Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health
Abstract
:1. Introduction
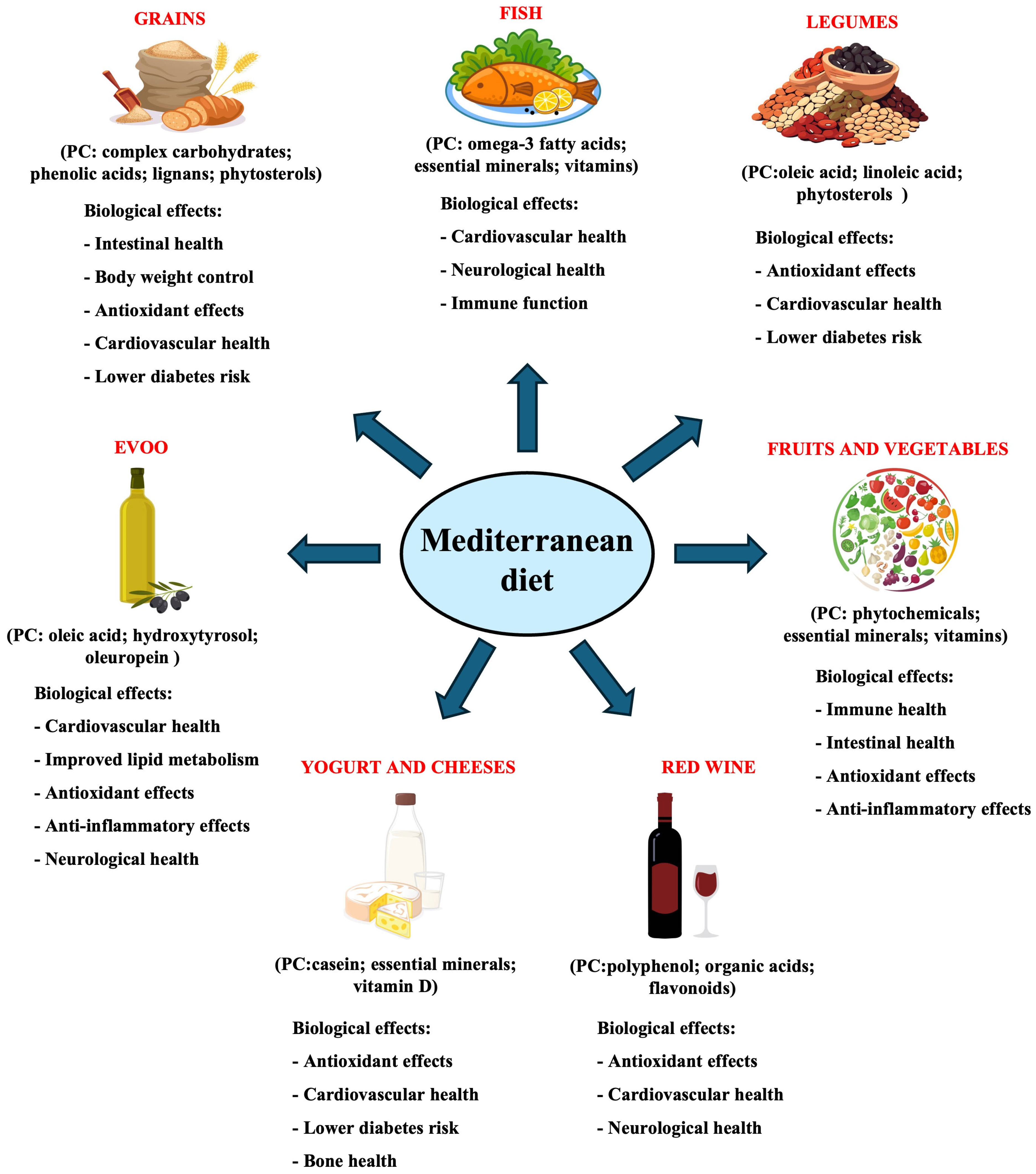
2. Main Components of the Mediterranean Diet
3. Intestinal Microbiota
4. Methodology
5. Effect of Mediterranean Diet on Gut Microbiota
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| EVOO | Extra virgin olive oil |
| FF | Fast-food diet |
| HT | Hydroxytyrosol |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| MD | Mediterranean diet |
| MUFA | Monounsaturated fatty acid |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acid |
| VD | Vegetarian diet |
References
- Unwin, N.; Alberti, K.G.M.M. Chronic Non-Communicable Diseases. Ann. Trop. Med. Parasitol. 2006, 100, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, M.; Guerriero, C.; Cornali, K.; Albanese, M.; Costacurta, M.; Mercuri, N.B.; Di Daniele, N.; Noce, A. Linking Migraine to Gut Dysbiosis and Chronic Non-Communicable Diseases. Nutrients 2023, 15, 4327. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; et al. Modern Vision of the Mediterranean Diet. J. Prev. Med. Hyg. 2022, 63, E36–E43. [Google Scholar] [CrossRef]
- D’Angelo, S.; Tafuri, D. Nutraceutical: Their Role in Improving Sports Performance. Sport. Sci. 2020, 13, 7–12. [Google Scholar]
- Boccellino, M.; Quagliuolo, L.; D’Angelo, S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Curr. Nutr. Food 2021, 17, 111–120. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 1170–1182. [Google Scholar] [CrossRef]
- Vuoso, D.; Porcelli, M.; Cacciapuoti, G.; D’Angelo, S. Biological Activity of MelAnnurca Flesh Apple Biophenols. Curr. Nutr. Food Sci. 2020, 16, 1149–1162. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.; Joksimovic, M.; D’Angelo, S. Could Polyphenolic Food Intake Help in the Control of Type 2 Diabetes? A Narrative Review of the Last Evidence. Curr. Nutr. Food Sci. 2022, 18, 785–798. [Google Scholar] [CrossRef]
- D’Angelo, S.; Pompilio, C. Adherence to the Mediterranean Diet in Athletes. Sport. Sci. 2020, 13, 58–63. [Google Scholar]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; Predimed Investigators. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Buil-Cosiales, P.; Toledo, E.; Salas-Salvadó, J.; Zazpe, I.; Farràs, M.; Basterra-Gortari, F.J.; Diez-Espino, J.; Estruch, R.; Corella, D.; Ros, E.; et al. Association between Dietary Fibre Intake and Fruit, Vegetable or Whole-Grain Consumption and the Risk of CVD: Results from the PREvención Con DIeta MEDiterránea (PREDIMED) Trial. Br. J. Nutr. 2016, 116, 534–546. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Basterra-Gortari, F.J.; Ruiz-Canela, M.; Martínez-González, M.A.; Babio, N.; Sorlí, J.V.; Fito, M.; Ros, E.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. Effects of a Mediterranean Eating Plan on the Need for Glucose-Lowering Medications in Participants With Type 2 Diabetes: A Subgroup Analysis of the PREDIMED Trial. Diabetes Care 2019, 42, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus Nutrition Intervention Randomized Trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef]
- Fernández-Lázaro, C.I.; Ruiz-Canela, M.; Martínez-González, M.Á. Deep Dive to the Secrets of the PREDIMED Trial. Curr. Opin. Lipidol. 2021, 32, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean Diet with Four Sustainable Benefits. Public. Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Clodoveo, M.L. Olive Oil: Processing Characterization, and Health Benefits. Foods 2020, 9, 1612. [Google Scholar] [CrossRef]
- Augimeri, G.; Avolio, E.; Caparello, G.; Galluccio, A.; De Rose, D.; Vivacqua, A.; Morelli, C.; Barone, I.; Catalano, S.; Andò, S.; et al. Serum from Adolescents with High Polyphenol Intake Exhibits Improved Lipid Profile and Prevents Lipid Accumulation in HepG2 Human Liver Cells. Oxid. Med. Cell Longev. 2023, 2023, 1555942. [Google Scholar] [CrossRef]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Original Article Extra Virgin Olive Oil as a Functional Food for Athletes: Recovery, Health, and Performance. J. Phys. Educ. Sport 2025, 25, 370–381. [Google Scholar] [CrossRef]
- Alonso, A.; Ruiz-Gutierrez, V.; Martínez-González, M.A. Monounsaturated Fatty Acids, Olive Oil and Blood Pressure: Epidemiological, Clinical and Experimental Evidence. Public. Health Nutr. 2006, 9, 251–257. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential Effect of Oleic and Palmitic Acid on Lipid Accumulation and Apoptosis in Cultured Hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef]
- Habán, P.; Zideková, E.; Klvanová, J. Oleic Acid Serum Phospholipid Content Is Linked with the Serum Total- and LDL-Cholesterol in Elderly Subjects. Med. Sci. Monit. 2000, 6, 1093–1097. [Google Scholar] [PubMed]
- Baró, L.; Fonollá, J.; Peña, J.L.; Martínez-Férez, A.; Lucena, A.; Jiménez, J.; Boza, J.J.; López-Huertas, E. N-3 Fatty Acids plus Oleic Acid and Vitamin Supplemented Milk Consumption Reduces Total and LDL Cholesterol, Homocysteine and Levels of Endothelial Adhesion Molecules in Healthy Humans. Clin. Nutr. 2003, 22, 175–182. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive Oil Intake and Risk of Cardiovascular Disease and Mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive Tree Derivatives and Hydroxytyrosol: Their Potential Effects on Human Health and Its Use as Functional Ingredient in Meat. Foods 2021, 10, 2611. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Suh, K.S.; Yun, S.J.; Park, J.; Park, S.Y.; Chin, S.O.; Chon, S. Oleuropein Attenuates the 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD)-Perturbing Effects on Pancreatic β-Cells. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2021, 56, 752–761. [Google Scholar] [CrossRef]
- Karakoç, M.D.; Sekkin, S. Effects of Oleuropein on Epirubicin and Cyclophosphamide Combination Treatment in Rats. Turk. J. Pharm. Sci. 2021, 18, 420–429. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol Reduces Intracellular Reactive Oxygen Species Levels in Vascular Endothelial Cells by Upregulating Catalase Expression through the AMPK-FOXO3a Pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef]
- Notariale, R.; Perrone, P.; Mele, L.; Lettieri, G.; Piscopo, M.; Manna, C. Olive Oil Phenols Prevent Mercury-Induced Phosphatidylserine Exposure and Morphological Changes in Human Erythrocytes Regardless of Their Different Scavenging Activity. Int. J. Mol. Sci. 2022, 23, 5693. [Google Scholar] [CrossRef]
- Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Notariale, R.; Lettieri, G.; Mele, L.; La Pietra, V.; Piscopo, M.; Manna, C. Protective Effects of Olive Oil Antioxidant Phenols on Mercury-Induced Phosphatidylserine Externalization in Erythrocyte Membrane: Insights into Scramblase and Flippase Activity. Free Radic. Biol. Med. 2024, 227, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Closse, C.; Dachary-Prigent, J.; Boisseau, M.R. Phosphatidylserine-Related Adhesion of Human Erythrocytes to Vascular Endothelium. Br. J. Haematol. 1999, 107, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Notariale, R.; Längst, E.; Perrone, P.; Crettaz, D.; Prudent, M.; Manna, C. Effect of Mercury on Membrane Proteins, Anionic Transport and Cell Morphology in Human Erythrocytes. Cell Physiol. Biochem. 2022, 56, 500–513. [Google Scholar] [CrossRef]
- Perrone, P.; Ortega-Luna, R.; Manna, C.; Álvarez-Ribelles, Á.; Collado-Diaz, V. Increased Adhesiveness of Blood Cells Induced by Mercury Chloride: Protective Effect of Hydroxytyrosol. Antioxidants 2024, 13, 1576. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean Diet Polyphenols Reduce Inflammatory Angiogenesis through MMP-9 and COX-2 Inhibition in Human Vascular Endothelial Cells: A Potentially Protective Mechanism in Atherosclerotic Vascular Disease and Cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Martinelli, R.; Massaro, M.; Calabriso, N.; Scoditti, E.; Maffia, M.; Verri, T.; Gatta, V.; De Caterina, R. Nutrigenomic Effect of Hydroxytyrosol in Vascular Endothelial Cells: A Transcriptomic Profile Analysis. Nutrients 2021, 13, 3990. [Google Scholar] [CrossRef]
- Setty, B.N.Y.; Betal, S.G. Microvascular Endothelial Cells Express a Phosphatidylserine Receptor: A Functionally Active Receptor for Phosphatidylserine-Positive Erythrocytes. Blood 2008, 111, 905–914. [Google Scholar] [CrossRef]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-Virgin Olive Oil and the Gut-Brain Axis: Influence on Gut Microbiota, Mucosal Immunity, and Cardiometabolic and Cognitive Health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and Immunologically Beneficial Impact of Extra Virgin Olive and Flaxseed Oils on Composition of Gut Microbiota in Mice. Eur. J. Nutr. 2020, 59, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Olid, M.C.; Hidalgo, M.; Prieto, I.; Cobo, A.; Martínez-Rodríguez, A.M.; Segarra, A.B.; Ramírez-Sánchez, M.; Gálvez, A.; Martínez-Cañamero, M. Evidence Supporting the Involvement of the Minority Compounds of Extra Virgin Olive Oil, through Gut Microbiota Modulation, in Some of the Dietary Benefits Related to Metabolic Syndrome in Comparison to Butter. Molecules 2023, 28, 2265. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef] [PubMed]
- Tabanez, M.; Santos, I.R.; Ikebara, J.M.; Camargo, M.L.M.; Dos Santos, B.A.; Freire, B.M.; Batista, B.L.; Takada, S.H.; Squitti, R.; Kihara, A.H.; et al. The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14950. [Google Scholar] [CrossRef]
- Katsirma, Z.; Dimidi, E.; Rodriguez-Mateos, A.; Whelan, K. Fruits and Their Impact on the Gut Microbiota, Gut Motility and Constipation. Food Funct. 2021, 12, 8850–8866. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-a Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca Apple Polyphenol Extract Selectively Kills MDA-MB-231 Cells through ROS Generation, Sustained JNK Activation and Cell Growth and Survival Inhibition. Sci. Rep. 2019, 9, 13045. [Google Scholar] [CrossRef]
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca Apple Polyphenol Extract Promotes Mesenchymal-to-Epithelial Transition and Inhibits Migration in Triple-Negative Breast Cancer Cells through ROS/JNK Signaling. Sci. Rep. 2020, 10, 15921. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.-Y.; Tang, F.; Liu, D.; Zhao, X.-L.; Zhang, J.-N.; Xia, J.; Wu, J.-J.; Yang, Y.; Peng, C.; et al. New Perspectives on the Therapeutic Potential of Quercetin in Non-Communicable Diseases: Targeting Nrf2 to Counteract Oxidative Stress and Inflammation. J. Pharm. Anal. 2024, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-Oxidant and pro-Apoptotic Activity of Polyphenol Extract from Annurca Apple and Its Underlying Mechanisms in Human Breast Cancer Cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, Y.-S.; Kim, K.-M.; Min, S.J.; Kim, Y. Β-Carotene Inhibits Neuroblastoma Tumorigenesis by Regulating Cell Differentiation and Cancer Cell Stemness. Biochem. Biophys. Res. Commun. 2014, 450, 1475–1480. [Google Scholar] [CrossRef]
- Duitsman, P.K.; Barua, A.B.; Becker, B.; Olson, J.A. Effects of Epoxycarotenoids, Beta-Carotene, and Retinoic Acid on the Differentiation and Viability of the Leukemia Cell Line NB4 in Vitro. Int. J. Vitam. Nutr. Res. 1999, 69, 303–308. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Lee, I.-M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and Design of a Large Randomized Controlled Trial of Vitamin D and Marine Omega-3 Fatty Acid Supplements for the Primary Prevention of Cancer and Cardiovascular Disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef]
- P., N.P.V.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef]
- Makhmudova, U.; Schulze, P.C.; Lütjohann, D.; Weingärtner, O. Phytosterols and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.B.; Oliveira Filho, J.G.d.; Salgaço, M.K.; Jesus, M.H.D.; Egea, M.B. Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review. Foods 2023, 12, 4440. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Lagarda, M.J.; Garcia-López, F.J.; Sánchez-Siles, L.M.; Blanco-Navarro, I.; Alegría, A.; Pérez-Sacristán, B.; Garcia-Llatas, G.; Donoso-Navarro, E.; Silvestre-Mardomingo, R.A.; et al. Effect of β-Cryptoxanthin plus Phytosterols on Cardiovascular Risk and Bone Turnover Markers in Post-Menopausal Women: A Randomized Crossover Trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1090–1096. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications after Myocardial Infarction: Final Report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Kuellenberg de Gaudry, D.; Lohner, S.; Bischoff, K.; Schmucker, C.; Hoerrlein, S.; Roeger, C.; Schwingshackl, L.; Meerpohl, J.J. A1- and A2 Beta-Casein on Health-Related Outcomes: A Scoping Review of Animal Studies. Eur. J. Nutr. 2022, 61, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.-I.S.; Delgado, S.; Mittal, J.; Eshraghi, R.S.; Mittal, R.; Eshraghi, A.A. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J. Nutr. 2021, 151, 1061–1072. [Google Scholar] [CrossRef]
- Zhao, K.; Pang, H.; Shao, K.; Yang, Z.; Li, S.; He, N. The Function of Human Milk Oligosaccharides and Their Substitute Oligosaccharides as Probiotics in Gut Inflammation. Food Funct. 2023, 14, 7780–7798. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.E.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis. Nutrients 2021, 13, 1329. [Google Scholar] [CrossRef]
- Jakala, P.; Pere, E.; Lehtinen, R.; Turpeinen, A.; Korpela, R.; Vapaatalo, H. Cardiovascular Activity of Milk Casein-Derived Tripeptides and Plant Sterols in Spontaneously Hypertensive Rats. J. Physiol. Pharmacol. 2009, 60, 11–20. [Google Scholar]
- Ferrara, L.; D’Angelo, S. Post-Exercise Fatigue, Lactate, and Natural Nutritional Strategy. J. Phys. Educ. Sport 2023, 23, 2274–2283. [Google Scholar] [CrossRef]
- Kataoka, K. The Intestinal Microbiota and Its Role in Human Health and Disease. J. Med. Invest. 2016, 63, 27–37. [Google Scholar] [CrossRef]
- Ronan, V.; Yeasin, R.; Claud, E.C. Childhood Development and the Microbiome-The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology 2021, 160, 495–506. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and Dysbiosis: The Two Sides of the Microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar] [PubMed]
- Ficara, M.; Pietrella, E.; Spada, C.; Della Casa Muttini, E.; Lucaccioni, L.; Iughetti, L.; Berardi, A. Changes of Intestinal Microbiota in Early Life. J. Matern. Fetal Neonatal Med. 2020, 33, 1036–1043. [Google Scholar] [CrossRef]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The Mode of Delivery Affects the Diversity and Colonization Pattern of the Gut Microbiota during the First Year of Infants’ Life: A Systematic Review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Salazar, N.; González, S.; Nogacka, A.M.; Rios-Covián, D.; Arboleya, S.; Gueimonde, M.; Reyes-Gavilán, C.G. de L. Microbiome: Effects of Ageing and Diet. Curr. Issues Mol. Biol. 2020, 36, 33–62. [Google Scholar] [CrossRef] [PubMed]
- Yersin, S.; Vonaesch, P. Small Intestinal Microbiota: From Taxonomic Composition to Metabolism. Trends Microbiol. 2024, 32, 970–983. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Pérez, J.C. Fungi of the Human Gut Microbiota: Roles and Significance. Int. J. Med. Microbiol. 2021, 311, 151490. [Google Scholar] [CrossRef]
- Olyaiee, A.; Sadeghi, A.; Yadegar, A.; Mirsamadi, E.S.; Mirjalali, H. Gut Microbiota Shifting in Irritable Bowel Syndrome: The Mysterious Role of Blastocystis sp. Front. Med. 2022, 9, 890127. [Google Scholar] [CrossRef]
- D’Angelo, S.; Donini, L. The Relationships between Microbiota and Exercise. Sport. Sci. 2020, 14, 24. [Google Scholar]
- Lukáčová, I.; Ambro, Ľ.; Dubayová, K.; Mareková, M. The Gut Microbiota, Its Relationship to the Immune System, and Possibilities of Its Modulation. Epidemiol. Mikrobiol. Imunol. 2023, 72, 40–53. [Google Scholar] [PubMed]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Canakis, A.; Haroon, M.; Weber, H.C. Irritable Bowel Syndrome and Gut Microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 28–35. [Google Scholar] [CrossRef]
- Caparrós, E.; Wiest, R.; Scharl, M.; Rogler, G.; Gutiérrez Casbas, A.; Yilmaz, B.; Wawrzyniak, M.; Francés, R. Dysbiotic Microbiota Interactions in Crohn’s Disease. Gut Microbes 2021, 13, 1949096. [Google Scholar] [CrossRef]
- Piccioni, A.; Rosa, F.; Manca, F.; Pignataro, G.; Zanza, C.; Savioli, G.; Covino, M.; Ojetti, V.; Gasbarrini, A.; Franceschi, F.; et al. Gut Microbiota and Clostridium Difficile: What We Know and the New Frontiers. Int. J. Mol. Sci. 2022, 23, 13323. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef]
- Jayachandran, M.; Qu, S. Non-Alcoholic Fatty Liver Disease and Gut Microbial Dysbiosis- Underlying Mechanisms and Gut Microbiota Mediated Treatment Strategies. Rev. Endocr. Metab. Disord. 2023, 24, 1189–1204. [Google Scholar] [CrossRef]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal Microbiota Transplantation: Current Challenges and Future Landscapes. Clin. Microbiol. Rev. 2024, 37, e0006022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Villarejo, A.B.; Ramírez-Sánchez, M.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Changes in Gut Microbiota Linked to a Reduction in Systolic Blood Pressure in Spontaneously Hypertensive Rats Fed an Extra Virgin Olive Oil-Enriched Diet. Plant Foods Hum. Nutr. 2018, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a Diet Enriched with Virgin Olive Oil or Butter on Mouse Gut Microbiota and Its Correlation to Physiological and Biochemical Parameters Related to Metabolic Syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean Diet Intervention in Overweight and Obese Subjects Lowers Plasma Cholesterol and Causes Changes in the Gut Microbiome and Metabolome Independently of Energy Intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary Intervention Impact on Gut Microbial Gene Richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- García-Gavilán, J.F.; Atzeni, A.; Babio, N.; Liang, L.; Belzer, C.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; et al. Effect of 1-Year Lifestyle Intervention with Energy-Reduced Mediterranean Diet and Physical Activity Promotion on the Gut Metabolome and Microbiota: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2024, 119, 1143–1154. [Google Scholar] [CrossRef]
- Haro, C.; García-Carpintero, S.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; López-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Ruiz-Limón, P.; Pilo, J.; Lisbona-Montañez, J.M.; Tinahones, F.J.; Moreno Indias, I.; Macías-González, M. Linking Serum Vitamin D Levels with Gut Microbiota after 1-Year Lifestyle Intervention with Mediterranean Diet in Patients with Obesity and Metabolic Syndrome: A Nested Cross-Sectional and Prospective Study. Gut Microbes 2023, 15, 2249150. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Pastor-Ibáñez, R.; Blanco-Heredia, J.; Etcheverry, F.; Sánchez-Palomino, S.; Díez-Fuertes, F.; Casas, R.; Navarrete-Muñoz, M.Á.; Castro-Barquero, S.; Lucero, C.; Fernández, I.; et al. Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals. Nutrients 2021, 13, 1141. [Google Scholar] [CrossRef]
- Zhu, C.; Sawrey-Kubicek, L.; Beals, E.; Rhodes, C.H.; Houts, H.E.; Sacchi, R.; Zivkovic, A.M. Human Gut Microbiome Composition and Tryptophan Metabolites Were Changed Differently by Fast Food and Mediterranean Diet in 4 Days: A Pilot Study. Nutr. Res. 2020, 77, 62–72. [Google Scholar] [CrossRef]
- Pagliai, G.; Russo, E.; Niccolai, E.; Dinu, M.; Di Pilato, V.; Magrini, A.; Bartolucci, G.; Baldi, S.; Menicatti, M.; Giusti, B.; et al. Influence of a 3-Month Low-Calorie Mediterranean Diet Compared to the Vegetarian Diet on Human Gut Microbiota and SCFA: The CARDIVEG Study. Eur. J. Nutr. 2020, 59, 2011–2024. [Google Scholar] [CrossRef]
- Galié, S.; García-Gavilán, J.; Papandreou, C.; Camacho-Barcía, L.; Arcelin, P.; Palau-Galindo, A.; Rabassa, A.; Bulló, M. Effects of Mediterranean Diet on Plasma Metabolites and Their Relationship with Insulin Resistance and Gut Microbiota Composition in a Crossover Randomized Clinical Trial. Clin. Nutr. 2021, 40, 3798–3806. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The Effects of the Green-Mediterranean Diet on Cardiometabolic Health Are Linked to Gut Microbiome Modifications: A Randomized Controlled Trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Ippolito, M.; Monge, T.; Violi, R.; Cappello, P.; Ferrocino, I.; Cocolin, L.S.; De Francesco, A.; Bo, S.; Finocchiaro, C. Gut Microbiota Composition after Diet and Probiotics in Overweight Breast Cancer Survivors: A Randomized Open-Label Pilot Intervention Trial. Nutrition 2020, 74, 110749. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.M.; Murphy, K.J.; Wade, A.T.; Wang, Y.; Bracci, E.L.; Davis, C.R.; Dyer, K.A.; Woodman, R.J.; Hodgson, J.M.; Rogers, G.B. Interactions between Mediterranean Diet Supplemented with Dairy Foods and the Gut Microbiota Influence Cardiovascular Health in an Australian Population. Nutrients 2023, 15, 3645. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral Microbiota in Human Systematic Diseases. Int. J. Oral. Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Caro, C.; Arce Retana, M.; Losada Amaya, S.; Arboleda, H.; Gallart-Palau, X.; Serra, A. APOE4, Alzheimer’s and Periodontal Disease: A Scoping Review. Ageing Res. Rev. 2025, 105, 102649. [Google Scholar] [CrossRef]
- Augimeri, G.; Caparello, G.; Caputo, I.; Reda, R.; Testarelli, L.; Bonofiglio, D. Mediterranean Diet: A Potential Player in the Link between Oral Microbiome and Oral Diseases. J. Oral Microbiol. 2024, 16, 2329474. [Google Scholar] [CrossRef]

| Group | Genus/Species | Quantity (%) | Main Location |
|---|---|---|---|
| Firmicutes | Lactobacillus | ~64% | Small intestine, colon |
| Clostridium | Colon | ||
| Faecalibacterium | Colon | ||
| Bacteroidetes | Bacteroides | ~23% | Colon |
| Prevotella | Colon | ||
| Actinobacteria | Bifidobacterium | ~3% | Small intestine, colon |
| Proteobacteria | Escherichia coli | Variable | Small intestine, colon |
| Helicobacter pylori | Low | Stomach | |
| Fusobacteria | Fusobacterium | ~2% | Colon |
| Verrucomicrobia | Akkermansia muciniphila | ~2% | Colon |
| Archaea | Methanobrevibacter smithii | <1% | Colon |
| Fungi | Candida | <1% | Small intestine, colon |
| Saccharomyces | <1% | Small intestine, colon | |
| Protozoa | Blastocystis | Variable | Colon |
| Viruses | Bacteriophages | Variable | Small intestine, colon |
| Model System | Associated Pathology | Key Beneficial Factors Observed | Reference |
|---|---|---|---|
| HFD mice | Obesity, inflammation, insulin resistance | HT improves gut barrier integrity and reduces inflammation | [112] |
| Hypertensive rats | Hypertension | EVOO increases microbial diversity and lowers blood pressure | [113] |
| Mice | Metabolic syndrome | EVOO promotes beneficial bacteria and reduces metabolic syndrome | [114] |
| 153 Italian individuals | Metabolic health and inflammation | MD increases SCFA-producing bacteria and lowers TMAO | [115] |
| Obese subjects | Systemic inflammation and metabolism | MD increases Akkermansia muciniphila and reduces inflammation | [116] |
| 27 healthy volunteers | Gut microbiota composition | MD increases Bifidobacterium and SCFA production | [117] |
| Clinical studies on humans | Inflammation and metabolism | Higher microbial diversity and reduction of pro-inflammatory bacteria | [118] |
| Clinical studies on humans | Lipid metabolism regulation | Favors SCFA-producing bacteria and reduces bacterial toxins | [119] |
| 400 participants (ages 55–75) | Cardiovascular diseases and metabolism | Weight loss and improvement in cardiovascular risk factors | [120] |
| 106 obese subjects | Obesity and gut dysbiosis | Increase in Bacteroides and Lactobacillus, reduction in pathogenic bacteria | [121] |
| Individuals with obesity and metabolic syndrome | Obesity and metabolic syndrome | Metabolic improvement and increase in vitamin D | [122] |
| Monozygotic and dizygotic twins | Metabolism and energy extraction | Greater microbial diversity in lean individuals compared to obese | [123] |
| 612 elderly Europeans | Cognition and aging | Increase in SCFA and improved cognitive function | [124] |
| Subjects with mild cognitive impairment | Cognitive decline and Alzheimer’s | Microbiota modulation and reduction of Alzheimer’s biomarkers | [125] |
| Humans with gastrointestinal symptoms | Gut health | Better balance between Bifidobacterium and Escherichia coli | [126] |
| Individuals with HIV | Microbiota and immune system | Increased microbial diversity and improved immune function | [127] |
| Comparison of diets (FF vs. MD) | Inflammation and dysbiosis | Increase in SCFA and reduction in pro-inflammatory bacteria | [128] |
| Comparison of MD vs. vegetarian diet | Gut microbiota modulation | Distinct effects of MD and vegetarian diet on microbial diversity | [129] |
| Multi-omics study on MD patients | Insulin sensitivity and metabolism | Improved insulin sensitivity and energy metabolism | [130] |
| Subjects with abdominal obesity | Abdominal obesity and microbiota | Green-MED increases beneficial taxa and reduces inflammation | [131] |
| Cancer survivors (breast cancer) | Insulin resistance and blood glucose | Probiotics with MD improve blood glucose and insulin resistance | [132] |
| Subjects on the MD integrated with dairy | Blood pressure and glucose metabolism | Modifies bacterial taxa and improves blood pressure | [133] |
| Effect of MD | Microorganisms Affected | Associated Dysbiosis | Related Human Diseases |
|---|---|---|---|
| Increased SCFA production | Roseburia, Bifidobacterium, Faecalibacterium | Reduced SCFA-producing bacteria | Metabolic syndrome, inflammation, obesity |
| Improved gut barrier function | Akkermansia muciniphila | Increased gut permeability | Irritable bowel syndrome (IBS), inflammatory bowel disease (IBD) |
| Reduced systemic inflammation | Bacteroides, Lactobacillus, Christensenellaceae | Higher levels of pro-inflammatory bacteria (E. coli) | Cardiovascular diseases, metabolic dysfunction |
| Enhanced microbial diversity | Clostridia cluster XIV, Bacteroides | Loss of microbial diversity | Obesity, metabolic syndrome, cognitive decline |
| Modulation of lipid metabolism | Bacteroides, Faecalibacterium | Disrupted bile acid metabolism | Cardiovascular diseases, obesity |
| Lower TMAO levels | Prevotella, Bacteroides | Higher levels of TMAO-producing bacteria | Cardiovascular diseases |
| Reduction of pathogenic bacteria | Bifidobacterium, Lactobacillus | Overgrowth of Bilophila wadsworthia, E. coli | Gut inflammation, metabolic disorders |
| Improved insulin sensitivity | Akkermansia muciniphila, Bifidobacterium | Gut microbiota imbalance | Type 2 diabetes, obesity |
| Neuroprotective effects | Bacteroidetes, Firmicutes | Dysbiosis affecting neurotransmitter production | Alzheimer’s, Parkinson’s, depression |
| Weight loss and metabolic health improvement | Bacteroides, Lactobacillus | Gut microbiota imbalance | Obesity, metabolic syndrome |
| Increased beneficial bacteria in cardiovascular health | Faecalibacterium prausnitzii, Bifidobacterium | Reduction in beneficial SCFA-producing bacteria | Hypertension, cardiovascular diseases |
| Reduced gut inflammation in HIV patients | Bifidobacterium, Lactobacillus | Microbial dysbiosis due to immune suppression | Gut inflammation in HIV patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. https://doi.org/10.3390/nu17060948
Perrone P, D’Angelo S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients. 2025; 17(6):948. https://doi.org/10.3390/nu17060948
Chicago/Turabian StylePerrone, Pasquale, and Stefania D’Angelo. 2025. "Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health" Nutrients 17, no. 6: 948. https://doi.org/10.3390/nu17060948
APA StylePerrone, P., & D’Angelo, S. (2025). Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients, 17(6), 948. https://doi.org/10.3390/nu17060948






