Highlights
- The clinical use of strain-specific Lactiplantibacillus plantarum demonstrates promising therapeutic effects across diverse health conditions.
- Evidence underscores the importance of selecting appropriate L. plantarum strains—such as 299V for IBS, IS-10506 for atopic dermatitis, K50 and EGCG 13110402 for metabolic syndrome, and PS128 for depression—for targeted clinical applications.
- Findings support the need for further investigation into mechanisms of action, optimal dosing, and long-term safety in human populations.
Abstract
Objectives: This systematically scoping review aims to evaluate the therapeutic potential and clinical benefits of specific Lactiplantibacillus plantarum (L. plantarum) strains in human health, identifying their strain-specific effects across various medical conditions. Methods: Following the PRISMA for Scoping Reviews (PRISMA-ScR) guidelines and employing the PICO framework, a comprehensive literature search was conducted in the PubMed and Embase databases to identify relevant studies published up to December 2023. Inclusion criteria were rigorously applied to ensure the selection of high-quality studies focusing on the clinical application of distinct L. plantarum stains. Results: This review analyzed several unique strains of L. plantarum across 69 studies, identifying several therapeutic benefits. L. plantarum 299v effectively improved gastrointestinal symptoms, enhanced oral health, and reduced systemic inflammation. L. plantarum IS-10506 exhibited notable immunomodulatory effects, especially in managing atopic dermatitis. L. plantarum LB931 showed promise in decreasing pathogenic colonization, supporting women’s vaginal health. Additionally, L. plantarum CCFM8724 demonstrated potential in reducing early childhood caries, highlighting its promise in pediatric oral care. Conclusions: The therapeutic potential of L. plantarum is extensive, with certain strains exhibiting promising clinical benefits for specific health concerns. The findings of this review advocate for the integration of L. plantarum strains into clinical practice, emphasizing the need for further research to elucidate their mechanisms of action, optimal dosages, and long-term safety profiles.
1. Introduction
Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits to the host [1]. They have been extensively studied due to their potential therapeutic roles in various clinical conditions, including immune disorders, cardiovascular diseases, hyperlipidemia, and hyperglycemia [2]. Among probiotic species, Lactiplantibacillus plantarum (L. plantarum), previously known as Lactobacillus plantarum [3], is recognized for its wide-ranging health benefits.
Lactobacillus species can be classified based on their fermentation capabilities into three functional groups: obligate homofermentative, obligate heterofermentative, and facultatively heterofermentative. Obligate homofermentative species, such as L. salivarius and L. acidophilus, produce primarily lactic acid. Obligate heterofermentative species, like L. reuteri and L. fermentum, generate lactic acid along with ethanol or acetic acid and carbon dioxide. Facultatively heterofermentative species, including L. casei and L. plantarum, produce lactic acid and carbon dioxide depending on environmental conditions [4,5,6,7].
L. plantarum is a Gram-positive lactic acid bacterium notable for its metabolic adaptability and ability to colonize diverse environments, such as fermented foods, meats, plants, and the gastrointestinal tract [8]. Its adaptability, environmental resilience, and extensive health-promoting effects distinguish it from other probiotics. Specific L. plantarum strains exhibit unique health-related functionalities. For instance, L. plantarum EGCG 13110402 demonstrates cholesterol-lowering effects [9], L. plantarum 299v improves vascular endothelial function [10], and L. plantarum HAC01 significantly reduces postprandial glucose and HbA1c levels, indicating potential benefits for individuals with prediabetes or type 2 diabetes [11].
The therapeutic potential of L. plantarum strains largely stems from their ability to modulate intestinal microbiota and immune responses, impacting overall health [12]. This strain-specific functionality is evident in clinical outcomes; L. plantarum 299v alleviates gastrointestinal inflammation by enhancing anti-inflammatory cytokines [13], L. plantarum PS128 supports neurological health and reduces oxidative stress in athletes [14,15,16], and L. plantarum Inducia positively influences metabolic and antioxidative responses related to cholesterol and BMI [17]. Thus, the precise selection of probiotic strains, similar to drug selection in pharmacology, is critical to effectively address specific clinical conditions, such as inflammatory bowel disease (IBD) [18] or neurological disorders [19].
In addition to therapeutic applications, L. plantarum is widely utilized in the food industry [3,20,21,22,23,24,25,26,27,28,29,30,31,32], particularly in fermented foods, due to its resilience to harsh conditions, low pH tolerance, and adherence to intestinal epithelial cells. These characteristics enhance its suitability for probiotic and therapeutic applications.
However, the clinical translation of L. plantarum benefits faces several limitations, including inconsistent study designs, small and heterogeneous samples, variable methodologies, and a lack of standardized outcome measures. Addressing these limitations through rigorous and targeted clinical investigations is essential for fully leveraging the therapeutic potential of L. plantarum. Therefore, bridging the gap between fundamental research and clinical application requires addressing these limitations through rigorous, targeted clinical investigations.
Therefore, this scoping review aims to address existing knowledge gaps by providing a comprehensive overview of current research on the therapeutic potential of individual L. plantarum strains across various health conditions. By summarizing strain-specific evidence, this review aims to inform future clinical guidelines, fostering the integration of L. plantarum into therapeutic or adjunct treatments and ultimately contributing to improved human health and well-being.
2. Materials and Methods
2.1. Registration of Protocols
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Review (PRISMA-ScR) guidelines. The protocol for this systematic review was retrospectively registered on INPLASY (INPLASY202520088) and is available on inplasy.com (accessed on 19 February 2025) (https://doi.org/10.37766/inplasy2025.2.0088).
2.2. Data Sources and Search Strategy
A systematic literature search was conducted using PubMed and Embase to identify relevant full-text articles published in English. The search strategy utilized the keyword “Lactiplantibacillus plantarum” or “Lactobacillus plantarum” or “L. plantarum” in “humans”. Additionally, the reference lists of included studies and relevant citations from other journals were screened via Google Scholar to identify additional studies.
2.3. Study Selection
A comprehensive literature search was conducted in two major biomedical databases, PubMed and Embase, to systematically identify peer-reviewed studies published up to December 2023. The search strategy involved targeted keyword combinations including “Lactiplantibacillus plantarum” or “Lactobacillus plantarum” or “L. plantarum” AND “humans”. Rigorous inclusion and exclusion criteria were applied during study selection, specifically selecting high-quality primary clinical studies involving distinct, clearly identifiable L. plantarum strains and reported therapeutic outcomes in human subjects. Screening was carried out in two stages (title/abstract screening and full-text screening) by at least two independent reviewers. Discrepancies were resolved through discussion and consensus or consultation with a third reviewer when necessary. Data extraction focused on strain-specific therapeutic outcomes, methodological quality, target population characteristics, study designs, and reported clinical endpoints. Data synthesis was qualitative, systematically summarizing findings to highlight strain-specific therapeutic benefits across identified health conditions.
Eligible studies included those that assessed L. plantarum as a monotherapy, without combining it with other probiotics species, in humans.
The study selection process involved four independent reviewers (OC, YS, CC, and PB), who screened articles for eligibility based on predefined inclusion and exclusion criteria. Any discrepancies between reviewers were resolved through group discussions in order to minimize the potential bias.
2.4. Data Extraction
Two independent reviewers (OC and NL) extracted data from the selected studies. The extracted information included the following: (1) study characteristics (L. plantarum strain, authors, year of publication, study type, source of publication, and country); (2) patient characteristics (sample size, type of disease, number of cases and controls); (3) outcomes (measurement methods for each disease and any additional relevant information).
All relevant text, tables, and figures in the included studies were reviewed for data extraction. The following types of studies were excluded: (1) non-original research (e.g., reviews, protocols, letters, comments, and guidelines); (2) studies that did not focus on L. plantarum monotherapy (i.e., mixing with other probiotic species); (3) non-human studies; (4) unpublished, gray literature or non-peer-reviewed studies; and (5) studies published in languages other than English in order to avoid any misunderstanding or language bias.
2.5. Data Synthesis and Analysis
The primary outcomes analyzed in this review included the following: the specific L. plantarum strains investigated and the types of diseases targeted by each strain. Since our study is a systematic scoping review, the checklist indicates that the risk of assessment bias is not liable to occur. The checklist can be found in the Supplementary files.
2.6. Patient and Public Involvement
No patients or members of the public were involved in the design or conduct of this study. However, the findings of this systematic scoping review on the strain-specific therapeutic potential of L. plantarum could provide valuable insights for optimizing probiotic-based treatments for specific health conditions.
2.7. Methods, Research Design and PRISMA Flow Diagram
Our systematic scoping review was designed to comprehensively evaluate the therapeutic potential and clinical benefits of specific L. plantarum strains only in human health. The study followed the PRISMA for Scoping Reviews (PRISMA-ScR) guidelines, ensuring methodological rigor in the selection and synthesis of evidence. A structured approach was employed using the PICO framework to define the study parameters such as Population (study in humans only), Intervention (no species mixed of L. plantarum strains), Comparison (study compare between groups), and Outcomes (clinical benefits across various health conditions). A comprehensive literature search was conducted in PubMed and Embase to identify relevant studies published up to December 2023. In fact, it was our own limitation that meant that we did not review papers from all four databases in this study: Scopus, Embase, PubMed, and Cochrane library. In our study, the search strategy we used only obtained papers from the Embase and PubMed databases because they are very well-known and well-accepted databases in biomedical fields. Therefore, we decided to use only these two databases for our studies.
The inclusion criteria were established to select high-quality studies reporting primary data on human clinical outcomes related to specific L. plantarum strains. Following a systematic screening and selection process, data were extracted and qualitatively synthesized. The results were categorized by targeted health conditions, enabling a thorough assessment of therapeutic efficacy.
Strict inclusion and exclusion criteria ensured the selection of scientifically rigorous studies focused explicitly on distinct L. plantarum strains and their strain-specific clinical outcomes. This strategy minimized bias and enhanced the reliability of findings, supporting evidence-based clinical applications and future research.
Inter-rater reliability was assessed by having multiple reviewers independently screen studies based on predefined criteria. Discrepancies were resolved through consensus discussions or consultation with a third reviewer. This method ensured consistency, reduced subjective bias, and strengthened methodological integrity.
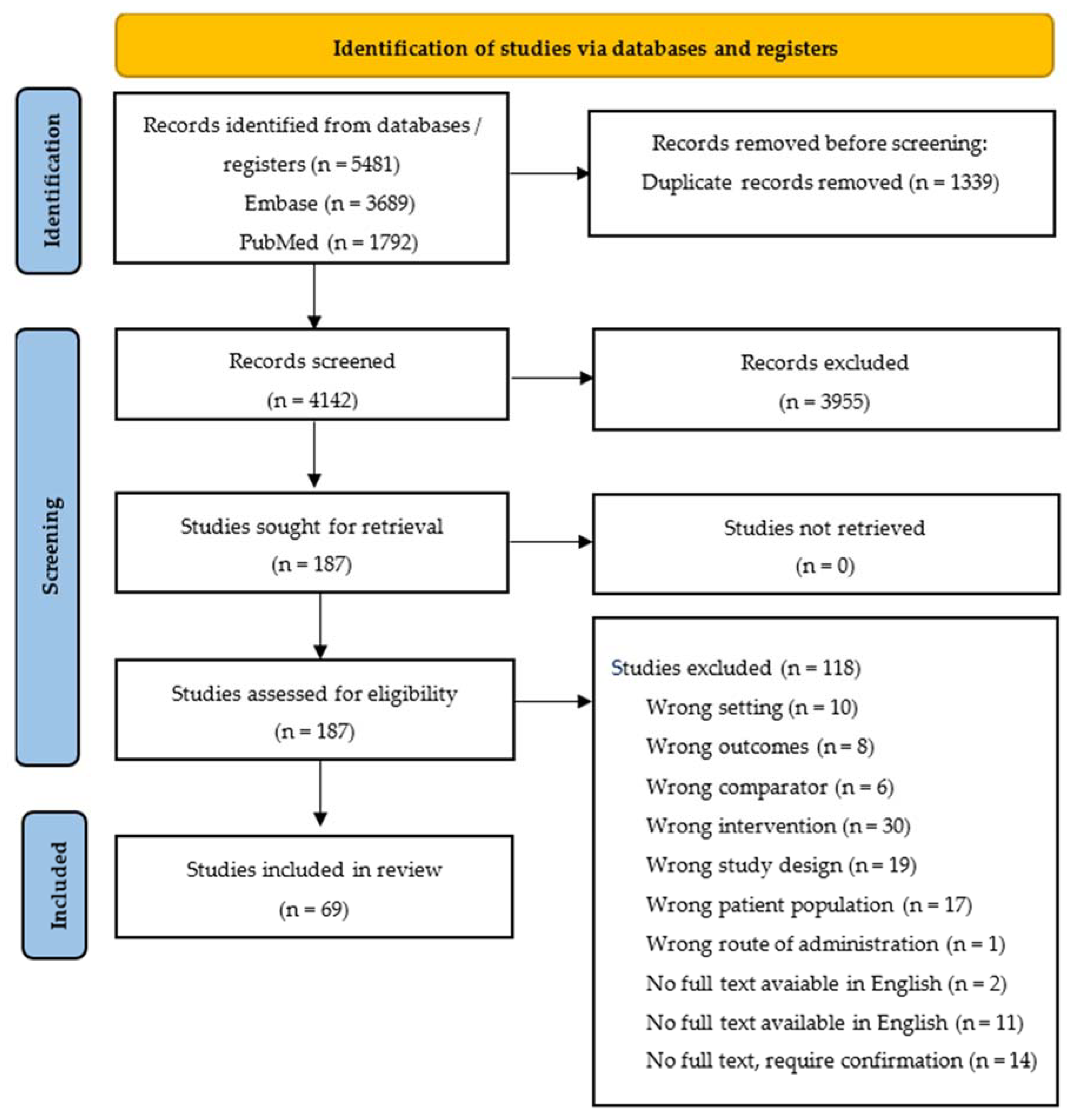
A total of 5481 studies were identified after a search across two databases. After excluding duplicates, 4142 studies’ titles and abstracts were screened. A total of 187 studies remained for full-text screening, and, finally, a total of 69 studies were included in our qualitative synthesis. The PRISMA 2020 flow diagram is shown in Figure 1.
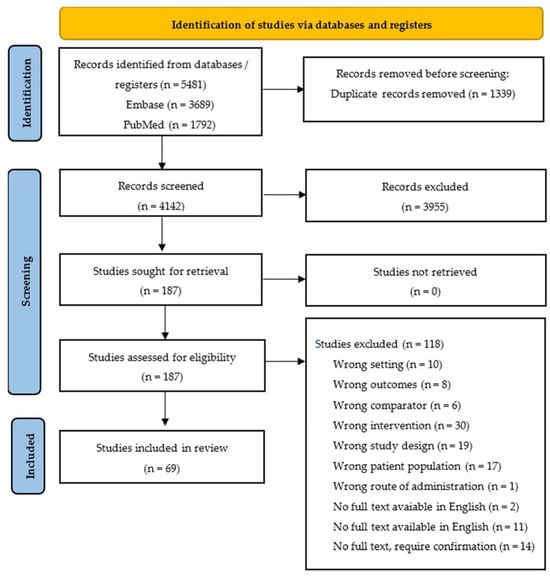
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews, which included the search of databases and registers only.
3. Results
3.1. Data Characteristics Table: Please See Table S1 in the Supplementary Materials for Data Characteristics Table
This systematic scoping review covered a wide array of randomized, double-blind, placebo-controlled trials. The result of the study was comprehensively summarized in Table S1, Data Characteristics Table, in the Supplementary Materials. The study included sample sizes ranging from 20 to greater than 400. Healthy adults, children, older populations, and patients suffering from gastrointestinal, metabolic, anxious, or immune-related disorders were tested within a subject sample composed of a wide variety of ages, sexes, and geographies. Treatments ranged from about a week to a total of six months, with most intervention periods being within four to twelve weeks. The study dosages had high heterogeneity, with some specifying concentrations while others did not. The observed effects were markedly different by strain and clinical target. Some strains significantly lower cholesterol, relieve anxiety, improve bowel function, and modulate immunity, while others report very little or no significant clinical outcomes when compared with their placebo counterparts. Methodological, participant background, intervention period, and outcome variability among the studies further emphasize the need for some definite probiotic strain to allow the reliable conclusion and application of the results.
Table 1 includes an overall classification of the symptoms with reference numbers. The gastrointestinal system was classified into five major symptoms such as IBS symptoms, bowel functions, microbiome diversity, diarrhea, and constipation. For IBS symptoms, there are seven studies included in this study. There were classified into seven major symptoms for the immune system, three symptoms for the central nervous system, two symptoms for the integumentary system, and three symptoms for the miscellaneous group.

Table 1.
Lactiplantibacillus plantarum’s classification by symptoms with reference numbers.
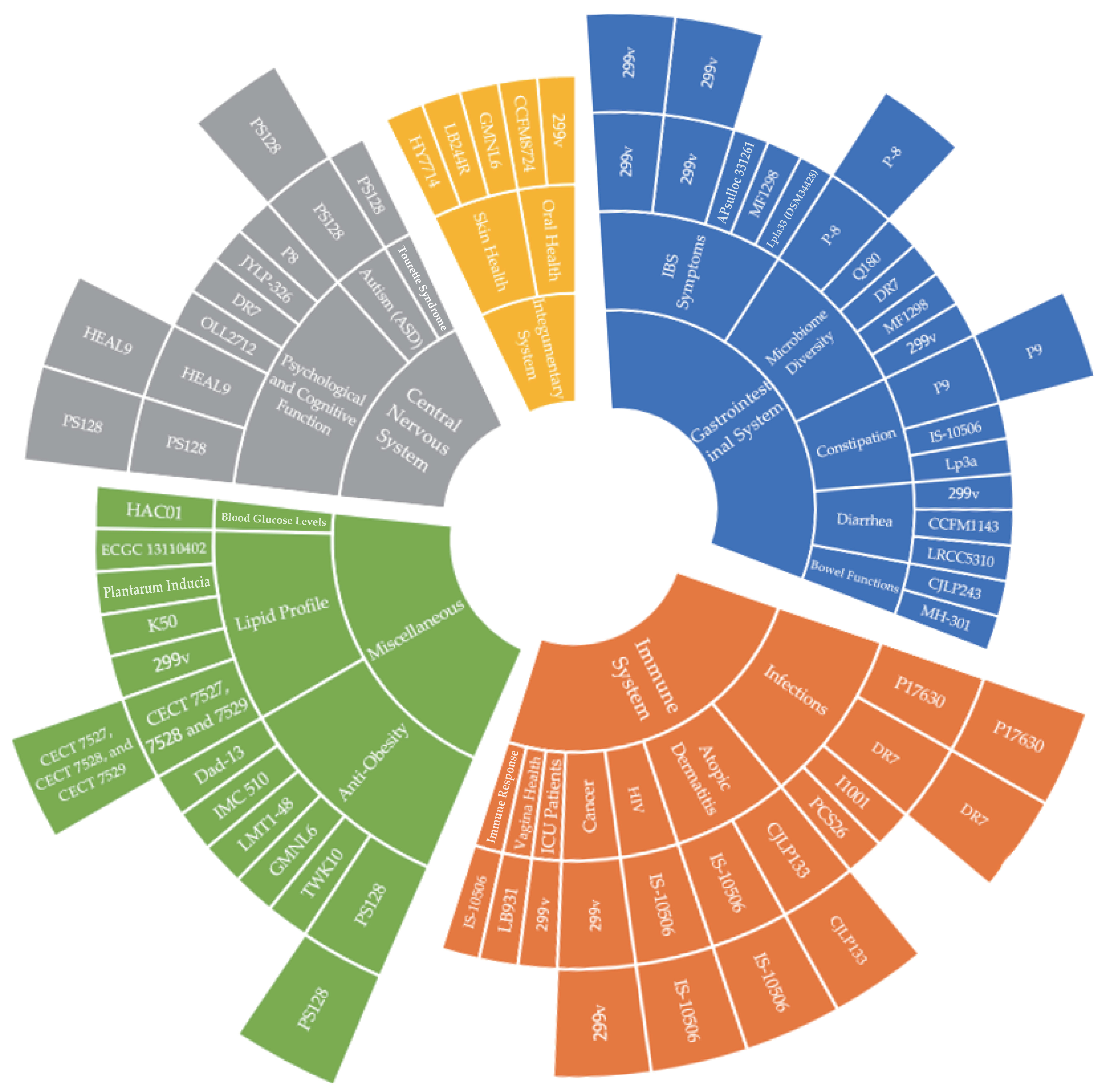
The graph of the L. plantarum strains classified by symptoms is also shown in Figure 2. The sunburst graph clearly presents the classification of L. plantarum strains by symptoms. Figure 2 shows that for infections under the subset of the immune system, there are several L. plantarum strains that help to alleviate infections, such as L. plantarum P17630 (two studies), L. plantarum DR7 (two studies), L. plantarum I1001, and L. plantarum PCS26. Therefore, this graph significantly helps readers to have an overall understanding of the studies at a glance.
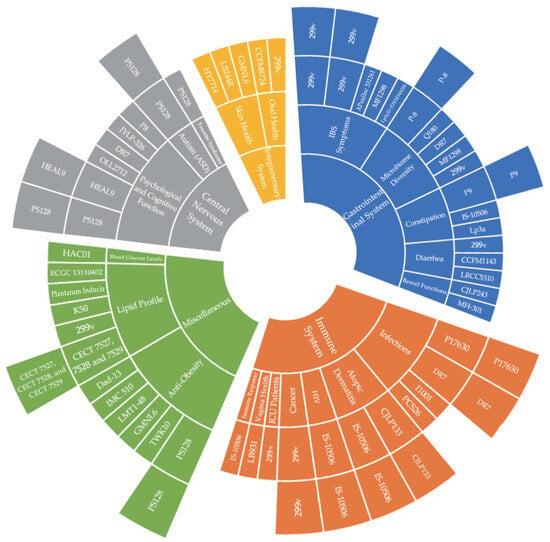
Figure 2.
Lactiplantibacillus plantarum strains classification and distribution graph.
The table of results, also shown in Table 2, are summarized from the Data Characteristics Table, Table S1 in the Supplementary Materials. From Table 2, L. plantarum CCFM1143 eases the symptoms of chronic diarrhea, while L. plantarum DR7 helps to reduce anxiety and improve cognition and URTI symptoms. However, L. plantarum 299v results in mixed results; some studies showed positive and some showed negative effects. There may be several related factors, but confounding factors may play a major role in this issue such as diet, age, genetic differences, and gut microbiome diversity, which may change the response to treatment; these have not been studied in this research.

Table 2.
Table of results: A summarization of the L. plantarum strains’ therapeutic potential on various health conditions (from Table S1 Data Characteristics Table in the Supplementary Materials).
3.2. Gastrointestinal System
3.2.1. Irritable Bowel Syndrome (IBS)
Several L. plantarum strains have been investigated for their effects on IBS, yielding mixed outcomes. L. plantarum Lpla33 (DM34428) [37] and L. plantarum APsulloc 331261 [35] demonstrated significant efficacy in improving diarrhea-related symptoms in IBS patients, offering potential relief. For Lpla33, SCFAs, particularly butyrate, may help to promote intestinal barrier function, a feature of several L. plantarum strains. Bile acid (BA) metabolism may also be implicated, as L. plantarum Lpla33 possesses significant bile salt hydrolase activity. Diarrhea and visceral hypersensitivity have been associated with the decreased 7α-dehydroxylation of primary BAs to secondary BAs. Additionally, the ability of L. plantarum Lpla33 to modulate intestinal barrier function, inhibit key pathogens, and moderate inflammatory markers may have played a key role in these observed effects.
In contrast, L. plantarum 299v [33,38] showed no significant effect on abdominal pain, bloating, or rectal emptying when compared to a placebo. However, some studies noted modest improvements in abdominal pain and bloating [34]. The IBS symptoms were evaluated using a composite score [39]; L. plantarum 299v failed to show significant efficacy. The IBS symptom scores were virtually unchanged in each subject. Fecal weights did not change significantly, and total hydrogen production over 24 h was not reduced. This suggests that, in this case, L. plantarum 299V does reduce hydrogen production in the colon, but not sufficiently enough in this group of patients to be clinically effective.
L. plantarum MF1298 [59] was associated with unfavorable effects on IBS symptoms, indicating potential strain-specific variability in therapeutic outcomes.
3.2.2. Bowel Function
The impact of L. plantarum strains on bowel function varies depending on the condition. In patients undergoing endoscopic sclerotherapy for internal hemorrhoids, L. plantarum MH-301 [49] demonstrated a positive effect on bowel movements. The oral L. Plantarum MH-301 has a beneficial effect on the improvement of both postoperative internal hemorrhoids and difficult defecation. It increased the relative abundance of Firmicutes at the phylum level. Firmicutes are mostly Gram-positive bacteria and are the dominant phylum in human intestinal microbiota. Firmicutes are health-promoting probiotics, including those such as Lactobacillus and Ruminococcus, which play a vital role in promoting health.
However, L. plantarum CJLP243 [48] did not improve bowel function in rectal cancer patients after ileostomy, suggesting that strain-specific benefits may not extend to all gastrointestinal conditions.
3.2.3. Microbiome Diversity
Several L. plantarum strains have shown their ability to modulate gut microbiota composition. In children, L. plantarum 299v [58] significantly enhanced microbiome diversity. L. plantarum DR7 [61] maintained a balanced gut bacterial profile, supporting gut homeostasis with Bacteroidia and Bacteroidales, which were maintained upon the administration of DR7. It reduced plasma cortisol levels and exerted changes along the pathways of two neurotransmitters, namely, serotonin and dopamine. Although changes in gut microbiota upon the administration of DR7 was consistent along different taxonomic levels, the correlations of the changes in these microbial groups with changes in neurotransmitter gene expression also showed such consistency.
L. plantarum P-8 [60] increased the richness of beneficial bacteria and reduced the presence of opportunistic pathogens [63]. However, L. plantarum MF1298 [59] had no significant effects on gut microbiota composition or gut wall health. L. plantarum Q180 [62] was able to maintain a healthy intestinal environment in postprandial conditions, reinforcing its role in digestive health. The only LDL-cholesterol and ApoB-100 levels were decreased by L. plantarum Q180 supplementation for 12 weeks; the postprandial blood levels of lipid markers such as TG, ApoB-48, and ApoB-100 were also significantly decreased. It also helped to maintain healthy postprandial lipid metabolisms in subjects with a higher level of enteric bacteria such as R. bromii, K. alysoides, B. intestinihominis, and F. plautii.
3.2.4. Diarrhea
The effects of L. plantarum strains on diarrhea varied across different populations and conditions. L. plantarum 299v [67] did not show beneficial effects in treating pediatric diarrhea, indicating limited efficacy for children. L. plantarum CCFM1143 [83] was effective in treating chronic diarrhea in adults, suggesting its potential for managing diarrhea in older populations.
L. plantarum LRCC5310 [82] significantly improved rotavirus-induced diarrhea in children, demonstrating its promise in treating infectious diarrhea in young patients. Rotavirus is one of the most common causes of Acute gastroenteritis (AGE) in children. Rotavirus consists of a segmented gene and can represent a variety of genes by a combination of G-P proteins. Generally, the genotypes of viruses that cause AGE in children are typically G1P, G2P, G3P, etc. Moreover, the intake of LRCC5310 was found to be effective in the suppression of viral symptoms as well as in prognosis and treatment via virus titer reduction.
3.2.5. Constipation
Several L. plantarum strains have demonstrated efficacy in relieving constipation. L. plantarum Lp3a [89] effectively improved functional constipation, making it a potential therapeutic option. Functional constipation (FC) is a common gastrointestinal (GI) disorder characterized by symptoms of constipation without a clear physiologic or anatomic cause. Gut microbiome dysbiosis has been postulated to be a factor in the development of FC, and treatment with probiotic regimens, including strains of Lactobacillus plantarum (L. plantarum), has demonstrated efficacy in managing symptoms. Lp3a relieves FC symptoms by enhancing intestinal motility and putatively modulating methane metabolism and bile acid synthesis. The bile acids serve as physiological laxatives; this suggests a role for the modulation of bile acid metabolism as an additional putative mechanism of Lp3a in FC.
L. plantarum IS10506 [87] specifically helped in treating functional constipation in women, suggesting gender-specific benefits. L. plantarum P9 [87,88] consistently relieved chronic constipation across studies, reinforcing its potential as a reliable treatment for this condition.
3.3. Immune System
3.3.1. Immune Response
L. plantarum IS-10506 [40] has been linked to an increase in secretory immunoglobulin A (sIgA) in children, a key component of mucosal immunity. This enhancement may improve the body’s ability to respond to pathogens and allergens. Lactobacillus plantarum IS-10506 supplementation significantly increased fecal secretory immunoglobulin A (sIgA) levels in children under two years old. The probiotic stimulates transforming growth factor-β1 (TGF-β1), which plays a crucial role in regulating immune responses and promoting sIgA production. The study found a strong correlation between higher TGF-β1/TNF-α ratios and increased sIgA levels, suggesting that IS-10506 enhances mucosal immunity, helping to protect against infections.
3.3.2. Infections
Probiotics have demonstrated potential in managing infections, including upper respiratory tract infections (URTI), urinary tract infections (UTI), and vulvovaginal candidiasis (VVC). L. plantarum DR7 [50,51] significantly reduced the severity and duration of URTI symptoms, indicating its potential as an immune-boosting probiotic.
L. plantatum PCS26 [87], Lp26, was found to alleviate UTI symptoms, suggesting it could serve as an alternative or adjunct to antimicrobial treatments. Urinary tract infections (UTI) are frequent bacterial infections in childhood. L. plantarum 26 was derived from local Slovenian cheese in the Pathogen Combat Project. Lp26 helps to relieve UTI symptoms primarily through its antimicrobial effect against E. coli, potentially reducing pathogenic bacteria in the gut and their migration to the urinary tract.
L. plantarum P17630 [52] was effective in reducing vaginal discomfort and preventing recurrent VVC infections [55]. L. plantarum I1001 [54] was identified as a compound that enhances the efficacy of treatment for recurrent VVC.
3.3.3. Atopic Dermatitis
Probiotics have shown promise in alleviating symptoms of atopic dermatitis, a chronic inflammatory skin condition. L. plantarum IS-10506 [66] demonstrated significant reduction in both adults and children [67], likely due to its immunomodulatory effects.
L. plantarum CJLP133 [65] also showed beneficial effects on atopic dermatitis in children [59], making it a promising probiotic for managing skin inflammation by modulating immune responses. In children with moderate to severe AD, those who responded well to probiotic treatment had high total IgE levels, increased transforming growth factor-beta (TGF-β) expression, and a high proportion of CD4+CD25+Foxp3+ regulatory T (Treg) cells. The supplementation of L. plantarum CJLP133 showed significant reductions in AD severity and an increase in Treg cells, indicating improved immune regulation.
3.3.4. Cancer
In cancer care, probiotics have been studied for their role in supporting gastrointestinal health and nutritional status. L. plantarum 299 [84] alleviated gastrointestinal symptoms in cancer patients, potentially improving their quality of life during chemotherapy or radiation treatments. L. plantarum 299v [85] demonstrated potential to improve the nutritional status of cancer patients, supporting overall health and recovery during cancer treatment. L. plantarum 299v (Lp299v) demonstrated potential to improve the nutritional status of cancer patients receiving home enteral nutrition (HEN) by preventing weight loss and enhancing nutrient absorption. It helps to maintain body weight and muscle mass, crucial for preventing cancer cachexia. L. plantarum 299v increased serum albumin, total protein, and transferrin levels, suggesting improved protein metabolism and reduced inflammation. It also modulated gut microbiota by increasing beneficial bacteria and enhancing mucin production (MUC2 and MUC3), which strengthen gut barrier function.
3.3.5. Human Immunodeficiency Virus (HIV)
The immune-modulating properties of L. plantarum have been examined in HIV-infected individuals with mixed results. L. plantarum IS-10506 [12] did not show a significant effect on overall immune responses in HIV-infected children. L. plantarum IS-10506 is a probiotic strain isolated from Indonesian fermented buffalo milk (dadih) with demonstrated gut barrier-enhancing properties. It has been shown to mitigate intestinal epithelial damage by inhibiting nuclear factor kappa B (NF-κB) activation and downregulating tumor necrosis factor receptor-1 (TNFR1), which play key roles in inflammatory responses. It significantly reduced blood lipopolysaccharide (LPS) levels, indicating reduced microbial translocation and improved gut integrity in HIV-infected children undergoing antiretroviral therapy (ARV). However, its effect on systemic and humoral immune responses, including CD4+/CD8+ T cell ratios and fecal secretory IgA (sIgA) levels, was not significant. It increased the number of regulatory T cells [90] in children receiving first-line antiretroviral therapy (ART), suggesting a potential role in immune regulation for managing HIV-related immune dysregulation.
3.3.6. Vaginal Health
L. plantarum LB931 [91] was observed to maintain a low vaginal pH, which helps to prevent harmful microbial overgrowth. This contributes to reducing the risk of bacterial vaginosis and yeast infections, promoting overall vaginal health. It helps to maintain a low vaginal pH, which is crucial for preventing harmful microbial overgrowth. The high numbers of lactobacilli, including LB931, had significantly lower vaginal pH levels, which is associated with a reduced prevalence of Group B streptococci (GBS) and yeast infections.
3.3.7. ICU Patients
The potential of probiotics in critically ill patients, particularly ICU patients, has been explored. L. plantarum 299v [92] did not show significant improvements in clinical outcomes for ICU patients, suggesting that while probiotics may offer benefits in other settings, their role in critically ill patients requires further investigation. L. plantarum 299v is a well-studied probiotic strain recognized for its resilience in the gastrointestinal tract, surviving gastric acidity and bile exposure. It has demonstrated antimicrobial properties, reducing bacterial translocation and systemic inflammation, particularly in critically ill patients. Despite its ability to attenuate systemic inflammation and support gut microbiota restoration, the study found no significant results.
3.4. Central Nervous System
3.4.1. Autistic Spectrum Disorders (ASD)
The specific probiotic strain L. plantarum PS128 [16,41] has shown significant improvement in symptoms associated with autism spectrum disorders (ASD). L. plantarum PS128 was also linked to improvements on the SNAP-IV scale, a widely used measure for ADHD and ASD symptoms, suggesting its potential adjunctive role in cognitive and behavioral therapy for ASD patients. L. plantarum PS128 has the ability to modulate gut microbiota and influence neurotransmitter levels, such as dopamine and serotonin, in the brain. Additionally, the probiotic was well-tolerated, with fewer side effects.
3.4.2. Tourette Syndrome
L. plantarum PS128 [19] did not exhibit tic-reducing effects in children with Tourette syndrome. Tourette syndrome results from a complex interaction between social–environmental factors, multiple genetic abnormalities, and neurotransmitter disturbances. The present study reflected that intervention with PS128 did not have a superior response in tic severity improvement. There are a few possible explanations for this. L. plantarum PS128 has been demonstrated in germ-free mice to increase concentrations of dopamine, serotonin, and their metabolites in the striatum. However, it is known that patients with Tourette syndrome may have dopamine hypersensitivity and, therefore, respond to dopamine blocking agents such as risperidone, pimozide, and haloperidol. Therefore, it may be explainable that the use of PS128 did not show a superior response in improving tic severity. This suggests that while L. plantarum PS128 may be beneficial for ASD, its therapeutic benefits may not extend to all neurological or psychological conditions.
3.4.3. Psychological and Cognitive Function
Several L. plantarum strains have been investigated for their impact on psychological well-being and cognitive function. L. plantarum HEAL9 [72] significantly reduced acute and chronic stress, indicating its stress-relieving potential. It showed a significant reduction in awakening cortisol levels, a key marker of stress response. Additionally, improvements were observed in mood, particularly in reducing confusion, anger, and depressive symptoms. The probiotic also enhanced cognitive function, particularly in memory and learning tasks, suggesting its role in modulating the gut–brain axis to alleviate stress and improve mental well-being.
L. plantarum P8 [88] has demonstrated broad benefits in stress management, including relief from stress, anxiety, and cognitive impairment in stressed adults, reinforcing its role in mental clarity enhancement. L. plantarum JYLP-326 [75] was found to alleviate anxiety, depression, and insomnia in college students experiencing test anxiety, highlighting its possible role in managing stress-induced psychological conditions. L. plantarum HEAL9 [73] was further linked to enhanced cognitive abilities in adults under moderate stress. L. plantarum DR7 [69] exhibited efficacy in reducing stress and anxiety in adults, supporting its role in mental well-being.
3.4.4. Cognitive Health and Aging
In elderly populations, L. plantarum OLL2712 [74] demonstrated memory function decline-protective effects, suggesting its potential in maintaining cognitive health in aging individuals by modulating neuroinflammation through the microbiota–gut–brain axis. The probiotic’s high interleukin-10 (IL-10)-inducing activity helped to suppress neuroinflammation by inhibiting pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β). Therefore, L. planatrum OLL2712’s immunomodulatory properties may help to mitigate age-related cognitive decline and neurodegenerative processes.
3.4.5. Depression and Sleep Regulation
L. plantarum PS128 [68] showed notable reductions in depressive severity among patients with major depressive disorder (MDD), reinforcing its potential as a probiotic for depression management. L. plantarum PS128 showed significant decreases in the Hamilton Depression Rating Scale-17 (HAMD-17) and Depression and Somatic Symptoms Scale (DSSS) scores, indicating improved mood and reduced somatic symptoms. It also influence neurotransmitter pathways and gut permeability markers, such as zonulin and intestinal fatty acid-binding protein (I-FABP), suggesting a role in regulating systemic inflammation and neuroimmune interactions in MDD.
Additionally, L. plantarum PS128 [70] was found to reduce depressive symptoms and improve sleep quality in patients with insomnia, suggesting its broader role in mood elevation and sleep regulation.
3.5. Integumentary System
3.5.1. Skin Health
Several L. plantarum strains have demonstrated potential benefits in skin health, particularly in melanin regulation, anti-aging effects, and skin rejuvenation. L. plantarum GMNL6 [44] has shown melanin-reducing effects, suggesting its potential in addressing hyperpigmentation and age spots. L. plantarum GMNL6 significantly decreased melanin synthesis, potentially through the inhibition of the mitogen-activated protein kinase (MAPK) signaling pathway and the activation of protein kinase B (AKT), which suppresses melanogenesis. Additionally, lipoteichoic acid (LTA) from L. plantarum GMNL6 was identified as a key component contributing to its skin-lightening effects. A clinical trial further confirmed that a heat-killed L. plantarum GMNL6 cream significantly reduced melanin index (M index).
L. plantarum HY7714 [42] has been proven to reduce visible signs of aging, likely due to its antioxidant properties and ability to regenerate skin cells. L. plantarum LB224R ointment [43] has demonstrated anti-aging benefits, enhancing skin elasticity and reducing wrinkles, making it a promising candidate for preserving youthful skin appearance.
3.5.2. Oral Health
L. plantarum 299v [56] was found to be as effective as chlorhexidine in reducing pathogenic bacteria in the oropharynx of chronically ill patients on mechanical ventilation, indicating its potential as an alternative strategy to prevent infections in vulnerable patients.
In pediatric oral health, L. plantarum CCFM8724 [57] was effective in preventing and treating early childhood caries, highlighting its role in dental health maintenance. It effectively prevented and treated early childhood caries (ECC) by modulating oral microbiota and reducing pathogens. L. plantarum CCFM8724 also significantly decreased Streptococcus mutans and Candida albicans while increasing beneficial genera like Granulicatella and Gemella. The probiotic also reduced caries-associated bacteria, helping to restore microbial balance and prevent biofilm formation, making it a promising ECC intervention.
3.6. Miscellaneous
3.6.1. Lipid Profile
Several L. plantarum strains and probiotic combinations have demonstrated significant effects on lipid metabolism, particularly in regulating cholesterol and triglyceride levels. L. plantarum EGCG 13110402 [9], significantly reduced LDL, increased HDL, and helped to regulate blood pressure in individuals with normal to mildly hypercholesterolemic conditions.
L. plantarum Inducia [17] showed a significant reduction in total cholesterol, LDL-c, and non-HDL cholesterol, supporting its role in lipid metabolism.
The combination of L. plantarum strains CECT 7527, CECT 7528, and CECT 7529 [45,46] effectively lowered cholesterol levels in hypercholesterolemic patients, highlighting the potential of probiotic combinations in lipid management.
L. plantarum K50 [47] demonstrated potency in reducing total cholesterol and triglycerides, making it a promising probiotic for cardiovascular health improvement. L. plantarum K50 altered gut microbiota by increasing Lactobacillus plantarum and reducing Actinobacteria, changes correlated with visceral adiposity modulation. These findings suggest L. plantarum K50’s potential as a microbiome-targeted intervention for dyslipidemia management.
L. plantarum 299v [10] improved vascular endothelial function and showed potential in cardiovascular disease prevention. However, it did not significantly alter plasma cholesterol, fasting glucose, or body mass index.
3.6.2. Blood Glucose Levels
L. plantarum HAC01 [11] significantly reduced 2 h postprandial glucose (2h-PPG) and HbA1c levels, suggesting its potential for managing blood glucose in individuals with prediabetes or type 2 diabetes. Despite no significant changes in fasting plasma glucose (FPG), the homeostasis model assessment of insulin resistance (HOMA-IR), and the quantitative insulin sensitivity check index (QUICKI), L. plantarum HAC01 may exert its effects via gut microbiota modulation rather than direct insulin sensitization.
3.6.3. Anti-Obesity
The impact of L. plantarum’s role on weight control and obesity management has been explored in various studies. L. plantarum Dad-13 [79] did not result in significant changes in body weight or lipid profiles, suggesting strain-dependent effects. L. plantarum IMC 510 [69] demonstrated positive effects on weight control, suggesting its potential use in obesity management.
L. plantarum LMT-48 [80] was shown to induce weight loss, indicating its possible application in obesity prevention and treatment. The probiotic also reduced serum insulin levels, the homeostasis model assessment of insulin resistance (HOMA-IR), and leptin levels, indicating improved metabolic regulation. Additionally, gut microbiota analysis revealed increased Lactobacillus and Oscillibacter levels, with the latter being negatively correlated with triglyceride and alanine transaminase (ALT) levels, suggesting a role in lipid metabolism and adiposity control. These findings support L. plantarum LMT1-48’s therapeutic potential as a microbiome-targeted intervention for obesity management.
3.6.4. Physiological Adaptations
Probiotics have also been investigated for their role in physical performance and post-exercise recovery. L. plantarum PS128 [14,15] significantly enhanced endurance performance in triathletes, particularly improving running performance and physiological adaptations related to athletic training. L. plantarum PS128 [77] was found to improve muscle recovery and renal function following a half marathon, highlighting its potential in post-exercise recovery and reducing exercise-induced damage.
L. plantarum TWK10 [78] demonstrated better physiological adaptation in non-athletic individuals, suggesting its broad application in enhancing physical performance and overall health. The probiotic also improved fatigue-associated biomarkers, reducing serum lactate and ammonia accumulation during exercise and recovery phases. Furthermore, L. plantarum TWK10 supplementation led to favorable body composition changes, including reduced fat mass and increased muscle mass. These effects may be linked to enhanced short-chain fatty acid (SCFA) production and gut microbiota modulation, suggesting L. plantarum TWK10 as a promising ergogenic aid.
4. Discussion
Overall, the findings show that L. plantarum strains have complicated and diverse functions for different physiological systems. Therapeutic efficacy is often different from one strain to another; thus, probiotic effects should be analyzed on a case-by-case basis with respect to the genetics of the microbes, interaction with hosts, and environmental influences. Such evidence indicates that some L. plantarum may greatly affect health outcomes because of its diverse modes of action; for instance, L. plantarum PS128 [16,68], confers protective effects against ASD, with beneficial effects in mitigating depression, stress, and sleep disturbances, probably by means of interactions with the gut–brain axis and neurotransmitter activity regulation. Another L. plantarum that may provide benefits to the vascular endothelial structure and microbial diversity, albeit not the best for IBS conditions, is L. plantarum 299v [10]. Inevitably, differences in the effectiveness of probiotics include contradictory results in many studies regarding the association with L. plantarum 299v [81]. This could be due to the heterogeneity of designs used in studies, the innate variation of the host response, and specific diseases as well. Those strains have a tendency to produce short-chain fatty acids and modulate inflammatory status; however, they often have insufficient strength against such complicated diseases as IBS. In such cases, effectiveness differs with IBS subtypes.
Among the lactic acid bacteria, L. plantarum 299v [13] turned out to be the most heterogeneous concerning their effects, which has been attributed to many factors, including differences in the design of studies. It has mainly to do with the difference in the approach taken by the different studies regarding the assessment of a range of symptoms. Also, the host is a factor determining the effect of L. plantarum 299v [92], as those with different gut microbiota and immune profiles respond differently. The effectiveness of L. plantarum 299v [38] may also depend on the subtype of IBS since its efficacy may be more pronounced for some subtypes than others. Just as L. plantarum MF1298 [59] fails to improve IBS symptoms, likely due to its poor colonization ability, L. plantarum P-8 enhances microbiome diversity through the reduction in opportunistic pathogens, underscoring that probiotic efficacy varies by strain.
Other factors that might influence probiotic efficacy include environmental and lifestyle elements such as diet, fiber intake, and prebiotics, which can shape the gut environment and affect the survival of a given probiotic. The ineffectiveness of L. plantarum CJLP243 [48] may be attributed to gut microbiome alterations following ileostomy in rectal cancer patients, whereas L. plantarum DR7 [69] may have alleviated stress and anxiety through gut neurotransmitter modulation, an effect influenced by dietary factors. The question remains open as to whether L. plantarum strains yield the best effects when administered alone or in combination with others. Some studies reported advantages for multi-strain formulations, as seen in the cholesterol-lowering effects of L. plantarum CECT 7527, CECT 7528, and CECT 7529 [46]. While other studies using the L. plantarum strain alone can demonstrate effective results as well.
Unfavorable or adverse effects from using L. plantarum are rare and not severe side effects. In one study [81], it was stated that the incidence of children with at least one adverse event was significantly higher in the placebo group compared with the L. plantarum 299V group. The most frequently recorded adverse events were pyrexia, headache, rash, anorexia, viral infection, and ear pain. There were no serious adverse events reported in the study.
5. Conclusions
Through highlighting the therapeutic versatility of L. plantarum strains across different physiological systems, it also observed how their inefficacies are expressed strain-specifically in the establishment of gastrointestinal health, immune modulation, central nervous system disorders, metabolic regulation, skin health, and physical performance. For example, irritable bowel syndrome, diarrhea, constipation, and bowel dysfunction symptoms have been observed to efficiently improve with strains such as L. plantarum Lpla33 and L. plantarum 299v, perhaps as a result of mechanisms involving gut barrier enhancement and inflammation modulation. Whereas L. plantarum IS-10506 increases mucosal immunity, L. plantarum PS128 seems to operate either via the gut–brain axis or closer peripheral sites to change certain neuropsychological functions. Other strains prove beneficial for improving metabolic health; for instance, L. plantarum EGCG 13110402 and L. plantarum HAC01 are implicated in downregulating lipid metabolism and reducing blood glucose levels. These findings empirically suggest that L. plantarum strains can be possible significant treatment adjuncts, particularly for applications in personalized medicine and integrative healthcare.
Regarding the limitations of our study, although this study tries to compile evidence from many sources, most findings are qualitative without effect size or confidence interval estimates. Study design variations, such as dosage, intervention period, and demographic characteristics in the endpoints above, also restrict external validity and render some findings not really applicable to the wider population. Confounding variables like diet, age, sex, genetic differences, and gut microbiome diversity, which can change the response to treatment, also have not been studied in this research. The potential confounding factors affect strain efficacy, such as creating differences in the host microbiome or dietary influences. Therefore, some L. plantarum strains shown inconsistent results, such as L. plantarum 299v, which shows mixed results; some studies show some positive and some negative effects. There may be several related factors, but confounding factors may play a major role in this issue.
Future research should employ randomized controlled trials (RCTs) with standardized dosages, treatment durations, adverse or side effects, and the validated therapeutic efficacy of L. plantarum strains, ensuring statistical significance with effect size and confidence intervals. Moreover, machine learning models could enhance personalized medicine by predicting patient-specific responses based on microbiome, genetic, and lifestyle factors. Furthermore, other promising L. plantarum strains in animal studies still require clinical trials to validate their role in precision medicine [18,75,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108].
While this study applied a rigorous and systematic methodology to evaluate the clinical applications of L. plantarum strains, it is acknowledged that certain claims may still lack a critical evaluation due to inherent limitations in the included studies. One such limitation is the decision to restrict the literature search to Embase and PubMed, although this was justified by their strong biomedical relevance. Additionally, while stringent inclusion criteria were applied to ensure high-quality evidence, variations in study design, sample size, and clinical endpoints among the included studies could impact the strength of the conclusions drawn. To mitigate these limitations, a cautious and evidence-based interpretation of findings was adopted, highlighting the need for further well-controlled clinical trials to validate the therapeutic potential of L. plantarum strains.
In summary, most of the studies reviewed utilized only a single strain of Lactobacillus plantarum as a therapeutic agent to improve specific aspects of human health and well-being. However, the present study uniquely compiles multiple individual strains of L. plantarum and their diverse health applications for humans into one comprehensive overview. Their use in therapies must still be well substantiated with more rigorously controlled clinical studies. The periodic advances in research are expected to include microbiome profiling, precision probiotic interventions, and probiotic multi-strain synergies to maximize efficacy in therapy. More direct treatments may be considered for use in the future, such as Fecal Microbiota Transplantation (FMT), which could represent a promising advanced strategy to restore the gut microbiome and enhance the cognitive functions of healthy people and patients with neurological disorders. With the current and continuing expansion of probiotic science, L. plantarum will surely have the spotlight in the personalized, technologically enhanced, and evidence-based probiotic therapies that mainstream healthcare will use in the future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu17071165/s1: Table S1: Data Characteristics.
Author Contributions
Y.S., P.B., C.-W.C. and O.C. paper screening, O.C. and N.L. data extraction, O.C. and N.L. writing—original draft preparation, O.C. and K.P. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Thailand Science Research and Innovation Fund Chulalongkorn University: FOODF67300006; Second Century Fund (C2F): N/A; Thai Traditional Medical Knowledge Fund: 1/2567.
Data Availability Statement
The data used and analyzed in this systematic scoping review were obtained from publicly available sources, including the PubMed and Embase databases. All relevant data supporting the findings of this study are included in the manuscript. No additional raw data were generated or used beyond those publicly accessible.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASD | Autism spectrum disorder; |
| ADHD | Attention-deficit/hyperactivity disorder; |
| CAD | Coronary artery disease; |
| CNS | Central nervous system; |
| CVD | Cardiovascular disease; |
| FC | Functional constipation; |
| GI Tract | Gastrointestinal tract; |
| LP | Lactiplantibacillus plantarum; |
| URTI | Upper Respiratory Tract Infections |
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, C.; Sakandar, H.A.; Kwok, L.Y.; Zhang, W. Meta-Analysis: Randomized Trials of Lactobacillus plantarum on Immune Regulation Over the Last Decades. Front. Immunol. 2021, 12, 643420. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, N.; Yilmaz, B.; Sharma, H.; Kumar, M.; Pateiro, M.; Ozogul, F.; Lorenzo, J.M. A novel approach to Lactiplantibacillus plantarum: From probiotic properties to the omics insights. Microbiol. Res. 2023, 268, 127289. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–Nomad and Ideal Probiotic [Review]. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef]
- Costabile, A.; Buttarazzi, I.; Kolida, S.; Quercia, S.; Baldini, J.; Swann, J.R.; Brigidi, P.; Gibson, G.R. An in vivo assessment of the cholesterol-lowering efficacy of L. plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS ONE 2017, 12, e0187964. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. L. plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Oh, M.-R.; Jang, H.-Y.; Lee, S.-Y.; Jung, S.-J.; Chae, S.-W.; Lee, S.-O.; Park, B.-H. L. plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2337. [Google Scholar] [CrossRef] [PubMed]
- Athiyyah, A.F.; Brahmantya, H.; Dwiastuti, S.; Darma, A.; Puspitasari, D.; Husada, D.; Ranuh, R.; Endaryanto, A.; Surono, I.; Sudarmo, S.M. Effect of L. plantarum IS-10506 on blood lipopolysaccharide level and immune response in HIV-infected children. Iran. J. Microbiol. 2019, 11, 137–144. [Google Scholar]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Folwarski, M.; Witkowski, J.M.; Bryl, E.; Makarewicz, W. The role of L. plantarum 299v in supporting treatment of selected diseases. Cent. Eur. J. Immunol. 2020, 45, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Pan, C.H.; Wei, C.C.; Huang, H.Y. L. plantarum PS128 Improves Physiological Adaptation and Performance in Triathletes through Gut Microbiota Modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wei, C.-C.; Huang, C.-C.; Chen, W.-L.; Huang, H.-Y. The Beneficial Effects of L. plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Mensi, M.M.; Rogantini, C.; Marchesi, M.; Borgatti, R.; Chiappedi, M.L. Lactobacillus plantarum PS128 and Other Probiotics in Children and Adolescents with Autism Spectrum Disorder: A Real-World Experience. Nutrients 2021, 13, 2036. [Google Scholar] [CrossRef]
- Štšepetova, J.; Rätsep, M.; Gerulis, O.; Jõesaar, A.; Mikelsaar, M.; Songisepp, E. Impact of Lactiplantibacillus plantarum Inducia on metabolic and antioxidative response in cholesterol and BMI variable indices: Randomised, double-blind, placebo-controlled trials. Benef. Microbes 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, M.; Yao, M.; Naseeb, J.; Sarwar, A.; Yang, Z.; Aziz, T.; Alhomrani, M.; Alsanie, W.F.; Alamri, A.S. Lactiplantibacillus plantarum NMGL2 exopolysaccharide ameliorates DSS-induced IBD in mice mainly by regulation of intestinal tight junction and NF-κB p65 protein expression. Front. Microbiol. 2024, 15, 1491727. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wong, L.-C.; Hsu, C.-J.; Yang, C.-W.; Tsai, Y.-C.; Cheng, F.-S.; Hu, H.-Y.; Lee, W.-T. Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients 2021, 13, 3698. [Google Scholar] [CrossRef]
- Zheng, S.S.; Wang, C.Y.; Hu, Y.Y.; Yang, L.; Xu, B.C. Enhancement of fermented sausage quality driven by mixed starter cultures: Elucidating the perspective of flavor profile and microbial communities. Food Res. Int. 2024, 178, 113951. [Google Scholar] [CrossRef]
- Zhou, R.Y.; Huang, X.; Liu, Z.; Chua, J.Y.; Liu, S.Q. Evaluating the effect of lactic acid bacterial fermentation on salted soy whey for development of a potential novel soy sauce-like condiment. Curr. Res. Food Sci. 2022, 5, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Wang, H.; Han, P.J.; Lan, Y.B. Identification of dominant functional microbes that contribute to the characteristic aroma of Msalais, traditional wine fermented from boiled local grape juice in China. Food Chem. X 2023, 19, 100778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Yang, K.; Shen, J.; Lu, Z.; Lv, F.; Wang, P. Comparative Transcriptome Analysis Revealing the Enhanced Volatiles of Cofermentation of Yeast and Lactic Acid Bacteria on Whole Wheat Steamed Bread Dough. J. Agric. Food Chem. 2023, 71, 19129–19141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic L. plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Ma, Q.; Zhang, X.Y.; Gong, G.Z.; Wang, Y.X.; Zeng, L.Z.; Li, R.T.; Xiao, C.; Zuo, Y. Functional yacon juice fermented by Lactiplantibacillus plantarum QS7T: Chemical composition, flavor volatiles, and gut microbiota modulation. J. Food Sci. 2025, 90, e17528. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Zhang, S.L.; Ma, Q.; Zuo, Y.; Li, R.T.; Yang, J.X.; Zhu, R.Y.; Ge, L.H. Global genome and comparative transcriptomic analysis reveal the inulin consumption strategy of Lactiplantibacillus plantarum QS7T. Food Res. Int. 2022, 151, 110846. [Google Scholar] [CrossRef]
- Zieliński, H.; Ciesarová, Z.; Kukurová, K.; Zielinska, D.; Szawara-Nowak, D.; Starowicz, M.; Wronkowska, M. Effect of fermented and unfermented buckwheat flour on functional properties of gluten-free muffins. J. Food Sci. Technol. 2017, 54, 1425–1432. [Google Scholar] [CrossRef]
- Zoghi, A.; Darani, K.K.; Hekmatdoost, A. Effects of Pretreatments on Patulin Removal from Apple Juices Using Lactobacilli: Binding Stability in Simulated Gastrointestinal Condition and Modeling. Probiotics Antimicrob. Proteins 2021, 13, 135–145. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S.; Attar, H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2019, 126, 1149–1160. [Google Scholar] [CrossRef]
- Zolghadrpour, M.A.; Jowshan, M.R.; Heidari Seyedmahalleh, M.; Karimpour, F.; Imani, H.; Asghari, S. The effect of a new developed synbiotic yogurt consumption on metabolic syndrome components in adults with metabolic syndrome: A randomized controlled clinical trial. Nutr. Diabetes 2024, 14, 97. [Google Scholar] [CrossRef]
- Zommara, M.; El-Ghaish, S.; Haertle, T.; Chobert, J.M.; Ghanimah, M. Probiotic and technological characterization of selected Lactobacillus strains isolated from different egyptian cheeses. BMC Microbiol. 2023, 23, 160. [Google Scholar] [CrossRef]
- Babar, A.N.; Hassan, M.K.; Ullah, F.; Ullah, A. Role of L. plantarum 299v versus Placebo in symptomatic improvement of irritable bowel syndrome patients. J. Pak. Med. Assoc. 2022, 72, 404–408. [Google Scholar] [CrossRef]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical trial: L. plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, A.; Lee, J.H.; Cho, D.; Seo, J.; Jung, E.S.; Kang, H.-J.; Roh, J.; Kim, W. Effect of Oral Intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1(TM)) on Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ligaarden, S.C.; Axelsson, L.; Naterstad, K.; Lydersen, S.; Farup, P.G. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: A randomised controlled trial. BMC Gastroenterol. 2010, 10, 16. [Google Scholar] [CrossRef]
- Martoni, C.J.; Srivastava, S.; Damholt, A.; Leyer, G.J. Efficacy and dose response of Lactiplantibacillus plantarum in diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2023, 29, 4451–4465. [Google Scholar] [CrossRef]
- Niedzielin, K.; Kordecki, H.; Birkenfeld, B. A controlled, double-blind, randomized study on the efficacy of L. plantarum 299v in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Mullan, M.M.; Parker, T.J.; Woolner, J.T.; Tarry, S.A.; Hunter, J.O. Effect of L. plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig. Dis. Sci. 2002, 47, 2615–2620. [Google Scholar] [CrossRef]
- Kusumo, P.D.; Bela, B.; Wibowo, H.; Munasir, Z.; Surono, I.S. L. plantarum IS-10506 supplementation increases faecal sIgA and immune response in children younger than two years. Benef. Microbes 2019, 10, 245–252. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liong, M.T.; Chung, Y.E.; Huang, H.Y.; Peng, W.S.; Cheng, Y.F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of L. plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical Evidence of Effects of L. plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Falholt Elvebakken, H.; Bruntse, A.B.; Vedel, C.; Kjaerulff, S. Topical Lactiplantibacillus plantarum LB244R® ointment alleviates skin aging: An exploratory trial. J. Cosmet. Dermatol. 2023, 22, 1911–1918. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of L. plantarum GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. A randomized clinical trial evaluating a proprietary mixture of L. plantarum strains for lowering cholesterol. Mediterr. J. Nutr. Metab. 2016, 9, 125–135. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. Cholesterol-lowering efficacy of L. plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 2013, 109, 1866–1872. [Google Scholar] [CrossRef]
- Sohn, M.; Na, G.Y.; Chu, J.; Joung, H.; Kim, B.K.; Lim, S. Efficacy and Safety of L. plantarum K50 on Lipids in Koreans With Obesity: A Randomized, Double-Blind Controlled Clinical Trial. Front. Endocrinol. 2021, 12, 790046. [Google Scholar] [CrossRef]
- Yoon, B.J.; Oh, H.-K.; Lee, J.; Cho, J.R.; Kim, M.J.; Kim, D.-W.; Kang, S. Effects of probiotics on bowel function restoration following ileostomy closure in rectal cancer patients: A randomized controlled trial. Color. Dis. 2021, 23, 901–910. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Liu, P.; Feng, Q.; Gan, L.; Yao, L.; Huang, G.; Fang, Z.; Chen, T.; Fang, N. Positive efficacy of Lactiplantibacillus plantarum MH-301 as a postoperative adjunct to endoscopic sclerotherapy for internal hemorrhoids: A randomized, double-blind, placebo-controlled trial. Food Funct. 2023, 14, 8521–8532. [Google Scholar] [CrossRef]
- Altadill, T.; Espadaler, J.; Liong, M.-T. Does Lactoplantibacillus plantarum DR7 Reduce Days of Upper Respiratory Tract Infections and Fever? A Post-Hoc Analysis of a Randomized, Placebo-Controlled Trial. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Chong, H.-X.; Yusoff, N.A.A.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.; Wahid, N.; Abdullah, M.F.I.; Zakaria, N.; et al. L. plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J. Dairy Sci. 2019, 102, 4783–4797. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Parazzini, F.; De Leo, R.; Banco, R.; Maso, G.P.; De Santo, D.; Sartore, A.; Stabile, G.; Inglese, S.; Tonon, M. L. plantarum P17630 for preventing Candida vaginitis recurrence: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Meštrović Popovič, K.; Povalej Bržan, P.; Langerholc, T.; Marčun Varda, N. The Impact of L. Plantarum PCS26 Supplementation on the Treatment and Recurrence of Urinary Tract Infections in Children—A Pilot Study. J. Clin. Med. 2022, 11, 7008. [Google Scholar] [CrossRef]
- Palacios, S.; Espadaler, J.; Fernández-Moya, J.M.; Prieto, C.; Salas, N. Is it possible to prevent recurrent vulvovaginitis? The role of L. plantarum I1001 (CECT7504). Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1701–1708. [Google Scholar] [CrossRef]
- Vladareanu, R.; Mihu, D.; Mitran, M.; Mehedintu, C.; Boiangiu, A.; Manolache, M.; Vladareanu, S. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 262–267. [Google Scholar] [CrossRef]
- Klarin, B.; Molin, G.; Jeppsson, B.; Larsson, A. Use of the probiotic L. plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: A randomised controlled open pilot study. Crit. Care 2008, 12, R136. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, B.; Xu, X.; Mao, B.; Tang, X.; Zhao, J.; Zhang, H.; Cui, S.; Chen, W. Lactiplantibacillus Plantarum CCFM8724 Reduces the Amounts of Oral Pathogens and Alters the Oral Microbiota in Children With Dental Caries: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Nutr. Assoc. 2023, 42, 361–370. [Google Scholar] [CrossRef]
- Berggren, A.; Söderberg, L.; Önning, G.; Hagslätt, M.-L.J.; Axelsson, I. Intestinal function, microflora and nutrient intake of children after administration of a fermented oat product containing L. plantarum DSM 9843 (299v). Microb. Ecol. Health Dis. 2003, 15, 160–168. [Google Scholar] [CrossRef]
- Farup, P.G.; Jacobsen, M.; Ligaarden, S.C.; Rudi, K. Probiotics, symptoms, and gut microbiota: What are the relations? A randomized controlled trial in subjects with irritable bowel syndrome. Gastroenterol. Res. Pract. 2012, 2012, 214102. [Google Scholar] [CrossRef]
- Kwok, L.Y.; Guo, Z.; Zhang, J.; Wang, L.; Qiao, J.; Hou, Q.; Zheng, Y.; Zhang, H. The impact of oral consumption of L. plantarum P-8 on faecal bacteria revealed by pyrosequencing. Benef. Microbes 2015, 6, 405–413. [Google Scholar] [CrossRef]
- Liu, G.; Chong, H.X.; Chung, F.Y.; Li, Y.; Liong, M.T. L. plantarum DR7 Modulated Bowel Movement and Gut Microbiota Associated with Dopamine and Serotonin Pathways in Stressed Adults. Int. J. Mol. Sci. 2020, 21, 4608. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, M.S.; Shim, K.W.; Kim, Y.I.; Chu, J.; Kim, B.K.; Choi, I.S.; Kim, J.Y. Effects of L. plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of oral consumption of probiotic L. planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014, 30, 776–783.e1. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, B.; Ban, J.; Lee, J.; Kim, B.J.; Choi, B.S.; Hwang, S.; Ahn, K.; Kim, J. A randomized trial of L. plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 667–673. [Google Scholar] [CrossRef]
- Kim, J.; Lee, B.S.; Kim, B.; Na, I.; Lee, J.; Lee, J.Y.; Park, M.; Kim, H.; Sohn, I.; Ahn, K. Identification of atopic dermatitis phenotypes with good responses to probiotics (L. plantarum CJLP133) in children. Benef. Microbes 2017, 8, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Prakoeswa, C.R.S.; Bonita, L.; Karim, A.; Herwanto, N.; Umborowati, M.A.; Setyaningrum, T.; Hidayati, A.N.; Surono, I.S. Beneficial effect of L. plantarum IS-10506 supplementation in adults with atopic dermatitis: A randomized controlled trial. J. Dermatol. Treat. 2022, 33, 1491–1498. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Herwanto, N.; Prameswari, R.; Astari, L.; Sawitri, S.; Hidayati, A.N.; Indramaya, D.; Kusumowidagdo, E.; Surono, I.S. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef. Microbes 2017, 8, 833–840. [Google Scholar] [CrossRef]
- Chen, H.M.; Kuo, P.H.; Hsu, C.Y.; Chiu, Y.H.; Liu, Y.W.; Lu, M.L.; Chen, C. Psychophysiological Effects of L. plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Bin Abdullah, M.F.I.L.; Zakaria, N.; et al. L. plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–374. [Google Scholar] [CrossRef]
- Ho, Y.-T.; Tsai, Y.-C.; Kuo, T.B.J.; Yang, C.C.H. Effects of L. plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.; Abdullah, M.F.I.; Zakaria, N.; et al. Probiotic L. plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Önning, G.; Hillman, M.; Hedin, M.; Montelius, C.; Eriksson, J.; Ahrné, S.; Jönsson, P. Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress: A randomized, double blind, placebo-controlled study. Physiol. Behav. 2020, 225, 113083. [Google Scholar] [CrossRef] [PubMed]
- Önning, G.; Montelius, C.; Hillman, M.; Larsson, N. Intake of Lactiplantibacillus plantarum HEAL9 Improves Cognition in Moderately Stressed Subjects: A Randomized Controlled Study. Nutrients 2023, 15, 3466. [Google Scholar] [CrossRef]
- Sakurai, K.; Toshimitsu, T.; Okada, E.; Anzai, S.; Shiraishi, I.; Inamura, N.; Kobayashi, S.; Sashihara, T.; Hisatsune, T. Effects of Lactiplantibacillus plantarum OLL2712 on Memory Function in Older Adults with Declining Memory: A Randomized Placebo-Controlled Trial. Nutrients 2022, 14, 4300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhao, X.; Wu, H.; Zeng, X.; Wei, J.; Chen, T. Psychobiotics Lactiplantibacillus plantarum JYLP-326: Antidepressant-like effects on CUMS-induced depressed mouse model and alleviation of gut microbiota dysbiosis. J. Affect. Disord. 2024, 354, 752–764. [Google Scholar] [CrossRef]
- Coman, M.M.; Miorelli, L.; Micioni Di Bonaventura, M.V.; Cifani, C.; Salvesi, C.; Amedei, A.; Silvi, S.; Verdenelli, M.C. Effects of probiotic Lactiplantibacillus plantarum IMC 510 supplementation on metabolic factors in otherwise healthy overweight and obese individuals. J. Appl. Microbiol. 2022, 133, 1956–1968. [Google Scholar] [CrossRef]
- Fu, S.-K.; Tseng, W.-C.; Tseng, K.-W.; Lai, C.-C.; Tsai, Y.-C.; Tai, H.-L.; Hsu, C.-C. Effect of Daily Oral L. plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon. Nutrients 2021, 13, 4023. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of L. plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Mariyatun, M.; Putri Manurung, N.E.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of probiotic L. plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J. Gastroenterol. 2021, 27, 107–128. [Google Scholar] [CrossRef]
- Sohn, M.; Jung, H.; Lee, W.S.; Kim, T.H.; Lim, S. Effect of L. plantarum LMT1-48 on Body Fat in Overweight Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. J. 2023, 47, 92–103. [Google Scholar] [CrossRef]
- Olek, A.; Woynarowski, M.; Ahrén, I.L.; Kierkuś, J.; Socha, P.; Larsson, N.; Önning, G. Efficacy and Safety of L. plantarum DSM 9843 (299v) in the Prevention of Antibiotic-Associated Gastrointestinal Symptoms in Children: Randomized, Double-Blind, Placebo-Controlled Study. J. Pediatr. 2017, 186, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.-H.; Kim, W.; Park, M.R.; Yoon, S.M.; Kim, Y.; Yang, S.; et al. Effect of a new L. plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192. [Google Scholar] [CrossRef]
- Yang, B.; Yue, Y.; Chen, Y.; Ding, M.; Li, B.; Wang, L.; Wang, Q.; Stanton, C.; Ross, R.P.; Zhao, J.; et al. L. plantarum CCFM1143 Alleviates Chronic Diarrhea via Inflammation Regulation and Gut Microbiota Modulation: A Double-Blind, Randomized, Placebo-Controlled Study. Front. Immunol. 2021, 12, 746585. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Folwarski, M.; Ruszkowski, J.; Skonieczna-Żydecka, K.; Szafrański, W.; Makarewicz, W. Effects of 4 weeks of L. plantarum 299v supplementation on nutritional status, enteral nutrition tolerance, and quality of life in cancer patients receiving home enteral nutrition—A double-blind, randomized, and placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9684–9694. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Folwarski, M.; Skonieczna-Żydecka, K.; Ruszkowski, J.; Makarewicz, W. The use of L. plantarum 299v (DSM 9843) in cancer patients receiving home enteral nutrition—Study protocol for a randomized, double-blind, and placebo-controlled trial. Nutr. J. 2020, 19, 98. [Google Scholar] [CrossRef]
- Kusumo, P.D.; Maulahela, H.; Utari, A.P.; Surono, I.S.; Soebandrio, A.; Abdullah, M. Probiotic L. plantarum IS 10506 supplementation increase SCFA of women with functional constipation. Iran. J. Microbiol. 2019, 11, 389–396. [Google Scholar]
- Liu, W.; Lu, N.H.; Zhou, X.; Li, Y.; Xie, Y.; Zheng, L.; Zhu, W.; Xiao, Q.; Yang, N.; Zuo, K.; et al. Effects of L. plantarum P9 Probiotics on Defecation and Quality of Life of Individuals with Chronic Constipation: Protocol for a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 4144321. [Google Scholar] [CrossRef]
- Ma, T.; Yang, N.; Xie, Y.; Li, Y.; Xiao, Q.; Li, Q.; Jin, H.; Zheng, L.; Sun, Z.; Zuo, K.; et al. Effect of the probiotic strain, Lactiplantibacillus plantarum P9, on chronic constipation: A randomized, double-blind, placebo-controlled study. Pharmacol. Res. 2023, 191, 106755. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Ma, K.; Wang, G.; Tang, M.; Wang, R.; Xia, Z.; Xu, Z.; Sun, M.; Bao, X.; et al. L. plantarum Lp3a improves functional constipation: Evidence from a human randomized clinical trial and animal model. Ann. Transl. Med. 2022, 10, 316. [Google Scholar] [CrossRef]
- Ranuh, R.G.; Radhiah, S.; Sumitro, K.R.; Darma, A.; Athiyyah, A.F.; Puspitasari, D.; Husada, D.; Endaryanto, A.; Surono, I.; Sudarmo, S.M. Regulation of T Helper and Regulatory T Cells by L. Plantarum IS-10506 Supplementation to Human Immunodeficiency Virus-Infected Children Under Antiretroviral Therapy. Int. J. Probiotics Prebiotics 2022, 17, 42–46. [Google Scholar] [CrossRef]
- Rönnqvist, P.D.; Forsgren-Brusk, U.B.; Grahn-Håkansson, E.E. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet. Gynecol. Scand. 2006, 85, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Litton, E.; Anstey, M.; Broadhurst, D.; Chapman, A.; Currie, A.; Ferrier, J.; Gummer, J.; Higgins, A.; Lim, J.; Manning, L.; et al. Early and sustained L. plantarum probiotic therapy in critical illness: The randomised, placebo-controlled, restoration of gut microflora in critical illness trial (ROCIT). Intensive Care Med. 2021, 47, 307–315. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Zhang, L.; Zhao, Y.; Duan, C.; Zhang, X.; Gao, L.; Li, S. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl. Microbiol. Biotechnol. 2019, 103, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of Dietary Lactobacillus plantarum on Growth Performance, Digestive Enzymes and Gut Morphology of Litopenaeus vannamei. Probiotics Antimicrob. Proteins 2018, 10, 504–510. [Google Scholar] [CrossRef]
- Zhong, B.; Liang, W.; Zhao, Y.; Li, F.; Zhao, Z.; Gao, Y.; Yang, G.; Li, S. Combination of Lactiplantibacillus Plantarum ELF051 and Astragalus Polysaccharides Improves Intestinal Barrier Function and Gut Microbiota Profiles in Mice with Antibiotic-Associated Diarrhea. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, Q.; He, Y.; Zhao, X.; Wang, Y.; He, L.; Wei, H.; Tao, X. Maternal supplementation with human milk-derived Lactiplantibacillus plantarum WLPL04 affects the immunity and gut microbiota of offspring rats. Food Funct. 2023, 14, 5326–5341. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Abdullah Hafeez, M.A.; Guan, R.; Feng, F. Lactobacillus plantarum ZJUFB2 Prevents High Fat Diet-Induced Insulin Resistance in Association With Modulation of the Gut Microbiota. Front. Nutr. 2021, 8, 754222. [Google Scholar] [CrossRef]
- Zhong, Y.; Nyman, M. Prebiotic and synbiotic effects on rats fed malted barley with selected bacteria strains. Food Nutr. Res. 2014, 58, 24848. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, B.; Gu, R.; Li, P.; Gu, Q. Lactobacillus plantarum ZJ316 Attenuates Helicobacter pylori-Induced Gastritis in C57BL/6 Mice. J. Agric. Food Chem. 2021, 69, 6510–6523. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, L.; Zeng, Y.; Niu, L.; Pu, Y.; Jacobs, J.P.; Chang, C.; Wang, J.; Khalique, A.; Pan, K.; et al. The Panda-Derived Lactobacillus plantarum G201683 Alleviates the Inflammatory Response in DSS-Induced Panda Microbiota-Associated Mice. Front. Immunol. 2021, 12, 747045. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, X.; Duan, L.; Niu, L.; Liu, Q.; Zeng, Y.; Wang, Q.; Wang, J.; Khalique, A.; Pan, K.; et al. Lactobacillus plantarum BSGP201683 Improves the Intestinal Barrier of Giant Panda Microbiota-Associated Mouse Infected by Enterotoxigenic Escherichia coli K88. Probiotics Antimicrob. Proteins 2021, 13, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.K.; Qin, H.L.; Zhang, M.; Shen, T.Y.; Chen, H.Q.; Ma, Y.L.; Chu, Z.X.; Zhang, P.; Liu, Z.H. Effects of Lactobacillus plantarum on gut barrier function in experimental obstructive jaundice. World J. Gastroenterol. 2012, 18, 3977–3991. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, M.; Zhang, R.; Sun, Z.; Wang, C.; Yang, F.; Huang, T.; Qu, S.; Zhao, L.; Li, Y.; et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Factories 2017, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, X.; Liu, N.; Zhao, R.; Wang, S. Lactobacillus plantarum alleviates high-fat diet-induced obesity by altering the structure of mice intestinal microbial communities and serum metabolic profiles. Front. Microbiol. 2024, 15, 1425764. [Google Scholar] [CrossRef]
- Zolfaghari, S.I.; Rabbani Khorasgani, M.; Noorbakhshnia, M. The effects of lactobacilli (L. rhamnosus, L. reuteri, L. Plantarum) on LPS-induced memory impairment and changes in CaMKII-α and TNF-α genes expression in the hippocampus of rat. Physiol. Behav. 2021, 229, 113224. [Google Scholar] [CrossRef]
- Zoumpourtikoudi, V.; Pyrgelis, N.; Chatzigrigoriou, M.; Tasakis, R.N.; Touraki, M. Interactions among yeast and probiotic bacteria enhance probiotic properties and metabolism offering augmented protection to Artemia franciscana against Vibrio anguillarum. Microb. Pathog. 2018, 125, 497–506. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, T.; Dong, X.; Liu, J.; Liu, Y. Identification of the key tryptophan metabolic characteristics of Lactiplantibacillus plantarum for aryl hydrocarbon receptor activation and ulcerative colitis alleviation. Food Res. Int. 2025, 203, 115766. [Google Scholar] [CrossRef]
- Zvanych, R.; Lukenda, N.; Kim, J.J.; Li, X.; Petrof, E.O.; Khan, W.I.; Magarvey, N.A. Small molecule immunomodulins from cultures of the human microbiome member Lactobacillus plantarum. J. Antibiot. 2014, 67, 85–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).