Risk Factors of Primary Dysmenorrhea in Female Adolescent Basketball Players Related to Dietary, Hormonal, and Immuno-Metabolic Factors and Disordered Eating Attitudes
Highlights
- Young female basketball players with dysmenorrhea showed higher EAT-26 scores, indicating a greater susceptibility to disordered eating attitudes (DEA).
- They also had elevated serum prolactin and cortisol levels compared to their non-dysmenorrheic counterparts.
- No significant differences in macronutrient and energy intake were found between the groups.
- The combination of higher prolactin, cortisol, and EAT-26 scores may be linked to an increased risk of premenstrual dysphoria (PD).
- The observed hormonal and behavioral changes suggest that dysmenorrheic young athletes may be more vulnerable to stress, depression, and gynecological health issues, potentially affecting their athletic performance.
- Targeted interventions are needed to improve their quality of life.
- Future research should include long-term dietary assessments, sleep patterns, psychological stress, and training intensity to better understand dysmenorrhea in female athletes.
- Larger, multi-center studies are required to confirm findings and determine causality between hormonal changes and PD.
Abstract
1. Introduction
1.1. Dysmenorrhea
1.2. Types of Dysmenorrhea
1.3. Risk Factors for Dysmenorrhea
1.4. Pathophysiology of Dysmenorrhea
1.5. Study Objectives
2. Materials and Methods
2.1. Study Participants and Design
2.2. Medical Interview
2.3. Anthropometric and Body Composition Assessment
2.4. Nutritional Evaluation
2.5. Eating Behavior Assessment
- The first subscale, Dieting, primarily reflects restrictive eating behaviors and body dissatisfaction.
- The second subscale, Bulimia and Food Preoccupation, encompasses bulimic tendencies and compulsive eating behaviors.
- The third subscale, Oral Control, assesses behaviors and beliefs related to self-imposed control over food intake, often linked to anorexic tendencies.
2.6. Biochemical Analysis
2.7. Statistical Analyses
- OR = 1: Indicates no association between the examined parameter and the occurrence of dysmenorrhea. The odds of experiencing dysmenorrhea are the same in both groups.
- OR > 1: Suggests an increased likelihood of dysmenorrhea as the parameter value increases. The higher the OR, the stronger the association.
- OR < 1: Suggests a decreased likelihood of dysmenorrhea as the parameter value increases. The lower the OR, the stronger the association.
3. Results
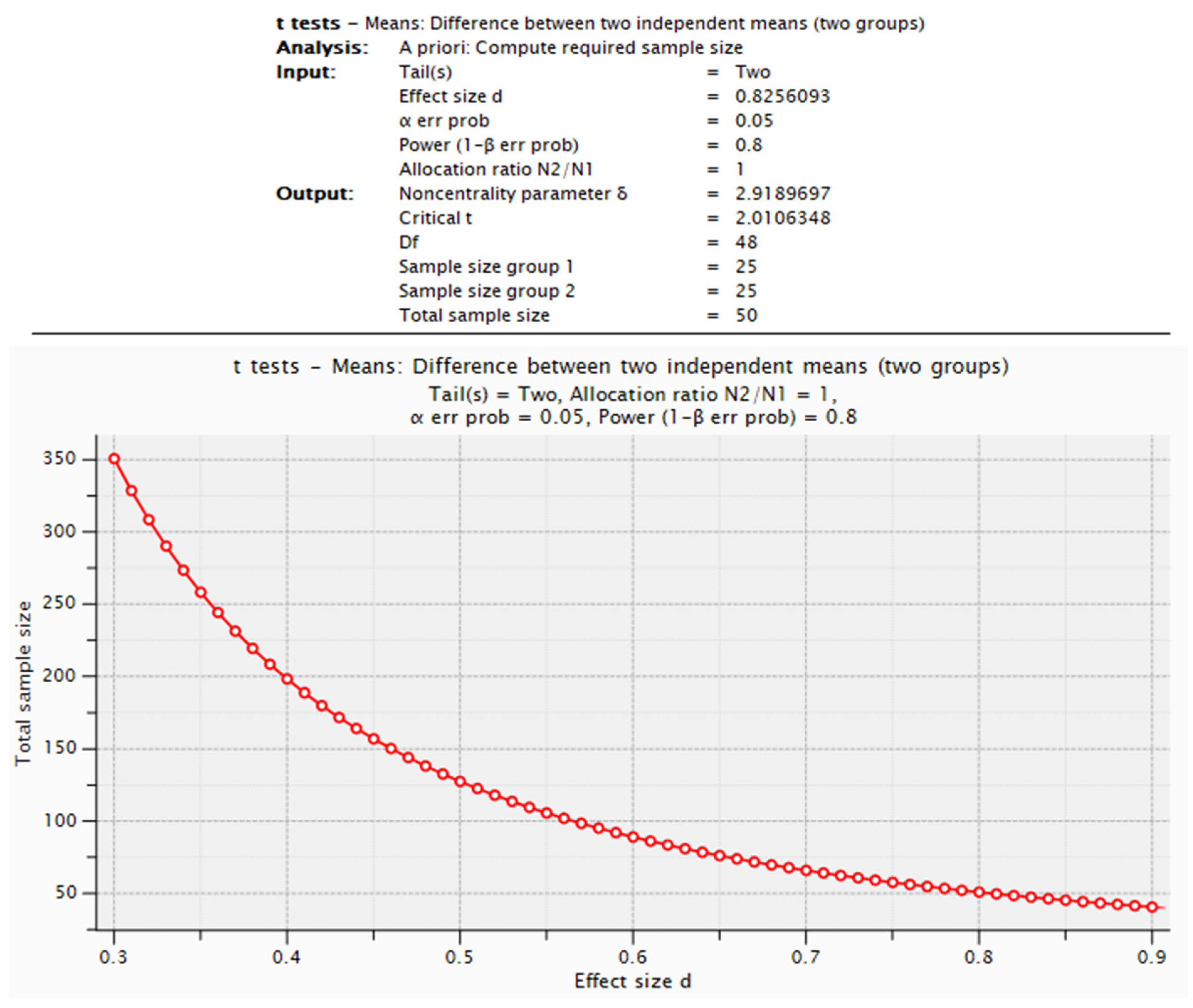
Required Sample Size in the Context of the Pilot Study
- BMI: To detect an effect size of d = 0.59127, 46 participants per group would be needed (Student’s t-test for two independent groups, α = 0.05, statistical power = 0.8).
- Age at menarche: To detect an effect size of d = 0.695648, 39 participants per group would be required (Wilcoxon–Mann–Whitney test, α = 0.05, statistical power = 0.8).
- HOMA-IR: To detect an effect size of d = 0.8256093, 25 participants per group would be necessary (Student’s t-test for two independent groups, α= 0.05, statistical power = 0.8).
- No-D group: 2.42 ± 0.59;
- D group: 1.89 ± 0.69 (Table 2).
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drejza, M.; Rylewicz, K.; Majcherek, E.; Barwińska, J.; Łopiński, G.; Mizgier, M.; Plagens-Rotman, K.; Pisarska-Krawczyk, M.; Jarząbek-Bielecka, G.; Kędzia, W. Dysmenorrhea in Polish Adolescent Girls: Impact on Physical, Mental, and Social Well-Being—Results from POLKA 18 Study. J. Clin. Med. 2024, 13, 6286. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Osayande, A.S.; Mehulic, S. Diagnosis and initial management of dysmenorrhea. Am. Fam. Physician 2014, 89, 341–346. [Google Scholar]
- Harel, Z. Dysmenorrhea in adolescents and young adults: An update on pharmacological treatments and management strategies. Expert. Opin. Pharmacother. 2012, 13, 2157–2170. [Google Scholar]
- Drosdzol, A.; Skrzypulec, V. Bolesne miesiaczkowanie w ginekologii dzieciecej i dziewczecej [Dysmenorrhea in pediatric and adolescent gynaecology]. Ginekol. Pol. 2008, 79, 499–503. (In Polish) [Google Scholar]
- Mendiratta, V.; Lentz, G.M. Primary and secondary dysmenorrhea, premenstrual syndrome, and premenstrual dysphoric disorder. In Comprehensive Gynecology, 7th ed.; Lobo, R.A., Gershenson, D.M., Lentz, G.M., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2017; pp. 815–828. [Google Scholar]
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and related disorders. F1000Res 2017, 6, 1645. [Google Scholar] [CrossRef]
- Ju, H.; Jones, M.; Mishra, G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar]
- American Psychiatric Association. DSM-5 Diagnostic Classification. In Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; p. 10. [Google Scholar]
- Alvero-Cruz, J.R.; Mathias, V.P.; García-Romero, J.C. Somatotype Components as Useful Predictors of Disordered Eating Attitudes in Young Female Ballet Dance Students. J. Clin. Med. 2020, 9, 2024. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar]
- Malina, R.M.; Spirduso, W.W.; Tate, C.; Baylor, A.M. Age at menarche and selected menstrual characteristics in athletes at different competitive levels and in different sports. Med. Sci. Sports 1978, 10, 218–222. [Google Scholar]
- Dusek, T. Influence of high intensity training on menstrual cycle disorders in athletes. Croat. Med. J. 2001, 42, 79–82. [Google Scholar] [PubMed]
- Millares Samperio, M.; Corrales Pardo, A. Effects of physical exercise on primary dysmenorrhea. Systematic review. MLS Sport. Res. 2021, 1, 51–68. [Google Scholar]
- Yang, M.-Y.; Chen, H.-Y.; Ho, C.-H.; Huang, W.-C. Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation. Nutrients 2025, 17, 622. [Google Scholar] [CrossRef]
- World Health Organization. Growth Reference 5–19. BMI-for-Age for Girls. 2007. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 21 January 2025).
- Mizgier, M.; Jarzabek-Bielecka, G.; Jakubek, E.; Kedzia, W. The relationship between body mass index, body composition and premenstrual syndrome prevalence in girls. Ginekol. Pol. 2019, 90, 256–261. [Google Scholar] [CrossRef]
- Wan, C.S.; Ward, L.C.; Halim, J.; Gow, M.L.; Ho, M.; Briody, J.N.; Leung, K.; Cowell, C.T.; Garnett, S.P. Bioelectrical impedance analysis to estimate body composition, and change in adiposity, in overweight and obese adolescents: Comparison with dual-energy X-ray absorptiometry. BMC Pediatr. 2014, 14, 249. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarząbek-Bielecka, G.; Opydo-Szymaczek, J.; Wendland, N.; Wieckowska, B.; Kędzia, W. Risk Factors of Overweight and Obesity Related to Diet and Disordered Eating Attitudes in Adolescent Girls with Clinical Features of Polycystic Ovary Syndrome. J. Clin. Med. 2020, 9, 3041. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarzabek-Bielecka, G.; Wendland, N.; Jodłowska-Siewert, E.; Nowicki, M.; Brozek, A.; Kedzia, W.; Formanowicz, D.; Opydo-Szymaczek, J. Relation between Inflammation, Oxidative Stress, and Macronutrient Intakes in Normal and Excessive Body Weight Adolescent Girls with Clinical Features of Polycystic Ovary Syndrome. Nutrients 2021, 13, 896. [Google Scholar] [CrossRef]
- Kowalkowska, J.; Slowinska, M.A.; Slowinski, D.; Dlugosz, A.; Niedzwiedzka, E.; Wadolowska, L. Comparison of a Full Food-Frequency Questionnaire with the Three-Day Unweighted Food Records in Young Polish Adult Women: Implications for Dietary Assessment. Nutrients 2013, 5, 2747–2776. [Google Scholar] [CrossRef]
- Gronowska-Senger, A. Przewodnik Metodyczny Badan Sposobu Żywienia; Komitet Nauki o Żywieniu Człowieka Polskiej Akademii Nauk: Warsaw, Poland, 2013. [Google Scholar]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album Fotografii Produktów i Potraw; Wydawnictwo IZiZ 2000: Warsaw, Poland, 2000. [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stos, K.; Wierzejska, R.; Wojtasik, A.; Charzewska, J.; Mojska, H.; Szponar, L.; Sajór, I.; Kłosiewicz-Latoszek, L.; et al. Normy Zywienia dla Populacji Polski; Instytut Zywnosci i Zywienia: Warsaw, Poland, 2017. [Google Scholar]
- Mizgier, M.; Jarzabek-Bielecka, G.; Mruczyk, K.; Kedzia, W. Comparison of dietary behaviour of a selected student population as regards their influence on fertility. Clin. Exp. Obstet. Gynecol. 2019, 46, 450–457. [Google Scholar] [CrossRef]
- Czech-Szczapa, B.; Szczapa, T.; Merritt, T.A.; Wysocki, J.; Gadzinowski, J.; Ptaszynski, T.; Drews, K. The Influence Of Disordered Eating Attitudes On Pregnancy And Neonatal Outcomes. Arch. Dis. Child. 2014, 99, A466. [Google Scholar] [CrossRef][Green Version]
- Włodarczyk-Bisaga, K.; Dolan, B.; McCluskey, S.; Lacey, H. Disordered eating behaviour and attitudes towards weight and shape in polish Women. Eur. Eat. Disord. Rev. 1995, 3, 205–216. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarzabek-Bielecka, G.; Formanowicz, D.; Jodłowska-Siewert, E.; Mruczyk, K.; Cisek-Wozniak, A.; Kedzia, W.; Opydo-Szymaczek, J. Dietary and Physical Activity Habits in Adolescent Girls with Polycystic Ovary Syndrome (PCOS)-HAstudy. J. Clin. Med. 2021, 10, 3469. [Google Scholar] [CrossRef] [PubMed]
- Wendland, N.; Opydo-Szymaczek, J.; Mizgier, M.; Jarzabek-Bielecka, G. Subgingival microflora in adolescent females with polycystic ovary syndrome and its association with oral hygiene, gingivitis, and selected metabolic and hormonal parameters. Clin. Oral. Investig. 2021, 25, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarząbek-Bielecka, G.; Mruczyk, K.; Kędzia, W. The role of obesity and environmental factors such as diet and physical activity in the etiopathogenesis of fertility disorders. Clin. Exp. Obstet. Gynecol. 2019, 46, 516–520. [Google Scholar] [CrossRef]
- Mizgier, M.; Watrowski, R.; Opydo-Szymaczek, J.; Jodłowska-Siewert, E.; Lombardi, G.; Kędzia, W.; Jarząbek-Bielecka, G. Association of Macronutrients Composition, Physical Activity and Serum Androgen Concentration in Young Women with Polycystic Ovary Syndrome. Nutrients 2022, 14, 73. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarząbek-Bielecka, G.; Drejza, M.; Luwański, D.; Wójcik, M.; Plagens-Rotman, K.; Gozdziewicz, T.; Pisarska-Krawczyk, M.; Kędzia, W. Associations between Diet and Changes in Pain Levels among Young Women with Premenstrual Syndrome—A Preliminary Study during the COVID-19 Pandemic. J. Clin. Med. 2023, 12, 4015. [Google Scholar] [CrossRef]
- Mizgier, M.; Więckowska, B.; Formanowicz, D.; Lombardi, G.; Brożek, A.; Nowicki, M.; Durkalec-Michalski, K.; Kędzia, W.; Jarząbek-Bielecka, G. Effects of AIDiet intervention to improve diet quality, immuno-metabolic health in normal and overweight PCOS girls: A pilot study. Sci. Rep. 2024, 14, 3525. [Google Scholar] [CrossRef]
- Rad, M.; Sabzevari, M.T.; Rastaghi, S.; Dehnavi, Z.M. The relationship between anthropometric index and primary dysmenorehea in female high school students. J. Educ. Health Promot. 2018, 7, 34. [Google Scholar] [CrossRef]
- Chauhan, M.; Kala, J. Relation between dysmenorrhea and body mass index in adolescents with rural versus urban variation. J. Obstet. Gynaecol. India 2012, 62, 442–445. [Google Scholar] [CrossRef]
- Bakhsh, H.; Algenaimi, E.; Aldhuwayhi, R.; AboWadaan, M. Prevalence of dysmenorrhea among reproductive age group in Saudi Women. BMC Women’s Health 2022, 22, 78. [Google Scholar] [CrossRef] [PubMed]
- Speroff, L.; Fritz, M.A. Clinical Gynecologic Endocrinology and Infertility, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Soliman, N.A.; Elalaily, R.; El Kholy, M. Dysmenorrhea in adolescents and young adults: A review in different countries. Acta Biomed. 2016, 87, 233–246. [Google Scholar] [PubMed]
- Marques, P.; Madeira, T.; Gama, A. Menstrual cycle among adolescents: Girls’ awareness and influence of age at menarche and overweight. Rev. Paul. Pediatr. 2022, 40, e2020494. [Google Scholar] [CrossRef]
- Moghaddam, H.G.; Gholami, N.; Esfahani, A.; Ghoreish, Z.; Khalaji, A. Serum vitamin D levels and their correlation with pro-inflammatory prostaglandins in Acute myeloid leukemia: A cross-sectional analysis. Sci. Rep. 2024, 14, 32069. [Google Scholar] [CrossRef]
- Abdul-Razzak, K.K.; Ayoub, N.M.; Abu-Taleb, A.A.; Obeidat, B.A. Influence of dietary intake of dairy products on dysmenorrhea. J. Obstet. Gynaecol. Res. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, Y.F.; Lin, Y.J.; Huang, K.C.; Chen, H.Y.; Hamdy, N.M.; Huang, T.C.; Chang, H.Y.; Shieh, T.M.; Huang, Y.J.; et al. Effect of vitamin D supplementation on primary dysmenorrhea: A systematic review and meta-analysis of randomized clinical trials. Nutrients 2023, 15, 2830. [Google Scholar] [CrossRef]
- Pakniat, H.; Chegini, V.; Ranjkesh, F.; Hosseini, M.A. Comparison of the effect of vitamin E, vitamin D and ginger on the severity of primary dysmenorrhea: A single-blind clinical trial. Obstet. Gynecol. Sci. 2019, 62, 462–468. [Google Scholar] [CrossRef]
- Sureda, A.; Del Mar Bibiloni, M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the mediterranean diet and inflammatory markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef]
- Mayr, H.L.; Tierney, A.C.; Thomas, C.J.; Ruiz-Canela, M.; Radcliffe, J.; Itsiopoulos, C. Mediterranean-type diets and inflammatory markers in patients with coronary heart disease: A systematic review and meta-analysis. Nutr. Res. 2018, 50, 10–24. [Google Scholar] [CrossRef]
- Lahoz, C.; Castillo, E.; Mostaza, J.M.; De Dios, O.; Salinero-Fort, M.A.; González-Alegre, T.; García-Iglesias, F.; Estirado, E.; Laguna, F.; Sanchez, V.; et al. Relationship of the adherence to a mediterranean diet and its main components with CRP levels in the Spanish population. Nutrients 2018, 10, 379. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. On behalf of the Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Fu, J.; Tan, L.J.; Lee, J.E.; Shin, S. Association between the mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 946361. [Google Scholar] [CrossRef]
- Dmitrović, R. Transvaginal color Doppler study of uterine blood flow in primary dysmenorrhea. Acta Obstet. Gynecol. Scand. 2000, 79, 1112–1116. [Google Scholar] [CrossRef]
- Ertiana, D.; Akhyar, M.; Budihastuti, U.R. Path analysis of factors which correlated with dysmenorrhea. J. Med. 2016, 1, 136–145. [Google Scholar]
- Emara, H.M.; Khalil, H.A.; Abo Elainin, M.F.; Osman, D.A. Effect of Low Level Laser Therapy Versus Pulsed Electromagnetic Field on Cortisol Levels in Primary Dysmenorrhea: A Randomized Controlled Trial. eJPT 2022, 9, 15–20. [Google Scholar]
- Pakpour, A.H.; Kazemi, F.; Alimoradi, Z.; Griffiths, M.D. Depression, anxiety, stress, and dysmenorrhea: A protocol for a systematic review. Syst. Rev. 2020, 9, 65. [Google Scholar] [CrossRef]
- Elgellaie, A.; Larkin, T.; Kaelle, J.; Mills, J.; Thomas, S. Plasma prolactin is higher in major depressive disorder and females, and associated with anxiety, hostility, somatization, psychotic symptoms and heart rate. Compr. Psychoneuroendocrinol. 2021, 6, 100049. [Google Scholar] [CrossRef]
- Litschgi, M.; Glatthaar, E. Primäre Dysmenorrhö und Hyperprolaktinämie [Primary dysmenorrhoea and hyperprolactinemia (author’s transl)]. Geburtshilfe Frauenheilkd. 1978, 38, 569–572. [Google Scholar]
- Litschgi, L.; Hauser, T.; Blum, C.A. Hyperprolactinemia and its association with severe primary dysmenorrhea. J. Endocrinol. Investig. 2019, 42, 1139–1148. [Google Scholar] [CrossRef]
- Bailer, U.F.; Kaye, W.H.; Guo, W. Abnormal neuroendocrine stress responses in eating disorders: Cortisol and beyond. Psychoneuroendocrinology 2017, 79, 79–91. [Google Scholar] [CrossRef]
| Parameters | Values | No-D Group (n = 11) | D Group (n = 14) | p | Test | OR [95% CI] |
|---|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 16 ± 0.54 | 16 ± 0.55 | 0.7026 | M-W | 0.72 [0.16, 3.28] |
| Median [Q1; Q3] | 16 [16; 16] | 16 [16; 16] | ||||
| Body height (cm) | Mean ± SD | 171.2 ± 6.95 | 171.4 ± 8.35 | 0.9379 | t-st | 1 [0.9, 1.12] |
| Median [Q1; Q3] | 173 [167; 175] | 170.5 [166.75; 175] | ||||
| Body weight (kg) | Mean ± SD | 63.4 ± 6.17 | 61.1 ± 9.76 | 0.4935 | t-st | 0.96 [0.87, 1.07] |
| Median [Q1; Q3] | 63.1 [58.35; 66.3] | 61.25 [55.43; 64.85] | ||||
| BMI | Mean ± SD | 21.6 ± 1.11 | 20.7 ± 1.9 | 0.1689 | t-st | 0.67 [0.38, 1.19] |
| Median [Q1; Q3] | 21.6 [20.7; 22.15] | 20.75 [19.73; 21.95] | ||||
| FM (%) | Mean ± SD | 23.9 ± 3.34 | 22.6 ± 5.49 | 0.5070 | t-st | 0.94 [0.78, 1.13] |
| Median [Q1; Q3] | 24.4 [22.85; 25.75] | 22 [19.8; 26.03] | ||||
| FM (kg) | Mean ± SD | 15.2 ± 3.09 | 14.2 ± 5.16 | 0.5445 | t-st | 0.94 [0.78, 1.14] |
| Median [Q1; Q3] | 15.2 [14.05; 16.6] | 12.85 [11.7; 16.68] | ||||
| WC (cm) | Mean ± SD | 69.6 ± 6.88 | 69.1 ± 12.75 | 0.8260 | M-W | 1 [0.92, 1.08] |
| Median [Q1; Q3] | 68 [66.5; 69.5] | 68.5 [60.25; 73.5] | ||||
| Menarche (years) | Mean ± SD | 12.7 ± 0.9 | 12 ± 1.18 | 0.0786 | M-W | 0.52 [0.23, 1.16] |
| Median [Q1; Q3] | 13 [12; 13] | 12 [11; 12] |
| Parameters | Values | No-D Group (n = 10) | D Group (n = 11) | p | Test | OR [95% CI] |
|---|---|---|---|---|---|---|
| CRP (mg/L) | Mean ± SD | 0.9 ± 0.9 | 1 ± 0.63 | 0.9719 | M-W | 1.09 [0.34, 3.55] |
| Median [Q1; Q3] | 0.54 [0.46; 0.85] | 0.68 [0.45; 1.34] | ||||
| 25-(OH)D3 (nmol/L) | Mean ± SD | 69.6 ± 23.82 | 62.8 ± 23.82 | 0.5035 | M-W | 0.97 [0.88, 1.07] |
| Median [Q1; Q3] | 61.77 [56.60; 73.75] | 58.20 [47.83; 71.05] | ||||
| TC (mg/dL) | Mean ± SD | 159.2 ± 35.65 | 162.5 ± 19.52 | 0.7885 | t-st | 1 [0.97, 1.04] |
| Median [Q1; Q3] | 153.4 [134.13; 182.18] | 165.4 [153.65; 174.9] | ||||
| LDL (mg/dL) | Mean ± SD | 80.7 ± 23.49 | 80 ± 14.22 | 0.9387 | t-st | 1 [0.95, 1.05] |
| Median [Q1; Q3] | 75.7 [63.33; 96.65] | 82 [73.25; 87.85] | ||||
| HDL (mg/dL) | Mean ± SD | 62.5 ± 14.04 | 63.3 ± 15.71 | 0.9072 | t-st | 1 [0.95, 1.07] |
| Median [Q1; Q3] | 62.15 [50.33; 71.1] | 61.7 [48.7; 74.7] | ||||
| TGs (mg/dL) | Mean ± SD | 76.1 ± 18.56 | 89.9 ± 31.05 | 0.2368 | t-st | 1.02 [0.99, 1.06] |
| Median [Q1; Q3] | 73.85 [65.4; 86.95] | 74.8 [66.15; 118.55] | ||||
| FG (mg/dL) | Mean ± SD | 93.3 ± 5.76 | 90.2 ± 7.69 | 0.3980 | M-W | 0.93 [0.8, 1.08] |
| Median [Q1; Q3] | 94.8 [89.08; 97.2] | 92.2 [89.05; 94.35] | ||||
| FI (μU/mL) | Mean ± SD | 10.4 ± 2.36 | 8.4 ± 2.68 | 0.1028 | t-st | 0.72 [0.49, 1.08] |
| Median [Q1; Q3] | 10.09 [8.06; 11.69] | 7.64 [6.87; 10.6] | ||||
| Homa-IR | Mean ± SD | 2.4 ± 0.59 | 1.9 ± 0.69 | 0.0868 | t-st | 0.26 [0.05, 1.29] |
| Median [Q1; Q3] | 2.43 [1.89; 2.76] | 1.74 [1.45; 2.4] |
| Parameters | Values | No-D Group (n = 10) | D Group (n = 11) | p | Test | OR [95% CI] |
|---|---|---|---|---|---|---|
| TT (ng/mL) | Mean ± SD | 0.4 ± 0.17 | 0.4 ± 0.11 | 0.7057 | t-st | 0.28 [0, 139.88] |
| Median [Q1; Q3] | 0.3 [0.26; 0.52] | 0.38 [0.28; 0.43] | ||||
| DHEA-S (µmol/L) | Mean ± SD | 6.6 ± 3.06 | 6.9 ± 2.98 | 0.8098 | t-st | 1.04 [0.76, 1.42] |
| Median [Q1; Q3] | 6.54 [4.7; 7.9] | 6.27 [4.87; 8.76] | ||||
| SHBG (nmol/L) | Mean ± SD | 60.1 ± 13.77 | 76.3 ± 50.2 | 0.3277 | C-C | 1.01 [0.98, 1.04] |
| Median [Q1; Q3] | 60.23 [50.72; 66.83] | 63.36 [44.69; 87.66] | ||||
| A (ng/mL) | Mean ± SD | 1.7 ± 0.54 | 1.9 ± 0.45 | 0.4120 | t-st | 2.24 [0.35, 14.26] |
| Median [Q1; Q3] | 1.65 [1.31; 1.92] | 2.03 [1.56; 2.23] | ||||
| FSH (mIU/mL) | Mean ± SD | 5.1 ± 2.21 | 4.9 ± 2.18 | 0.8353 | t-st | 0.95 [0.63, 1.44] |
| Median [Q1; Q3] | 4.63 [3.55; 6.19] | 5.18 [3.71; 6.13] | ||||
| LH (mIU/mL) | Mean ± SD | 8.5 ± 6.09 | 9.9 ± 7.88 | 0.7513 | M-W | 1.03 [0.9, 1.17] |
| Median [Q1; Q3] | 6.72 [4.55; 10.52] | 9.83 [4.21; 12.46] | ||||
| Estradiol (pg/mL) | Mean ± SD | 121.8 ± 106.03 | 84.5 ± 49.63 | 0.6471 | M-W | 0.99 [0.98, 1.01] |
| Median [Q1; Q3] | 88.64 [41.82; 153.93] | 73.78 [62.69; 113.76] | ||||
| Prolactin (ng/mL) | Mean ± SD | 12.2 ± 2.13 | 18.5 ± 6.5 | 0.0108 | C-C | 1.75 [1.03, 2.97] |
| Median [Q1; Q3] | 11.77 [10.88; 13.87] | 16.45 [13.91; 20.45] | ||||
| Cortisol (nmol/L) | Mean ± SD | 304.2 ± 85.58 | 433 ± 180.63 | 0.0035 | M-W | 1.02 [1, 1.04] |
| Median [Q1; Q3] | 307.1 [254.75; 334.93] | 379.4 [358.3; 418.05] | ||||
| 17-OHP (ng/mL) | Mean ± SD | 1.3 ± 0.77 | 1.2 ± 0.33 | 0.3416 | M-W | 0.78 [0.16, 3.66] |
| Median [Q1; Q3] | 0.94 [0.84; 1.25] | 1.15 [1; 1.25] |
| Parameters | Values | No-D Group (n = 10) | D Group (n = 13) | p | Test | OR [95% CI] |
|---|---|---|---|---|---|---|
| Energy (kcal) | Mean ± SD | 2310.9 ± 286.45 | 2101.1 ± 492.94 | 0.2452 | t-st | 1 [1, 1] |
| Median [Q1; Q3] | 2306.87 [2139.54; 2514.27] | 1997 [1812.4; 2264] | ||||
| Protein (g) | Mean ± SD | 93.3 ± 20.42 | 85.2 ± 19.23 | 0.3422 | t-st | 0.98 [0.94, 1.02] |
| Media [Q1; Q3] | 93.52 [76.91; 103.52] | 79.41 [75.66; 100] | ||||
| Fat (g) | Mean ± SD | 92.9 ± 29.36 | 75.8 ± 25.35 | 0.1497 | t-st | 0.98 [0.94, 1.01] |
| Media [Q1; Q3] | 91.02 [66.25; 113.07] | 66 [63.88; 92.01] | ||||
| SFAs (g) | Mean ± SD | 34.3 ± 10.98 | 26.2 ± 8.05 | 0.0539 | (t-st) | 0.9 [0.81, 1.01] |
| Median [Q1; Q3] | 32.63 [25.79; 40.14] | 26.38 [21.65; 29.84] | ||||
| MUFAs (g) | Mean ± SD | 28.7 ± 8.25 | 23.6 ± 8.1 | 0.1483 | (t-st) | 0.92 [0.83, 1.03] |
| Median [Q1; Q3] | 29.47 [21.45; 35.59] | 24.04 [17; 28.1] | ||||
| PUFAs (g) | Mean ± SD | 14 ± 8.78 | 10.8 ± 4.86 | 0.3521 | (M-W) | 0.93 [0.8, 1.07] |
| Median [Q1; Q3] | 11.17 [9.07; 15.18] | 9.97 [8; 11.73] | ||||
| Cholesterol (mg) | Mean ± SD | 366.8 ± 207.77 | 258.7 ± 103.68 | 0.1450 | (M-W) | 0.99 [0.99, 1] |
| Median [Q1; Q3] | 322.72 [251.34; 409.39] | 225 [194; 341.4] | ||||
| Carbohydrates (g) | Mean ± SD | 281.6 ± 36.44 | 276.4 ± 74.06 | 0.8280 | (C-C) | 1 [0.98, 1.01] |
| Median [Q1; Q3] | 271.92 [250.85; 306.82] | 258 [209.9; 326.02] | ||||
| Fiber (g) | Mean ± SD | 23.1 ± 6.6 | 20.2 ± 5.74 | 0.2810 | (t-st) | 0.92 [0.79, 1.07] |
| Median [Q1; Q3] | 22 [19.65; 22.52] | 20 [18.01; 24.21] | ||||
| Saccharose (g) | Mean ± SD | 51.9 ± 23.32 | 36.1 ± 18.48 | 0.0586 | (M-W) | 0.96 [0.92, 1.01] |
| Median [Q1; Q3] | 45.39 [42.03; 69.68] | 33 [24; 41.63] | ||||
| EAT-26 score | Mean ± SD | 6.1 ± 4.99 | 9.2 ± 5.32 | 0.0284 | (M-W) | 1.14 [0.95, 1.36] |
| Median [Q1; Q3] | 3 [3; 7.5] | 7 [5; 12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizgier, M.; Więckowska, B.; Sansoni, V.; Malvandi, A.M.; Jarząbek-Bielecka, G.; Drejza, M.; Mruczyk, K.; Cisek-Woźniak, A.; Lombardi, G. Risk Factors of Primary Dysmenorrhea in Female Adolescent Basketball Players Related to Dietary, Hormonal, and Immuno-Metabolic Factors and Disordered Eating Attitudes. Nutrients 2025, 17, 1190. https://doi.org/10.3390/nu17071190
Mizgier M, Więckowska B, Sansoni V, Malvandi AM, Jarząbek-Bielecka G, Drejza M, Mruczyk K, Cisek-Woźniak A, Lombardi G. Risk Factors of Primary Dysmenorrhea in Female Adolescent Basketball Players Related to Dietary, Hormonal, and Immuno-Metabolic Factors and Disordered Eating Attitudes. Nutrients. 2025; 17(7):1190. https://doi.org/10.3390/nu17071190
Chicago/Turabian StyleMizgier, Małgorzata, Barbara Więckowska, Veronica Sansoni, Amir Mohammad Malvandi, Grażyna Jarząbek-Bielecka, Michalina Drejza, Kinga Mruczyk, Angelika Cisek-Woźniak, and Giovanni Lombardi. 2025. "Risk Factors of Primary Dysmenorrhea in Female Adolescent Basketball Players Related to Dietary, Hormonal, and Immuno-Metabolic Factors and Disordered Eating Attitudes" Nutrients 17, no. 7: 1190. https://doi.org/10.3390/nu17071190
APA StyleMizgier, M., Więckowska, B., Sansoni, V., Malvandi, A. M., Jarząbek-Bielecka, G., Drejza, M., Mruczyk, K., Cisek-Woźniak, A., & Lombardi, G. (2025). Risk Factors of Primary Dysmenorrhea in Female Adolescent Basketball Players Related to Dietary, Hormonal, and Immuno-Metabolic Factors and Disordered Eating Attitudes. Nutrients, 17(7), 1190. https://doi.org/10.3390/nu17071190










