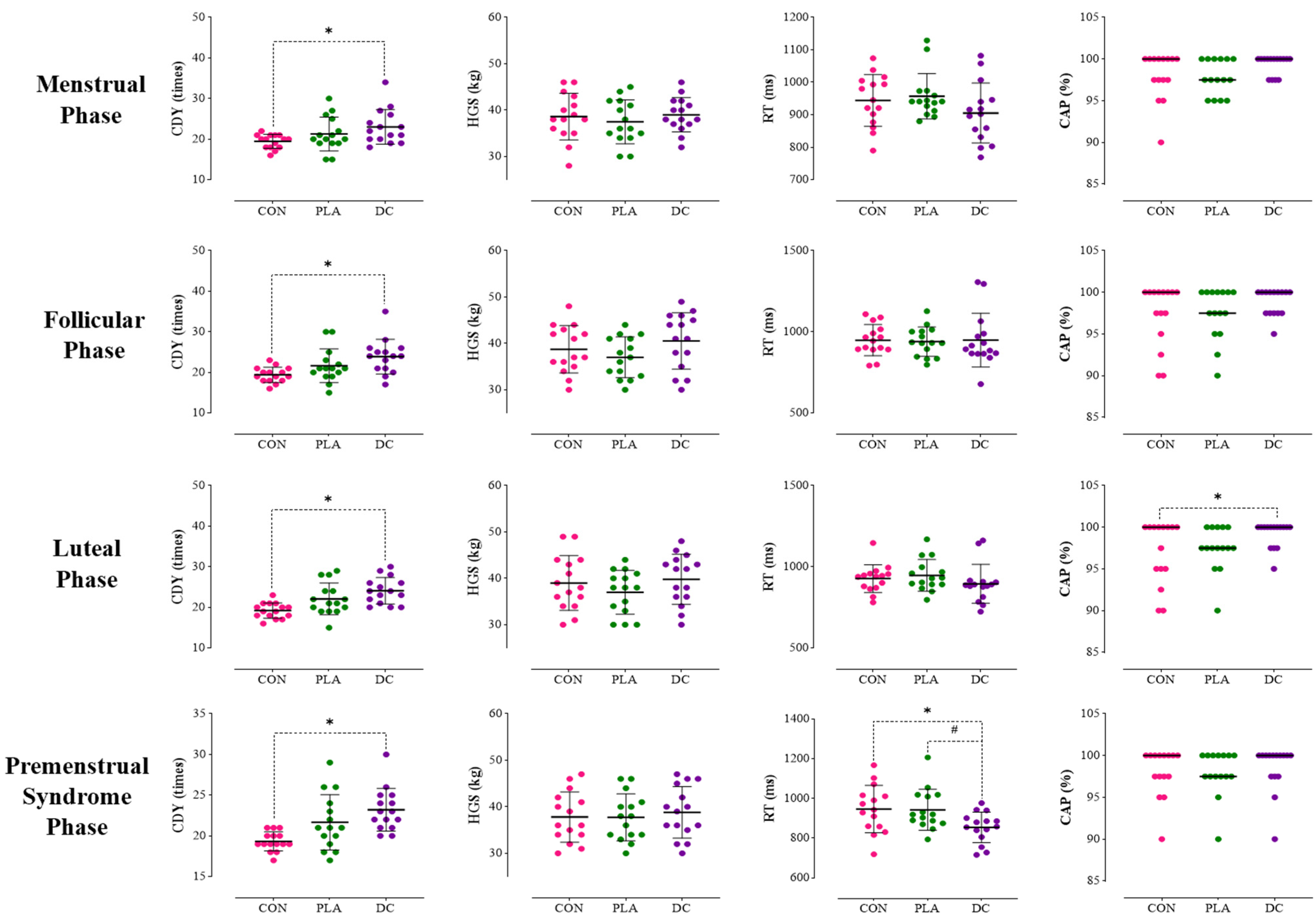
3.1. Menstrual Phase
3.2. Follicular Phase
3.3. Luteal Phase
3.4. Premenstrual Phase
4. Discussion
4.1. Overview of Main Findings
4.2. Phase-Specific Performance Across Menstrual Cycle Phases
4.3. Cognitive Performance Across Phases
4.4. Mechanistic Insights and Possible Explanations
4.5. Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| CAP | Correct Answer Percentage |
| CDY | CINDY WOD (Workout of the Day) |
| CK | Creatine Kinase |
| CON | Control |
| CRP | C-Reactive Protein |
| DC | Dark Chocolate |
| DOMS | Delayed-Onset Muscle Soreness |
| EIMD | Exercise-Induced Muscle Damage |
| F | Follicular Phase |
| HGS | Handgrip Strength |
| IL | Interleukin |
| L | Luteal Phase |
| LDH | Lactate Dehydrogenase |
| M | Menstrual Phase |
| Mb | Myoglobin |
| NO | Nitric Oxide |
| PLA | Placebo |
| PMS | Premenstrual Syndrome |
| PSST | Premenstrual Symptoms Screening Tool |
| RT | Reaction Time |
| TNF-α | Tumor Necrosis Factor-alpha |
| VAS | Visual Analog Scale |
| WOD | Workout of the Day |
References
- de Souza, R.A.S.; da Silva, A.G.; de Souza, M.F.; Souza, L.K.F.; Roschel, H.; da Silva, S.F.; Saunders, B. A systematic review of CrossFit® workouts and dietary and supplementation interventions to guide nutritional strategies and future research in CrossFit®. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-intensity functional training (HIFT): Definition and research implications for improved fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Claudino, J.G.; Gabbett, T.J.; Bourgeois, F.; Souza, H.d.S.; Miranda, R.C.; Mezêncio, B.; Soncin, R.; Cardoso Filho, C.A.; Bottaro, M.; Hernandez, A.J. CrossFit overview: Systematic review and meta-analysis. Sports Med.-Open 2018, 4, 11. [Google Scholar] [CrossRef]
- Drum, S.N.; Bellovary, B.N.; Jensen, R.L.; Moore, M.T.; Donath, L. Perceived demands and postexercise physical dysfunction in CrossFit® compared to an ACSM based training session. J. Sports Med. Phys. Fit. 2017, 57, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Markert, C.D.; Bechke, E.; Williamson, C.; Clemons, K.N.; Snarr, R.L.; McKenzie, M.J. Acute inflammatory responses to high-intensity functional training programming: An observational study. J. Hum. Sport Exerc. 2019, 14, 906–917. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Pearce, E.; Jolly, K.; Jones, L.L.; Matthewman, G.; Zanganeh, M.; Daley, A. Exercise for premenstrual syndrome: A systematic review and meta-analysis of randomised controlled trials. BJGP Open 2020, 4, bjgpopen20X101032. [Google Scholar] [CrossRef]
- Nakamura, Y.; Aizawa, K.; Imai, T.; Kono, I.; Mesaki, N. Hormonal responses to resistance exercise during different menstrual cycle states. Med. Sci. Sports Exerc. 2011, 43, 967–973. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Drescher, C.; Englbrecht, J.S.; Klein, T.; Magerl, W.; Zahn, P.K. Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. Pain 2019, 160, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Paludo, A.C.; Paravlic, A.; Dvořáková, K.; Gimunová, M. The effect of menstrual cycle on perceptual responses in athletes: A systematic review with meta-analysis. Front. Psychol. 2022, 13, 926854. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, F.; Messina, G.; Polito, R.; Porro, C.; Monda, V.; Monda, M.; Scarinci, A.; Dipace, A.; Cibelli, G.; Messina, A. Aerobic and anaerobic effect of CrossFit training: A narrative review. Sport Mont 2023, 21, 123–128. [Google Scholar] [CrossRef]
- Tibana, R.A.; De Almeida, L.M.; Frade de Sousa, N.M.; Nascimento Dda, C.; Neto, I.; De Almeida, J.A.; De Souza, V.C.; Lopes Mde, F.; Nobrega Ode, T.; Vieira, D. Two consecutive days of crossfit training affects pro and anti-inflammatory cytokines and osteoprotegerin without impairments in muscle power. Front. Physiol. 2016, 7, 260. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of Beetroot Juice Supplementation on Muscle Soreness and Performance Recovery after Exercise-Induced Muscle Damage in Female Volleyball Players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef]
- Molaeikhaletabadi, M.; Bagheri, R.; Hemmatinafar, M.; Nemati, J.; Wong, A.; Nordvall, M.; Namazifard, M.; Suzuki, K. Short-Term effects of Low-Fat chocolate milk on delayed onset muscle soreness and performance in players on a women’s university badminton team. Int. J. Environ. Res. Public Health 2022, 19, 3677. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hoorang, N.; Imanian, B.; Hemmatinafar, M.; Rezaei, R.; Nemati, J.; Eftekhari, F.; Alkasasbeh, W.J. Boosting Recovery: Omega-3 and Whey Protein Enhance Strength and Ease Muscle Soreness in Female Futsal Players. Nutrients 2024, 16, 4263. [Google Scholar] [CrossRef]
- Hemmati, H.; Alkasasbeh, W.J.; Hemmatinafar, M.; Salesi, M.; Pirmohammadi, S.; Imanian, B.; Rezaei, R. Effect of a honey-sweetened beverage on muscle soreness and recovery of performance after exercise-induced muscle damage in strength-trained females. Front. Physiol. 2024, 15, 1426872. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Kaltsatou, A.; Cicchella, A. Effect of cocoa products and its polyphenolic constituents on exercise performance and exercise-induced muscle damage and inflammation: A review of clinical trials. Nutrients 2019, 11, 1471. [Google Scholar] [CrossRef]
- Goya, L.; Kongor, J.E.; de Pascual-Teresa, S. From cocoa to chocolate: Effect of processing on flavanols and methylxanthines and their mechanisms of action. Int. J. Mol. Sci. 2022, 23, 14365. [Google Scholar] [CrossRef]
- Decroix, L.; Soares, D.D.; Meeusen, R.; Heyman, E.; Tonoli, C. Cocoa flavanol supplementation and exercise: A systematic review. Sports Med. 2018, 48, 867–892. [Google Scholar] [CrossRef]
- Daussin, F.N.; Cuillerier, A.; Touron, J.; Bensaid, S.; Melo, B.; Al Rewashdy, A.; Vasam, G.; Menzies, K.J.; Harper, M.-E.; Heyman, E. Dietary cocoa flavanols enhance mitochondrial function in skeletal muscle and modify whole-body metabolism in healthy mice. Nutrients 2021, 13, 3466. [Google Scholar] [CrossRef]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kadam, A. Does Dark Chocolate Relieve Menstrual Pain in Adult Women?: A Study Among Indian Population. Int. J. Physiol. 2019, 7, 16–21. [Google Scholar] [CrossRef]
- Ferina, F.; Hadianti, D.N.; Fatimah, Y.U. Dark chocolate as a non-pharmacological alternative to reduce dysmenorrhea in adolescents. Healthc. Low Resour. Settings 2023, 11, 11809. [Google Scholar] [CrossRef]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010, 24, 1505–1514. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C. Dark chocolate intake positively modulates redox status and markers of muscular damage in elite football athletes: A randomized controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef]
- Sanchez Diaz, M.; Martín-Castellanos, A.; Fernández-Elías, V.E.; Lopez Torres, O.; Lorenzo Calvo, J. Effects of polyphenol consumption on recovery in team sport athletes of both sexes: A systematic review. Nutrients 2022, 14, 4085. [Google Scholar] [CrossRef]
- Hariri, F.Z.; Moghaddam-Banaem, L.; Siah Bazi, S.; Saki Malehi, A.; Montazeri, A. The Iranian version of the Premenstrual Symptoms Screening Tool (PSST): A validation study. Arch. Women’s Ment. Health 2013, 16, 531–537. [Google Scholar] [CrossRef]
- Le, J.; Thomas, N.; Gurvich, C. Cognition, the menstrual cycle, and premenstrual disorders: A review. Brain Sci. 2020, 10, 198. [Google Scholar] [CrossRef]
- De Jonge, X.J.; Thompson, B.; Han, A. Methodological recommendations for menstrual cycle research in sports and exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef]
- Hooshmand Moghadam, B.; Bagheri, R.; Ghanavati, M.; Khodadadi, F.; Cheraghloo, N.; Wong, A.; Nordvall, M.; Suzuki, K.; Shabkhiz, F. The combined effects of 6 weeks of jump rope interval exercise and dark chocolate consumption on antioxidant markers in obese adolescent boys. Antioxidants 2021, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar]
- Ryu, K.; Kim, J.; Ali, A.; Choi, S.; Kim, H.; Radlo, S.J. Comparison of athletes with and without burnout using the stroop color and word test. Percept. Mot. Ski. 2015, 121, 413–430. [Google Scholar] [CrossRef]
- Cronin, J.; Lawton, T.; Harris, N.; Kilding, A.; McMaster, D.T. A brief review of handgrip strength and sport performance. J. Strength Cond. Res. 2017, 31, 3187–3217. [Google Scholar] [CrossRef]
- Forte, L.D.; Freire, Y.G.; Júnior, J.S.; Melo, D.A.; Meireles, C.L. Physiological responses after two different CrossFit workouts. Biol. Sport 2022, 39, 231–236. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. JAAOS Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Bijur, P.E.; Latimer, C.; Silver, W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am. J. Emerg. Med. 2002, 20, 287–290. [Google Scholar] [CrossRef]
- Pirmohammadi, S.; Hemmatinafar, M.; Nemati, J.; Imanian, B.; Abdollahi, M.H. Early absorption sources of caffeine can be a useful strategy for improving female table tennis players-specific performance. J. Int. Soc. Sports Nutr. 2023, 20, 2282051. [Google Scholar] [CrossRef] [PubMed]
- Nurazizah, E.; Tih, F.; Suwindere, W. Black chocolate consumption reduces subjective symptoms in 18-22 years old females with premenstrual syndrome. J. Med. Health 2015, 1, 76–84. [Google Scholar] [CrossRef]
- Kalantarzadeh, E.; Radahmadi, M.; Reisi, P. Effects of different dark chocolate diets on memory functions and brain corticosterone levels in rats under chronic stress. Physiol. Pharmacol. 2020, 24, 185–196. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Snarr, R.L.; Esco, M.R. Metabolic and cardiovascular response to the CrossFit workout ‘Cindy’. J. Sport Hum. Perform. 2014, 2, 1–9. [Google Scholar]
- Rashki, M.; Hemmatinafar, M.; Safari, K.; Imanian, B.; Rezaei, R.; Koushkie Jahromi, M.; Suzuki, K. Capsaicin’s Role in Mitigating Muscle Soreness and Enhancing Futsal Players’ Recovery After Exercise-Induced Muscle Damage. Nutrients 2025, 17, 813. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Guerra, R.; Amaral, T.F. Comparison of hand dynamometers in elderly people. J. Nutr. Health Aging 2009, 13, 907–912. [Google Scholar] [CrossRef]
- Migliaccio, G.M.; Di Filippo, G.; Russo, L.; Orgiana, T.; Ardigò, L.P.; Casal, M.Z.; Peyré-Tartaruga, L.A.; Padulo, J. Effects of mental fatigue on reaction time in sportsmen. Int. J. Environ. Res. Public Health 2022, 19, 14360. [Google Scholar] [CrossRef]
- MacLeod, C.M. Half a century of research on the Stroop effect: An integrative review. Psychol. Bull. 1991, 109, 163. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Corr, L.D.; Field, A.; Pufal, D.; Clifford, T.; Harper, L.D.; Naughton, R.J. The effects of cocoa flavanols on indices of muscle recovery and exercise performance: A narrative review. BMC Sports Sci. Med. Rehabil. 2021, 13, 90. [Google Scholar] [CrossRef]
- Dasa, M.S.; Kristoffersen, M.; Ersvær, E.; Bovim, L.P.; Bjørkhaug, L.; Moe-Nilssen, R.; Sagen, J.V.; Haukenes, I. The female menstrual cycles effect on strength and power parameters in high-level female team athletes. Front. Physiol. 2021, 12, 600668. [Google Scholar] [CrossRef]
- de Jonge, X.A.J. Effects of the menstrual cycle on exercise performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Presler, K.M.; Webster, M.J. Dark Chocolate Supplementation Elevates Resting Energy Expenditure in Exercise Trained Females. Int. J. Exerc. Sci. 2021, 14, 250–259. [Google Scholar] [CrossRef]
- Baker, F.C.; Siboza, F.; Fuller, A. Temperature regulation in women: Effects of the menstrual cycle. Temperature 2020, 7, 226–262. [Google Scholar] [CrossRef] [PubMed]
- Minuzzi, L.G.; Lira, F.S.; de Poli, R.A.B.; Lopes, V.H.F.; Zagatto, A.M.; Suzuki, K.; Antunes, B.M. High-intensity intermittent exercise induces a potential anti-inflammatory response in healthy women across the menstrual cycle. Cytokine 2022, 154, 155872. [Google Scholar] [CrossRef]
- Scholey, A.; Owen, L. Effects of chocolate on cognitive function and mood: A systematic review. Nutr. Rev. 2013, 71, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Goya, L.; de Pascual-Teresa, S. Effect of Cocoa and Cocoa Products on Cognitive Performance in Young Adults. Nutrients 2020, 12, 3691. [Google Scholar] [CrossRef]
- Schwarz, N.A.; Theodore, A.P.; Funderburg, B.R.; Waldhelm, A.; McKinley-Barnard, S.K.; Hudson, G.M. Acute (−)-Epicatechin Consumption: Effects on Local Vasodilation Following Resistance Exercise and High-Intensity Exercise Performance. Sports 2020, 8, 22. [Google Scholar] [CrossRef]
- Gröne, M.; Duse, D.A.; Kramser, N.; Ophoff, N.; Schweers, H.; Voß, F.; Quast, C.; Sansone, R.; Heiss, C.; Jung, C. Cocoa flavanols improve peakVO2 and exercise capacity in a randomized double blinded clinical trial in healthy elderly people. Food Funct. 2023, 14, 7562–7573. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef]
- Sung, E.; Han, A.; Hinrichs, T.; Vorgerd, M.; Manchado, C.; Platen, P. Effects of follicular versus luteal phase-based strength training in young women. Springerplus 2014, 3, 668. [Google Scholar] [CrossRef]
- Yonkers, K.A.; O’Brien, P.S.; Eriksson, E. Premenstrual syndrome. Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37, 2445–2453. [Google Scholar] [CrossRef]
- Bruinsma, K.; Taren, D.L. Chocolate: Food or Drug? J. Am. Diet. Assoc. 1999, 99, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Garbarino, E.; Lanteri, P. Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions. Nutrients 2022, 14, 3113. [Google Scholar] [CrossRef]
- De Souza, M.C.; Walker, A.F.; Robinson, P.A.; Bolland, K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: A randomized, double-blind, crossover study. J. Women’s Health Gend. Based Med. 2000, 9, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef]
- Poredoš, D.; Jenko Pražnikar, Z.; Kozinc, Ž. Acute Effects of Beetroot Juice Supplementation on Isometric Muscle Strength, Rate of Torque Development and Isometric Endurance in Young Adult Men and Women: A Randomized, Double-Blind, Controlled Cross-Over Pilot Study. Nutrients 2022, 14, 4759. [Google Scholar] [CrossRef]




| Characteristic | Mean ± SD (n = 15) |
|---|---|
| Age (years) | 22.9 ± 0.5 |
| Height (cm) | 159.6 ± 1.1 |
| Weight (kg) | 56.9 ± 1.7 |
| BMI (kg/m2) | 22.2 ± 0.5 |
| Phase | Variables | CON | PLA | DC |
|---|---|---|---|---|
| Menstrual Phase | CDY (Times) | 19.46 ± 1.72 | 21.26 ± 4.18 | 23.00 ± 4.27 |
| HGS (kg) | 38.60 ± 5.03 | 37.46 ± 4.71 | 39.00 ± 3.70 | |
| RT (ms) | 944.06 ± 79.62 | 957.00 ± 69.92 | 905.33 ± 92.14 | |
| CAP (%) | 98.00 ± 2.86 | 97.83 ± 2.08 | 99.33 ± 1.14 | |
| Follicular Phase | CDY (Times) | 19.40 ± 1.88 | 21.60 ± 4.13 | 23.86 ± 4.27 |
| HGS (kg) | 38.73 ± 5.10 | 37.00 ± 4.39 | 40.53 ± 6.08 | |
| RT (ms) | 948.13 ± 95.94 | 939.53 ± 90.29 | 948.86 ± 165.62 | |
| CAP (%) | 97.33 ± 3.71 | 97.50 ± 3.13 | 98.83 ± 1.59 | |
| Luteal Phase | CDY (Times) | 19.20 ± 1.89 | 22.06 ± 3.91 | 24.06 ± 3.26 |
| HGS (kg) | 39.00 ± 5.90 | 37.00 ± 4.72 | 39.80 ± 5.38 | |
| RT (ms) | 926.93 ± 85.30 | 946.06 ± 97.91 | 894.86 ± 119.78 | |
| CAP (%) | 91.00 ± 3.80 | 97.50 ± 2.67 | 99.16 ± 1.54 | |
| Premenstrual Syndrome Phase | CDY (Times) | 19.33 ± 1.17 | 21.66 ± 3.39 | 23.20 ± 2.62 |
| HGS (kg) | 37.80 ± 5.40 | 37.73 ± 5.04 | 38.80 ± 5.54 | |
| RT (ms) | 946.73 ± 119.49 | 943.06 ± 103.00 | 854.33 ± 76.86 | |
| CAP (%) | 98.00 ± 2.86 | 98.00 ± 2.70 | 98.50 ± 2.80 |
| Phase | Variables | CON | PLA | %RCPLA/CON | DC | %RCDC/CON | %RCDC/PLA |
|---|---|---|---|---|---|---|---|
| Menstrual Phase | DOMS Baseline (mm) | 1.66 ± 4.49 | 2.00 ± 3.68 | 20.48% | 1.66 ± 3.61 | 0.00% | −17.00% |
| DOMS 0 h (mm) | 15.00 ± 17.32 | 12.53 ± 16.20 | −16.47% | 13.33 ± 14.71 | −11.13% | 6.38% | |
| DOMS 12 h (mm) | 22.00 ± 19.06 | 23.66 ± 16.19 | 7.55% | 10.33 ± 13.15 | −53.05% | −56.34% | |
| DOMS 24 h (mm) | 18.66 ± 15.40 | 21.73 ± 20.26 | 16.45% | 9.33 ± 15.33 | −50.00% | −57.06% | |
| DOMS 48 h (mm) | 7.66 ± 9.42 | 11.80 ± 11.68 | 54.05% | 4.53 ± 10.60 | −40.86% | −61.61% | |
| DOMS 72 h (mm) | 3.33 ± 6.17 | 6.53 ± 9.61 | 96.10% | 3.00 ± 5.91 | −9.91% | −54.06% | |
| Follicular Phase | DOMS Baseline (mm) | 3.66 ± 8.12 | 2.66 ± 4.16 | −27.32% | 2.33 ± 3.71 | −36.34% | −12.41% |
| DOMS 0 h (mm) | 9.66 ± 11.41 | 19.33 ± 24.48 | 100.10% | 13.66 ± 23.10 | 41.41% | −29.33% | |
| DOMS 12 h (mm) | 21.66 ± 16.54 | 25.66 ± 22.18 | 18.47% | 13.00 ± 16.77 | −39.98% | −49.34% | |
| DOMS 24 h (mm) | 26.33 ± 16.95 | 16.66 ± 15.54 | −36.73% | 9.33 ± 16.78 | −64.57% | −44.00% | |
| DOMS 48 h (mm) | 11.66 ± 12.34 | 7.00 ± 9.96 | −39.97% | 7.66 ± 15.79 | −34.31% | 9.43% | |
| DOMS 72 h (mm) | 5.33 ± 8.12 | 3.33 ± 8.38 | −37.52% | 2.00 ± 3.68 | −62.48% | −39.94% | |
| Luteal Phase | DOMS Baseline (mm) | 1.00 ± 2.80 | 12.66 ± 13.34 | 1166.00% | 1.66 ± 5.23 | 66.00% | −86.89% |
| DOMS 0 h (mm) | 12.33 ± 13.87 | 22.33 ± 16.88 | 81.10% | 13.66 ± 17.77 | 10.79% | −38.83% | |
| DOMS 12 h (mm) | 29.00 ± 15.49 | 23.00 ± 17.60 | −20.69% | 16.33 ± 14.32 | −43.69% | −29.00% | |
| DOMS 24 h (mm) | 23.66 ± 13.94 | 14.33 ± 13.47 | −39.43% | 10.66 ± 9.03 | −54.95% | −25.61% | |
| DOMS 48 h (mm) | 14.66 ± 12.60 | 7.66 ± 9.42 | −47.75% | 5.66 ± 8.83 | −61.39% | −26.11% | |
| DOMS 72 h (mm) | 10.33 ± 11.72 | 1.66 ± 5.23 | −83.93% | 0.66 ± 1.75 | −93.61% | −60.24% | |
| Premenstrual Syndrome Phase | DOMS Baseline (mm) | 1.00 ± 2.80 | 1.66 ± 3.61 | 66.00% | 2.00 ± 3.68 | 100.00% | 20.48% |
| DOMS 0 h (mm) | 14.66 ± 17.67 | 14.33 ± 14.37 | −2.25% | 15.66 ± 13.99 | 6.82% | 9.28% | |
| DOMS 12 h (mm) | 19.33 ± 16.78 | 16.00 ± 16.49 | −17.23% | 15.00 ± 12.81 | −22.40% | −6.25% | |
| DOMS 24 h (mm) | 14.66 ± 13.15 | 17.66 ± 18.88 | 20.46% | 11.66 ± 12.77 | −20.46% | −33.98% | |
| DOMS 48 h (mm) | 7.33 ± 8.63 | 9.66 ± 12.31 | 31.79% | 3.33 ± 5.87 | −54.57% | −65.53% | |
| DOMS 72 h (mm) | 4.33 ± 5.93 | 4.00 ± 7.12 | −7.62% | 0.66 ± 2.58 | −84.76% | −83.50% |
| Phases | PLA | DC | ||||
|---|---|---|---|---|---|---|
| CON | DC | CON | PLA | |||
| Menstrual Phase | CDY (Times) | MD | 1.80 | −1.73 | 3.53 | 1.73 |
| Sig | 0.433 | 0.513 | 0.017 | 0.513 | ||
| 95%CI | −1.36–4.96 | −4.99–1.53 | 0.581–6.48 | −1.53–4.99 | ||
| HGS (kg) | MD | −1.13 | −1.53 | 0.400 | 1.53 | |
| Sig | 0.943 | 0.634 | 1.000 | 0.634 | ||
| 95%CI | −4.08–1.81 | −4.71–1.64 | −3.53–4.33 | −1.64–4.71 | ||
| RT (ms) | MD | 12.93 | 51.66 | −38.73 | −51.66 | |
| Sig | 1.000 | 0.175 | 0.740 | 0.175 | ||
| 95%CI | −49.41–75.27 | −16.49–119.82 | −125.80–48.33 | −119.82–16.49 | ||
| CAP (%) | MD | −0.16 | −1.50 | 1.33 | 1.50 | |
| Sig | 1.000 | 0.042 | 0.359 | 0.042 | ||
| 95%CI | −2.41–2.07 | −2.95–−0.04 | −0.85–3.51 | 0.04–2.95 | ||
| Follicular Phase | CDY (Times) | MD | −1.73 | −3.53 | 1.80 | 3.53 |
| Sig | 0.307 | 0.077 | 0.008 | 0.077 | ||
| 95%CI | −1.21–5.61 | −4.73–0.200 | −1.14–7.78 | −0.200–4.73 | ||
| HGS (kg) | MD | 12.49 | −4.14 | 16.64 | 4.14 | |
| Sig | 0.91 | 0.114 | 0.797 | 0.114 | ||
| 95%CI | −3.69–0.224 | −7.72–0.659 | −2.42–6.02 | −0.65–7.72 | ||
| RT (ms) | MD | −8.60 | −9.33 | 0.73 | 9.33 | |
| Sig | 1.000 | 1.000 | 1.000 | 1.000 | ||
| 95%CI | −56.57–39.37 | −118.23–99.57 | −111.64–113.11 | −99.571–118.238 | ||
| CAP (%) | MD | 0.167 | −1.33 | 1.50 | 1.33 | |
| Sig | 1.000 | 0.493 | 0.323 | 0.493 | ||
| 95%CI | −3.04–3.37 | −3.80–1.13 | −0.872–3.87 | −1.13–3.80 | ||
| Luteal Phase | CDY (Times) | MD | 2.86 | −2.00 | 4.86 | 2.00 |
| Sig | 0.096 | 0.213 | 0.000 | 0.213 | ||
| 95%CI | −0.40–6.13 | −4.78–0.782 | 2.32–7.40 | −0.782–4.782 | ||
| HGS (kg) | MD | −2.00 | −2.80 | 0.800 | 2.80 | |
| Sig | 0.605 | 0.207 | 1.000 | 0.207 | ||
| 95%CI | −6.05–2.05 | −6.66–1.06 | −3.58–5.18 | −1.06–6.66 | ||
| RT (ms) | MD | 19.13 | 51.20 | −32.06 | −51.20 | |
| Sig | 1.000 | 0.611 | 1.000 | 0.611 | ||
| 95%CI | −40.36–78.63 | −53.13–155.53 | −131.47–67.34 | −155.53–53.13 | ||
| CAP (%) | MD | 0.50 | −1.66 | 2.16 | 1.66 | |
| Sig | 1.000 | 0.108 | 0.080 | 0.108 | ||
| 95%CI | −1.90–2.90 | −3.61–0.285 | −0.21–4.54 | −0.28–3.61 | ||
| Premenstrual Syndrome Phase | CDY (Times) | MD | 2.33 | −1.53 | 3.86 | 1.53 |
| Sig | 0.087 | 0.157 | 0.002 | 0.157 | ||
| 95%CI | −0.274–4.94 | −3.49–0.431 | 1.48–6.25 | −0.431–3.49 | ||
| HGS (kg) | MD | −0.06 | −1.06 | 1.00 | 1.06 | |
| Sig | 1.000 | 1.000 | 1.000 | 1.000 | ||
| 95%CI | −2.77–2.64 | −4.32–2.18 | −3.24–5.24 | −2.18–4.32 | ||
| RT (ms) | MD | −3.66 | 88.73 | −92.40 | −88.73 | |
| Sig | 1.000 | 0.002 | 0.010 | 0.002 | ||
| 95%CI | −71.17–63.83 | 32.14–145.32 | −163.12–−21.67 | −145.32–−32.14 | ||
| CAP (%) | MD | 0.00 | −0.50 | 0.50 | 0.50 | |
| Sig | 1.000 | 1.000 | 1.000 | 1.000 | ||
| 95%CI | −2.39–2.39 | −2.99–1.99 | −2.32–3.32 | −1.99–2.99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safari, K.; Hemmatinafar, M.; Suzuki, K.; Koushkie Jahromi, M.; Imanian, B. Dark Chocolate Mitigates Premenstrual Performance Impairments and Muscle Soreness in Female CrossFit® Athletes: Evidence from a Menstrual-Phase-Specific Trial. Nutrients 2025, 17, 1374. https://doi.org/10.3390/nu17081374
Safari K, Hemmatinafar M, Suzuki K, Koushkie Jahromi M, Imanian B. Dark Chocolate Mitigates Premenstrual Performance Impairments and Muscle Soreness in Female CrossFit® Athletes: Evidence from a Menstrual-Phase-Specific Trial. Nutrients. 2025; 17(8):1374. https://doi.org/10.3390/nu17081374
Chicago/Turabian StyleSafari, Kousar, Mohammad Hemmatinafar, Katsuhiko Suzuki, Maryam Koushkie Jahromi, and Babak Imanian. 2025. "Dark Chocolate Mitigates Premenstrual Performance Impairments and Muscle Soreness in Female CrossFit® Athletes: Evidence from a Menstrual-Phase-Specific Trial" Nutrients 17, no. 8: 1374. https://doi.org/10.3390/nu17081374
APA StyleSafari, K., Hemmatinafar, M., Suzuki, K., Koushkie Jahromi, M., & Imanian, B. (2025). Dark Chocolate Mitigates Premenstrual Performance Impairments and Muscle Soreness in Female CrossFit® Athletes: Evidence from a Menstrual-Phase-Specific Trial. Nutrients, 17(8), 1374. https://doi.org/10.3390/nu17081374








