Nutritional Support of Chronic Obstructive Pulmonary Disease
Abstract
1. Background
2. Assessment Methods and Relevance of Body Composition in COPD
3. Impact of Different Dietary Components on COPD Patient Outcomes
4. Effects of Vitamins on the Quality of Life of Patients with COPD
4.1. Vitamin A
4.2. B Vitamins
4.3. Vitamin C
4.4. Vitamin D
4.5. Vitamin E
4.6. Vitamin K
5. Effects of Inorganic Elements on the Prognosis of Patients with COPD
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deng, M.; Lu, Y.; Zhang, Q.; Bian, Y.; Zhou, X.; Hou, G. Global prevalence of malnutrition in patients with chronic obstructive pulmonary disease: Systemic review and meta-analysis. Clin. Nutr. 2023, 42, 848–858. [Google Scholar] [CrossRef]
- Shen, X.; Qian, R.; Wei, Y.; Tang, Z.; Zhong, H.; Huang, J.; Zhang, X. Prediction model and assessment of malnutrition in patients with stable chronic obstructive pulmonary disease. Sci. Rep. 2024, 14, 6508. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, Z.; Li, K.; Liu, L.; Xu, L. Association between nutrition-related indicators with the risk of chronic obstructive pulmonary disease and all-cause mortality in the elderly population: Evidence from NHANES. Front. Nutr. 2024, 11, 1380791. [Google Scholar] [CrossRef]
- Schols, A.M.; Slangen, J.; Volovics, L.; Wouters, E.F. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Guleria, R.; Arora, S.; Kumar, G.; Mohan, A. Does Systemic Inflammation Effects Nutritional Status and Severity of COPD? Obstr. Lung Dis. 2016, 150, 850A. [Google Scholar]
- Montalcini, T.; Romeo, S.; Ferro, Y.; Migliaccio, V.; Gazzaruso, C.; Pujia, A. Osteoporosis in chronic inflammatory disease: The role of malnutrition. Endocrine 2013, 43, 59–64. [Google Scholar] [CrossRef]
- Morales, F.; Montserrat-de la Paz, S.; Leon, M.J.; Rivero-Pino, F. Effects of Malnutrition on the Immune System and Infection and the Role of Nutritional Strategies Regarding Improvements in Children’s Health Status: A Literature Review. Nutrients 2023, 16, 1. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef]
- Collins, P.F.; Elia, M.; Stratton, R.J. Nutritional support and functional capacity in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respirology 2013, 18, 616–629. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Brooks, D.; White, J.; Goldstein, R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 12, CD000998. [Google Scholar] [CrossRef]
- Fekete, M.; Csipo, T.; Fazekas-Pongor, V.; Feher, A.; Szarvas, Z.; Kaposvari, C.; Horvath, K.; Lehoczki, A.; Tarantini, S.; Varga, J.T. The Effectiveness of Supplementation with Key Vitamins, Minerals, Antioxidants and Specific Nutritional Supplements in COPD-A Review. Nutrients 2023, 15, 2741. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Csipo, T.; Fazekas-Pongor, V.; Szappanos, A.; Major, D.; Mozes, N.; Dosa, N.; Varga, J.T. The Role of Trace Elements in COPD: Pathogenetic Mechanisms and Therapeutic Potential of Zinc, Iron, Magnesium, Selenium, Manganese, Copper, and Calcium. Nutrients 2024, 16, 4118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, Q.; Li, Z.; Li, F. Body Composition and COPD: A New Perspective. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 79–97. [Google Scholar] [CrossRef]
- Kim, T.; Shin, S.H.; Kim, H.; Im, Y.; Cho, J.; Kang, D.; Park, H.Y. Longitudinal BMI change and outcomes in Chronic Obstructive Pulmonary Disease: A nationwide population-based cohort study. Respir. Res. 2024, 25, 150. [Google Scholar] [CrossRef] [PubMed]
- Mete, B.; Pehlivan, E.; Gulbas, G.; Gunen, H. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3307–3312. [Google Scholar] [CrossRef]
- Arora, N.S.; Rochester, D.F. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am. Rev. Respir. Dis. 1982, 126, 5–8. [Google Scholar] [CrossRef]
- Sahebjami, H.; Doers, J.T.; Render, M.L.; Bond, T.L. Anthropometric and pulmonary function test profiles of outpatients with stable chronic obstructive pulmonary disease. Am. J. Med. 1993, 94, 469–474. [Google Scholar] [CrossRef]
- Wilson, D.O.; Rogers, R.M.; Wright, E.C.; Anthonisen, N.R. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am. Rev. Respir. Dis. 1989, 139, 1435–1438. [Google Scholar] [CrossRef]
- Gunay, E.; Kaymaz, D.; Selcuk, N.T.; Ergun, P.; Sengul, F.; Demir, N. Effect of nutritional status in individuals with chronic obstructive pulmonary disease undergoing pulmonary rehabilitation. Respirology 2013, 18, 1217–1222. [Google Scholar] [CrossRef]
- Hoong, J.M.; Ferguson, M.; Hukins, C.; Collins, P.F. Economic and operational burden associated with malnutrition in chronic obstructive pulmonary disease. Clin. Nutr. 2017, 36, 1105–1109. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Collins, P.F.; Pavey, T.G.; Nguyen, N.V.; Pham, T.D.; Gallegos, D.L. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 215–226. [Google Scholar] [CrossRef]
- Schols, A.M.; Broekhuizen, R.; Weling-Scheepers, C.A.; Wouters, E.F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2005, 82, 53–59. [Google Scholar] [CrossRef]
- Mador, M.J. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 787–789. [Google Scholar] [CrossRef]
- Zou, R.H.; Nouraie, S.M.; Karoleski, C.; Zhang, Y.; Sciurba, F.C.; Forman, D.E.; Bon, J. Incident low muscle mass is associated with greater lung disease and lower circulating leptin in a tobacco-exposed longitudinal cohort. Respir. Res. 2023, 24, 224. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, L.; Li, Y.; Guo, S.; Li, X.; Zheng, J.; Zhu, Z.; Chen, Y.; Huang, Y.; Chen, R.; et al. Fat-Free Mass Index for Evaluating the Nutritional Status and Disease Severity in COPD. Respir. Care 2016, 61, 680–688. [Google Scholar] [CrossRef]
- McDonald, M.N.; Diaz, A.A.; Rutten, E.; Lutz, S.M.; Harmouche, R.; San Jose Estepar, R.; Kinney, G.; Hokanson, J.E.; Gower, B.A.; Wouters, E.F.M.; et al. Chest computed tomography-derived low fat-free mass index and mortality in COPD. Eur. Respir. J. 2017, 50, 1701134. [Google Scholar] [CrossRef]
- Marco, E.; Sanchez-Rodriguez, D.; Davalos-Yerovi, V.N.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Rodriguez, D.A.; Aguilera-Zubizarreta, A.; Escalada, F.; Duarte, E. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin. Nutr. 2019, 38, 2180–2186. [Google Scholar] [CrossRef]
- Kawabata, R.; Soma, Y.; Kudo, Y.; Yokoyama, J.; Shimizu, H.; Akaike, A.; Suzuki, D.; Katsuragi, Y.; Totsuka, M.; Nakaji, S. Relationships between body composition and pulmonary function in a community-dwelling population in Japan. PLoS ONE 2020, 15, e0242308. [Google Scholar] [CrossRef]
- Hasselgren, P.O.; Alamdari, N.; Aversa, Z.; Gonnella, P.; Smith, I.J.; Tizio, S. Corticosteroids and muscle wasting: Role of transcription factors, nuclear cofactors, and hyperacetylation. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 423–428. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Du, H.; Yang, L.; Chen, Y.; Zhou, Y.; et al. Association between adiposity measures and COPD risk in Chinese adults. Eur. Respir. J. 2020, 55, 1901899. [Google Scholar] [CrossRef]
- He, H.; Wang, B.; Zhou, M.; Cao, L.; Qiu, W.; Mu, G.; Chen, A.; Yang, S.; Chen, W. Systemic Inflammation Mediates the Associations Between Abdominal Obesity Indices and Lung Function Decline in a Chinese General Population. Diabetes Metab. Syndr. Obes. 2020, 13, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.K.; Kang, H.Y.; Lee, Y.; Choi, S.H.; Kim, H.J.; Kim, J.S. The longitudinal association between changes in lung function and changes in abdominal visceral obesity in Korean non-smokers. PLoS ONE 2018, 13, e0193516. [Google Scholar] [CrossRef]
- Elliot, J.G.; Donovan, G.M.; Wang, K.C.W.; Green, F.H.Y.; James, A.L.; Noble, P.B. Fatty airways: Implications for obstructive disease. Eur. Respir. J. 2019, 54, 1900857. [Google Scholar] [CrossRef]
- Tkacova, R. Systemic inflammation in chronic obstructive pulmonary disease: May adipose tissue play a role? Review of the literature and future perspectives. Mediat. Inflamm. 2010, 2010, 585989. [Google Scholar] [CrossRef]
- Bunk, S.A.O.; Ipema, J.; Sidorenkov, G.; Bennink, E.; Vliegenthart, R.; de Jong, P.A.; Pompe, E.; Charbonnier, J.P.; Luijk, B.H.D.; Aerts, J.; et al. The relationship of fat and muscle measurements with emphysema and bronchial wall thickening in smokers. ERJ Open Res. 2024, 10, 00749–2023. [Google Scholar] [CrossRef]
- Engelen, M.P.; Schols, A.M.; Lamers, R.J.; Wouters, E.F. Different patterns of chronic tissue wasting among patients with chronic obstructive pulmonary disease. Clin. Nutr. 1999, 18, 275–280. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Shidfar, F.; Moosavi, A.J.; Heidarnazhad, H.; Haghani, H. Energy and protein intake and its relationship with pulmonary function in chronic obstructive pulmonary disease (COPD) patients. Acta Medica Iran. 2010, 48, 374–379. [Google Scholar]
- Ji, J.; Fotros, D.; Sohouli, M.H.; Velu, P.; Fatahi, S.; Liu, Y. The effect of a ketogenic diet on inflammation-related markers: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2025, 83, 40–58. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Baker, E.H.; Janaway, C.H.; Philips, B.J.; Brennan, A.L.; Baines, D.L.; Wood, D.M.; Jones, P.W. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 2006, 61, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, A.E. Chronic obstructive pulmonary disease and glucose metabolism: A bitter sweet symphony. Cardiovasc. Diabetol. 2012, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Qiu, Y.; Shen, N.; Chen, H.; Zhang, J.; Wang, Y.; Shi, X.; Li, M. Effects of Low-Carbohydrate and Low-Fat Diets on Morbidity and Mortality of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 2443–2455. [Google Scholar] [CrossRef]
- Jiang, R.; Paik, D.C.; Hankinson, J.L.; Barr, R.G. Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults. Am. J. Respir. Crit. Care Med. 2007, 175, 798–804. [Google Scholar] [CrossRef]
- Okubo, H.; Shaheen, S.O.; Ntani, G.; Jameson, K.A.; Syddall, H.E.; Sayer, A.A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Hertfordshire Cohort Study, G. Processed meat consumption and lung function: Modification by antioxidants and smoking. Eur. Respir. J. 2014, 43, 972–982. [Google Scholar] [CrossRef]
- Cornell, K.; Alam, M.; Lyden, E.; Wood, L.; LeVan, T.D.; Nordgren, T.M.; Bailey, K.; Hanson, C. Saturated Fat Intake Is Associated with Lung Function in Individuals with Airflow Obstruction: Results from NHANES 2007–2012. Nutrients 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djousse, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H.; On behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Jeansen, S.; Witkamp, R.F.; Garthoff, J.A.; van Helvoort, A.; Calder, P.C. Fish oil LC-PUFAs do not affect blood coagulation parameters and bleeding manifestations: Analysis of 8 clinical studies with selected patient groups on omega-3-enriched medical nutrition. Clin. Nutr. 2018, 37, 948–957. [Google Scholar] [CrossRef]
- Broekhuizen, R.; Wouters, E.F.; Creutzberg, E.C.; Weling-Scheepers, C.A.; Schols, A.M. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005, 60, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Lehoczki, A.; Tarantini, S.; Varga, J.T. Effects of omega-3 supplementation on quality of life, nutritional status, inflammatory parameters, lipid profile, exercise tolerance and inhaled medications in chronic obstructive pulmonary disease. Ann. Palliat. Med. 2022, 11, 2819–2829. [Google Scholar] [CrossRef]
- Piao, Z.; Chai, B.; Wu, Y.; Diao, H.; He, Q.; Zheng, Q.; Yan, F.; Cui, W. The association between polyunsaturated fatty acids and chronic obstructive pulmonary disease: A meta-analysis. Food Funct. 2024, 15, 5929–5941. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Su, X.; Lei, T.; Zhang, C.; Zhang, M.; Wang, Y.; Zhu, L.; Liu, J. Effect of Omega-3 Fatty Acids on Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2677–2686. [Google Scholar] [CrossRef]
- Engelen, M.; Jonker, R.; Sulaiman, H.; Fisk, H.L.; Calder, P.C.; Deutz, N.E.P. omega-3 polyunsaturated fatty acid supplementation improves postabsorptive and prandial protein metabolism in patients with chronic obstructive pulmonary disease: A randomized clinical trial. Am. J. Clin. Nutr. 2022, 116, 686–698. [Google Scholar] [CrossRef]
- Engelen, M.; Simbo, S.Y.; Ruebush, L.E.; Thaden, J.J.; Ten Have, G.A.M.; Harrykissoon, R.I.; Zachria, A.J.; Calder, P.C.; Pereira, S.L.; Deutz, N.E.P. Functional and metabolic effects of omega-3 polyunsaturated fatty acid supplementation and the role of beta-hydroxy-beta-methylbutyrate addition in chronic obstructive pulmonary disease: A randomized clinical trial. Clin. Nutr. 2024, 43, 2263–2278. [Google Scholar] [CrossRef]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Ohlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachexia Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Fuld, J.P.; Kilduff, L.P.; Neder, J.A.; Pitsiladis, Y.; Lean, M.E.; Ward, S.A.; Cotton, M.M. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005, 60, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Deacon, S.J.; Vincent, E.E.; Greenhaff, P.L.; Fox, J.; Steiner, M.C.; Singh, S.J.; Morgan, M.D. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Faager, G.; Soderlund, K.; Skold, C.M.; Rundgren, S.; Tollback, A.; Jakobsson, P. Creatine supplementation and physical training in patients with COPD: A double blind, placebo-controlled study. Int. J. Chronic Obstr. Pulm. Dis. 2006, 1, 445–453. [Google Scholar] [CrossRef]
- De Benedetto, F.; Pastorelli, R.; Ferrario, M.; de Blasio, F.; Marinari, S.; Brunelli, L.; Wouters, E.F.M.; Polverino, F.; Celli, B.R.; Interdisciplinary Association for Research in Lung Disease (AIMAR) Study Group. Supplementation with Qter((R)) and Creatine improves functional performance in COPD patients on long term oxygen therapy. Respir. Med. 2018, 142, 86–93. [Google Scholar] [CrossRef]
- Al-Ghimlas, F.; Todd, D.C. Creatine supplementation for patients with COPD receiving pulmonary rehabilitation: A systematic review and meta-analysis. Respirology 2010, 15, 785–795. [Google Scholar] [CrossRef]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term consumption of fruits and vegetables and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Int. J. Epidemiol. 2018, 47, 1897–1909. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, Y.; Jiang, W. Fruit and Vegetable Intake and the Risk of Chronic Obstructive Pulmonary Disease: A Dose-Response Meta-Analysis of Observational Studies. Biomed. Res. Int. 2020, 2020, 3783481. [Google Scholar] [CrossRef]
- Kaluza, J.; Larsson, S.C.; Orsini, N.; Linden, A.; Wolk, A. Fruit and vegetable consumption and risk of COPD: A prospective cohort study of men. Thorax 2017, 72, 500–509. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Hertfordshire Cohort Study, G. The relationship of dietary patterns with adult lung function and COPD. Eur. Respir. J. 2010, 36, 277–284. [Google Scholar] [CrossRef]
- Keranis, E.; Makris, D.; Rodopoulou, P.; Martinou, H.; Papamakarios, G.; Daniil, Z.; Zintzaras, E.; Gourgoulianis, K.I. Impact of dietary shift to higher-antioxidant foods in COPD: A randomised trial. Eur. Respir. J. 2010, 36, 774–780. [Google Scholar] [CrossRef]
- Kaluza, J.; Harris, H.; Wallin, A.; Linden, A.; Wolk, A. Dietary Fiber Intake and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Epidemiology 2018, 29, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Willett, W.C.; Camargo, C.A., Jr. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am. J. Epidemiol. 2010, 171, 776–784. [Google Scholar] [CrossRef]
- Kan, H.; Stevens, J.; Heiss, G.; Rose, K.M.; London, S.J. Dietary fiber, lung function, and chronic obstructive pulmonary disease in the atherosclerosis risk in communities study. Am. J. Epidemiol. 2008, 167, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Szmidt, M.K.; Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Eur. J. Nutr. 2020, 59, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, S.H.; Chang, J.H.; Lee, H.S.; Kang, E.H.; Lee, S.W. The Impact of Changes in the Intake of Fiber and Antioxidants on the Development of Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 580. [Google Scholar] [CrossRef]
- Valisoltani, N.; Ghoreishy, S.M.; Imani, H.; Rajabi Harsini, A.; Jowshan, M.; Travica, N.; Mohammadi, H. Fiber intake and risk of chronic obstructive pulmonary disease: A systematic review and dose response meta-analysis. Food Sci. Nutr. 2023, 11, 6775–6788. [Google Scholar] [CrossRef]
- Vaughan, A.; Frazer, Z.A.; Hansbro, P.M.; Yang, I.A. COPD and the gut-lung axis: The therapeutic potential of fibre. J. Thorac. Dis. 2019, 11, S2173–S2180. [Google Scholar] [CrossRef]
- Hildebrand, C.B.; Lichatz, R.; Pich, A.; Muhlfeld, C.; Woltemate, S.; Vital, M.; Brandenberger, C. Short-chain fatty acids improve inflamm-aging and acute lung injury in old mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L480–L492. [Google Scholar] [CrossRef]
- Baye, K.; Guyot, J.P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Sello, C.T.; Qin, G.X.; Che, D.; Han, R. Does Dietary Fiber Affect the Levels of Nutritional Components after Feed Formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef]
- Ghasemi, A. Quantitative aspects of nitric oxide production from nitrate and nitrite. EXCLI J. 2022, 21, 470–486. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef]
- Berry, M.J.; Justus, N.W.; Hauser, J.I.; Case, A.H.; Helms, C.C.; Basu, S.; Rogers, Z.; Lewis, M.T.; Miller, G.D. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide 2015, 48, 22–30. [Google Scholar] [CrossRef]
- Pavitt, M.J.; Tanner, R.J.; Lewis, A.; Buttery, S.; Mehta, B.; Jefford, H.; Curtis, K.J.; Banya, W.A.S.; Husain, S.; Satkunam, K.; et al. Oral nitrate supplementation to enhance pulmonary rehabilitation in COPD: ON-EPIC a multicentre, double-blind, placebo-controlled, randomised parallel group study. Thorax 2020, 75, 547–555. [Google Scholar] [CrossRef]
- Kerley, C.P.; James, P.E.; McGowan, A.; Faul, J.; Cormican, L. Dietary nitrate improved exercise capacity in COPD but not blood pressure or pulmonary function: A 2 week, double-blind randomised, placebo-controlled crossover trial. Int. J. Food Sci. Nutr. 2019, 70, 222–231. [Google Scholar] [CrossRef]
- Pavitt, M.J.; Lewis, A.; Buttery, S.C.; Fernandez, B.O.; Mikus-Lelinska, M.; Banya, W.A.S.; Feelisch, M.; Polkey, M.I.; Hopkinson, N.S. Dietary nitrate supplementation to enhance exercise capacity in hypoxic COPD: EDEN-OX, a double-blind, placebo-controlled, randomised cross-over study. Thorax 2022, 77, 968–975. [Google Scholar] [CrossRef]
- Gilani, P.S.; Fesahat, M.; Shariatifar, N. Nitrosamine in meat and meat products: A review. J. Food Saf. Hyg. 2023, 9, 217–226. [Google Scholar] [CrossRef]
- Hecht, S.S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.M.; Chang, Y.J.; Cooke, M.S.; Pan, C.H.; Hu, C.H.; Chao, M.R.; Hu, C.W. Alkylating and oxidative stresses in smoking and non-smoking patients with COPD: Implications for lung carcinogenesis. Free Radic. Biol. Med. 2021, 164, 99–106. [Google Scholar] [CrossRef]
- Edwards, S.H.; Rossiter, L.M.; Taylor, K.M.; Holman, M.R.; Zhang, L.; Ding, Y.S.; Watson, C.H. Tobacco-Specific Nitrosamines in the Tobacco and Mainstream Smoke of U.S. Commercial Cigarettes. Chem. Res. Toxicol. 2017, 30, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Vikram, H.P.R.; Kumar, T.P.; Kumar, G.; Beeraka, N.M.; Deka, R.; Suhail, S.M.; Jat, S.; Bannimath, N.; Padmanabhan, G.; Chandan, R.S.; et al. Nitrosamines crisis in pharmaceuticals—Insights on toxicological implications, root causes and risk assessment: A systematic review. J. Pharm. Anal. 2024, 14, 100919. [Google Scholar] [CrossRef]
- Li, L.; Shao, J.; Zhu, X.; Zhou, G.; Xu, X. Effect of plant polyphenols and ascorbic acid on lipid oxidation, residual nitrite and N-nitrosamines formation in dry-cured sausage. Int. J. Food Sci. Technol. 2013, 48, 1157–1164. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Wishnok, J.S.; Leaf, C.D. Inhibition of nitrosamine formation by ascorbic acid. Am. J. Clin. Nutr. 1991, 53, 247S–250S. [Google Scholar] [CrossRef]
- Bayne, A.V.; Misic, Z.; Stemmler, R.T.; Wittner, M.; Frerichs, M.; Bird, J.K.; Besheer, A. N-nitrosamine Mitigation with Nitrite Scavengers in Oral Pharmaceutical Drug Products. J. Pharm. Sci. 2023, 112, 1794–1800. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Baydar, T. Evaluation of the protective effect of ascorbic acid on nitrite- and nitrosamine-induced cytotoxicity and genotoxicity in human hepatoma line. Toxicol. Mech. Methods 2010, 20, 45–52. [Google Scholar] [CrossRef]
- Waly, M.I.; Al-Bulushi, I.M.; Al-Hinai, S.; Guizani, N.; Al-Malki, R.N.; Rahman, M.S. The Protective Effect of Curcumin against Nitrosamine-Induced Gastric Oxidative Stress in Rats. Prev. Nutr. Food Sci. 2018, 23, 288–293. [Google Scholar] [CrossRef]
- Demkowicz-Dobrzanski, K.; Castonguay, A. Modulation by glutathione of DNA strand breaks induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and its aldehyde metabolites in rat hepatocytes. Carcinogenesis 1992, 13, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xu, Y.; Li, K.; Liu, L. Heavy metal levels and flavonoid intakes are associated with chronic obstructive pulmonary disease: An NHANES analysis (2007–2010 to 2017–2018). BMC Public Health 2023, 23, 2335. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Parmenter, B.H.; Dalgaard, F.; Murray, K.; Rasmussen, D.B.; Kyro, C.; Cassidy, A.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; et al. Flavonoid intakes inversely associate with COPD in smokers. Eur. Respir. J. 2022, 60, 2102604. [Google Scholar] [CrossRef]
- Culpitt, S.V.; Rogers, D.F.; Fenwick, P.S.; Shah, P.; De Matos, C.; Russell, R.E.; Barnes, P.J.; Donnelly, L.E. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax 2003, 58, 942–946. [Google Scholar] [CrossRef]
- Patel, S.; Marchetti, N.; Ganjian, H.; Kelsen, S.G.; Criner, G.J.; Sajjan, U. Oral Treatment with Quercetin Reduces Markers of Inflammation in COPD Patients. Am. J. Respir. Crit. Care Med. 2023, 207, A5001. [Google Scholar]
- Cobb, K.; Payne, C.; Lavender, R.; Simovic, T.; Harris, R.A.; Pollock, J.; Baban, B.; Mannino, D.; Nana-Sinkam, P.; Rodriguez Miguelez, P. Resveratrol Reduces Arterial Stiffness and Improves Functional Capacity in Patients with COPD. FASEB J. Physiol. 2022, 36. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef]
- Shin, S.Y.; Woo, Y.; Hyun, J.; Yong, Y.; Koh, D.; Lee, Y.H.; Lim, Y. Relationship between the structures of flavonoids and their NF-kappaB-dependent transcriptional activities. Bioorg. Med. Chem. Lett. 2011, 21, 6036–6041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, G.H.; Zhou, M.X.; Xiang, L.; Ren, D.M.; Lou, H.X.; Wang, X.N.; Shen, T. Discovery of natural flavonoids as activators of Nrf2-mediated defense system: Structure-activity relationship and inhibition of intracellular oxidative insults. Bioorg. Med. Chem. 2018, 26, 5140–5150. [Google Scholar] [CrossRef]
- Perez-Cano, F.J.; Castell, M. Flavonoids, Inflammation and Immune System. Nutrients 2016, 8, 659. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Gemes, N.; Balog, J.A.; Neuperger, P.; Schlegl, E.; Barta, I.; Fillinger, J.; Antus, B.; Zvara, A.; Hegedus, Z.; Czimmerer, Z.; et al. Single-cell immunophenotyping revealed the association of CD4+ central and CD4+ effector memory T cells linking exacerbating chronic obstructive pulmonary disease and NSCLC. Front. Immunol. 2023, 14, 1297577. [Google Scholar] [CrossRef]
- Szalontai, K.; Gemes, N.; Furak, J.; Varga, T.; Neuperger, P.; Balog, J.A.; Puskas, L.G.; Szebeni, G.J. Chronic Obstructive Pulmonary Disease: Epidemiology, Biomarkers, and Paving the Way to Lung Cancer. J. Clin. Med. 2021, 10, 2889. [Google Scholar] [CrossRef]
- Babu, P.V.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Wyeth, R.P.; Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W., Sr. Quercetin acutely relaxes airway smooth muscle and potentiates beta-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCbeta and PDE4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L396–L403. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir. Res. 2010, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Farazuddin, M.; Mishra, R.; Jing, Y.; Srivastava, V.; Comstock, A.T.; Sajjan, U.S. Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS ONE 2018, 13, e0199612. [Google Scholar] [CrossRef]
- Tabak, C.; Arts, I.C.; Smit, H.A.; Heederik, D.; Kromhout, D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGEN Study. Am. J. Respir. Crit. Care Med. 2001, 164, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhu, X.; Guan, G.; Cui, Q.; Zhu, L.; Xing, Y.; Zhao, J. Association of dietary flavonoid intakes with prevalence of chronic respiratory diseases in adults. J. Transl. Med. 2024, 22, 205. [Google Scholar] [CrossRef]
- Pereira, G.S.; Percebom, I.; Mendes, S.; Souza, P.S.S.; Diniz, L.F.A.; Costa, M.F.; Lopes, B.R.P.; Toledo, K.A. Quercetin inhibits neutrophil extracellular traps release and their cytotoxic effects on A549 cells, as well the release and enzymatic activity of elastase and myeloperoxidase. Braz. J. Biol. 2022, 84, e252936. [Google Scholar] [CrossRef]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef]
- Lv, P.; Han, P.; Cui, Y.; Chen, Q.; Cao, W. Quercetin attenuates inflammation in LPS-induced lung epithelial cells via the Nrf2 signaling pathway. Immun. Inflamm. Dis. 2024, 12, e1185. [Google Scholar] [CrossRef]
- Li, N.; Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. The effect of quercetin on human neutrophil elastase-induced mucin5AC expression in human airway epithelial cells. Int. Immunopharmacol. 2012, 14, 195–201. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Beijers, R.J.; Gosker, H.R.; Sanders, K.J.; de Theije, C.; Kelders, M.; Clarke, G.; Cryan, J.F.; van den Borst, B.; Schols, A.M. Resveratrol and metabolic health in COPD: A proof-of-concept randomized controlled trial. Clin. Nutr. 2020, 39, 2989–2997. [Google Scholar] [CrossRef]
- Cerda, B.; Soto, C.; Albaladejo, M.D.; Martinez, P.; Sanchez-Gascon, F.; Tomas-Barberan, F.; Espin, J.C. Pomegranate juice supplementation in chronic obstructive pulmonary disease: A 5-week randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2006, 60, 245–253. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Otaki, F.; Bonsu, P.; Dang, D.T.; Teater, M.; Strulovici-Barel, Y.; Salit, J.; Harvey, B.G.; Crystal, R.G. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell. Mol. Life Sci. 2011, 68, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.D.; Bentzel, C.J.; Riecken, E.O.; Schulzke, J.D. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastian, V.P.; Alvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef]
- Song, X.; Dou, X.; Chang, J.; Zeng, X.; Xu, Q.; Xu, C. The role and mechanism of gut-lung axis mediated bidirectional communication in the occurrence and development of chronic obstructive pulmonary disease. Gut Microbes 2024, 16, 2414805. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Garssen, J.; Henricks, P.A.J.; Folkerts, G.; Braber, S. The Bidirectional Gut-Lung Axis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2023, 207, 1145–1160. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Rodsgaard, P.A.; Weinreich, U.M. Chronic obstructive pulmonary disease as comorbidity in patients admitted to a university hospital: A cross-sectional study. Clin. Respir. J. 2014, 8, 274–280. [Google Scholar] [CrossRef]
- Ekbom, A.; Brandt, L.; Granath, F.; Lofdahl, C.G.; Egesten, A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung 2008, 186, 167–172. [Google Scholar] [CrossRef]
- Rutten, E.P.; Spruit, M.A.; Franssen, F.M.; Buurman, W.A.; Wouters, E.F.; Lenaerts, K. GI symptoms in patients with COPD. Chest 2014, 145, 1437–1438. [Google Scholar] [CrossRef]
- Carvalho, J.L.; Miranda, M.; Fialho, A.K.; Castro-Faria-Neto, H.; Anatriello, E.; Keller, A.C.; Aimbire, F. Oral feeding with probiotic Lactobacillus rhamnosus attenuates cigarette smoke-induced COPD in C57Bl/6 mice: Relevance to inflammatory markers in human bronchial epithelial cells. PLoS ONE 2020, 15, e0225560. [Google Scholar] [CrossRef]
- Wenger, N.M.; Qiao, L.; Nicola, T.; Nizami, Z.; Martin, I.; Halloran, B.A.; Tanaka, K.; Evans, M.; Xu, X.; Dinan, T.G.; et al. Clinical trial of a probiotic and herbal supplement for lung health. Front. Nutr. 2023, 10, 1168582. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Vaughan, A.; Amorim, N.; Horvat, J.C.; Hansbro, N.G.; Wood, D.L.A.; Hugenholtz, P.; Dennis, P.G.; Wark, P.A.B.; et al. Probiotic Bifidobacterium longum subsp. longum Protects against Cigarette Smoke-Induced Inflammation in Mice. Int. J. Mol. Sci. 2022, 24, 252. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Qiu, R.; Zhou, J.; Ren, L.; Qu, Y.; Zhang, G. Fecal Microbiota Transplantation Alleviates Airway Inflammation in Asthmatic Rats by Increasing the Level of Short-Chain Fatty Acids in the Intestine. Inflammation 2025. [Google Scholar] [CrossRef]
- Budden, K.F.; Shukla, S.D.; Bowerman, K.L.; Vaughan, A.; Gellatly, S.L.; Wood, D.L.A.; Lachner, N.; Idrees, S.; Rehman, S.F.; Faiz, A.; et al. Faecal microbial transfer and complex carbohydrates mediate protection against COPD. Gut 2024, 73, 751–769. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Klamt, F.; Dal-Pizzol, F.; Gelain, D.P.; Dalmolin, R.S.; Birnfeld de Oliveira, R.; Bastiani, M.; Horn, F.; Fonseca Moreira, J.C. Vitamin A treatment induces apoptosis through an oxidant-dependent activation of the mitochondrial pathway. Cell Biol. Int. 2008, 32, 100–106. [Google Scholar] [CrossRef]
- Blomhoff, H.K.; Smeland, E.B.; Erikstein, B.; Rasmussen, A.M.; Skrede, B.; Skjonsberg, C.; Blomhoff, R. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J. Biol. Chem. 1992, 267, 23988–23992. [Google Scholar]
- Ross, A.C.; Gardner, E.M. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv. Exp. Med. Biol. 1994, 352, 187–200. [Google Scholar] [CrossRef]
- Yang, F.C.; Xu, F.; Wang, T.N.; Chen, G.X. Roles of vitamin A in the regulation of fatty acid synthesis. World J. Clin. Cases 2021, 9, 4506–4519. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G. The Roles of Vitamin A in the Regulation of Carbohydrate, Lipid, and Protein Metabolism. J. Clin. Med. 2014, 3, 453–479. [Google Scholar] [CrossRef]
- Tierney, M.T.; Polak, L.; Yang, Y.; Abdusselamoglu, M.D.; Baek, I.; Stewart, K.S.; Fuchs, E. Vitamin A resolves lineage plasticity to orchestrate stem cell lineage choices. Science 2024, 383, eadi7342. [Google Scholar] [CrossRef] [PubMed]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Guo, Y.; Brown, C.; Ortiz, C.; Noelle, R.J. Leukocyte homing, fate, and function are controlled by retinoic acid. Physiol. Rev. 2015, 95, 125–148. [Google Scholar] [CrossRef]
- Sirisinha, S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015, 33, 71–89. [Google Scholar] [PubMed]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Checkley, W.; West, K.P., Jr.; Wise, R.A.; Baldwin, M.R.; Wu, L.; LeClerq, S.C.; Christian, P.; Katz, J.; Tielsch, J.M.; Khatry, S.; et al. Maternal vitamin A supplementation and lung function in offspring. N. Engl. J. Med. 2010, 362, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Penkert, R.R.; Sealy, R.E.; Jones, B.G.; Marion, T.N.; Vogel, P.; Hurwitz, J.L. Consequences of Vitamin A Deficiency: Immunoglobulin Dysregulation, Squamous Cell Metaplasia, Infectious Disease, and Death. Int. J. Mol. Sci. 2020, 21, 5570. [Google Scholar] [CrossRef]
- Baybutt, R.C.; Hu, L.; Molteni, A. Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J. Nutr. 2000, 130, 1159–1165. [Google Scholar] [CrossRef]
- McGowan, S.E.; Takle, E.J.; Holmes, A.J. Vitamin A deficiency alters the pulmonary parenchymal elastic modulus and elastic fiber concentration in rats. Respir. Res. 2005, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- McGowan, S.E.; Holmes, A.J. Vitamin A deficiency alters pulmonary parenchymal collagen and tissue mechanics. Respir. Physiol. Neurobiol. 2007, 156, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Pretel, G.; Marin, M.P.; Renau-Piqueras, J.; Barber, T.; Timoneda, J. Vitamin A deficiency alters rat lung alveolar basement membrane: Reversibility by retinoic acid. J. Nutr. Biochem. 2010, 21, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Huang, H.M.; Li, T.Y.; Qu, P.; Liu, Y.X.; Chen, J. Marginal vitamin A deficiency affects lung maturation in rats from prenatal to adult stage. J. Nutr. Sci. Vitaminol. 2009, 55, 208–214. [Google Scholar] [CrossRef]
- McGowan, S.E.; Holmes, A.J.; Smith, J. Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L437–L444. [Google Scholar] [CrossRef]
- Esteban-Pretel, G.; Marin, M.P.; Renau-Piqueras, J.; Sado, Y.; Barber, T.; Timoneda, J. Vitamin A deficiency disturbs collagen IV and laminin composition and decreases matrix metalloproteinase concentrations in rat lung. Partial reversibility by retinoic acid. J. Nutr. Biochem. 2013, 24, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Molteni, A.; Latkovich, P.; Castellani, W.; Baybutt, R.C. Vitamin A depletion induced by cigarette smoke is associated with the development of emphysema in rats. J. Nutr. 2003, 133, 2629–2634. [Google Scholar] [CrossRef]
- van Eijl, S.; Mortaz, E.; Versluis, C.; Nijkamp, F.P.; Folkerts, G.; Bloksma, N. A low vitamin A status increases the susceptibility to cigarette smoke-induced lung emphysema in C57BL/6J mice. J. Physiol. Pharmacol. 2011, 62, 175–182. [Google Scholar]
- Sklan, D.; Rappaport, R.; Vered, M. Inhibition of the activity of human leukocyte elastase by lipids particularly oleic acid and retinoic acid. Lung 1990, 168, 323–332. [Google Scholar] [CrossRef]
- Ng-Blichfeldt, J.P.; Alcada, J.; Montero, M.A.; Dean, C.H.; Griesenbach, U.; Griffiths, M.J.; Hind, M. Deficient retinoid-driven angiogenesis may contribute to failure of adult human lung regeneration in emphysema. Thorax 2017, 72, 510–521. [Google Scholar] [CrossRef]
- Chen, Y.; Vasquez, M.M.; Zhu, L.; Lizarraga, R.E.; Krutzsch, M.; Einspahr, J.; Alberts, D.S.; Di, P.Y.P.; Martinez, F.D.; Guerra, S. Effects of Retinoids on Augmentation of Club Cell Secretory Protein. Am. J. Respir. Crit. Care Med. 2017, 196, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Chuwers, P.; Barnhart, S.; Blanc, P.; Brodkin, C.A.; Cullen, M.; Kelly, T.; Keogh, J.; Omenn, G.; Williams, J.; Balmes, J.R. The protective effect of beta-carotene and retinol on ventilatory function in an asbestos-exposed cohort. Am. J. Respir. Crit. Care Med. 1997, 155, 1066–1071. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, X.; Xia, Z.; Guo, Y.; Dai, Y. The Associations Between Serum Vitamins and Carotenoids with Chronic Obstructive Pulmonary Disease: Results from the NHANES. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2985–2997. [Google Scholar] [CrossRef]
- Hu, G.; Cassano, P.A. Antioxidant nutrients and pulmonary function: The Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Epidemiol. 2000, 151, 975–981. [Google Scholar] [CrossRef]
- McKeever, T.M.; Lewis, S.A.; Smit, H.A.; Burney, P.; Cassano, P.A.; Britton, J. A multivariate analysis of serum nutrient levels and lung function. Respir. Res. 2008, 9, 67. [Google Scholar] [CrossRef]
- Guenegou, A.; Leynaert, B.; Pin, I.; Le Moel, G.; Zureik, M.; Neukirch, F. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax 2006, 61, 320–326. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Meyer, K.A.; Smith, L.J.; Beckett, W.S.; Williams, O.D.; Gross, M.D.; Jacobs, D.R., Jr. Serum carotenoid concentrations predict lung function evolution in young adults: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Clin. Nutr. 2011, 94, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Bimali, M.; Faramawi, M.; Orloff, M.S. Consumption of Vitamin K and Vitamin A Are Associated with Reduced Risk of Developing Emphysema: NHANES 2007-2016. Front. Nutr. 2020, 7, 47. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hung, M.S. Associations between vitamin A and K intake and lung function in the general US population: Evidence from NHANES 2007-2012. Front. Nutr. 2024, 11, 1417489. [Google Scholar] [CrossRef]
- McKeever, T.M.; Scrivener, S.; Broadfield, E.; Jones, Z.; Britton, J.; Lewis, S.A. Prospective study of diet and decline in lung function in a general population. Am. J. Respir. Crit. Care Med. 2002, 165, 1299–1303. [Google Scholar] [CrossRef]
- Kelly, Y.; Sacker, A.; Marmot, M. Nutrition and respiratory health in adults: Findings from the health survey for Scotland. Eur. Respir. J. 2003, 21, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Adeloye, D.; Li, S.; Zhao, D.; Ye, X.; Pan, Q.; Qiu, Y.; Zhang, R.; Rudan, I.; Global Health Epidemiology Research, G. The prevalence of vitamin A deficiency and its public health significance in children in low- and middle-income countries: A systematic review and modelling analysis. J. Glob. Health 2023, 13, 04084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, S.; Zhang, R.; Zhao, Z.; Yu, H.; Pu, L.; Wang, L.; Han, L. Global Burden of Vitamin A Deficiency in 204 Countries and Territories from 1990–2019. Nutrients 2022, 14, 950. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Van Loo-Bouwman, C.A.; Naber, T.H.; Schaafsma, G. A review of vitamin A equivalency of beta-carotene in various food matrices for human consumption. Br. J. Nutr. 2014, 111, 2153–2166. [Google Scholar] [CrossRef]
- Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.K.; Golczak, M. The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158571. [Google Scholar] [CrossRef]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468S–1473S. [Google Scholar] [CrossRef]
- Redlich, C.A.; Grauer, J.N.; Van Bennekum, A.M.; Clever, S.L.; Ponn, R.B.; Blaner, W.S. Characterization of carotenoid, vitamin A, and alpha-tocopheral levels in human lung tissue and pulmonary macrophages. Am. J. Respir. Crit. Care Med. 1996, 154, 1436–1443. [Google Scholar] [CrossRef]
- Genaro Pde, S.; Martini, L.A. Vitamin A supplementation and risk of skeletal fracture. Nutr. Rev. 2004, 62, 65–67. [Google Scholar] [CrossRef]
- Melhus, H.; Michaelsson, K.; Kindmark, A.; Bergstrom, R.; Holmberg, L.; Mallmin, H.; Wolk, A.; Ljunghall, S. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann. Intern. Med. 1998, 129, 770–778. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y. Relation of serum vitamin A levels to all-cause and cause-specific mortality among older adults in the NHANES III population. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1197–1203. [Google Scholar] [CrossRef]
- Rohde, C.M.; Manatt, M.; Clagett-Dame, M.; DeLuca, H.F. Vitamin A antagonizes the action of vitamin D in rats. J. Nutr. 1999, 129, 2246–2250. [Google Scholar] [CrossRef]
- Bastie, J.N.; Balitrand, N.; Guidez, F.; Guillemot, I.; Larghero, J.; Calabresse, C.; Chomienne, C.; Delva, L. 1 alpha,25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol. Endocrinol. 2004, 18, 2685–2699. [Google Scholar] [CrossRef]
- MacDonald, P.N.; Dowd, D.R.; Nakajima, S.; Galligan, M.A.; Reeder, M.C.; Haussler, C.A.; Ozato, K.; Haussler, M.R. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Mol. Cell. Biol. 1993, 13, 5907–5917. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lara, A.M.; Aranda, A. Vitamin D represses retinoic acid-dependent transactivation of the retinoic acid receptor-beta2 promoter: The AF-2 domain of the vitamin D receptor is required for transrepression. Endocrinology 1999, 140, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Polly, P.; Carlberg, C.; Eisman, J.A.; Morrison, N.A. 1α,25-dihydroxyvitamin D3 receptor as a mediator of transrepression of retinoid signaling. J. Cell. Biochem. 1997, 67, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.S.; Yang, S.W.; Song, B.C.; Yeum, K.J. Vitamin A intake, serum vitamin D and bone mineral density: Analysis of the Korea National Health and Nutrition Examination Survey (KNHANES, 2008–2011). Nutrients 2015, 7, 1716–1727. [Google Scholar] [CrossRef]
- Ruiter, B.; Patil, S.U.; Shreffler, W.G. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin. Exp. Allergy 2015, 45, 1214–1225. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Buchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Freedman, D.M.; Tangrea, J.A.; Virtamo, J.; Albanes, D. The effect of beta-carotene supplementation on serum vitamin D metabolite concentrations. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 1115–1116. [Google Scholar]
- Kritchevsky, S.B.; Schwartz, G.G.; Morris, D.L. beta-Carotene supplementation, vitamin D, and cancer risk: A hypothesis. Epidemiology 1995, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Serini, S.; Trombino, S.; Lauriola, L.; Ranelletti, F.O.; Calviello, G. Dual role of beta-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: Dependence on pO2. Carcinogenesis 2006, 27, 2383–2391. [Google Scholar] [CrossRef]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef]
- Alpha-Tocopherol, B.C.C.P.S.G. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Malouf, M.; Grimley, E.J.; Areosa, S.A. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst. Rev. 2003, 4, CD004514. [Google Scholar] [CrossRef]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Pancharuniti, N.; Lewis, C.A.; Sauberlich, H.E.; Perkins, L.L.; Go, R.C.; Alvarez, J.O.; Macaluso, M.; Acton, R.T.; Copeland, R.B.; Cousins, A.L.; et al. Plasma homocyst(e)ine, folate, and vitamin B-12 concentrations and risk for early-onset coronary artery disease. Am. J. Clin. Nutr. 1994, 59, 940–948. [Google Scholar] [CrossRef]
- Fenech, M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat. Res. 2012, 733, 21–33. [Google Scholar] [CrossRef]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of Vitamin B6, B9, and B12 Supplementation on Homocysteine Level and Cardiovascular Outcomes in Stroke Patients: A Meta-Analysis of Randomized Controlled Trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Shen, S.Y.; Ho, C.S.; Huang, C.C. A functional evaluation of anti-fatigue and exercise performance improvement following vitamin B complex supplementation in healthy humans, a randomized double-blind trial. Int. J. Med. Sci. 2023, 20, 1272–1281. [Google Scholar] [CrossRef]
- Wu, N.C.; Wang, J.J. Niacin Pretreatment Attenuates Lung Ischemia and Reperfusion-Induced Pulmonary Barrier Function Impairment by Reducing Oxidative Stress and Activating SIRT1 in an Isolated-Perfused Rat Lung Model. Transplant. Proc. 2018, 50, 2834–2838. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B12, Folic Acid, and the Immune System. In Nutrition and Immunity; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Prasad, R.; Lakshmi, A.V.; Bamji, M.S. Impaired collagen maturity in vitamins B2 and B6 deficiency--probable molecular basis of skin lesions. Biochem. Med. 1983, 30, 333–341. [Google Scholar] [CrossRef]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics 2015, 2, 146–161. [Google Scholar] [CrossRef]
- Rezavanimehr, M.M.; Kakhki, S.; Pahlavani, H.; Khosropour, M.; Khatibi, S.R.; Beheshti, F. Vitamin B(12) supplementation improved memory impairment following nicotine withdrawal in adolescent male rats: The role of oxidative stress, inflammatory, BDNF, GFAP, and AChE activity. Behav. Brain Res. 2024, 474, 115180. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef]
- Hannibal, L.; Lysne, V.; Bjorke-Monsen, A.L.; Behringer, S.; Grunert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef]
- Kerns, J.C.; Arundel, C.; Chawla, L.S. Thiamin deficiency in people with obesity. Adv. Nutr. 2015, 6, 147–153. [Google Scholar] [CrossRef]
- Nix, W.A.; Zirwes, R.; Bangert, V.; Kaiser, R.P.; Schilling, M.; Hostalek, U.; Obeid, R. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 2015, 107, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ding, H.; Chen, H.; Ye, X.; Li, H.; Lin, X.; Ke, Z. Thiamine nutritional status and depressive symptoms are inversely associated among older Chinese adults. J. Nutr. 2013, 143, 53–58. [Google Scholar] [CrossRef]
- Benton, D.; Griffiths, R.; Haller, J. Thiamine supplementation mood and cognitive functioning. Psychopharmacology 1997, 129, 66–71. [Google Scholar] [CrossRef]
- Smidt, L.J.; Cremin, F.M.; Grivetti, L.E.; Clifford, A.J. Influence of thiamin supplementation on the health and general well-being of an elderly Irish population with marginal thiamin deficiency. J. Gerontol. 1991, 46, M16–M22. [Google Scholar] [CrossRef]
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Hanger, H.C.; George, P.M.; Sainsbury, R. Is thiamine deficiency in elderly people related to age or co-morbidity? Age Ageing 2000, 29, 111–116. [Google Scholar] [CrossRef]
- Nichols, H.K.; Basu, T.K. Thiamin status of the elderly: Dietary intake and thiamin pyrophosphate response. J. Am. Coll. Nutr. 1994, 13, 57–61. [Google Scholar] [CrossRef]
- Clarke, R.; Grimley Evans, J.; Schneede, J.; Nexo, E.; Bates, C.; Fletcher, A.; Prentice, A.; Johnston, C.; Ueland, P.M.; Refsum, H.; et al. Vitamin B12 and folate deficiency in later life. Age Ageing 2004, 33, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Linardakis, M.K.; Hatzis, C.M.; Malliaraki, N.; Saris, W.H.; Kafatos, A.G. Smoking status in relation to serum folate and dietary vitamin intake. Tob. Induc. Dis. 2008, 4, 8. [Google Scholar] [CrossRef]
- Ulvik, A.; Ebbing, M.; Hustad, S.; Midttun, O.; Nygard, O.; Vollset, S.E.; Bonaa, K.H.; Nordrehaug, J.E.; Nilsen, D.W.; Schirmer, H.; et al. Long- and short-term effects of tobacco smoking on circulating concentrations of B vitamins. Clin. Chem. 2010, 56, 755–763. [Google Scholar] [CrossRef]
- Vermaak, W.J.; Ubbink, J.B.; Barnard, H.C.; Potgieter, G.M.; van Jaarsveld, H.; Groenewald, A.J. Vitamin B-6 nutrition status and cigarette smoking. Am. J. Clin. Nutr. 1990, 51, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, M.S.; Al-Oun, M.A.; Abusahyoun, F.Y.; Abualarja, M.I.; Al Smadi, A.; Al-Trad, B.; Awadin, S.A.; Al-Batayneh, K.; Elaarag, M.; Al-Zoubi, R.M. Exploring the Impact of Cigarette Smoke Extracts on Vitamin B12: Insights into the Transformation of Methylcobalamin and Hydroxycobalamin to Cyanocobalamin through In Vitro Evaluation. Biochem. Res. Int. 2024, 2024, 8827402. [Google Scholar] [CrossRef]
- Laudisio, A.; Costanzo, L.; Di Gioia, C.; Delussu, A.S.; Traballesi, M.; Gemma, A.; Antonelli Incalzi, R. Dietary intake of elderly outpatients with chronic obstructive pulmonary disease. Arch. Gerontol. Geriatr. 2016, 64, 75–81. [Google Scholar] [CrossRef]
- Li, W.W.; Ren, K.L.; Yu, J.; Guo, H.S.; Liu, B.H.; Sun, Y. Association of dietary niacin intake with the prevalence and incidence of chronic obstructive pulmonary disease. Sci. Rep. 2024, 14, 2863. [Google Scholar] [CrossRef]
- Chambaneau, A.; Filaire, M.; Jubert, L.; Bremond, M.; Filaire, E. Nutritional Intake, Physical Activity and Quality of Life in COPD Patients. Int. J. Sports Med. 2016, 37, 730–737. [Google Scholar] [CrossRef]
- Hirayama, F.; Lee, A.H.; Terasawa, K.; Kagawa, Y. Folate intake associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease. Asia Pac. J. Clin. Nutr. 2010, 19, 103–109. [Google Scholar]
- Andersson, I.; Gronberg, A.; Slinde, F.; Bosaeus, I.; Larsson, S. Vitamin and mineral status in elderly patients with chronic obstructive pulmonary disease. Clin. Respir. J. 2007, 1, 23–29. [Google Scholar] [CrossRef]
- Kim, T.; Choi, H.; Kim, J. Association Between Dietary Nutrient Intake and Chronic Obstructive Pulmonary Disease Severity: A Nationwide Population-Based Representative Sample. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 49–58. [Google Scholar] [CrossRef]
- Kim, T.; Oak, C.H.; Jung, M.H.; Jang, T.W.; Kim, J. High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey. Nutrients 2020, 12, 2219. [Google Scholar] [CrossRef]
- Gariballa, S.; Forster, S.; Powers, H. Riboflavin status in acutely ill patients and response to dietary supplements. JPEN J. Parenter. Enteral Nutr. 2009, 33, 656–661. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, Y.; Ruan, Z.; Zang, G.; Chen, X.; Qiu, Z. Association between B-vitamins intake and frailty among patients with chronic obstructive pulmonary disease. Aging Clin. Exp. Res. 2023, 35, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, F.L.; Loffredo, L.; Di Simone, S.; Sampietro, F.; Pastorelli, R.; Monaldo, M.; Violi, F.; D’Angelo, A. Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Mangoni, A.A. Arginine, Transsulfuration, and Folic Acid Pathway Metabolomics in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 2180. [Google Scholar] [CrossRef]
- Horadagoda, C.; Dinihan, T.; Roberts, M.; Kairaitis, K. Body composition and micronutrient deficiencies in patients with an acute exacerbation of chronic obstructive pulmonary disease. Intern. Med. J. 2017, 47, 1057–1063. [Google Scholar] [CrossRef]
- Paulin, F.V.; Zagatto, A.M.; Chiappa, G.R.; Muller, P.T. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: A randomized and controlled study. Respir. Med. 2017, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Dosedel, M.; Jirkovsky, E.; Macakova, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remiao, F.; Novakova, L.; Mladenka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- See, X.Z.; Yeo, W.S.; Saptoro, A. A comprehensive review and recent advances of vitamin C: Overview, functions, sources, applications, market survey and processes. Chem. Eng. Res. Des. 2024, 206, 108–129. [Google Scholar]
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Ward, S.; Cramer, D.; Barnes, P.J. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 162, 369–373. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.; Aben, K.K.; Dekker, I.; Aarts, L.P.; Wielders, P.L.; van Herwaarden, C.L.; Bast, A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 154, 813–816. [Google Scholar] [CrossRef]
- Ansarin, K.; Chatkin, J.M.; Ferreira, I.M.; Gutierrez, C.A.; Zamel, N.; Chapman, K.R. Exhaled nitric oxide in chronic obstructive pulmonary disease: Relationship to pulmonary function. Eur. Respir. J. 2001, 17, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Packer, J.E.; Slater, T.F.; Willson, R.L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979, 278, 737–738. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Dietrich, M.; Block, G.; Benowitz, N.L.; Morrow, J.D.; Hudes, M.; Jacob, P., 3rd; Norkus, E.P.; Packer, L. Vitamin C supplementation decreases oxidative stress biomarker f2-isoprostanes in plasma of nonsmokers exposed to environmental tobacco smoke. Nutr. Cancer 2003, 45, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ma, W.; Dong, Z. Inhibitory effects of ascorbic acid on AP-1 activity and transformation of JB6 cells. Int. J. Oncol. 1996, 8, 389–393. [Google Scholar] [CrossRef]
- Mostafavi-Pour, Z.; Ramezani, F.; Keshavarzi, F.; Samadi, N. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol. Lett. 2017, 13, 1965–1973. [Google Scholar] [CrossRef]
- Vineetha, R.C.; Binu, P.; Arathi, P.; Nair, R.H. L-ascorbic acid and α-tocopherol attenuate arsenic trioxide-induced toxicity in H9c2 cardiomyocytes by the activation of Nrf2 and Bcl2 transcription factors. Toxicol. Mech. Methods 2018, 28, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013, 19, 2054–2067. [Google Scholar] [CrossRef]
- Prasad, A.; Rathi, D.; Sedlarova, M.; Manoharan, R.R.; Prudkova, E.; Pospisil, P. Differential effects of ascorbic acid on monocytic cell morphology and protein modification: Shifting from pro-oxidative to antioxidant properties. Biochem. Biophys. Rep. 2024, 37, 101622. [Google Scholar] [CrossRef]
- Gegotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Flashman, E.; Davies, S.L.; Yeoh, K.K.; Schofield, C.J. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 2010, 427, 135–142. [Google Scholar] [CrossRef]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef]
- Siegel, B.V. Enhancement of interferon production by poly(rI)-poly(rC) in mouse cell cultures by ascorbic acid. Nature 1975, 254, 531–532. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat. Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef]
- Dahl, H.; Degre, M. The effect of ascorbic acid on production of human interferon and the antiviral activity in vitro. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. 1976, 84B, 280–284. [Google Scholar] [CrossRef]
- Huang, H.Y.; Appel, L.J.; Croft, K.D.; Miller, E.R., 3rd; Mori, T.A.; Puddey, I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: Results of a randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 549–555. [Google Scholar] [CrossRef]
- Booth, B.A.; Uitto, J. Collagen biosynthesis by human skin fibroblasts. III. The effects of ascorbic acid on procollagen production and prolyl hydroxylase activity. Biochim. Biophys. Acta 1981, 675, 117–122. [Google Scholar] [CrossRef]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar] [PubMed]
- Ito, J.T.; Lourenco, J.D.; Righetti, R.F.; Tiberio, I.; Prado, C.M.; Lopes, F. Extracellular Matrix Component Remodeling in Respiratory Diseases: What Has Been Found in Clinical and Experimental Studies? Cells 2019, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Kononov, S.; Brewer, K.; Sakai, H.; Cavalcante, F.S.; Sabayanagam, C.R.; Ingenito, E.P.; Suki, B. Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am. J. Respir. Crit. Care Med. 2001, 164, 1920–1926. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Regmi, B.; Ottaviani, M.M.; Kahles, F.; Giannoni, A.; Borrelli, C.; Passino, C.; Macefield, V.; Dreher, M. Sympathetic and Vagal Nerve Activity in COPD: Pathophysiology, Presumed Determinants and Underappreciated Therapeutic Potential. Front. Physiol. 2022, 13, 919422. [Google Scholar] [CrossRef]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. [Google Scholar] [CrossRef]
- Levine, M. Ascorbic acid specifically enhances dopamine beta-monooxygenase activity in resting and stimulated chromaffin cells. J. Biol. Chem. 1986, 261, 7347–7356. [Google Scholar]
- Levine, M.; Morita, K.; Pollard, H. Enhancement of norepinephrine biosynthesis by ascorbic acid in cultured bovine chromaffin cells. J. Biol. Chem. 1985, 260, 12942–12947. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.P.; Kirshner, N. Effects of ascorbic acid, dexamethasone, and insulin on the catecholamine and opioid peptide stores of cultured adrenal medullary chromaffin cells. J. Neurosci. 1983, 3, 1971–1978. [Google Scholar] [CrossRef]
- Elsammak, M.; Attia, A.; Suleman, M. Carnitine Deficiency in Chronic Obstructive Pulmonary Disease Patients. J. Pulm. Respir. Med. 2011, 1, 2. [Google Scholar]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Huber-Lang, M.; Sarma, J.V.; Ward, P.A. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Mol. Med. 2008, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. Mucolysis by ascorbic acid and hydrogen peroxide on compact mucin secreted in pseudomyxoma peritonei. J. Surg. Res. 2012, 174, e69–e73. [Google Scholar] [CrossRef]
- Adewale, A.T.; Falk Libby, E.; Fu, L.; Lenzie, A.; Boitet, E.R.; Birket, S.E.; Petty, C.F.; Johns, J.D.; Mazur, M.; Tearney, G.J.; et al. Novel Therapy of Bicarbonate, Glutathione, and Ascorbic Acid Improves Cystic Fibrosis Mucus Transport. Am. J. Respir. Cell Mol. Biol. 2020, 63, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Daoud, K.M.; El Ayyadi, M.A. The relation between vitamin C and adrenaline. Biochem. J. 1938, 32, 1424–1434. [Google Scholar] [CrossRef]
- Farasati Far, B.; Behnoush, A.H.; Ghondaghsaz, E.; Habibi, M.A.; Khalaji, A. The interplay between vitamin C and thyroid. Endocrinol. Diabetes Metab. 2023, 6, e432. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, E.; Que, H. Association of circulating vitamin levels with thyroid diseases: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1360851. [Google Scholar] [CrossRef]
- Fogarty, A.; Lewis, S.A.; Scrivener, S.L.; Antoniak, M.; Pacey, S.; Pringle, M.; Britton, J. Corticosteroid sparing effects of vitamin C and magnesium in asthma: A randomised trial. Respir. Med. 2006, 100, 174–179. [Google Scholar] [CrossRef]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Tribble, D.L.; Giuliano, L.J.; Fortmann, S.P. Reduced plasma ascorbic acid concentrations in nonsmokers regularly exposed to environmental tobacco smoke. Am. J. Clin. Nutr. 1993, 58, 886–890. [Google Scholar] [CrossRef]
- Schectman, G.; Byrd, J.C.; Gruchow, H.W. The influence of smoking on vitamin C status in adults. Am. J. Public Health 1989, 79, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Schectman, G.; Byrd, J.C.; Hoffmann, R. Ascorbic acid requirements for smokers: Analysis of a population survey. Am. J. Clin. Nutr. 1991, 53, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.M.; Rodriguez, C.; Rivera, C.E.; Sahai, H. Influence of environmental tobacco smoke on vitamin C status in children. Am. J. Clin. Nutr. 2003, 77, 167–172. [Google Scholar] [CrossRef]
- Abuhajar, S.M.; Taleb, M.H.; Ellulu, M.S. Vitamin C deficiency and risk of metabolic complications among adults with chronic respiratory diseases: A case-control study. Clin. Nutr. ESPEN 2021, 43, 448–455. [Google Scholar] [CrossRef]
- Salo, P.M.; Mendy, A.; Wilkerson, J.; Molsberry, S.A.; Feinstein, L.; London, S.J.; Fessler, M.B.; Thorne, P.S.; Zeldin, D.C. Serum antioxidant vitamins and respiratory morbidity and mortality: A pooled analysis. Respir. Res. 2022, 23, 150. [Google Scholar] [CrossRef]
- Schwartz, J.; Weiss, S.T. Dietary factors and their relation to respiratory symptoms. The Second National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1990, 132, 67–76. [Google Scholar] [CrossRef]
- Pearson, P.; Britton, J.; McKeever, T.; Lewis, S.A.; Weiss, S.; Pavord, I.; Fogarty, A. Lung function and blood levels of copper, selenium, vitamin C and vitamin E in the general population. Eur. J. Clin. Nutr. 2005, 59, 1043–1048. [Google Scholar] [CrossRef]
- Ochs-Balcom, H.M.; Grant, B.J.; Muti, P.; Sempos, C.T.; Freudenheim, J.L.; Browne, R.W.; McCann, S.E.; Trevisan, M.; Cassano, P.A.; Iacoviello, L.; et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur. J. Clin. Nutr. 2006, 60, 991–999. [Google Scholar] [CrossRef]
- Sargeant, L.A.; Jaeckel, A.; Wareham, N.J. Interaction of vitamin C with the relation between smoking and obstructive airways disease in EPIC Norfolk. European Prospective Investigation into Cancer and Nutrition. Eur. Respir. J. 2000, 16, 397–403. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Chen, J.; Peto, R.; Campbell, T.C.; Cassano, P.A. Dietary vitamin C intake and lung function in rural China. Am. J. Epidemiol. 1998, 148, 594–599. [Google Scholar] [CrossRef]
- Grievink, L.; Smit, H.A.; Ocke, M.C.; van’t Veer, P.; Kromhout, D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: The MORGEN study. Thorax 1998, 53, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Byun, M.K.; Kim, H.J.; Kim, J.Y.; Kim, Y.I.; Yoo, K.H.; Chun, E.M.; Jung, J.Y.; Lee, S.H.; Ahn, C.M. Dietary vitamin C intake protects against COPD: The Korea National Health and Nutrition Examination Survey in 2012. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2721–2728. [Google Scholar] [CrossRef]
- Schwartz, J.; Weiss, S.T. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). Am. J. Clin. Nutr. 1994, 59, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Teng, H.; Zhang, L.; Wu, L. Association between dietary antioxidant intakes and chronic respiratory diseases in adults. World Allergy Organ. J. 2024, 17, 100851. [Google Scholar] [CrossRef]
- Butland, B.K.; Fehily, A.M.; Elwood, P.C. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax 2000, 55, 102–108. [Google Scholar] [CrossRef]
- Cook, D.G.; Carey, I.M.; Whincup, P.H.; Papacosta, O.; Chirico, S.; Bruckdorfer, K.R.; Walker, M. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax 1997, 52, 628–633. [Google Scholar] [CrossRef]
- Tabak, C.; Smit, H.A.; Rasanen, L.; Fidanza, F.; Menotti, A.; Nissinen, A.; Feskens, E.J.; Heederik, D.; Kromhout, D. Dietary factors and pulmonary function: A cross sectional study in middle aged men from three European countries. Thorax 1999, 54, 1021–1026. [Google Scholar] [CrossRef]
- Wu, T.C.; Huang, Y.C.; Hsu, S.Y.; Wang, Y.C.; Yeh, S.L. Vitamin E and vitamin C supplementation in patients with chronic obstructive pulmonary disease. Int. J. Vitam. Nutr. Res. 2007, 77, 272–279. [Google Scholar] [CrossRef]
- Dey, D.; Sengupta, S.; Bhattacharyya, P. Long-term use of Vitamin-C in chronic obstructive pulmonary disease: Early pilot observation. Lung India 2021, 38, 500–501. [Google Scholar] [CrossRef]
- Gouzi, F.; Maury, J.; Heraud, N.; Molinari, N.; Bertet, H.; Ayoub, B.; Blaquiere, M.; Bughin, F.; De Rigal, P.; Poulain, M.; et al. Additional Effects of Nutritional Antioxidant Supplementation on Peripheral Muscle during Pulmonary Rehabilitation in COPD Patients: A Randomized Controlled Trial. Oxidative Med. Cell. Longev. 2019, 2019, 5496346. [Google Scholar] [CrossRef]
- Hureau, T.J.; Weavil, J.C.; Sidhu, S.K.; Thurston, T.S.; Reese, V.R.; Zhao, J.; Nelson, A.D.; Birgenheier, N.M.; Richardson, R.S.; Amann, M. Ascorbate attenuates cycling exercise-induced neuromuscular fatigue but fails to improve exertional dyspnea and exercise tolerance in COPD. J. Appl. Physiol. 2021, 130, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Isbaniah, F.; Wiyono, W.H.; Yunus, F.; Setiawati, A.; Totzke, U.; Verbruggen, M.A. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: Results from a randomized controlled trial. J. Clin. Pharm. Ther. 2011, 36, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Pirabbasi, E.; Shahar, S.; Manaf, Z.A.; Rajab, N.F.; Manap, R.A. Efficacy of Ascorbic Acid (Vitamin C) and/N-Acetylcysteine (NAC) Supplementation on Nutritional and Antioxidant Status of Male Chronic Obstructive Pulmonary Disease (COPD) Patients. J. Nutr. Sci. Vitaminol. 2016, 62, 54–61. [Google Scholar] [CrossRef]
- Lei, T.; Lu, T.; Yu, H.; Su, X.; Zhang, C.; Zhu, L.; Yang, K.; Liu, J. Efficacy of Vitamin C Supplementation on Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2201–2216. [Google Scholar] [CrossRef]
- Kallner, A.B.; Hartmann, D.; Hornig, D.H. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. Am. J. Clin. Nutr. 1981, 34, 1347–1355. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Kallner, A.; Hartmann, D.; Hornig, D. Steady-state turnover and body pool of ascorbic acid in man. Am. J. Clin. Nutr. 1979, 32, 530–539. [Google Scholar] [CrossRef]
- Zhang, P.; Omaye, S.T. Antioxidant and prooxidant roles for beta-carotene, alpha-tocopherol and ascorbic acid in human lung cells. Toxicol. In Vitr. 2001, 15, 13–24. [Google Scholar] [CrossRef]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin C exhibits pro-oxidant properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Oliveri, B.; Mastaglia, S.R.; Brito, G.M.; Seijo, M.; Keller, G.A.; Somoza, J.; Diez, R.A.; Di Girolamo, G. Vitamin D3 seems more appropriate than D2 to sustain adequate levels of 25OHD: A pharmacokinetic approach. Eur. J. Clin. Nutr. 2015, 69, 697–702. [Google Scholar] [CrossRef]
- Nimitphong, H.; Saetung, S.; Chanprasertyotin, S.; Chailurkit, L.O.; Ongphiphadhanakul, B. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D3 or D2supplementation. Nutr. J. 2013, 12, 39. [Google Scholar] [CrossRef]
- Hollis, B.W. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J. Steroid Biochem. 1984, 21, 81–86. [Google Scholar] [CrossRef]
- Holick, M.F.; MacLaughlin, J.A.; Doppelt, S.H. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science 1981, 211, 590–593. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderon, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed]
- El Abd, A.; Dasari, H.; Dodin, P.; Trottier, H.; Ducharme, F.M. The effects of vitamin D supplementation on inflammatory biomarkers in patients with asthma: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 2024, 15, 1335968. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Ramirez-Mejia, J.M.; Urcuqui-Inchima, S. Vitamin D boosts immune response of macrophages through a regulatory network of microRNAs and mRNAs. J. Nutr. Biochem. 2022, 109, 109105. [Google Scholar] [CrossRef]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Plaza Cajide, A.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef]
- Asghari, S.; Hamedi-Shahraki, S.; Amirkhizi, F. Vitamin D status and systemic redox biomarkers in adults with obesity. Clin. Nutr. ESPEN 2021, 45, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Alamro, A.A.; Alsulami, E.A.; Almutlaq, M.; Alghamedi, A.; Alokail, M.; Haq, S.H. Therapeutic Potential of Vitamin D and Curcumin in an In Vitro Model of Alzheimer Disease. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520924311. [Google Scholar] [CrossRef]
- Gimenez, V.M.M.; Sanz, R.L.; Maron, F.J.M.; Ferder, L.; Manucha, W. Vitamin D-RAAS Connection: An Integrative Standpoint into Cardiovascular and Neuroinflammatory Disorders. Curr. Protein Pept. Sci. 2020, 21, 948–954. [Google Scholar] [CrossRef]
- Han, L.; Xu, X.J.; Zhang, J.S.; Liu, H.M. Association between Vitamin D Deficiency and Levels of Renin and Angiotensin in Essential Hypertension. Int. J. Clin. Pract. 2022, 2022, 8975396. [Google Scholar] [CrossRef]
- Heulens, N.; Korf, H.; Janssens, W. Innate immune modulation in chronic obstructive pulmonary disease: Moving closer toward vitamin D therapy. J. Pharmacol. Exp. Ther. 2015, 353, 360–368. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, J.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parekh, D.; Gao-Smith, F.; Thickett, D.R.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-beta induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 177, 113955. [Google Scholar] [CrossRef]
- Mandell, E.W.; Ryan, S.; Seedorf, G.J.; Gonzalez, T.; Smith, B.J.; Fleet, J.C.; Abman, S.H. Maternal Vitamin D Deficiency Causes Sustained Impairment of Lung Structure and Function and Increases Susceptibility to Hyperoxia-induced Lung Injury in Infant Rats. Am. J. Respir. Cell Mol. Biol. 2020, 63, 79–91. [Google Scholar] [CrossRef]
- Saadoon, A.; Ambalavanan, N.; Zinn, K.; Ashraf, A.P.; MacEwen, M.; Nicola, T.; Fanucchi, M.V.; Harris, W.T. Effect of Prenatal versus Postnatal Vitamin D Deficiency on Pulmonary Structure and Function in Mice. Am. J. Respir. Cell Mol. Biol. 2017, 56, 383–392. [Google Scholar] [CrossRef]
- Lykkedegn, S.; Sorensen, G.L.; Beck-Nielsen, S.S.; Pilecki, B.; Duelund, L.; Marcussen, N.; Christesen, H.T. Vitamin D Depletion in Pregnancy Decreases Survival Time, Oxygen Saturation, Lung Weight and Body Weight in Preterm Rat Offspring. PLoS ONE 2016, 11, e0155203. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.E.; Bosco, A.; Jones, A.C.; Gout, A.; Gorman, S.; Hart, P.H.; Zosky, G.R. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am. J. Respir. Cell Mol. Biol. 2015, 53, 664–675. [Google Scholar] [CrossRef]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, e167–e173. [Google Scholar] [CrossRef] [PubMed]
- Heulens, N.; Korf, H.; Cielen, N.; De Smidt, E.; Maes, K.; Gysemans, C.; Verbeken, E.; Gayan-Ramirez, G.; Mathieu, C.; Janssens, W. Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice. Respir. Res. 2015, 16, 110. [Google Scholar] [CrossRef]
- Cielen, N.; Heulens, N.; Maes, K.; Carmeliet, G.; Mathieu, C.; Janssens, W.; Gayan-Ramirez, G. Vitamin D deficiency impairs skeletal muscle function in a smoking mouse model. J. Endocrinol. 2016, 229, 97–108. [Google Scholar] [CrossRef]
- Sundar, I.K.; Hwang, J.W.; Wu, S.; Sun, J.; Rahman, I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem. Biophys. Res. Commun. 2011, 406, 127–133. [Google Scholar] [CrossRef]
- Kaneko, I.; Sabir, M.S.; Dussik, C.M.; Whitfield, G.K.; Karrys, A.; Hsieh, J.C.; Haussler, M.R.; Meyer, M.B.; Pike, J.W.; Jurutka, P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023–4035. [Google Scholar] [CrossRef]
- Cui, X.; Pertile, R.; Liu, P.; Eyles, D.W. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 2015, 304, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Guillet, C.; Vaes, A.M.; Brouwer-Brolsma, E.M.; Rocher, C.; Giraudet, C.; Patrac, V.; Meugnier, E.; Montaurier, C.; et al. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: Role in sarcopenia. Commun. Biol. 2022, 5, 1288. [Google Scholar] [CrossRef]
- Acharya, M.; Singh, N.; Gupta, G.; Tambuwala, M.M.; Aljabali, A.A.A.; Chellappan, D.K.; Dua, K.; Goyal, R. Vitamin D, Calbindin, and calcium signaling: Unraveling the Alzheimer’s connection. Cell. Signal. 2024, 116, 111043. [Google Scholar] [CrossRef]
- Kido, S.; Kaneko, I.; Tatsumi, S.; Segawa, H.; Miyamoto, K. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib. Nephrol. 2013, 180, 86–97. [Google Scholar] [CrossRef]
- Farzaneh-Far, A.; Weissberg, P.L.; Proudfoot, D.; Shanahan, C.M. Transcriptional regulation of matrix gla protein. Z. Kardiol. 2001, 90 (Suppl. S3), 38–42. [Google Scholar] [CrossRef]
- Han, Y.Y.; Hsu, S.H.; Su, T.C. Association between Vitamin D Deficiency and High Serum Levels of Small Dense LDL in Middle-Aged Adults. Biomedicines 2021, 9, 464. [Google Scholar] [CrossRef]
- Sabico, S.; Wani, K.; Grant, W.B.; Al-Daghri, N.M. Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients 2023, 15, 551. [Google Scholar] [CrossRef]
- Tishkoff, D.X.; Nibbelink, K.A.; Holmberg, K.H.; Dandu, L.; Simpson, R.U. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008, 149, 558–564. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.D.; Berry, J.E.; Jarvis, A.K.; Somerman, M.J.; Simpson, R.U. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am. J. Physiol. 1997, 272, H1751–H1758. [Google Scholar] [CrossRef]
- Heberden, C.; Denis, I.; Pointillart, A.; Mercier, T. TGF-beta and calcitriol. Gen. Pharmacol. 1998, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Alnimer, A.; Bhamidimarri, P.M.; Talaat, I.M.; Alkhayaal, N.; Eltayeb, A.; Ali, N.; Abusnana, S.; Hamoudi, R.; Bendardaf, R. Association Between Expression of Vitamin D Receptor and Insulin-Like Growth Factor 1 Receptor Among Breast Cancer Patients. World J. Oncol. 2023, 14, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Li, F.; Qiao, F.; Maimaititusvn, G.; Liu, F. Causal associations between insulin-like growth factor 1 and vitamin D levels: A two-sample bidirectional Mendelian randomization study. Front. Nutr. 2023, 10, 1162442. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Tang, J.; Kasiappan, R.; Jinwal, U.; Li, P.; Hann, S.; Nicosia, S.V.; Wu, J.; Zhang, X.; et al. The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells. Mol. Cell. Endocrinol. 2011, 338, 58–67. [Google Scholar] [CrossRef]
- Duque, G.; El Abdaimi, K.; Henderson, J.E.; Lomri, A.; Kremer, R. Vitamin D inhibits Fas ligand-induced apoptosis in human osteoblasts by regulating components of both the mitochondrial and Fas-related pathways. Bone 2004, 35, 57–64. [Google Scholar] [CrossRef]
- Gkotinakou, I.M.; Kechagia, E.; Pazaitou-Panayiotou, K.; Mylonis, I.; Liakos, P.; Tsakalof, A. Calcitriol Suppresses HIF-1 and HIF-2 Transcriptional Activity by Reducing HIF-1/2alpha Protein Levels via a VDR-Independent Mechanism. Cells 2020, 9, 2440. [Google Scholar] [CrossRef]
- Guzey, M.; Kitada, S.; Reed, J.C. Apoptosis induction by 1α,25-dihydroxyvitamin D3 in prostate cancer. Mol. Cancer Ther. 2002, 1, 667–677. [Google Scholar] [PubMed]
- Chu, C.; Tsuprykov, O.; Chen, X.; Elitok, S.; Kramer, B.K.; Hocher, B. Relationship Between Vitamin D and Hormones Important for Human Fertility in Reproductive-Aged Women. Front. Endocrinol. 2021, 12, 666687. [Google Scholar] [CrossRef] [PubMed]
- Kolcsar, M.; Berecki, B.; Gall, Z. Relationship between Serum 25-Hydroxyvitamin D Levels and Hormonal Status in Infertile Women: A Retrospective Study. Diagnostics 2023, 13, 3024. [Google Scholar] [CrossRef]
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Deleskog, A.; Hilding, A.; Brismar, K.; Hamsten, A.; Efendic, S.; Ostenson, C.G. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia 2012, 55, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Babic Leko, M.; Juresko, I.; Rozic, I.; Pleic, N.; Gunjaca, I.; Zemunik, T. Vitamin D and the Thyroid: A Critical Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 3586. [Google Scholar] [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Liu, K.; Wang, Y.; Liu, Q.; Sun, T.; Chen, S.; Ren, L. Smoking behavior and circulating vitamin D levels in adults: A meta-analysis. Food Sci. Nutr. 2021, 9, 5820–5832. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Patel, D.R.; Nicol, M.J.; Field, C.J.; Restori, K.H.; Wang, J.; Froelich, N.E.; Katkere, B.; Terwilliger, J.A.; Weaver, V.; et al. Vitamin D and the Ability to Produce 1,25(OH)(2)D Are Critical for Protection from Viral Infection of the Lungs. Nutrients 2022, 14, 3061. [Google Scholar] [CrossRef]
- Zhu, Z.; Wan, X.; Liu, J.; Zhang, D.; Luo, P.; Du, W.; Chen, L.; Su, J.; Hang, D.; Zhou, J.; et al. Vitamin D status and chronic obstructive pulmonary disease risk: A prospective UK Biobank study. BMJ Open Respir. Res. 2023, 10, e001684. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bartz, T.M.; Chittoor, G.; Eiriksdottir, G.; Manichaikul, A.W.; Sun, F.; Terzikhan, N.; Zhou, X.; Booth, S.L.; Brusselle, G.G.; et al. Meta-analysis across Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium provides evidence for an association of serum vitamin D with pulmonary function. Br. J. Nutr. 2018, 120, 1159–1170. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, T.; Wang, C.; Ji, Y. The association between vitamin D and COPD risk, severity, and exacerbation: An updated systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2597–2607. [Google Scholar] [CrossRef]
- Hu, G.; Dong, T.; Wang, S.; Jing, H.; Chen, J. Vitamin D(3)-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. eBioMedicine 2019, 45, 563–577. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, T.; Yao, L.; Xing, Y.; Zhao, X.; Fu, J.; Xue, X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci. Rep. 2017, 7, 3312. [Google Scholar] [CrossRef]
- Ghosh, A.J.; Moll, M.; Hayden, L.P.; Bon, J.; Regan, E.; Hersh, C.P.; Investigators, C.O. Vitamin D deficiency is associated with respiratory symptoms and airway wall thickening in smokers with and without COPD: A prospective cohort study. BMC Pulm. Med. 2020, 20, 123. [Google Scholar] [CrossRef]
- Zhao, G.; Ford, E.S.; Tsai, J.; Li, C.; Croft, J.B. Low concentrations of serum 25-hydroxyvitamin D associated with increased risk for chronic bronchitis among US adults. Br. J. Nutr. 2012, 107, 1386–1392. [Google Scholar] [CrossRef]
- Yang, M.; Pang, B.; Wang, Q.; Zhang, Z.; Niu, W. The causal association between genetically regulated 25OHD and chronic obstructive pulmonary disease: A meta-analysis and Mendelian randomization study. Front. Genet. 2022, 13, 932764. [Google Scholar] [CrossRef]
- Khanna, R.; Nandy, D.; Senapati, S. Systematic Review and Meta-Analysis to Establish the Association of Common Genetic Variations in Vitamin D Binding Protein with Chronic Obstructive Pulmonary Disease. Front. Genet. 2019, 10, 413. [Google Scholar] [CrossRef]
- Wang, Y.L.; Kong, H.; Xie, W.P.; Wang, H. Association of vitamin D-binding protein variants with chronic obstructive pulmonary disease: A meta-analysis. Genet. Mol. Res. 2015, 14, 10774–10785. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Wang, T.; Zhu, T.; Wen, F. Dual role of vitamin D-binding protein 1F allele in chronic obstructive pulmonary disease susceptibility: A meta-analysis. Genet. Mol. Res. 2015, 14, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Horita, N.; Miyazawa, N.; Tomaru, K.; Inoue, M.; Ishigatsubo, Y.; Kaneko, T. Vitamin D binding protein genotype variants and risk of chronic obstructive pulmonary disease: A meta-analysis. Respirology 2015, 20, 219–225. [Google Scholar] [CrossRef]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Yu, M.; Sun, J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020, 9, 286–297. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; Global Initiative for Chronic Obstructive Lung Disease: Deer Park, IL, USA, 2024.
- Hua, Y.; Jiang, T.; Feng, J.; Zou, M. Negligible effect of vitamin D supplementation on exacerbation in patients with chronic obstructive pulmonary disease: Meta-analysis. Biochem. Med. 2023, 33, 030703. [Google Scholar] [CrossRef]
- Bleizgys, A. Zinc, Magnesium and Vitamin K Supplementation in Vitamin D Deficiency: Pathophysiological Background and Implications for Clinical Practice. Nutrients 2024, 16, 834. [Google Scholar] [CrossRef] [PubMed]
- Melissa Crooks, S.B. Parathyroid hormone and vitamin D: From bench to bedside. Orthop. Trauma 2021, 35, 267–273. [Google Scholar]
- Hunt, C.D.; Herbel, J.L.; Idso, J.P. Dietary boron modifies the effects of vitamin D3 nutrition on indices of energy substrate utilization and mineral metabolism in the chick. J. Bone Miner. Res. 1994, 9, 171–182. [Google Scholar] [CrossRef]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv. Exp. Med. Biol. 2008, 624, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Chan, P.C.; MacFarlane, G.D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001, 73, 288–294. [Google Scholar] [CrossRef]
- Wakeman, M. A Literature Review of the Potential Impact of Medication on Vitamin D Status. Risk Manag. Healthc. Policy 2021, 14, 3357–3381. [Google Scholar] [CrossRef]
- Schrumpf, J.A.; Amatngalim, G.D.; Veldkamp, J.B.; Verhoosel, R.M.; Ninaber, D.K.; Ordonez, S.R.; van der Does, A.M.; Haagsman, H.P.; Hiemstra, P.S. Proinflammatory Cytokines Impair Vitamin D-Induced Host Defense in Cultured Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2017, 56, 749–761. [Google Scholar] [CrossRef]
- Noyola-Martinez, N.; Diaz, L.; Zaga-Clavellina, V.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Regulation of CYP27B1 and CYP24A1 gene expression by recombinant pro-inflammatory cytokines in cultured human trophoblasts. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 106–109. [Google Scholar] [CrossRef]
- Hummel, D.M.; Fetahu, I.S.; Groschel, C.; Manhardt, T.; Kallay, E. Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 91–95. [Google Scholar] [CrossRef]
- Raymond-Lezman, J.R.; Riskin, S.I. Benefits and Risks of Sun Exposure to Maintain Adequate Vitamin D Levels. Cureus 2023, 15, e38578. [Google Scholar] [CrossRef] [PubMed]
- Rubsamen, D.; Kunze, M.M.; Buderus, V.; Brauss, T.F.; Bajer, M.M.; Brune, B.; Schmid, T. Inflammatory conditions induce IRES-dependent translation of cyp24a1. PLoS ONE 2014, 9, e85314. [Google Scholar] [CrossRef]
- Wu, Z.; Camargo, C.A., Jr.; Reid, I.R.; Beros, A.; Sluyter, J.D.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.T.; Scragg, R. What factors modify the effect of monthly bolus dose vitamin D supplementation on 25-hydroxyvitamin D concentrations? J. Steroid Biochem. Mol. Biol. 2020, 201, 105687. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar]
- Kaiser, S.; Di Mascio, P.; Murphy, M.E.; Sies, H. Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Arch. Biochem. Biophys. 1990, 277, 101–108. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.V.; Franke, A.A.; Harwood, P.J.; Hatch-Pigott, V.; Custer, L.J.; Mordan, L.J. Gamma-tocopherol detoxification of nitrogen dioxide: Superiority to alpha-tocopherol. Proc. Natl. Acad. Sci. USA 1993, 90, 1771–1775. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content. Stresses 2022, 2, 213–230. [Google Scholar] [CrossRef]
- Yan, M.; Xu, S.; Wang, H.; Dong, S.; Mo, C. Ferroptosis in chronic obstructive pulmonary disease: From cellular mechanisms to therapeutic applications. Chin. Med. J. 2024, 137, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, L.M.; Zhang, X.J.; Chu, B. Targeting ferroptosis as a vulnerability in pulmonary diseases. Cell Death Dis. 2022, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, Y.; Lou, H.; Ou, Z.; Liu, J.; Duan, W.; Wang, H.; Ge, Y.; Min, J.; Wang, F.; et al. GPX4 and vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis. 2021, 12, 706. [Google Scholar] [CrossRef]
- Meng, D.; Zhu, C.; Jia, R.; Li, Z.; Wang, W.; Song, S. The molecular mechanism of ferroptosis and its role in COPD. Front. Med. 2022, 9, 1052540. [Google Scholar] [CrossRef]
- Marko, M.G.; Ahmed, T.; Bunnell, S.C.; Wu, D.; Chung, H.; Huber, B.T.; Meydani, S.N. Age-associated decline in effective immune synapse formation of CD4+ T cells is reversed by vitamin E supplementation. J. Immunol. 2007, 178, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464–20469. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef]
- Wallert, M.; Kluge, S.; Schubert, M.; Koeberle, A.; Werz, O.; Birringer, M.; Lorkowski, S. Diversity of Chromanol and Chromenol Structures and Functions: An Emerging Class of Anti-Inflammatory and Anti-Carcinogenic Agents. Front. Pharmacol. 2020, 11, 362. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Manu, K.A.; Shanmugam, M.K.; Loo, S.Y.; Kumar, A.P.; Sethi, G. gamma-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br. J. Pharmacol. 2011, 163, 283–298. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Tasinato, A.; Clement, S.; Ozer, N.K.; Boscoboinik, D.; Azzi, A. alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 1998, 334 Pt 1, 243–249. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Kline, K.; Lawson, K.A.; Yu, W.; Sanders, B.G. Vitamin E and cancer. Vitam. Horm. 2007, 76, 435–461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Deshmukh, P.; Kumar, M.; Bhatt, A.; Sinha, A.H.; Chawla, P. Vitamin E Supplementation and Cardiovascular Health: A Comprehensive Review. Cureus 2023, 15, e48142. [Google Scholar] [CrossRef]
- Princen, H.M.; van Duyvenvoorde, W.; Buytenhek, R.; van der Laarse, A.; van Poppel, G.; Gevers Leuven, J.A.; van Hinsbergh, V.W. Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kiyose, C.; Yoshimura, H.; Ueda, T.; Kondo, K.; Igarashi, O. Gamma-tocotrienol, a vitamin E homolog, is a natriuretic hormone precursor. J. Lipid Res. 2003, 44, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M. Vitamin E: More than an antioxidant. Clin. Cardiol. 1993, 16, I16–I18. [Google Scholar] [CrossRef]
- Theriault, A.; Chao, J.T.; Gapor, A. Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis 2002, 160, 21–30. [Google Scholar] [CrossRef]
- Manolescu, B.; Atanasiu, V.; Cercasov, C.; Stoian, I.; Oprea, E.; Busu, C. So many options but one choice: The human body prefers alpha-tocopherol. A matter of stereochemistry. J. Med. Life 2008, 1, 376–382. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Aspects Med. 2007, 28, 692–728. [Google Scholar] [CrossRef]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New perspectives on vitamin E: γ-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef]
- Malik, A.; Eggersdorfer, M.; Trilok-Kumar, G. Vitamin E status in healthy population in Asia: A review of current literature. Int. J. Vitam. Nutr. Res. 2021, 91, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L., 3rd; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Maras, J.E.; Bermudez, O.I.; Qiao, N.; Bakun, P.J.; Boody-Alter, E.L.; Tucker, K.L. Intake of α-tocopherol is limited among US adults. J. Am. Diet. Assoc. 2004, 104, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Traber, M.G. Cigarette smoke alters human vitamin E requirements. J. Nutr. 2005, 135, 671–674. [Google Scholar] [CrossRef]
- Sobczak, A.; Golka, D.; Szoltysek-Boldys, I. The effects of tobacco smoke on plasma alpha- and gamma-tocopherol levels in passive and active cigarette smokers. Toxicol. Lett. 2004, 151, 429–437. [Google Scholar] [CrossRef]
- Karademirci, M.; Kutlu, R.; Kilinc, I. Relationship between smoking and total antioxidant status, total oxidant status, oxidative stress index, vit C, vit E. Clin. Respir. J. 2018, 12, 2006–2012. [Google Scholar] [CrossRef]
- Leonard, S.W.; Bruno, R.S.; Paterson, E.; Schock, B.C.; Atkinson, J.; Bray, T.M.; Cross, C.E.; Traber, M.G. 5-nitro-gamma-tocopherol increases in human plasma exposed to cigarette smoke in vitro and in vivo. Free Radic. Biol. Med. 2003, 35, 1560–1567. [Google Scholar] [CrossRef]
- al Senaidy, A.M.; al Zahrany, Y.A.; al Faqeeh, M.B. Effects of smoking on serum levels of lipid peroxides and essential fat-soluble antioxidants. Nutr. Health 1997, 12, 55–65. [Google Scholar] [CrossRef]
- Tug, T.; Karatas, F.; Terzi, S.M. Antioxidant vitamins (A, C and E) and malondialdehyde levels in acute exacerbation and stable periods of patients with chronic obstructive pulmonary disease. Clin. Investig. Med. 2004, 27, 123–128. [Google Scholar]
- Britton, J.R.; Pavord, I.D.; Richards, K.A.; Knox, A.J.; Wisniewski, A.F.; Lewis, S.A.; Tattersfield, A.E.; Weiss, S.T. Dietary antioxidant vitamin intake and lung function in the general population. Am. J. Respir. Crit. Care Med. 1995, 151, 1383–1387. [Google Scholar] [CrossRef]
- Rautalahti, M.; Virtamo, J.; Haukka, J.; Heinonen, O.P.; Sundvall, J.; Albanes, D.; Huttunen, J.K. The effect of alpha-tocopherol and beta-carotene supplementation on COPD symptoms. Am. J. Respir. Crit. Care Med. 1997, 156, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Campos, H.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin. Nutr. 2016, 35, 169–174. [Google Scholar] [CrossRef]
- Marchese, M.E.; Kumar, R.; Colangelo, L.A.; Avila, P.C.; Jacobs, D.R., Jr.; Gross, M.; Sood, A.; Liu, K.; Cook-Mills, J.M. The vitamin E isoforms alpha-tocopherol and gamma-tocopherol have opposite associations with spirometric parameters: The CARDIA study. Respir. Res. 2014, 15, 31. [Google Scholar] [CrossRef]
- Nadeem, A.; Raj, H.G.; Chhabra, S.K. Effect of vitamin E supplementation with standard treatment on oxidant-antioxidant status in chronic obstructive pulmonary disease. Indian. J. Med. Res. 2008, 128, 705–711. [Google Scholar]
- Habib, M.P.; Tank, L.J.; Lane, L.C.; Garewal, H.S. Effect of vitamin E on exhaled ethane in cigarette smokers. Chest 1999, 115, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Agler, A.H.; Kurth, T.; Gaziano, J.M.; Buring, J.E.; Cassano, P.A. Randomised vitamin E supplementation and risk of chronic lung disease in the Women’s Health Study. Thorax 2011, 66, 320–325. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.D.; Chan, T.K.; Pow, C.W.; Foster, P.S.; Wong, W.S.F. Vitamin E isoform gamma-tocotrienol protects against emphysema in cigarette smoke-induced COPD. Free Radic. Biol. Med. 2017, 110, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.; Conrad, W.; Adhya, A.; Zhang, S.; Vong, C.I.; Ji, X. Ability of γ-tocotrienol to Mitigate Inflammation and Preserve Lung Function in a Model of E-Cigarette Induced Chronic Obstructive Pulmonary Disease. Curr. Dev. Nutr. 2020, 4, nzaa045_070. [Google Scholar]
- Pei, R.; Mah, E.; Leonard, S.W.; Traber, M.G.; Bruno, R.S. alpha-Tocopherol supplementation reduces 5-nitro-gamma-tocopherol accumulation by decreasing gamma-tocopherol in young adult smokers. Free Radic. Res. 2015, 49, 1114–1121. [Google Scholar] [CrossRef]
- Pandey, R.; Singh, M.; Singhal, U.; Gupta, K.B.; Aggarwal, S.K. Oxidative/Nitrosative stress and the pathobiology of chronic obstructive pulmonary disease. J. Clin. Diagn. Res. 2013, 7, 580–588. [Google Scholar] [CrossRef]
- Huang, H.Y.; Appel, L.J. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J. Nutr. 2003, 133, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Vitamin E Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 20 January 2025).
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [CrossRef]
- Conly, J.M.; Stein, K.; Worobetz, L.; Rutledge-Harding, S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am. J. Gastroenterol. 1994, 89, 915–923. [Google Scholar] [PubMed]
- Hao, Z.; Jin, D.Y.; Stafford, D.W.; Tie, J.K. Vitamin K-dependent carboxylation of coagulation factors: Insights from a cell-based functional study. Haematologica 2020, 105, 2164–2173. [Google Scholar] [CrossRef]
- Mishima, E.; Wahida, A.; Seibt, T.; Conrad, M. Diverse biological functions of vitamin K: From coagulation to ferroptosis. Nat. Metab. 2023, 5, 924–932. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cancela, M.L.; Price, P.A. Retinoic acid induces matrix Gla protein gene expression in human cells. Endocrinology 1992, 130, 102–108. [Google Scholar] [CrossRef]
- Galunska, B.; Yotov, Y.; Nikolova, M.; Angelov, A. Extrahepatic Vitamin K-Dependent Gla-Proteins-Potential Cardiometabolic Biomarkers. Int. J. Mol. Sci. 2024, 25, 3517. [Google Scholar] [CrossRef]
- Khavandgar, Z.; Roman, H.; Li, J.; Lee, S.; Vali, H.; Brinckmann, J.; Davis, E.C.; Murshed, M. Elastin haploinsufficiency impedes the progression of arterial calcification in MGP-deficient mice. J. Bone Miner. Res. 2014, 29, 327–337. [Google Scholar] [CrossRef]
- Koshihara, Y.; Hoshi, K.; Okawara, R.; Ishibashi, H.; Yamamoto, S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J. Endocrinol. 2003, 176, 339–348. [Google Scholar] [CrossRef]
- Urayama, S.; Kawakami, A.; Nakashima, T.; Tsuboi, M.; Yamasaki, S.; Hida, A.; Ichinose, Y.; Nakamura, H.; Ejima, E.; Aoyagi, T.; et al. Effect of vitamin K2 on osteoblast apoptosis: Vitamin K2 inhibits apoptotic cell death of human osteoblasts induced by Fas, proteasome inhibitor, etoposide, and staurosporine. J. Lab. Clin. Med. 2000, 136, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, H.; Otsuki, T.; Tomita, M.; Fukunaga, M.; Fukunaga, T.; Suzuki, N.; Saijoh, K.; Fushimi, S.; Sunami, S. Menaquinone-7 regulates the expressions of osteocalcin, OPG, RANKL and RANK in osteoblastic MC3T3E1 cells. Int. J. Mol. Med. 2005, 15, 231–236. [Google Scholar] [PubMed]
- Akbari, S.; Rasouli-Ghahroudi, A.A. Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. Biomed. Res. Int. 2018, 2018, 4629383. [Google Scholar] [CrossRef]
- Saputra, W.D.; Aoyama, N.; Komai, M.; Shirakawa, H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 2317. [Google Scholar] [CrossRef]
- Xia, J.; Matsuhashi, S.; Hamajima, H.; Iwane, S.; Takahashi, H.; Eguchi, Y.; Mizuta, T.; Fujimoto, K.; Kuroda, S.; Ozaki, I. The role of PKC isoforms in the inhibition of NF-kappaB activation by vitamin K2 in human hepatocellular carcinoma cells. J. Nutr. Biochem. 2012, 23, 1668–1675. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-kappaB activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar] [CrossRef]
- Pan, M.H.; Maresz, K.; Lee, P.S.; Wu, J.C.; Ho, C.T.; Popko, J.; Mehta, D.S.; Stohs, S.J.; Badmaev, V. Inhibition of TNF-alpha, IL-1alpha, and IL-1beta by Pretreatment of Human Monocyte-Derived Macrophages with Menaquinone-7 and Cell Activation with TLR Agonists In Vitro. J. Med. Food 2016, 19, 663–669. [Google Scholar] [CrossRef]
- Shibayama-Imazu, T.; Aiuchi, T.; Nakaya, K. Vitamin K2-mediated apoptosis in cancer cells: Role of mitochondrial transmembrane potential. Vitam. Horm. 2008, 78, 211–226. [Google Scholar] [CrossRef]
- Vervoort, L.M.; Ronden, J.E.; Thijssen, H.H. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem. Pharmacol. 1997, 54, 871–876. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, X.; Li, R. Ferroptosis Induced by Pollutants: An Emerging Mechanism in Environmental Toxicology. Environ. Sci. Technol. 2024, 58, 2166–2184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, D.; Chen, X.; Li, X.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Chen, X.; Tang, D.; Liu, J. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy 2023, 19, 1982–1996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, S.; He, G.; Wang, C.; Ou, C. Ferroptosis at the crossroads of manganese-induced neurotoxicity: A retrospective study. Toxicology 2024, 502, 153727. [Google Scholar] [CrossRef]
- Choi, J.A.; Lee, E.H.; Cho, H.; Kim, J.H. High-Dose Selenium Induces Ferroptotic Cell Death in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 1918. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Jespersen, T.; Kampmann, F.B.; Dantoft, T.M.; Jorgensen, N.R.; Karhus, L.L.; Madsen, F.; Linneberg, A.; Thysen, S.M. The association of vitamin K status with lung function and disease in a general population. ERJ Open Res. 2023, 9, 00208–2023. [Google Scholar] [CrossRef]
- Piscaer, I.; van den Ouweland, J.M.W.; Vermeersch, K.; Reynaert, N.L.; Franssen, F.M.E.; Keene, S.; Wouters, E.F.M.; Janssens, W.; Vermeer, C.; Janssen, R. Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 1116. [Google Scholar] [CrossRef]
- Piscaer, I.; Janssen, R.; Franssen, F.M.E.; Schurgers, L.J.; Wouters, E.F.M. The Pleiotropic Role of Vitamin K in Multimorbidity of Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2023, 12, 1261. [Google Scholar] [CrossRef]
- Piscaer, I.; Wouters, E.F.M.; Vermeer, C.; Janssens, W.; Franssen, F.M.E.; Janssen, R. Vitamin K deficiency: The linking pin between COPD and cardiovascular diseases? Respir. Res. 2017, 18, 189. [Google Scholar] [CrossRef]
- Booth, S.L.; Al Rajabi, A. Determinants of vitamin K status in humans. Vitam. Horm. 2008, 78, 1–22. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moyses, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef]
- Riphagen, I.J.; Keyzer, C.A.; Drummen, N.E.A.; de Borst, M.H.; Beulens, J.W.J.; Gansevoort, R.T.; Geleijnse, J.M.; Muskiet, F.A.J.; Navis, G.; Visser, S.T.; et al. Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study. Nutrients 2017, 9, 1334. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.; Hamulyak, K.; Knapen, M.H.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef]
- Dowd, P.; Zheng, Z.B. On the mechanism of the anticlotting action of vitamin E quinone. Proc. Natl. Acad. Sci. USA 1995, 92, 8171–8175. [Google Scholar] [CrossRef]
- Woolley, D.W. Some Biological Effects Produced by α-Tocopherol Quinone. J. Biol. Chem. 1945, 159, 59–66. [Google Scholar]
- Booth, S.L.; Golly, I.; Sacheck, J.M.; Roubenoff, R.; Dallal, G.E.; Hamada, K.; Blumberg, J.B. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am. J. Clin. Nutr. 2004, 80, 143–148. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E and K interactions--a 50-year-old problem. Nutr. Rev. 2008, 66, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Gell, D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018, 70, 13–42. [Google Scholar] [CrossRef]
- Galy, B.; Ferring-Appel, D.; Sauer, S.W.; Kaden, S.; Lyoumi, S.; Puy, H.; Kolker, S.; Grone, H.J.; Hentze, M.W. Iron regulatory proteins secure mitochondrial iron sufficiency and function. Cell Metab. 2010, 12, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xu, B.; Xu, Y.; Zhang, K.; Zhu, W.; Lian, X.; Chen, Z.; Wang, M.; Liu, L.; Guo, Z. Serum iron level is independently associated with sarcopenia: A retrospective study. Sci. Rep. 2024, 14, 10554. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Karakus, Y.Y. Typical Catalases: Function and Structure. In Glutathione System and Oxidative Stress in Health and Disease; BoD—Books on Demand: Norderstedt, Germany, 2020. [Google Scholar]
- Isaac, I.S.; Dawson, J.H. Haem iron-containing peroxidases. Essays Biochem. 1999, 34, 51–69. [Google Scholar] [CrossRef]
- Myllyharju, J.; Kivirikko, K.I. Characterization of the iron- and 2-oxoglutarate-binding sites of human prolyl 4-hydroxylase. EMBO J. 1997, 16, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Scietti, L.; Chiapparino, A.; De Giorgi, F.; Fumagalli, M.; Khoriauli, L.; Nergadze, S.; Basu, S.; Olieric, V.; Cucca, L.; Banushi, B.; et al. Molecular architecture of the multifunctional collagen lysyl hydroxylase and glycosyltransferase LH3. Nat. Commun. 2018, 9, 3163. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Lukianova, O.A.; David, S.S. A role for iron-sulfur clusters in DNA repair. Curr. Opin. Chem. Biol. 2005, 9, 145–151. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef]
- Jacobs, A.; Rhodes, J.; Eakins, J.D. Gastric Factors Influencing Iron Absorption in Anaemic Patients. Scand. J. Haematol. 1967, 4, 105–110. [Google Scholar] [CrossRef]
- Tandara, L.; Grubisic, T.Z.; Ivan, G.; Jurisic, Z.; Tandara, M.; Gugo, K.; Mladinov, S.; Salamunic, I. Systemic inflammation up-regulates serum hepcidin in exacerbations and stabile chronic obstructive pulmonary disease. Clin. Biochem. 2015, 48, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Nickol, A.H.; Frise, M.C.; Cheng, H.Y.; McGahey, A.; McFadyen, B.M.; Harris-Wright, T.; Bart, N.K.; Curtis, M.K.; Khandwala, S.; O’Neill, D.P.; et al. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open 2015, 5, e007911. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Renal Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Martin-Ontiyuelo, C.; Rodo-Pin, A.; Sancho-Munoz, A.; Martinez-Llorens, J.M.; Admetllo, M.; Molina, L.; Gea, J.; Barreiro, E.; Chiaradia, D.A.R. Is iron deficiency modulating physical activity in COPD? Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 211–214. [Google Scholar] [CrossRef]
- Perez-Peiro, M.; Martin-Ontiyuelo, C.; Rodo-Pi, A.; Piccari, L.; Admetllo, M.; Duran, X.; Rodriguez-Chiaradia, D.A.; Barreiro, E. Iron Replacement and Redox Balance in Non-Anemic and Mildly Anemic Iron Deficiency COPD Patients: Insights from a Clinical Trial. Biomedicines 2021, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Grasmuk-Siegl, E.; Urban, M.H.; Scherrer, S.; Funk, G.C. Effect of intravenous ferric carboxymaltose on exercise capacity and quality of life in patients with COPD: A pilot study. Wien. Klin. Wochenschr. 2023, 135, 35–44. [Google Scholar] [CrossRef]
- Martin-Ontiyuelo, C.; Rodo-Pin, A.; Echeverria-Esnal, D.; Admetllo, M.; Duran-Jorda, X.; Alvarado, M.; Gea, J.; Barreiro, E.; Rodriguez-Chiaradia, D.A. Intravenous Iron Replacement Improves Exercise Tolerance in COPD: A Single-Blind Randomized Trial. Arch. Bronconeumol. 2022, 58, 689–698. [Google Scholar] [CrossRef]
- Puntarulo, S. Iron, oxidative stress and human health. Mol. Aspects Med. 2005, 26, 299–312. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A.; Kubicki, J.D.; Meskhidze, N. A perspective on iron (Fe) in the atmosphere: Air quality, climate, and the ocean. Environ. Sci. Process Impacts 2023, 25, 151–164. [Google Scholar] [CrossRef]
- Thompson, A.B.; Bohling, T.; Heires, A.; Linder, J.; Rennard, S.I. Lower respiratory tract iron burden is increased in association with cigarette smoking. J. Lab. Clin. Med. 1991, 117, 493–499. [Google Scholar]
- Ghio, A.J.; Hilborn, E.D.; Stonehuerner, J.G.; Dailey, L.A.; Carter, J.D.; Richards, J.H.; Crissman, K.M.; Foronjy, R.F.; Uyeminami, D.L.; Pinkerton, K.E. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am. J. Respir. Crit. Care Med. 2008, 178, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Mumby, S.; Saito, J.; Adcock, I.M.; Chung, K.F.; Quinlan, G.J. Decreased breath excretion of redox active iron in COPD: A protective failure? Eur. Respir. J. 2016, 47, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Philippot, Q.; Deslee, G.; Adair-Kirk, T.L.; Woods, J.C.; Byers, D.; Conradi, S.; Dury, S.; Perotin, J.M.; Lebargy, F.; Cassan, C.; et al. Increased iron sequestration in alveolar macrophages in chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e96285. [Google Scholar] [CrossRef]
- Betul Altinisik, H.; Kirdemir, P.; Altinisik, U.; Gokalp, O. Effects of magnesium sulfate on airway smooth muscle contraction in rats. Med. Glas. 2016, 13, 68–74. [Google Scholar] [CrossRef]
- Janssen, R. Magnesium to counteract elastin degradation and vascular calcification in chronic obstructive pulmonary disease. Med. Hypotheses 2017, 107, 74–77. [Google Scholar] [CrossRef]
- Kistemaker, L.E.; Gosens, R. Acetylcholine beyond bronchoconstriction: Roles in inflammation and remodeling. Trends Pharmacol. Sci. 2015, 36, 164–171. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxidative Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, L.; Tian, C.; Qian, B. Magnesium isoglycyrrhizinate inhibits airway inflammation in rats with chronic obstructive pulmonary disease. BMC Pulm. Med. 2021, 21, 371. [Google Scholar] [CrossRef]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef]
- Calviello, G.; Ricci, P.; Lauro, L.; Palozza, P.; Cittadini, A. Mg deficiency induces mineral content changes and oxidative stress in rats. Biochem. Mol. Biol. Int. 1994, 32, 903–911. [Google Scholar]
- Shivakumar, K.; Kumar, B.P. Magnesium deficiency enhances oxidative stress and collagen synthesis in vivo in the aorta of rats. Int. J. Biochem. Cell Biol. 1997, 29, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Kuzniar, A.; Mitura, P.; Kurys, P.; Szymonik-Lesiuk, S.; Florianczyk, B.; Stryjecka-Zimmer, M. The influence of hypomagnesemia on erythrocyte antioxidant enzyme defence system in mice. Biometals 2003, 16, 349–357. [Google Scholar] [CrossRef]
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007, 458, 73–89. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joosten, M.M.; de Borst, M.H.; Bakker, S.J.L. Magnesium and Blood Pressure: A Physiology-Based Approach. Adv. Chronic Kidney Dis. 2018, 25, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C. Magnesium and disturbances in carbohydrate metabolism. Diabetes Obes. Metab. 2015, 17, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, J.; Wang, L.; Li, S.; Zhang, Q.; Xiao, P.; Wang, K.; Zhuang, M.; Jiang, Y. Association between serum magnesium and blood lipids: Influence of type 2 diabetes and central obesity. Br. J. Nutr. 2018, 120, 250–258. [Google Scholar] [CrossRef]
- Sgrignani, J.; Magistrato, A. The structural role of Mg2+ ions in a class I RNA polymerase ribozyme: A molecular simulation study. J. Phys. Chem. B 2012, 116, 2259–2268. [Google Scholar] [CrossRef]
- Yang, L.; Arora, K.; Beard, W.A.; Wilson, S.H.; Schlick, T. Critical Role of Magnesium Ions in DNA Polymerase β’s Closing and Active Site Assembly. J. Am. Chem. Soc. 2004, 126, 8441–8453. [Google Scholar] [CrossRef]
- Zhou, H.; Bi, G.Q.; Liu, G. Intracellular magnesium optimizes transmission efficiency and plasticity of hippocampal synapses by reconfiguring their connectivity. Nat. Commun. 2024, 15, 3406. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Agricultural Research Service. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat in America, NHANES 2013–2016; Food Surveys Research Group: Beltsville, MD, USA, 2019. [Google Scholar]
- Ab Rahim, S.N.; Nordin, N.; Wan Omar, W.F.A.; Zulkarnain, S.; Kumar, S.; Sinha, S.; Haque, M. The Laboratory and Clinical Perspectives of Magnesium Imbalance. Cureus 2023, 15, e49835. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, S194–S198. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Kshirsagar, K.; Patil, V.C. Chronic obstructive pulmonary disease: Is serum magnesium level a risk factor for its acute exacerbation? Caspian J. Intern. Med. 2021, 12, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Hashim Ali Hussein, S.; Nielsen, L.P.; Konow Bogebjerg Dolberg, M.; Dahl, R. Serum magnesium and not vitamin D is associated with better QoL in COPD: A cross-sectional study. Respir. Med. 2015, 109, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.; Hoorn, E.J.; Florentin, M.; Milionis, H. An overview of diagnosis and management of drug-induced hypomagnesemia. Pharmacol. Res. Perspect. 2021, 9, e00829. [Google Scholar] [CrossRef]
- Grober, U. Magnesium and Drugs. Int. J. Mol. Sci. 2019, 20, 2094. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, A.F.; Rodrigues-Junior, A.L.; Terra Filho, J.; Vannucchi, H.; Martinez, J.A. Effects of acute magnesium loading on pulmonary function of stable COPD patients. Med. Sci. Monit. 2008, 14, CR524–CR529. [Google Scholar]
- Amaral, A.F.; Gallo, L., Jr.; Vannucchi, H.; Crescencio, J.C.; Vianna, E.O.; Martinez, J.A. The effect of acute magnesium loading on the maximal exercise performance of stable chronic obstructive pulmonary disease patients. Clinics 2012, 67, 615–622. [Google Scholar] [CrossRef]
- Zanforlini, B.M.; Ceolin, C.; Trevisan, C.; Alessi, A.; Seccia, D.M.; Noale, M.; Maggi, S.; Guarnieri, G.; Vianello, A.; Sergi, G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin. Exp. Res. 2022, 34, 167–174. [Google Scholar] [CrossRef]
- Ni, H.; Aye, S.Z.; Naing, C. Magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2022, 5, CD013506. [Google Scholar] [CrossRef]
- Edwards, L.; Shirtcliffe, P.; Wadsworth, K.; Healy, B.; Jefferies, S.; Weatherall, M.; Beasley, R.; Magnesium, C.S.T. Use of nebulised magnesium sulphate as an adjuvant in the treatment of acute exacerbations of COPD in adults: A randomised double-blind placebo-controlled trial. Thorax 2013, 68, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Nouira, S.; Bouida, W.; Grissa, M.H.; Beltaief, K.; Trimech, M.N.; Boubaker, H.; Marghli, S.; Letaief, M.; Boukef, R. Magnesium sulfate versus ipratropium bromide in chronic obstructive pulmonary disease exacerbation: A randomized trial. Am. J. Ther. 2014, 21, 152–158. [Google Scholar] [CrossRef]
- Liebscher, D.H.; Liebscher, D.E. About the misdiagnosis of magnesium deficiency. J. Am. Coll. Nutr. 2004, 23, 730S–731S. [Google Scholar] [CrossRef]
- Fanta, C.H. Role of calcium in airway smooth muscle contraction and mast cell secretion. J. Asthma 1984, 21, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, L.; Pabelick, C.M. Airway smooth muscle in contractility and remodeling of asthma: Potential drug target mechanisms. Expert. Opin. Ther. Targets 2023, 27, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Brandi, M.L. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13, 1059369. [Google Scholar] [CrossRef]
- Villa-Bellosta, R. Impact of magnesium:calcium ratio on calcification of the aortic wall. PLoS ONE 2017, 12, e0178872. [Google Scholar] [CrossRef]
- Britton, W.M.; Stokstad, E.L. Aorta and other soft tissue calcification in the magnesium-deficient rat. J. Nutr. 1970, 100, 1501–1505. [Google Scholar] [CrossRef]
- Centeio, R.; Ousingsawat, J.; Schreiber, R.; Kunzelmann, K. CLCA1 Regulates Airway Mucus Production and Ion Secretion Through TMEM16A. Int. J. Mol. Sci. 2021, 22, 5133. [Google Scholar] [CrossRef]
- Benson, B.J.; Williams, M.C.; Sueishi, K.; Goerke, J.; Sargeant, T. Role of calcium ions the structure and function of pulmonary surfactant. Biochim. Biophys. Acta 1984, 793, 18–27. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef]
- Hu, L.; Ji, J.; Li, D.; Meng, J.; Yu, B. The combined effect of vitamin K and calcium on bone mineral density in humans: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 592. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef] [PubMed]
- Bristow, S.M.; Bolland, M.J.; Gamble, G.D.; Leung, W.; Reid, I.R. Dietary calcium intake and change in bone mineral density in older adults: A systematic review of longitudinal cohort studies. Eur. J. Clin. Nutr. 2022, 76, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.L.; Lee, C.C.; Chen, C.Y. Evaluating the association of osteoporosis with inhaled corticosteroid use in chronic obstructive pulmonary disease in Taiwan. Sci. Rep. 2021, 11, 724. [Google Scholar] [CrossRef]
- Chee, C.; Sellahewa, L.; Pappachan, J.M. Inhaled corticosteroids and bone health. Open Respir. Med. J. 2014, 8, 85–92. [Google Scholar] [CrossRef]
- Passarelli, S.; Free, C.M.; Shepon, A.; Beal, T.; Batis, C.; Golden, C.D. Global estimation of dietary micronutrient inadequacies: A modelling analysis. Lancet Glob. Health 2024, 12, e1590–e1599. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Gold, M.E.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G.; Ignarro, L.J. Antagonistic modulatory roles of magnesium and calcium on release of endothelium-derived relaxing factor and smooth muscle tone. Circ. Res. 1990, 66, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Chen, L.; Zhu, Z.; Luo, P.; Hang, D.; Su, J.; Tao, R.; Zhou, J.; Fan, X. Association of Serum Calcium with the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Study from UK Biobank. Nutrients 2023, 15, 3439. [Google Scholar] [CrossRef] [PubMed]
- Rastoder, E.; Sivapalan, P.; Eklof, J.; Achir Alispahic, I.; Jordan, A.S.; Laursen, C.B.; Vestbo, J.; Jenkins, C.; Nielsen, R.; Bakke, P.; et al. Calcium Channel Blockers and the Risk of Exacerbation in Patients with Chronic Obstructive Pulmonary Disease: A Nationwide Study of 48,488 Outpatients. Biomedicines 2023, 11, 1974. [Google Scholar] [CrossRef]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Bao, S.; Knoell, D.L. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1132–L1141. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The Role of Zinc in Thyroid Hormones Metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc transporters and insulin resistance: Therapeutic implications for type 2 diabetes and metabolic disease. J. Biomed. Sci. 2017, 24, 87. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- El-Attar, M.; Said, M.; El-Assal, G.; Sabry, N.A.; Omar, E.; Ashour, L. Serum trace element levels in COPD patient: The relation between trace element supplementation and period of mechanical ventilation in a randomized controlled trial. Respirology 2009, 14, 1180–1187. [Google Scholar] [CrossRef]
- Kirkil, G.; Hamdi Muz, M.; Seckin, D.; Sahin, K.; Kucuk, O. Antioxidant effect of zinc picolinate in patients with chronic obstructive pulmonary disease. Respir. Med. 2008, 102, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Chambers, I.G.; Ratan, R.R. Selenium abandons selenoproteins to inhibit ferroptosis rapidly. Nat. Metab. 2024, 6, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Proneth, B. Selenium: Tracing Another Essential Element of Ferroptotic Cell Death. Cell Chem. Biol. 2020, 27, 409–419. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Bera, S.; De Rosa, V.; Rachidi, W.; Diamond, A.M. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis 2013, 28, 127–134. [Google Scholar] [CrossRef]
- Kafai, M.R.; Ganji, V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: Third National Health and Nutrition Examination Survey, 1988–1994. J. Trace Elem. Med. Biol. 2003, 17, 13–18. [Google Scholar] [CrossRef]
- Kocyigit, A.; Erel, O.; Gur, S. Effects of tobacco smoking on plasma selenium, zinc, copper and iron concentrations and related antioxidative enzyme activities. Clin. Biochem. 2001, 34, 629–633. [Google Scholar] [CrossRef]
- Ates Alkan, F.; Karis, D.; Cakmak, G.; Ercan, A.M. Analysis of the Relationship Between Hemorheologic Parameters, Aluminum, Manganese, and Selenium in Smokers. Biol. Trace Elem. Res. 2019, 187, 22–31. [Google Scholar] [CrossRef]
- Santos, M.C.; Oliveira, A.L.; Viegas-Crespo, A.M.; Vicente, L.; Barreiros, A.; Monteiro, P.; Pinheiro, T.; Bugalho De Almeida, A. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers 2004, 9, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Huang, X.; Zhang, C.; Liu, C.; Cui, X.; Zhou, Y.; Sun, H.; Qiu, G.; Guo, H.; He, M.; et al. The dose-response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: A population-based study in China. BMJ Open 2015, 5, e007643. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hu, X.; Jarrell, Z.R.; He, X.; Orr, M.; Fernandes, J.; Chandler, J.D.; Walker, D.I.; Esper, A.; Marts, L.; et al. Study on the relationship between selenium and cadmium in diseased human lungs. Adv. Redox Res. 2023, 7, 100065. [Google Scholar] [CrossRef] [PubMed]
- Agler, A.H.; Crystal, R.G.; Mezey, J.G.; Fuller, J.; Gao, C.; Hansen, J.G.; Cassano, P.A. Differential expression of vitamin E and selenium-responsive genes by disease severity in chronic obstructive pulmonary disease. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 450–458. [Google Scholar] [CrossRef]
- Guertin, K.A.; Grant, R.K.; Arnold, K.B.; Burwell, L.; Hartline, J.; Goodman, P.J.; Minasian, L.M.; Lippman, S.M.; Klein, E.; Cassano, P.A. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic. Biol. Med. 2016, 95, 349–356. [Google Scholar] [CrossRef]
- Goyer, R.A. Toxic and essential metal interactions. Annu. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef]
- Caruso, R.V.; O’Connor, R.J.; Stephens, W.E.; Cummings, K.M.; Fong, G.T. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: Results from the International Tobacco Control (ITC) United States survey cohort. Int. J. Environ. Res. Public Health 2013, 11, 202–217. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An Alarming Decline in the Nutritional Quality of Foods: The Biggest Challenge for Future Generations’ Health. Foods 2024, 13, 877. [Google Scholar] [CrossRef]
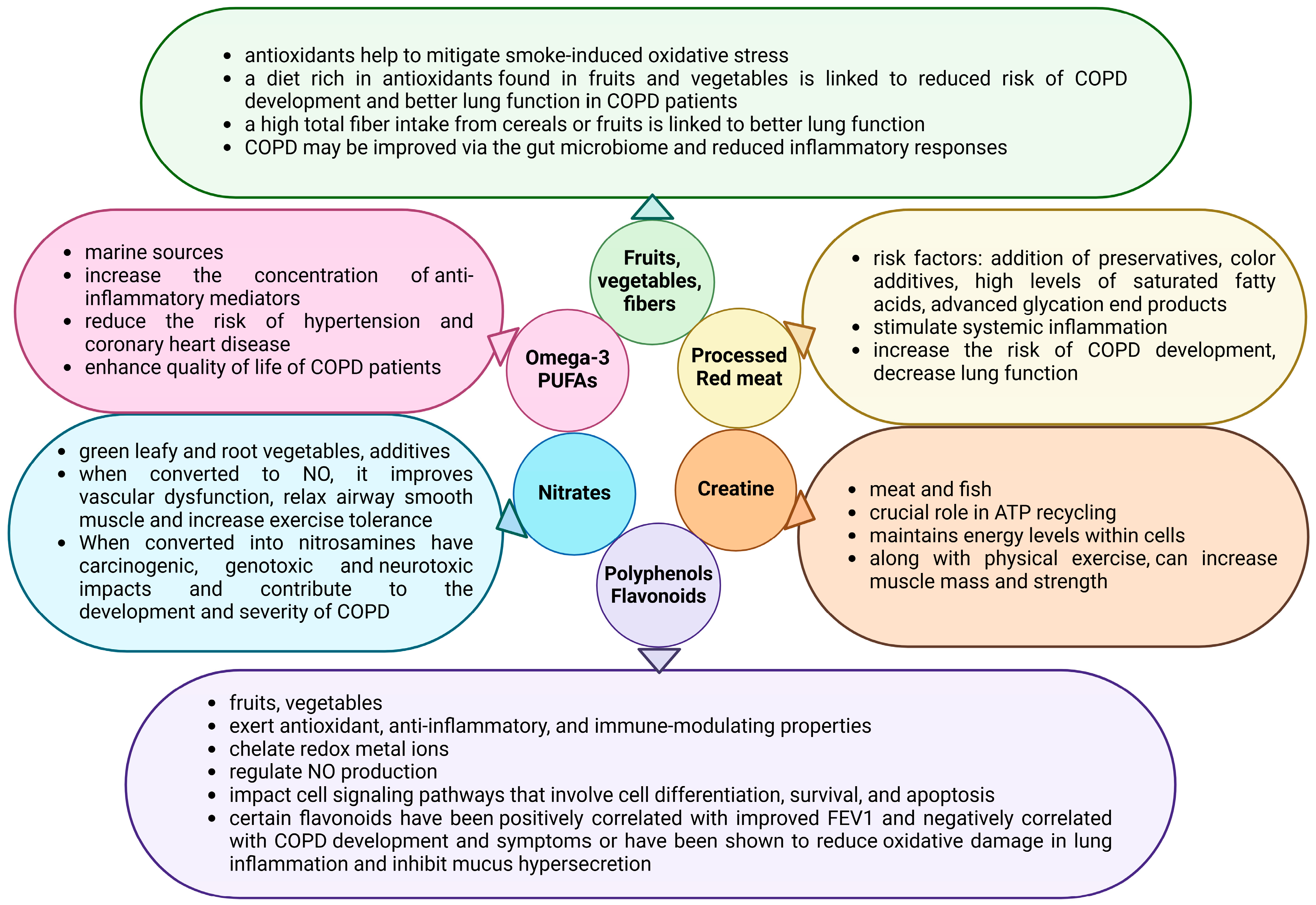
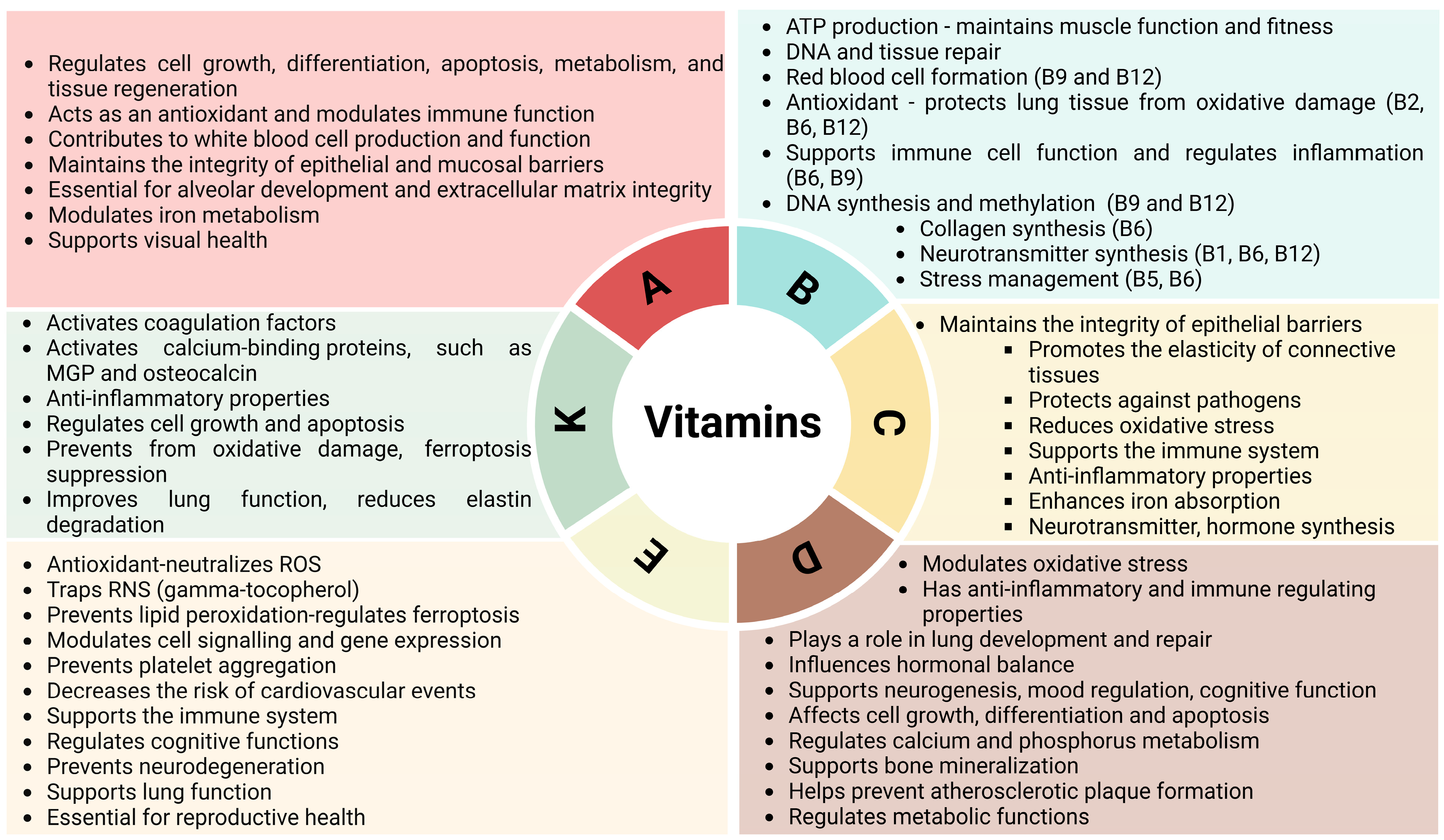
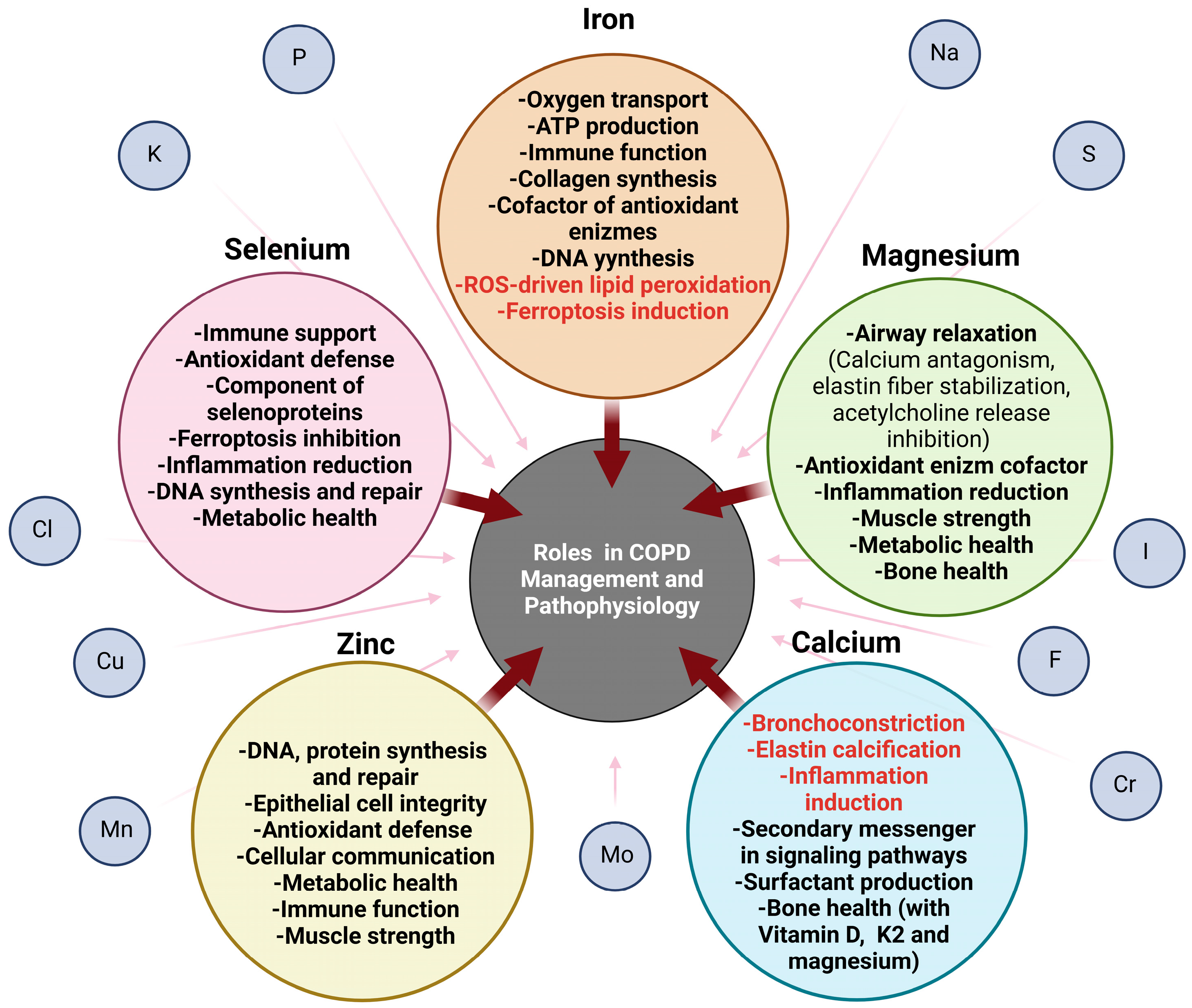
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, P.; Török, É.; Szalontai, K.; Kari, B.; Neuperger, P.; Zavala, N.; Kanizsai, I.; Puskás, L.G.; Török, S.; Szebeni, G.J. Nutritional Support of Chronic Obstructive Pulmonary Disease. Nutrients 2025, 17, 1149. https://doi.org/10.3390/nu17071149
Simon P, Török É, Szalontai K, Kari B, Neuperger P, Zavala N, Kanizsai I, Puskás LG, Török S, Szebeni GJ. Nutritional Support of Chronic Obstructive Pulmonary Disease. Nutrients. 2025; 17(7):1149. https://doi.org/10.3390/nu17071149
Chicago/Turabian StyleSimon, Péter, Éva Török, Klára Szalontai, Beáta Kari, Patrícia Neuperger, Norma Zavala, Iván Kanizsai, László G. Puskás, Szilvia Török, and Gabor J. Szebeni. 2025. "Nutritional Support of Chronic Obstructive Pulmonary Disease" Nutrients 17, no. 7: 1149. https://doi.org/10.3390/nu17071149
APA StyleSimon, P., Török, É., Szalontai, K., Kari, B., Neuperger, P., Zavala, N., Kanizsai, I., Puskás, L. G., Török, S., & Szebeni, G. J. (2025). Nutritional Support of Chronic Obstructive Pulmonary Disease. Nutrients, 17(7), 1149. https://doi.org/10.3390/nu17071149






