Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer’s Mice: A Pilot Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dietary Supplementation
2.3. Tissue Sampling and Processing
2.4. Platelet Activation
2.5. Microglia Isolation
2.6. Microglia Phagocytosis
2.7. Quantification of Endocannabinoids, Endocannabinoid-like Amides, and Free Oxylipins/Eicosanoids
2.8. Microbiome
2.9. RNA Isolation of Retinal Tissue and Gene Expression Analysis
2.10. Immunohistochemistry of Retinal Tissue
2.11. Immunohistochemistry of Brain Tissue
2.12. Microscopy and Analysis
2.13. Statistics
3. Results
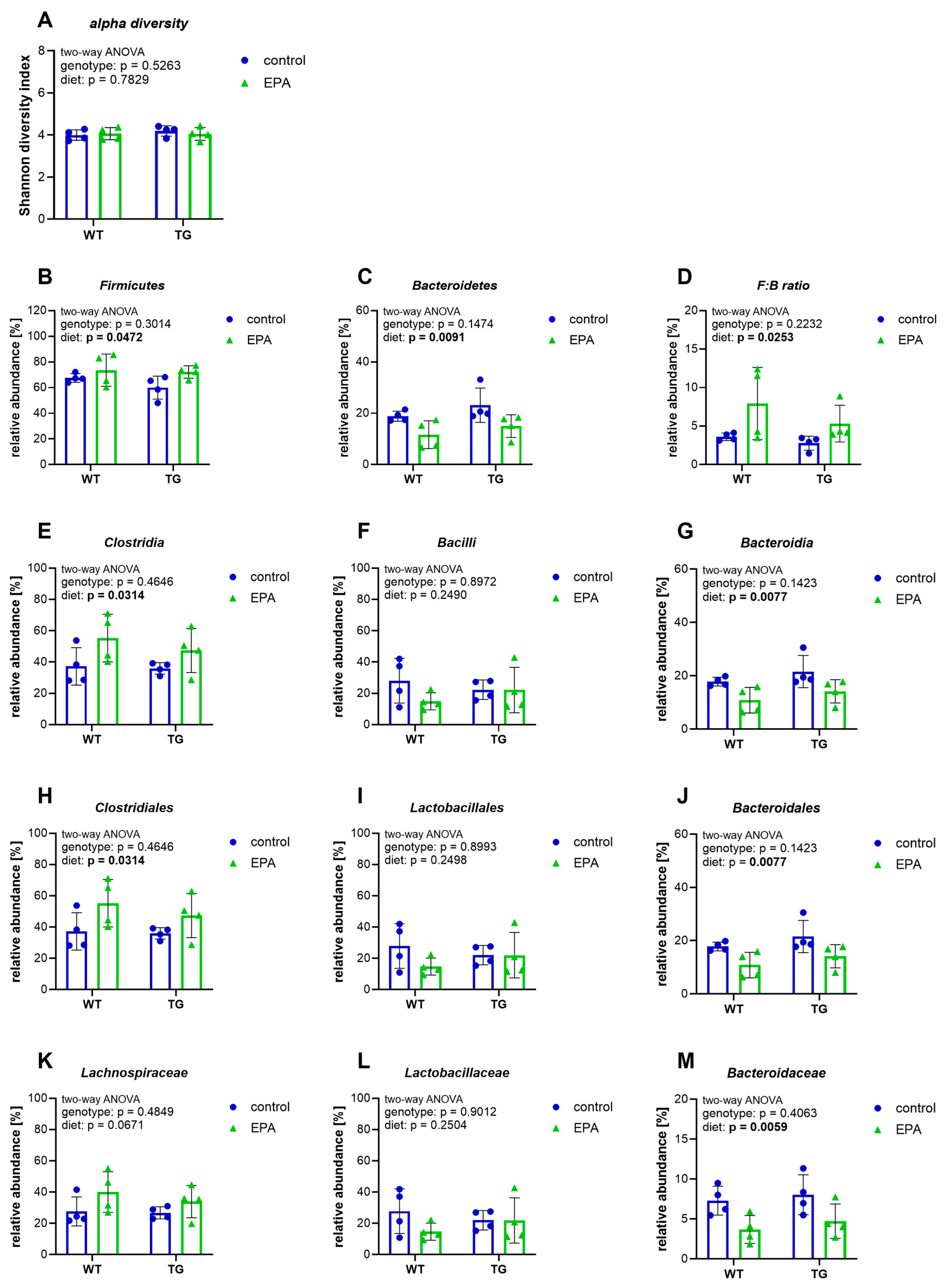
3.1. EPA Supplementation Shapes the Gut Microbiota by Increasing the Firmicutes-to-Bacteroidetes Ratio
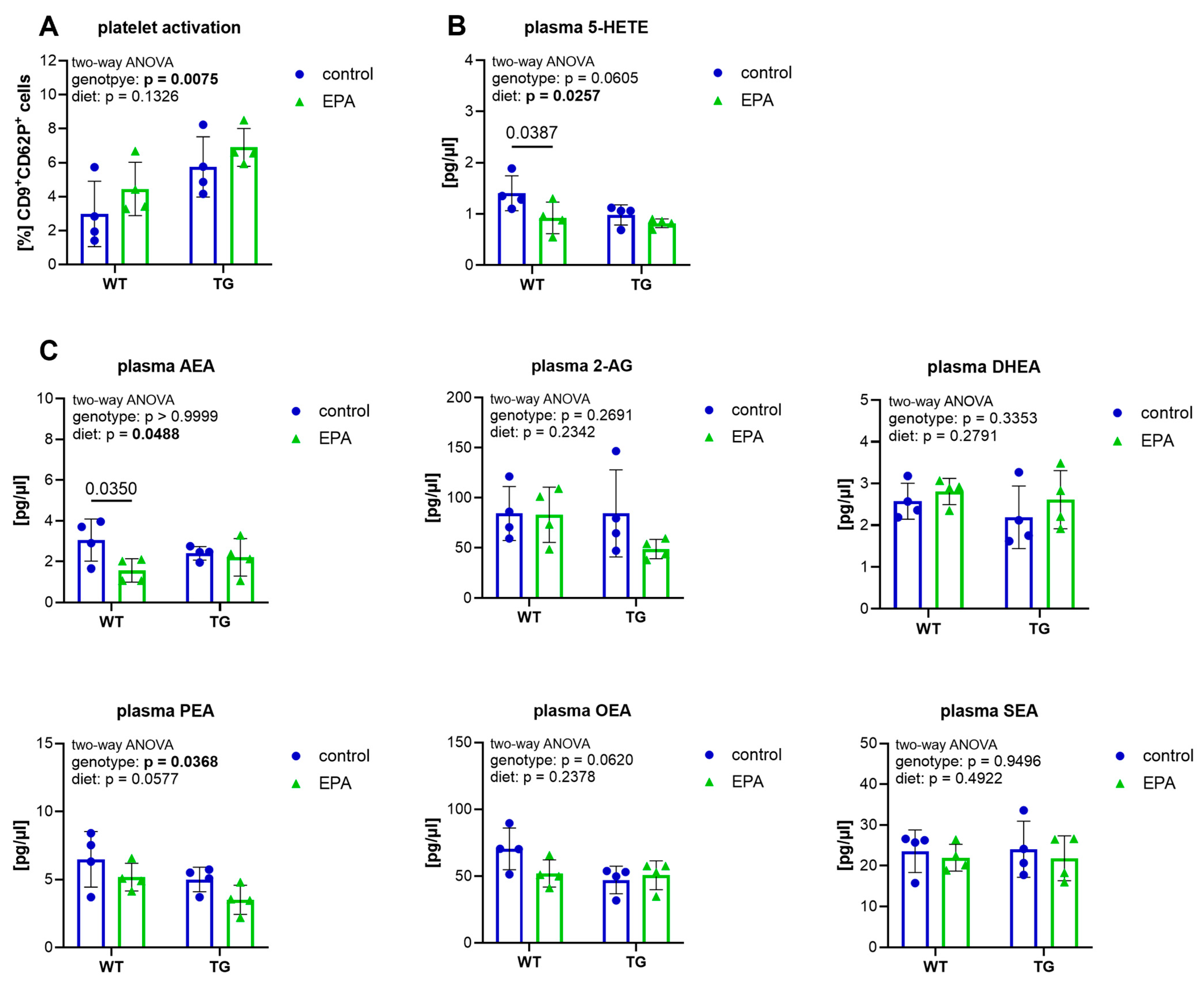
3.2. EPA Supplementation Does Not Affect Platelet Activation, but Reduces Plasma 5-HETE and AEA in Aged WT Mice
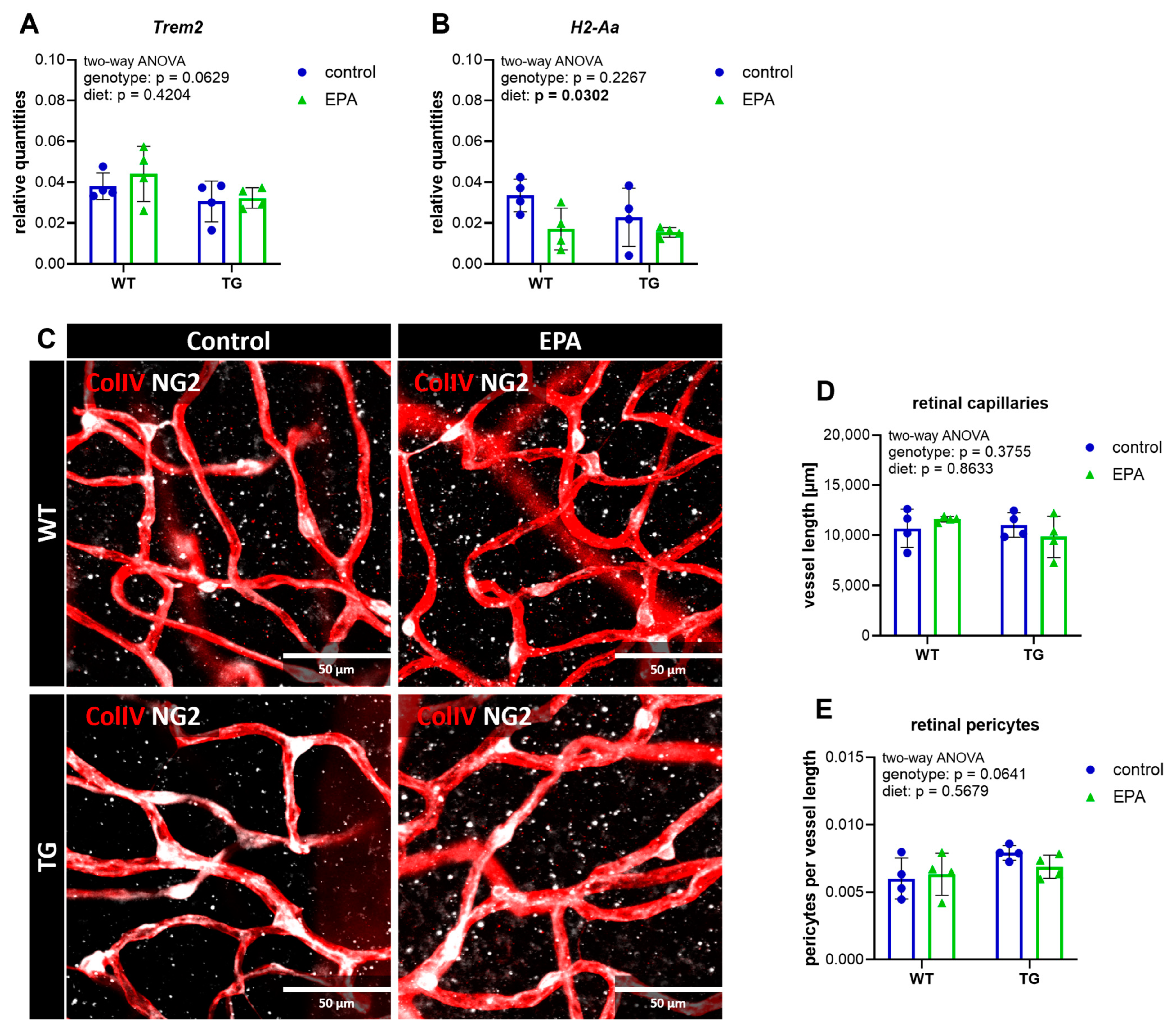
3.3. Retinal Gene Expression of the Inflammatory Marker H2-Aa Is Downregulated by EPA Supplementation
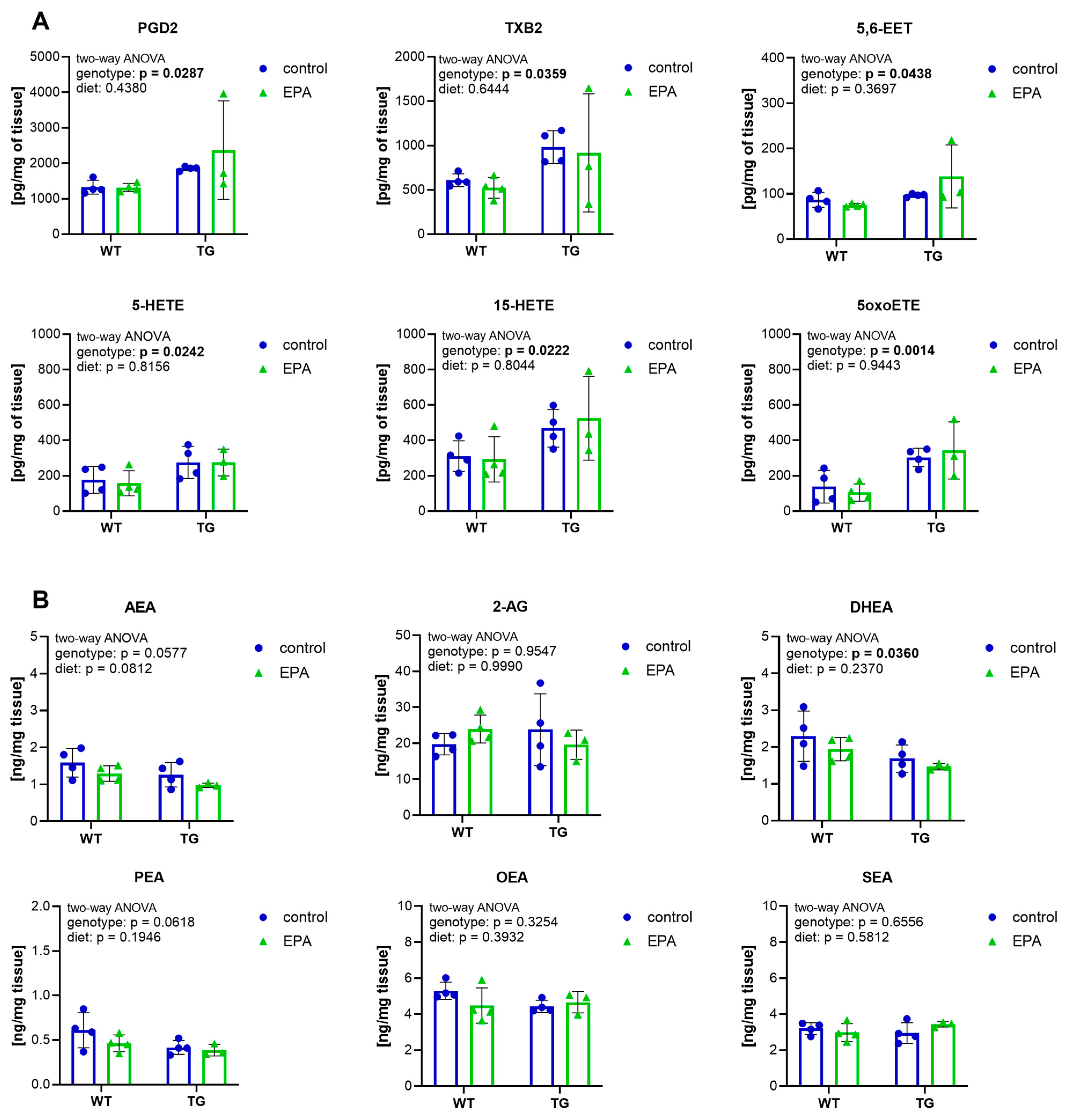
3.4. Higher Inflammatory Eicosanoids and Lower Endocannabinoids in the Hippocampus of APP/PS1 Mice
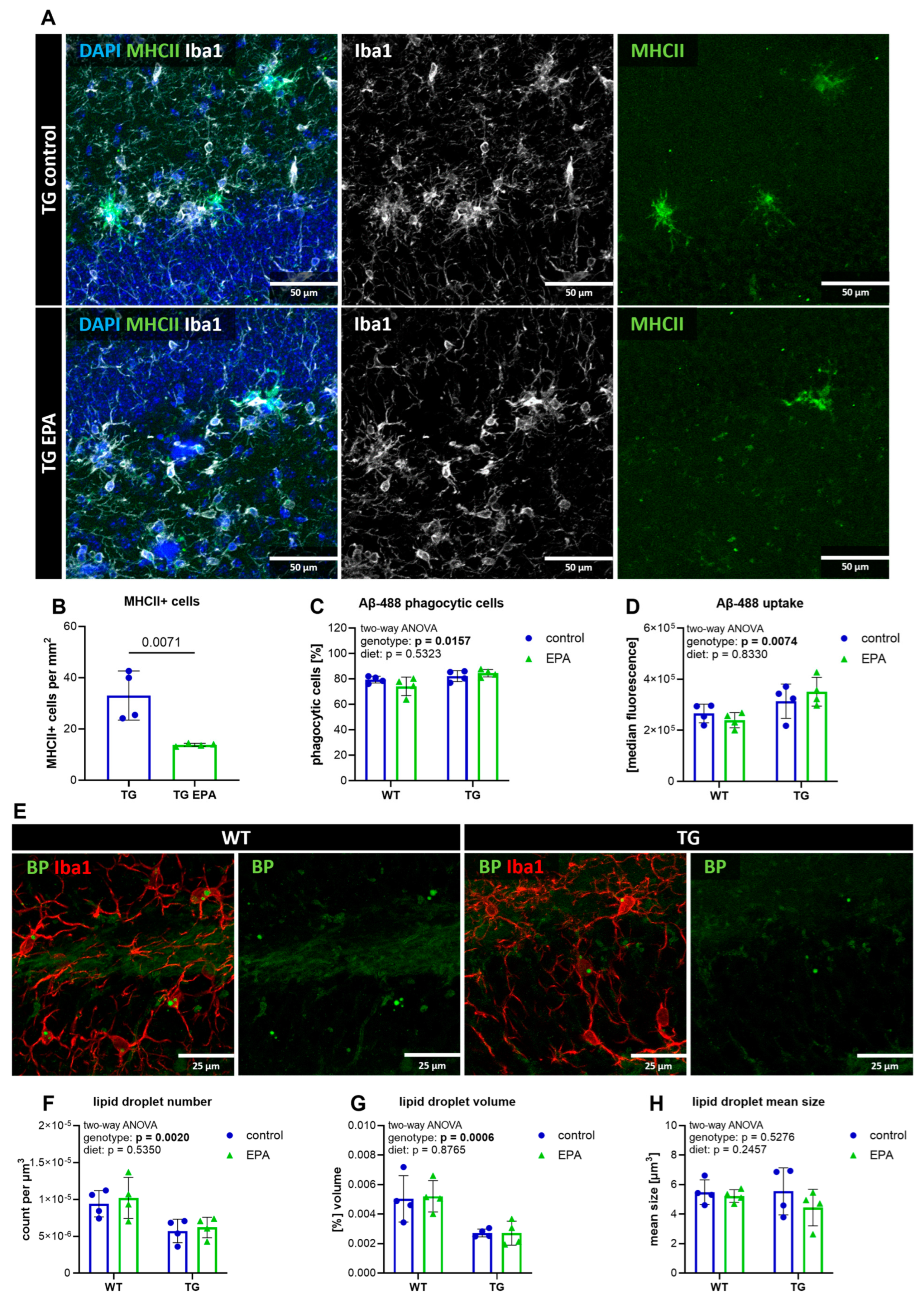
3.5. EPA Reduces Hippocampal MHCII+ Cells, but Does Not Influence Microglial Phagocytosis, Aβ-Pathology, or Lipid Droplet Formation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| AD | Alzheimer’s disease |
| AEA | N-arachidonoylethanolamine |
| ANOVA | analysis of variance |
| Aβ | amyloid-beta |
| Aβ-488 | Beta-Amyloid (1-42), HiLyte™ Fluor 488-labeled |
| BSA | bovine serum albumin |
| CD | cluster of differentiation |
| CNS | central nervous system |
| ColIV | collagen IV |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DHA | docosahexaenoic acid |
| DHEA | docosahexaenoyl ethanolamide |
| DiHETE | dihydroxy-eicosatetraenoic acid |
| EDTA | ethylenediamine tetraacetic acid |
| EET | epoxyeicosatrienoic acid |
| EPA | eicosapentaenoic acid |
| EPEA | eicosapentaenoyl ethanolamide |
| HDoHE | hydroxydocosahexaenoic acid |
| HEPE | hydroxyeicosapentaenoic acid |
| HETE | eicosatetraenoic acid |
| HODE | hydroxyoctadecadienoic acid |
| Iba1 | ionized calcium-binding adaptor molecule 1 |
| Lamp1 | lysosomal-associated membrane protein 1 |
| LPS | lipopolysaccharide |
| LSM | laser scanning microscope |
| LT | leukotriene |
| LX | lipoxin |
| MaR | maresin |
| MHCII | major histocompatibility complex class II |
| NG2 | neural/glial antigen 2 |
| OEA | oleoylethanolamide |
| OTU | operational taxonomic unit |
| oxoETE | oxoeicosatetraenoic acid |
| P | protectin |
| PBS | phosphate-buffered saline |
| PDGFRb | platelet-derived growth factor receptor beta |
| PEA | palmitoylethanolamide |
| PFA | paraformaldehyde |
| PG | prostaglandin |
| PRP | platelet rich plasma |
| PUFAs | polyunsaturated fatty acids |
| RT | room temperature |
| RT-qPCR | real-time quantitative PCR |
| Rv | resolvin |
| SEA | stearoylethanolamide |
| TG | transgenic |
| TX | thromboxane |
| WT | wild-type |
| ω-3 | omega-3 |
References
- World Alzheimer Report 2024: Global Changes in Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2024.
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Li, Z.; Noori, A.; Nguyen, H.N.; Mezlini, A.; Li, L.; Hudry, E.; Jackson, R.J.; Hyman, B.T.; Das, S. Effect of APOE Alleles on the Glial Transcriptome in Normal Aging and Alzheimer’s Disease. Nat. Aging 2021, 1, 919–931. [Google Scholar] [CrossRef]
- Ayodele, T.; Rogaeva, E.; Kurup, J.T.; Beecham, G.; Reitz, C. Early-Onset Alzheimer’s Disease: What Is Missing in Research? Curr. Neurol. Neurosci. Rep. 2021, 21, 4. [Google Scholar] [CrossRef]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer Disease. Nat. Rev. Immunol. 2024. [Google Scholar] [CrossRef]
- Lohitaksha, K.; Kumari, D.; Shukla, M.; Byagari, L.; Ashireddygari, V.R.; Tammineni, P.; Reddanna, P.; Gorla, M. Eicosanoid Signaling in Neuroinflammation Associated with Alzheimer’s Disease. Eur. J. Pharmacol. 2024, 976, 176694. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Wyss-Coray, T. Microglial Dysfunction in Brain Aging and Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 594–604. [Google Scholar] [CrossRef]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging Role of Gut Microbiota in Modulation of Neuroinflammation and Neurodegeneration with Emphasis on Alzheimer’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef]
- Cox, L.M.; Schafer, M.J.; Sohn, J.; Vincentini, J.; Weiner, H.L.; Ginsberg, S.D.; Blaser, M.J. Calorie Restriction Slows Age-Related Microbiota Changes in an Alzheimer’s Disease Model in Female Mice. Sci. Rep. 2019, 9, 17904. [Google Scholar] [CrossRef]
- Wasen, C.; Beauchamp, L.C.; Vincentini, J.; Li, S.Q.; Leserve, D.S.; Gauthier, C.; Lopes, J.R.; Moreira, T.G.; Ekwudo, M.N.; Yin, Z.R.; et al. Bacteroidota Inhibit Microglia Clearance of Amyloid-Beta and Promote Plaque Deposition in Alzheimer’s Disease Mouse Models. Nat. Commun. 2024, 15, 3872. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.M.; Xia, Y.Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K.Q. Gut Microbiota Regulate Alzheimer’s Disease Pathologies and Cognitive Disorders via PUFA-Associated Neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Poggioli, R.; Hirani, K.; Jogani, V.G.; Ricordi, C. Modulation of Inflammation and Immunity by Omega-3 Fatty Acids: A Possible Role for Prevention and to Halt Disease Progression in Autoimmune, Viral, and Age-Related Disorders. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7380–7400. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Pasker-de Jong, P.C.M.; de Vries, R.B.M.; Ritskes-Hoitinga, M. The Effects of Long-Term Omega-3 Fatty Acid Supplementation on Cognition and Alzheimer’s Pathology in Animal Models of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2012, 28, 191–209. [Google Scholar] [CrossRef]
- Burckhardt, M.; Herke, M.; Wustmann, T.; Watzke, S.; Langer, G.; Fink, A. Omega-3 Fatty Acids for the Treatment of Dementia. Cochrane Database Syst. Rev. 2016, 4, CD009002. [Google Scholar] [CrossRef]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef]
- Martí Del Moral, A.; Fortique, F. Omega-3 Fatty Acids and Cognitive Decline: A Systematic Review. Nutr. Hosp. 2019, 36, 939–949. [Google Scholar] [CrossRef]
- Cisbani, G.; Metherel, A.H.; Smith, M.E.; Bazinet, R.P. Murine and Human Microglial Cells Are Relatively Enriched with Eicosapentaenoic Acid Compared to the Whole Brain. Neurochem. Int. 2021, 150, 105154. [Google Scholar] [CrossRef]
- Chen, C.T.; Bazinet, R.P. β-Oxidation and Rapid Metabolism, but Not Uptake Regulate Brain Eicosapentaenoic Acid Levels. Prostaglandins. Leukot. Essent. Fatty Acids 2015, 92, 33–40. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-Analysis of the Effects of Eicosapentaenoic Acid (EPA) in Clinical Trials in Depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef]
- Kerman, B.E.; Self, W.; Yassine, H.N. Can the Gut Microbiome Inform the Effects of Omega-3 Fatty Acid Supplementation Trials on Cognition? Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 116–124. [Google Scholar]
- Cao, S.; Fisher, D.W.; Rodriguez, G.; Yu, T.; Dong, H. Comparisons of Neuroinflammation, Microglial Activation, and Degeneration of the Locus Coeruleus-Norepinephrine System in APP/PS1 and Aging Mice. J. Neuroinflamm. 2021, 18, 10. [Google Scholar] [CrossRef]
- Qian, X.; Hai, W.; Chen, S.; Zhang, M.; Jiang, X.; Tang, H. Multi-Omics Data Reveals Aberrant Gut Microbiota-Host Glycerophospholipid Metabolism in Association with Neuroinflammation in APP/PS1 Mice. Gut Microbes 2023, 15, 2282790. [Google Scholar] [CrossRef] [PubMed]
- Kniewallner, K.M.; de Sousa, D.M.B.; Unger, M.S.; Mrowetz, H.; Aigner, L. Platelets in Amyloidogenic Mice Are Activated and Invade the Brain. Front. Neurosci. 2020, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.S.; Li, E.; Scharnagl, L.; Poupardin, R.; Altendorfer, B.; Mrowetz, H.; Hutter-Paier, B.; Weiger, T.M.; Heneka, M.T.; Attems, J.; et al. CD8+ T-Cells Infiltrate Alzheimer’s Disease Brains and Regulate Neuronal- and Synapse-Related Gene Expression in APP-PS1 Transgenic Mice. Brain. Behav. Immun. 2020, 89, 67–86. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Benhar, I.; Schwartz, M. The Retina as a Window to the Brain—From Eye Research to CNS Disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Preishuber-Pflügl, J.; Mayr, D.; Altinger, V.; Brunner, S.M.; Koller, A.; Runge, C.; Ladek, A.-M.; Lenzhofer, M.; Rivera, F.J.; Tempfer, H.; et al. Pericyte-Derived Cells Participate in Optic Nerve Scar Formation. Front. Physiol. 2023, 14, 1151495. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.M.B.; Benedetti, A.; Altendorfer, B.; Mrowetz, H.; Unger, M.S.; Schallmoser, K.; Aigner, L.; Kniewallner, K.M. Immune-Mediated Platelet Depletion Augments Alzheimer’s Disease Neuropathological Hallmarks in APP-PS1 Mice. Aging 2023, 15, 630–649. [Google Scholar] [CrossRef]
- de Sousa, D.M.B.; Poupardin, R.; Villeda, S.A.; Schroer, A.B.; Fröhlich, T.; Frey, V.; Staffen, W.; Mrowetz, H.; Altendorfer, B.; Unger, M.S.; et al. The Platelet Transcriptome and Proteome in Alzheimer’s Disease and Aging: An Exploratory Cross-Sectional Study. Front. Mol. Biosci. 2023, 10, 1196083. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Fitzgerald, D.; Cox, D. Platelet Function: Assessment, Diagnosis, and Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 1592599176. [Google Scholar]
- Sepulveda-Rodriguez, A.; Li, P.; Khan, T.; Ma, J.D.; Carlone, C.A.; Bozzelli, P.L.; Conant, K.E.; Forcelli, P.A.; Vicini, S. Electroconvulsive Shock Enhances Responsive Motility and Purinergic Currents in Microglia in the Mouse Hippocampus. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Le Faouder, P.; Baillif, V.; Spreadbury, I.; Motta, J.-P.; Rousset, P.; Chêne, G.; Guigné, C.; Tercé, F.; Vanner, S.; Vergnolle, N.; et al. LC-MS/MS Method for Rapid and Concomitant Quantification of pro-Inflammatory and pro-Resolving Polyunsaturated Fatty Acid Metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 932, 123–133. [Google Scholar] [CrossRef]
- Koller, A.; Preishuber-Pflügl, J.; Mayr, D.; Brunner, S.M.; Ladek, A.-M.; Runge, C.; Aigner, L.; Reitsamer, H.A.; Trost, A. Cysteinyl Leukotriene Receptor 1 Modulates Retinal Immune Cells, Vascularity and Proteolytic Activity in Aged Mice. Aging 2025, 17, 308–328. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-Droplet-Accumulating Microglia Represent a Dysfunctional and Proinflammatory State in the Aging Brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of Platelet Function and Thrombosis by Omega-3 and Omega-6 Polyunsaturated Fatty Acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xia, Q.; Zheng, F.; Zhao, X.; Ge, F.; Xiao, J.; Liu, Z.; Shen, Y.; Ye, K.; Wang, D.; et al. Microglia-Mediated Neurovascular Unit Dysfunction in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, S335–S354. [Google Scholar] [CrossRef]
- Nelson, A.R.; Sweeney, M.D.; Sagare, A.P.; Zlokovic, B. V Neurovascular Dysfunction and Neurodegeneration in Dementia and Alzheimer’s Disease. Biochim. Biophys. Acta—Mol. Basis Dis. 2016, 1862, 887–900. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Fuchs, D.-T.; Sheyn, J.; Wawrowsky, K.; Lahiri, S.; Black, K.L.; Koronyo-Hamaoui, M. Retinal Capillary Degeneration and Blood-Retinal Barrier Disruption in Murine Models of Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 202. [Google Scholar] [CrossRef]
- Bettadahalli, S.; Acharya, P.; Talahalli, R. Evidence on N-3 Fatty Acids and Oleic Acid Role in Retinal Inflammation and Microvascular Integrity: Insight from a Hyperlipidemic Rat Model. Inflammation 2020, 43, 868–877. [Google Scholar] [CrossRef]
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Larion, M.; Bild, W.; Stanciu, G.D.; Solcan, C.; Bild, V. Endocannabinoid Modulation in Neurodegenerative Diseases: In Pursuit of Certainty. Biology 2022, 11, 440. [Google Scholar] [CrossRef]
- Chu, J.; Praticò, D. 5-Lipoxygenase as an Endogenous Modulator of Amyloid β Formation in Vivo. Ann. Neurol. 2011, 69, 34–46. [Google Scholar] [CrossRef]
- Piro, J.R.; Benjamin, D.I.; Duerr, J.M.; Pi, Y.; Gonzales, C.; Wood, K.M.; Schwartz, J.W.; Nomura, D.K.; Samad, T.A. A Dysregulated Endocannabinoid-Eicosanoid Network Supports Pathogenesis in a Mouse Model of Alzheimer’s Disease. Cell Rep. 2012, 1, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Herbst-Robinson, K.J.; Liu, L.; James, M.; Yao, Y.; Xie, S.X.; Brunden, K.R. Inflammatory Eicosanoids Increase Amyloid Precursor Protein Expression via Activation of Multiple Neuronal Receptors. Sci. Rep. 2015, 5, 18286. [Google Scholar] [CrossRef]
- Praticò, D.; Zhukareva, V.; Yao, Y.; Uryu, K.; Funk, C.D.; Lawson, J.A.; Trojanowski, J.Q.; Lee, V.M.-Y. 12/15-Lipoxygenase Is Increased in Alzheimer’s Disease: Possible Involvement in Brain Oxidative Stress. Am. J. Pathol. 2004, 164, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Oakes, E.G.; Vlasakov, I.; Kotler, G.; Bubes, V.; Mora, S.; Tatituri, R.; Cook, N.R.; Manson, J.E.; Costenbader, K.H. Joint Effects of One Year of Marine Omega-3 Fatty Acid Supplementation and Participant Dietary Fish Intake upon Circulating Lipid Mediators of Inflammation Resolution in a Randomized Controlled Trial. Nutrition 2024, 123, 112413. [Google Scholar] [CrossRef]
- Watanabe, S.; Doshi, M.; Hamazaki, T. N-3 Polyunsaturated Fatty Acid (PUFA) Deficiency Elevates and n-3 PUFA Enrichment Reduces Brain 2-Arachidonoylglycerol Level in Mice. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 51–59. [Google Scholar] [CrossRef]
- Wood, J.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary Docosahexaenoic Acid Supplementation Alters Select Physiological Endocannabinoid-System Metabolites in Brain and Plasma. J. Lipid Res. 2010, 51, 1416–1423. [Google Scholar] [CrossRef]
- Oddi, S.; Scipioni, L.; Totaro, A.; Giacovazzo, G.; Ciaramellano, F.; Tortolani, D.; Leuti, A.; Businaro, R.; Armeli, F.; Bilkei-Gorzo, A.; et al. Fatty-Acid Amide Hydrolase Inhibition Mitigates Alzheimer’s Disease Progression in Mouse Models of Amyloidosis. FEBS J. 2025; early view. [Google Scholar] [CrossRef]
- Jung, K.M.; Astarita, G.; Yasar, S.; Vasilevko, V.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. An Amyloid β42-Dependent Deficit in Anandamide Mobilization Is Associated with Cognitive Dysfunction in Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 1522–1532. [Google Scholar] [CrossRef]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 Is Linked to Damaging Lipid Droplets in Alzheimer’s Disease Microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef]
- Liu, L.; MacKenzie, K.R.; Putluri, N.; Maletić-Savatić, M.; Bellen, H.J. The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab. 2017, 26, 719–737.e6. [Google Scholar] [CrossRef]
- Lewandowski, C.T.; Maldonado Weng, J.; LaDu, M.J. Alzheimer’s Disease Pathology in APOE Transgenic Mouse Models: The Who, What, When, Where, Why, and How. Neurobiol. Dis. 2020, 139, 104811. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Zhao, Y.; Gong, X.K.; Liang, Z.; Yu, J.; Wang, J.Q.; Zhang, Y.H.; Wang, X.C.; Shu, X.J.; Bao, J. Intermittent Fasting Ameliorates β-Amyloid Deposition and Cognitive Impairment Accompanied by Decreased Lipid Droplet Aggregation Within Microglia in an Alzheimer’s Disease Model. Mol. Nutr. Food Res. 2025, 69, e202400660. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Paul, S.; Banerjee, S.; Banerjee, S. Lipid Droplets and Neurodegeneration. Neuroscience 2024, 549, 13–23. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, P.Y.; Deng, S.J.; Zhu, L.W.; Cao, X.; Bao, X.Y.; Xia, S.N.; Xu, Y.; Zhang, B. Pharmacological Upregulation of Microglial Lipid Droplet Alleviates Neuroinflammation and Acute Ischemic Brain Injury. Inflammation 2023, 46, 1832–1848. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Seo, D.; Holtzman, D.M. Current Understanding of the Alzheimer’s Disease-Associated Microbiome and Therapeutic Strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef]
- Jemimah, S.; Chabib, C.M.M.; Hadjileontiadis, L.; AlShehhi, A. Gut Microbiome Dysbiosis in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0285346. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Chang, C.-C.; Huang, C.-W.; Nouchi, R.; Cheng, C.-H. Gut Microbiota in Patients with Alzheimer’s Disease Spectrum: A Systematic Review and Meta-Analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, X.; Zhu, Y.; Shi, W.; Kong, L. Gut Microbiome Characteristics in Subjective Cognitive Decline, Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Eur. J. Neurol. 2023, 30, 3568–3580. [Google Scholar] [CrossRef]
- Killingsworth, J.; Sawmiller, D.; Shytle, R.D. Propionate and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 12, 580001. [Google Scholar] [CrossRef]
- Komodromou, I.; Andreou, E.; Vlahoyiannis, A.; Christofidou, M.; Felekkis, K.; Pieri, M.; Giannaki, C.D. Exploring the Dynamic Relationship between the Gut Microbiome and Body Composition across the Human Lifespan: A Systematic Review. Nutrients 2024, 16, 660. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Tai, W.-C.; Liang, C.-M.; Wu, C.-K.; Tsai, M.-C.; Hu, W.-H.; Huang, P.-Y.; Chen, C.-H.; Kuo, Y.-H.; Yao, C.-C.; et al. Alternations of the Gut Microbiota and the Firmicutes/Bacteroidetes Ratio after Biologic Treatment in Inflammatory Bowel Disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, L.; Chen, S.; Zhou, H.; Fan, Y.; Lin, L.; Li, J.; Xu, J.; Chen, Y.; Ma, Y.; et al. Gut Microbiome Alterations Precede Cerebral Amyloidosis and Microglial Pathology in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2020, 2020, 8456596. [Google Scholar] [CrossRef]
- La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Iorio, C.D.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A Randomised Trial of the Effect of Omega-3 Polyunsaturated Fatty Acid Supplements on the Human Intestinal Microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016, 3089303. [Google Scholar] [CrossRef]
- DiCarlo, G.; Wilcock, D.; Henderson, D.; Gordon, M.; Morgan, D. Intrahippocampal LPS Injections Reduce Aβ Load in APP+PS1 Transgenic Mice. Neurobiol. Aging 2001, 22, 1007–1012. [Google Scholar] [CrossRef]
- Hopperton, K.; Mohammad, D.; Trépanier, M.; Giuliano, V.; Bazinet, R.P. Markers of Microglia in Post-Mortem Brain Samples from Patients with Alzheimer’s Disease: A Systematic Review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Lue, L.-F.; Brachova, L.; Civin, W.H.; Rogers, J. Inflammation, Aβ Deposition, and Neurofibrillary Tangle Formation as Correlates of Alzheimer’s Disease Neurodegeneration. J. Neuropathol. Exp. Neurol. 1996, 55, 1083–1088. [Google Scholar] [CrossRef]
- Lu, J.; Wu, K.X.; Sha, X.D.; Lin, J.Y.; Chen, H.Z.; Yu, Z.H. TRPV1 Alleviates APOE4-Dependent Microglial Antigen Presentation and T Cell Infiltration in Alzheimer’s Disease. Transl. Neurodegener. 2024, 13, 52. [Google Scholar] [CrossRef]
- Jantzen, P.T.; Connor, K.E.; DiCarlo, G.; Wenk, G.L.; Wallace, J.L.; Rojiani, A.M.; Coppola, D.; Morgan, D.; Gordon, M.N. Microglial Activation and β-Amyloid Deposit Reduction Caused by a Nitric Oxide-Releasing Nonsteroidal Anti-Inflammatory Drug in Amyloid Precursor Protein plus Presenilin-1 Transgenic Mice. J. Neurosci. 2002, 22, 2246–2254. [Google Scholar] [CrossRef]
- Lau, S.F.; Chen, C.P.; Fu, W.Y.; Qu, J.N.Y.; Cheung, T.H.; Fu, A.K.Y.; Ip, N.Y. IL-33-PU.1 Transcriptome Reprogramming Drives Functional State Transition and Clearance Activity of Microglia in Alzheimer’s Disease. Cell Rep. 2020, 31, 107530. [Google Scholar] [CrossRef] [PubMed]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tanila, H.; Puoliväli, J.; Kadish, I.; van Groen, T. Gender Differences in the Amount and Deposition of Amyloidbeta in APPswe and PS1 Double Transgenic Mice. Neurobiol. Dis. 2003, 14, 318–327. [Google Scholar] [CrossRef] [PubMed]
| Group (n = 4) | Weight at Start [g] | Weight at End [g] | Mean Food Consumption/Day [g] | Increase in Weight [g] | Increase in Weight [%] |
|---|---|---|---|---|---|
| WT | 26.4 (1.1) | 29.4 (2.5) | 4.9 (0.8) | 3.1 (1.5) | 11.4 (5.5) |
| WT + EPA | 26.9 (4.9) | 30.2 (6.5) | 4.4 (0.5) | 3.3 (1.7) | 11.7 (5.1) |
| TG | 25.8 (0.4) | 27.4 (1.0) | 4.1 (0.4) | 1.6 (0.9) | 6.2 (3.6) |
| TG + EPA | 25.1 (2.4) | 26.3 (2.5) | 4.3 (0.5) | 1.2 (0.2) | 5.0 (0.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altendorfer, B.; Benedetti, A.; Mrowetz, H.; Bernegger, S.; Bretl, A.; Preishuber-Pflügl, J.; Bessa de Sousa, D.M.; Ladek, A.M.; Koller, A.; Le Faouder, P.; et al. Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer’s Mice: A Pilot Experimental Study. Nutrients 2025, 17, 1108. https://doi.org/10.3390/nu17071108
Altendorfer B, Benedetti A, Mrowetz H, Bernegger S, Bretl A, Preishuber-Pflügl J, Bessa de Sousa DM, Ladek AM, Koller A, Le Faouder P, et al. Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer’s Mice: A Pilot Experimental Study. Nutrients. 2025; 17(7):1108. https://doi.org/10.3390/nu17071108
Chicago/Turabian StyleAltendorfer, Barbara, Ariane Benedetti, Heike Mrowetz, Sabine Bernegger, Alina Bretl, Julia Preishuber-Pflügl, Diana Marisa Bessa de Sousa, Anja Maria Ladek, Andreas Koller, Pauline Le Faouder, and et al. 2025. "Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer’s Mice: A Pilot Experimental Study" Nutrients 17, no. 7: 1108. https://doi.org/10.3390/nu17071108
APA StyleAltendorfer, B., Benedetti, A., Mrowetz, H., Bernegger, S., Bretl, A., Preishuber-Pflügl, J., Bessa de Sousa, D. M., Ladek, A. M., Koller, A., Le Faouder, P., Bertrand-Michel, J., Trost, A., & Aigner, L. (2025). Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer’s Mice: A Pilot Experimental Study. Nutrients, 17(7), 1108. https://doi.org/10.3390/nu17071108






