The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders
Abstract
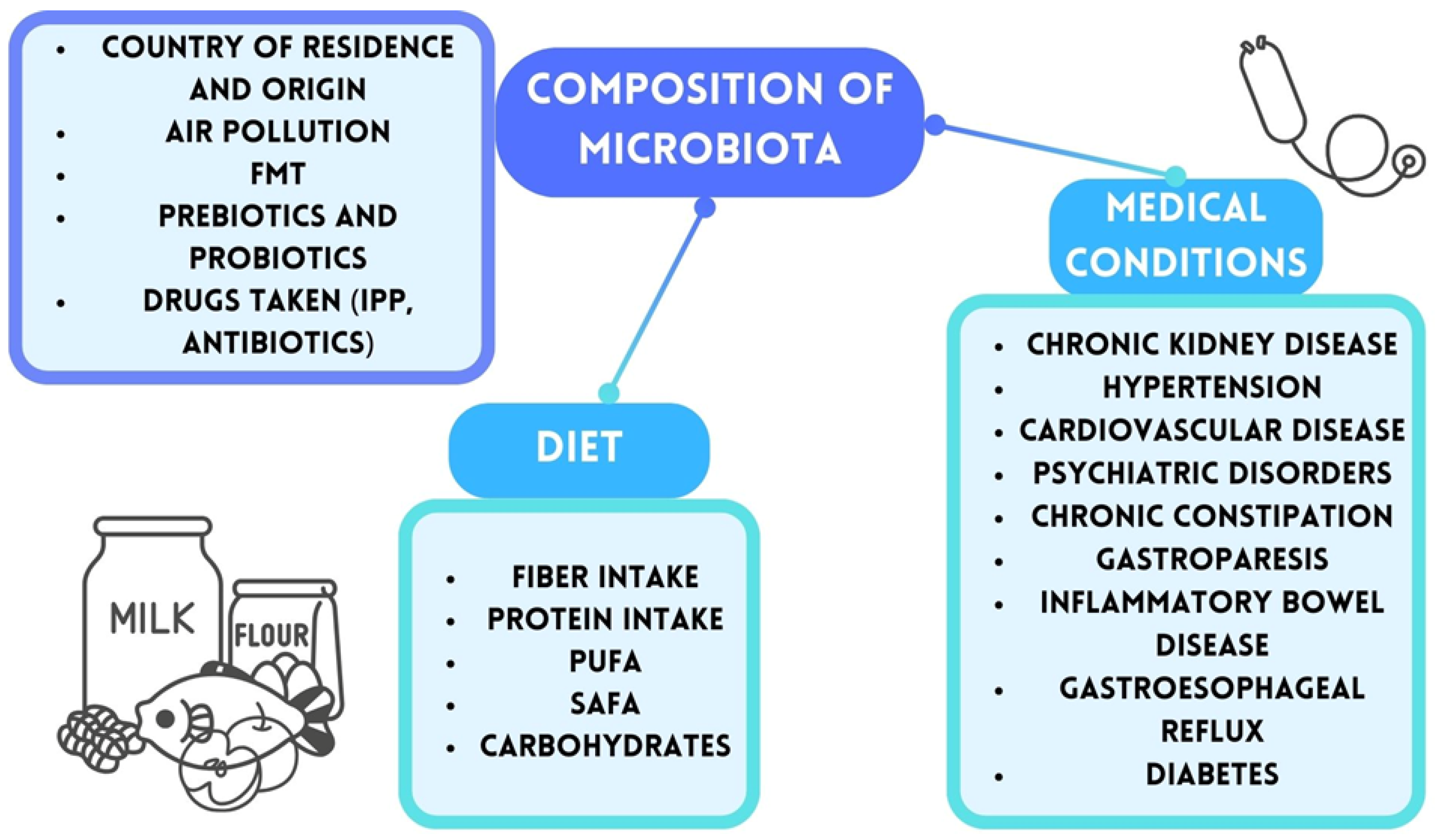
:1. Introduction
2. Gut Microbiota and Gut–Brain Axis
2.1. Gut–Brain Axis and Bacterial Metabolites
2.2. Neurotransmitters
3. The Association Between Gut Microbiota and the Development and Progression of Autism Spectrum Disorders (ASD)
3.1. The Basic Elements of Gut Microbiota and the Characteristics of Their Metabolic Products
3.2. A Comparative Analysis of the Gut Microbiota in Patiens Diagnosed withASD and a Healthy Population
4. Microbiota-Targeted Therapies in Autism Spectrum Disorders
4.1. Dietary Interventions
4.2. Probiotic Interventions
4.3. Exploring Fecal Microbiota Transplantation as a Novel Therapy for Autism
5. Challenges and Future Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, M.-C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Wiggins, L.D.; Rice, C.E.; Barger, B.; Soke, G.N.; Lee, L.-C.; Moody, E.; Edmondson-Pretzel, R.; Levy, S.E. DSM-5 Criteria for Autism Spectrum Disorder Maximizes Diagnostic Sensitivity and Specificity in Preschool Children. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 693–701. [Google Scholar] [CrossRef]
- Cakir, J.; Frye, R.E.; Walker, S.J. The Lifetime Social Cost of Autism: 1990–2029. Res. Autism Spectr. Disord. 2020, 72, 101502. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Chaste, P.; Leboyer, M. Autism Risk Factors: Genes, Environment, and Gene-Environment Interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [CrossRef]
- Carpita, B.; Marazziti, D.; Palego, L.; Giannaccini, G.; Betti, L.; Dell’Osso, L. Microbiota, Immune System and Autism Spectrum Disorders: An Integrative Model towards Novel Treatment Options. Curr. Med. Chem. 2020, 27, 5119–5136. [Google Scholar] [CrossRef]
- Lungba, R.M.; Khan, S.Z.A.; Ajibawo-Aganbi, U.; Perez Bastidas, M.V.; Veliginti, S.; Saleem, S.; Cancarevic, I. The Role of the Gut Microbiota and the Immune System in the Development of Autism. Cureus 2020, 12, e11226. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Chawner, S.J.R.A.; Doherty, J.L.; Anney, R.J.L.; Antshel, K.M.; Bearden, C.E.; Bernier, R.; Chung, W.K.; Clements, C.C.; Curran, S.R.; Cuturilo, G.; et al. A Genetics-First Approach to Dissecting the Heterogeneity of Autism: Phenotypic Comparison of Autism Risk Copy Number Variants. Am. J. Psychiatry 2021, 178, 77–86. [Google Scholar] [CrossRef]
- Warrier, V.; Zhang, X.; Reed, P.; Havdahl, A.; Moore, T.M.; Cliquet, F.; Leblond, C.S.; Rolland, T.; Rosengren, A.; Rowitch, D.H.; et al. Genetic Correlates of Phenotypic Heterogeneity in Autism. Nat. Genet. 2022, 54, 1293–1304. [Google Scholar] [CrossRef]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental Risk Factors for Autism: An Evidence-Based Review of Systematic Reviews and Meta-Analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Khachadourian, V.; Mahjani, B.; Sandin, S.; Kolevzon, A.; Buxbaum, J.D.; Reichenberg, A.; Janecka, M. Comorbidities in Autism Spectrum Disorder and Their Etiologies. Transl. Psychiatry 2023, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism—Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Indika, N.-L.R.; Deutz, N.E.P.; Engelen, M.P.K.J.; Peiris, H.; Wijetunge, S.; Perera, R. Sulfur Amino Acid Metabolism and Related Metabotypes of Autism Spectrum Disorder: A Review of Biochemical Evidence for a Hypothesis. Biochimie 2021, 184, 143–157. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota Revolution: How Gut Microbes Regulate Our Lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From Fiction to Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Ballini, A.; Boccellino, M.; Scacco, S.; Lovero, R.; Charitos, I.A.; Santacroce, L. The Intestinal Microbiota May Be a Potential Theranostic Tool for Personalized Medicine. J. Pers. Med. 2022, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Młynarska, E.; Budny, E.; Saar, M.; Wojtanowska, E.; Jankowska, J.; Marciszuk, S.; Mazur, M.; Rysz, J.; Franczyk, B. Does the Composition of Gut Microbiota Affect Chronic Kidney Disease? Molecular Mechanisms Contributed to Decreasing Glomerular Filtration Rate. Int. J. Mol. Sci. 2024, 25, 10429. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Yang, Y.; Han, B.; Wang, Q.; Du, S. Investigating the Causal Relationship of Gut Microbiota with GERD and BE: A Bidirectional Mendelian Randomization. BMC Genom. 2024, 25, 471. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Ke, H. Investigating the Causal Relationship Between Gut Microbiota and Crohn’s Disease: A Mendelian Randomization Study. Gastroenterology 2024, 166, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The Gut Microbiota and the Brain–Gut–Kidney Axis in Hypertension and Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; Harun-Or-Rashid, M.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut Microbiota in the Pathogenesis and Therapeutic Approaches of Diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Rodriguez, J. Nutrition and Microbiome. In From Obesity to Diabetes; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2022; Volume 274, pp. 57–73. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Grosso, G. Nutritional Psychiatry: How Diet Affects Brain through Gut Microbiota. Nutrients 2021, 13, 1282. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Charitos, I.A.; Inchingolo, A.M.; Ferrante, L.; Inchingolo, F.; Inchingolo, A.D.; Castellaneta, F.; Cotoia, A.; Palermo, A.; Scacco, S.; Dipalma, G. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients 2024, 16, 4404. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. Intestinal Permeability Defects: Is It Time to Treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, J.; Gawlik-Kotelnicka, O. Intestinal Permeability and Its Significance in Psychiatric Disorders—A Narrative Review and Future Perspectives. Behav. Brain Res. 2023, 448, 114459. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary Fiber and SCFAs in the Regulation of Mucosal Immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.F.; Miao, J.; Zheng, R.F.; Li, J.Y. Short-Chain Fatty Acids: Important Components of the Gut-Brain Axis against AD. Biomed. Pharmacother. 2024, 175, 116601. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective Unconscious: How Gut Microbes Shape Human Behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Gao, Z.; Liu, X.; Rong, Z.; Jia, J.; Kang, K.; Guo, W.; Li, J. Intravenous Administration of Sodium Propionate Induces Antidepressant or Prodepressant Effect in a Dose Dependent Manner. Sci. Rep. 2020, 10, 19917. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the Gut Microbiome and Brain Function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Osman, A.; Mervosh, N.L.; Strat, A.N.; Euston, T.J.; Zipursky, G.; Pollak, R.M.; Meckel, K.R.; Tyler, S.R.; Chan, K.L.; Buxbaum Grice, A.; et al. Acetate Supplementation Rescues Social Deficits and Alters Transcriptional Regulation in Prefrontal Cortex of Shank3 Deficient Mice. Brain Behav. Immun. 2023, 114, 311–324. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-Cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary Fructose Feeds Hepatic Lipogenesis via Microbiota-Derived Acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-Inflammatory Properties of the Short-Chain Fatty Acids Acetate and Propionate: A Study with Relevance to Inflammatory Bowel Disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Boini, K.M.; Hussain, T.; Li, P.-L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell. Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Xia, J. Stroke and Vascular Cognitive Impairment: The Role of Intestinal Microbiota Metabolite TMAO. CNS Neurol. Disord. Drug Targets 2024, 23, 102–121. [Google Scholar] [CrossRef]
- Ge, P.; Duan, H.; Tao, C.; Niu, S.; Hu, Y.; Duan, R.; Shen, A.; Sun, Y.; Sun, W. TMAO Promotes NLRP3 Inflammasome Activation of Microglia Aggravating Neurological Injury in Ischemic Stroke Through FTO/IGF2BP2. J. Inflamm. Res. 2023, 16, 3699–3714. [Google Scholar] [CrossRef]
- Yaqub, A.; Vojinovic, D.; Vernooij, M.W.; Slagboom, P.E.; Ghanbari, M.; Beekman, M.; van der Grond, J.; Hankemeier, T.; van Duijn, C.M.; Ikram, M.A.; et al. Plasma Trimethylamine N-Oxide (TMAO): Associations with Cognition, Neuroimaging, and Dementia. Alzheimer’s Res. Ther. 2024, 16, 113. [Google Scholar] [CrossRef]
- Açıkel, S.B.; Kara, A.; Bağcı, Z.; Can, Ü. Serum Trimethylamine N-Oxide and Lipopolysaccharide Binding Protein Levels among Children Diagnosed with Autism Spectrum Disorder. Int. J. Dev. Neurosci. 2023, 83, 571–577. [Google Scholar] [CrossRef]
- Quan, L.; Yi, J.; Zhao, Y.; Zhang, F.; Shi, X.-T.; Feng, Z.; Miller, H.L. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated with Autism Spectrum Disorders. Neurotoxicology 2020, 76, 93–98. [Google Scholar] [CrossRef]
- Wang, T.; Chen, B.; Luo, M.; Xie, L.; Lu, M.; Lu, X.; Zhang, S.; Wei, L.; Zhou, X.; Yao, B.; et al. Microbiota-Indole 3-Propionic Acid-Brain Axis Mediates Abnormal Synaptic Pruning of Hippocampal Microglia and Susceptibility to ASD in IUGR Offspring. Microbiome 2023, 11, 245. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. From “Leaky Gut” to Impaired Glia-Neuron Communication in Depression. In Major Depressive Disorder: Rethinking and Understanding Recent Discoveries; Kim, Y.-K., Ed.; Springer: Singapore, 2021; pp. 129–155. ISBN 978-981-336-044-0. [Google Scholar]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine Pathway Metabolism and the Microbiota-Gut-Brain Axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan Metabolism: Mechanism-Oriented Therapy for Neurological and Psychiatric Disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J.; Greene, C.; Campbell, M. Blood-Brain Barrier Regulation in Psychiatric Disorders. Neurosci. Lett. 2020, 726, 133664. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Barriers to Neurotoxic Inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, Q.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Layé, S. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016, 2016, 3597209. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Cecil, J.D.; O’Brien-Simpson, N.M.; Lenzo, J.C.; Holden, J.A.; Singleton, W.; Perez-Gonzalez, A.; Mansell, A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef]
- Xie, J.; Cools, L.; Van Imschoot, G.; Van Wonterghem, E.; Pauwels, M.J.; Vlaeminck, I.; De Witte, C.; El Andaloussi, S.; Wierda, K.; De Groef, L.; et al. Helicobacter Pylori-Derived Outer Membrane Vesicles Contribute to Alzheimer’s Disease Pathogenesis via C3-C3aR Signalling. J. Extracell. Vesicles 2023, 12, e12306. [Google Scholar] [CrossRef]
- Arentsen, T.; Qian, Y.; Gkotzis, S.; Femenia, T.; Wang, T.; Udekwu, K.; Forssberg, H.; Diaz Heijtz, R. The Bacterial Peptidoglycan-Sensing Molecule Pglyrp2 Modulates Brain Development and Behavior. Mol. Psychiatry 2017, 22, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santana, A.; Diaz Heijtz, R. Bacterial Peptidoglycans from Microbiota in Neurodevelopment and Behavior. Trends Mol. Med. 2020, 26, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, Z.; Song, S.; Zhang, H.; Zhang, W.; Liu, L.; Chen, Y.; Sun, J. Gut Microbiota Modulates Stress-Induced Hypertension through the HPA Axis. Brain Res. Bull. 2020, 162, 49–58. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA Axis Dysregulation in Severe Mental Illness: Can We Shift the Blame to Gut Microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- Tomaszek, N.; Urbaniak, A.D.; Bałdyga, D.; Chwesiuk, K.; Modzelewski, S.; Waszkiewicz, N. Unraveling the Connections: Eating Issues, Microbiome, and Gastrointestinal Symptoms in Autism Spectrum Disorder. Nutrients 2025, 17, 486. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal Barrier Dysfunction and Microbiota–Gut–Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef]
- Suprunowicz, M.; Tomaszek, N.; Urbaniak, A.; Zackiewicz, K.; Modzelewski, S.; Waszkiewicz, N. Between Dysbiosis, Maternal Immune Activation and Autism: Is There a Common Pathway? Nutrients 2024, 16, 549. [Google Scholar] [CrossRef]
- De Sales-Millán, A.; Aguirre-Garrido, J.F.; González-Cervantes, R.M.; Velázquez-Aragón, J.A. Microbiome-Gut-Mucosal-Immune-Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav. Sci. 2023, 13, 548. [Google Scholar] [CrossRef]
- Caputi, V.; Hill, L.; Figueiredo, M.; Popov, J.; Hartung, E.; Margolis, K.G.; Baskaran, K.; Joharapurkar, P.; Moshkovich, M.; Pai, N. Functional Contribution of the Intestinal Microbiome in Autism Spectrum Disorder, Attention Deficit Hyperactivity Disorder, and Rett Syndrome: A Systematic Review of Pediatric and Adult Studies. Front. Neurosci. 2024, 18, 1341656. [Google Scholar] [CrossRef]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef] [PubMed]
- Poupard, L.; Page, G.; Thoreau, V.; Kaouah, Z. Relationships between Gut Microbiota and Autism Spectrum Disorders: Development and Treatment. Clin. Psychopharmacol. Neurosci. 2024, 22, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Zarimeidani, F.; Rahmati, R.; Mostafavi, M.; Darvishi, M.; Khodadadi, S.; Mohammadi, M.; Shamlou, F.; Bakhtiyari, S.; Alipourfard, I. Gut Microbiota and Autism Spectrum Disorder: A Neuroinflammatory Mediated Mechanism of Pathogenesis? Inflammation 2024. [Google Scholar] [CrossRef]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism Spectrum Disorders and Intestinal Microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef]
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of Regressive Autism. Anaerobe 2012, 18, 260–262. [Google Scholar] [CrossRef]
- Finegold, S.M. Desulfovibrio Species Are Potentially Important in Regressive Autism. Med. Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing Study of Fecal Microflora of Autistic and Control Children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of Novel PCR-Based Methods for Detection, Quantitation, and Phylogenetic Characterization of Sutterella Species in Intestinal Biopsy Samples from Children with Autism and Gastrointestinal Disturbances. mBio 2012, 3, e00261-11. [Google Scholar] [CrossRef]
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; De Caro, C.; Comegna, M.; et al. Gut Microbiota Features in Young Children with Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Kang, D.-W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered Composition and Function of Intestinal Microbiota in Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2019, 9, 43. [Google Scholar] [CrossRef]
- Wang, M.; Wan, J.; Rong, H.; He, F.; Wang, H.; Zhou, J.; Cai, C.; Wang, Y.; Xu, R.; Yin, Z.; et al. Alterations in Gut Glutamate Metabolism Associated with Changes in Gut Microbiota Composition in Children with Autism Spectrum Disorder. mSystems 2019, 4, e00321-18. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal Involvement of Autism Spectrum Disorder: Focus on Gut Microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut Microbiota in Autism and Mood Disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Korteniemi, J.; Karlsson, L.; Aatsinki, A. Systematic Review: Autism Spectrum Disorder and the Gut Microbiota. Acta Psychiatr. Scand. 2023, 148, 242–254. [Google Scholar] [CrossRef]
- Kushak, R.I.; Winter, H.S.; Buie, T.M.; Cox, S.B.; Phillips, C.D.; Ward, N.L. Analysis of the Duodenal Microbiome in Autistic Individuals: Association with Carbohydrate Digestion. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e110–e116. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal Microflora Studies in Late-Onset Autism. Clin. Infect. Dis. 2002, 35, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Soltysova, M.; Tomova, A.; Ostatnikova, D. Gut Microbiota Profiles in Children and Adolescents with Psychiatric Disorders. Microorganisms 2022, 10, 2009. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Liu, F.; Dai, N.; Liang, R.; Lv, S.; Bao, L. Gut Microbiota and Autism Spectrum Disorders: A Bidirectional Mendelian Randomization Study. Front. Cell. Infect. Microbiol. 2023, 13, 1267721. [Google Scholar] [CrossRef]
- Pérez-Cabral, I.D.; Bernal-Mercado, A.T.; Islas-Rubio, A.R.; Suárez-Jiménez, G.M.; Robles-García, M.Á.; Puebla-Duarte, A.L.; Del-Toro-Sánchez, C.L. Exploring Dietary Interventions in Autism Spectrum Disorder. Foods 2024, 13, 3010. [Google Scholar] [CrossRef]
- Baspinar, B.; Hulya Yardimci, H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J. Med. 2020, 52, 292–297. [Google Scholar] [CrossRef]
- Zafirovski, K.; Aleksoska, M.T.; Thomas, J.; Hanna, F. Impact of Gluten-Free and Casein-Free Diet on Behavioural Outcomes and Quality of Life of Autistic Children and Adolescents: A Scoping Review. Children 2024, 11, 862. [Google Scholar] [CrossRef]
- Quan, L.; Xu, X.; Cui, Y.; Han, H.; Hendren, R.L.; Zhao, L.; You, X. A Systematic Review and Meta-Analysis of the Benefits of a Gluten-Free Diet and/or Casein-Free Diet for Children with Autism Spectrum Disorder. Nutr. Rev. 2022, 80, 1237–1246. [Google Scholar] [CrossRef]
- Olivito, I.; Avolio, E.; Minervini, D.; Soda, T.; Rocca, C.; Angelone, T.; Iaquinta, F.S.; Bellizzi, D.; De Rango, F.; Bruno, R.; et al. Ketogenic Diet Ameliorates Autism Spectrum Disorders-like Behaviors via Reduced Inflammatory Factors and Microbiota Remodeling in BTBR T+ Itpr3tf/J Mice. Exp. Neurol. 2023, 366, 114432. [Google Scholar] [CrossRef]
- Li, Q.; Liang, J.; Fu, N.; Han, Y.; Qin, J. A Ketogenic Diet and the Treatment of Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 650624. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Du, Y.; Shi, S.; Cheng, Y. Antioxidant Interventions in Autism Spectrum Disorders: A Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 113, 110476. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Gibson, E.L.; Nielsen, D.S.; Costabile, A. A Prebiotic Intervention Study in Children with Autism Spectrum Disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, S.; Zhang, Y.; Li, E. A Review of Probiotics in the Treatment of Autism Spectrum Disorders: Perspectives from the Gut–Brain Axis. Front. Microbiol. 2023, 14, 1123462. [Google Scholar] [CrossRef]
- Meguid, N.A.; Mawgoud, Y.I.A.; Bjørklund, G.; Mehanne, N.S.; Anwar, M.; Effat, B.A.E.-K.; Chirumbolo, S.; Elrahman, M.M.A. Molecular Characterization of Probiotics and Their Influence on Children with Autism Spectrum Disorder. Mol. Neurobiol. 2022, 59, 6896–6902. [Google Scholar] [CrossRef]
- Kong, X.-J.; Liu, J.; Liu, K.; Koh, M.; Sherman, H.; Liu, S.; Tian, R.; Sukijthamapan, P.; Wang, J.; Fong, M.; et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 1552. [Google Scholar] [CrossRef]
- Billeci, L.; Callara, A.L.; Guiducci, L.; Prosperi, M.; Morales, M.A.; Calderoni, S.; Muratori, F.; Santocchi, E. A Randomized Controlled Trial into the Effects of Probiotics on Electroencephalography in Preschoolers with Autism. Autism 2023, 27, 117–132. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.-T.s.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef]
- Njotto, L.L.; Simin, J.; Fornes, R.; Odsbu, I.; Mussche, I.; Callens, S.; Engstrand, L.; Bruyndonckx, R.; Brusselaers, N. Maternal and Early-Life Exposure to Antibiotics and the Risk of Autism and Attention-Deficit Hyperactivity Disorder in Childhood: A Swedish Population-Based Cohort Study. Drug Saf. 2023, 46, 467–478. [Google Scholar] [CrossRef]
- Yang, K.-L.; Yen, T.-A.; Lin, F.-J.; Hsu, C.-N.; Wang, C.-C. Antibiotic Use and Risk of Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder: A Population-Based Cohort Study. Child Adolesc. Psychiatry Ment. Health 2024, 18, 82. [Google Scholar] [CrossRef]
- Łukasik, J.; Patro-Gołąb, B.; Horvath, A.; Baron, R.; Szajewska, H.; on behalf of the SAWANTI Working Group. Early Life Exposure to Antibiotics and Autism Spectrum Disorders: A Systematic Review. J. Autism Dev. Disord. 2019, 49, 3866–3876. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Masirah M Zain, N.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef] [PubMed]
- Dossaji, Z.; Khattak, A.; Tun, K.M.; Hsu, M.; Batra, K.; Hong, A.S. Efficacy of Fecal Microbiota Transplant on Behavioral and Gastrointestinal Symptoms in Pediatric Autism: A Systematic Review. Microorganisms 2023, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.d.l.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Anaclerio, F.; Minelli, M.; Antonucci, I.; Gatta, V.; Stuppia, L. Microbiota and Autism: A Review on Oral and Gut Microbiome Analysis Through 16S rRNA Sequencing. Biomedicines 2024, 12, 2686. [Google Scholar] [CrossRef]
- Agarwala, S.; Naik, B.; Ramachandra, N.B. Mucosa-Associated Specific Bacterial Species Disrupt the Intestinal Epithelial Barrier in the Autism Phenome. Brain Behav. Immun. Health 2021, 15, 100269. [Google Scholar] [CrossRef]
- Wang, X.; Hu, R.; Lin, F.; Yang, T.; Lu, Y.; Sun, Z.; Li, T.; Chen, J. Lactobacillus reuteri or Lactobacillus rhamnosus GG Intervention Facilitates Gut Barrier Function, Decreases Corticosterone and Ameliorates Social Behavior in LPS-Exposed Offspring. Food Res. Int. 2024, 197, 115212. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, M.; Santocchi, E.; Guiducci, L.; Frinzi, J.; Morales, M.A.; Tancredi, R.; Muratori, F.; Calderoni, S. Interventions on Microbiota: Where Do We Stand on a Gut–Brain Link in Autism? A Systematic Review. Nutrients 2022, 14, 462. [Google Scholar] [CrossRef] [PubMed]
| Phylum | Class | Order | Family | Genus | Gram Classification | Presence in ASD |
|---|---|---|---|---|---|---|
| Actinomycetota | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Collinsella | Positive | Higher [18] |
| Actinomycetota | Coriobacteriia | Eggerthellales | Eggerthellaceae | Eggerthella | Positive | Higher [104] |
| Bacillota | Clostridia | Clostridiales | Clostridiaceae | Caloramator | Positive | Higher [8,50,96,97,98] |
| Bacillota | Clostridia | Clostridiales | Clostridiaceae | Sarcina | Positive | Higher [8,50,96,97,98] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | - | Positive | Higher [105] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Dorea | Positive | Higher [18,52] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Anaerofilum | Positive | Higher [52] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Faecalibacterium | Positive | Higher [52,89,101,107,109,113] |
| Bacillota | Clostridia | Eubacteriales | Peptostreptococcaceae | Peptostreptococcus | Positive | Higher [112] |
| Bacteroidota | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | Negative | Higher [101,103,108,109,113] |
| Bacteroidota | Bacteroidia | Bacteroidales | Barnesiellaceae | Barnesiella | Negative | Higher [52] |
| Bacteroidota | Bacteroidia | Bacteroidales | Odoribacteraceae | Odirobacter | Negative | Higher [52] |
| Bacteroidota | Bacteroidia | Bacteroidales | Porphyromonadaceae | Porphyromonas | Negative | Higher [52] |
| Bacteroidota | Bacteroidia | Bacteroidales | Rikenellaceae | Alistipes | Negative | Higher [50,89,96,97,98] |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | Positive | Higher [8,18,50,96,97,98,102,103,106,109,110,111] |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | - | Positive | Higher [8,101] |
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Clostridium | Positive | Higher [8,50,52,96,97,98,99,100,101,102,103,104,105,106,107,108,109] |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | Negative | Higher [50,96,97,98,103,106,107,109] |
| Pseudomonadota | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | - | Negative | Higher [8,50,52,96,97,98,101,111] |
| Pseudomonadota | Gammaproteobacteria | Pasteurellales | Pasteurellaceae | - | Negative | Higher [8,101] |
| Pseudomonadota | Gammaproteobacteria | Pasteurellales | Pasteurellaceae | Haemophilus | Negative | Higher [102] |
| Pseudomonadota | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Klebsiella | Negative | Higher [104] |
| Pseudomonadota | Betaproteobacteria | Burkholderiales | Sutterellaceae | Parasutterella | Negative | Higher [52] |
| Pseudomonadota | Gammaproteobacteria | Aeromonadales | Aeromonadaceae | Aeromonas | Negative | Higher [52] |
| Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | Negative | Higher [52] |
| Pseudomonadota | Betaproteobacteria | Burkholderiales | Alcaligenaceae | - | Negative | Higher [52,110] |
| Pseudomonadota | Betaproteobacteria | Burkholderiales | Burkholderiaceae | Burkholderia | Negative | Higher [112] |
| Pseudomonadota | Betaproteobacteria | Burkholderiales | Burkholderiaceae | Ralstonia | Negative | Higher [112] |
| Pseudomonadota | Betaproteobacteria | Burkholderiales | Sutterellaceae | Sutterella | Negative | Higher [89,107,109,110] |
| Pseudomonadota | Alphaproteobacteria | Hyphomicrobiales | Phyllobacteriaceae | Phyllobacterium | Negative | Higher [111] |
| Verrucomicrobiota | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia | Negative | Higher [52,89,107] |
| Phylum | Class | Order | Family | Genus | Gram Classification | Presence in ASD |
|---|---|---|---|---|---|---|
| Actinobacteria | Coriobacteriia | Coriobacteriale | Coriobacteriaceae | - | Positive | Lower [8,101] |
| Actinomycetota | Actinomycetia | Actinomycetales | Actinomycetaceae | - | Positive | Lower [8,101] |
| Actinomycetota | Actinomycetia | Bifidobacteriales | Bifidobacteriaceae | - | Positive | Lower [8,101] |
| Actinomycetota | Actinomycetia | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | Positive | Lower [52,89,101,102,103,106,107,113] |
| Bacillota | Bacilli | Caryophanales | Staphylococcaceae | Staphylococcus | Positive | Lower [107] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Coprococcus | Positive | Lower [8,52,107,111,114,115] |
| Bacillota | Negativicutes | Vellionellales | Veillonellaceae | Veillonella | Negative | Lower [8,18,52,114,115] |
| Bacillota | Bacilli | Lactobacillales | Streptococcaceae | Streptococcus | Positive | Lower [8,52,101,107,112] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Blautia | Positive | Lower [103] |
| Bacillota | Negativicutes | Veillonellales | Veillonellaceae | Dialister | Negative | Lower [18,103] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Subdoligranulum | Positive | Lower [52] |
| Bacillota | Bacilli | Lactobacillales | Streptococcaceae | Lactococcus | Positive | Lower [52,107] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Flavonifractor | Positive | Lower [111] |
| Bacillota | Clostridia | Clostridiales | Eubacteriaceae | Eubacterium | Positive | Lower [109] |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Sporobacter | Positive | Lower [52] |
| Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | Positive | Lower [52,107,109] |
| Fusobacteriota | Fusobacteriia | Fusobacteriales | Fusobacteriaceae | Fusobacterium | Negative | Lower [52] |
| Pseudomonadota | Alphaproteobacteria | Hyphomicrobiales | Devosiaceae | Devosia | Negative | Lower [112] |
| Pseudomonadota | Betaproteobacteria | Neisseriales | Neisseriaceae | Neisseria | Negative | Lower [112] |
| Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | Escherichia | Negative | Lower [52,112] |
| Thermodesulfobacteriota | Desulfovibrionia | Desulfovibrionales | Desulfovibrionaceae | Bilophila | Negative | Lower [18] |
| Phylum | Class | Order | Family | Genus | Gram Classification | Higher Presence in ASD | Lower Presence in ASD |
|---|---|---|---|---|---|---|---|
| Actinomycetota | Actinomycetia | Actinomycetales | Actinomycetaceae | Actinomyces | Positive | [89,112] | [101] |
| Actinomycetota | Actinomycetia | Mycobacteriales | Corynebacteriaceae | Corynebacterium | Positive | [18] | [101] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Oscillospira | Positive | [101,111,112] | [52] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Roseburia | Positive | [52,89] | [111] |
| Bacillota | Erysipelotrichia | Erysipelotrichales | Turicibacteraceae | Turicibacter | Positive | [52,89] | [103] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | Negative | [110,114,115] | [102,103,107,112] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Parabacteroides | Negative | [52,89,113] | [18] |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | Positive | [101,109,110] | [107] |
| Diet Type | Key Features | Mechanism of Action | Reported Benefits | Key References |
|---|---|---|---|---|
| Gluten-Free Diet | Elimination of gluten, a protein found in wheat, barley, and rye | Prevention of gluten-derived opioid-like peptides; reduction in intestinal permeability and systemic inflammation | Reduction in GI symptoms and hyperactivity, and improvements in social behavior and focus | [119,120,121] |
| Casein-Free Diet | Elimination of casein, a protein found in dairy products | Reduction in casein-derived opioid-like peptides; minimization of GI inflammation and intestinal permeability | Improvement in GI disturbances and potential behavioral benefits | [120,121] |
| Ketogenic Diet | High-fat, low-carbohydrate intake | Ketone production as alternative brain energy source; reduction in neuroinflammation and oxidative stress | Seizure reduction (in co-occurring epilepsy), improved attention, and behavioral enhancements | [122,123] |
| High-Antioxidant Diet | Emphasis on antioxidant-rich foods such as fruits, vegetables, and nuts | Neutralization of reactive oxygen species; reduction in oxidative stress and systemic/neural inflammation | Behavioral enhancement, oxidative damage reduction, and neuroprotection | [118,124] |
| Prebiotic-Rich Diet | Incorporation of fermented foods like yogurt, kefir, and sauerkraut | Restoration of microbial balance; enhancement of gut integrity; production of metabolites influencing brain function | Reduction in GI symptoms, anxiety, and improved social interactions | [118,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Barszcz, E.; Budny, E.; Gajewska, A.; Kopeć, K.; Wasiak, J.; Rysz, J.; Franczyk, B. The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients 2025, 17, 1135. https://doi.org/10.3390/nu17071135
Młynarska E, Barszcz E, Budny E, Gajewska A, Kopeć K, Wasiak J, Rysz J, Franczyk B. The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients. 2025; 17(7):1135. https://doi.org/10.3390/nu17071135
Chicago/Turabian StyleMłynarska, Ewelina, Ewelina Barszcz, Emilian Budny, Agata Gajewska, Kacper Kopeć, Jakub Wasiak, Jacek Rysz, and Beata Franczyk. 2025. "The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders" Nutrients 17, no. 7: 1135. https://doi.org/10.3390/nu17071135
APA StyleMłynarska, E., Barszcz, E., Budny, E., Gajewska, A., Kopeć, K., Wasiak, J., Rysz, J., & Franczyk, B. (2025). The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients, 17(7), 1135. https://doi.org/10.3390/nu17071135








