Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
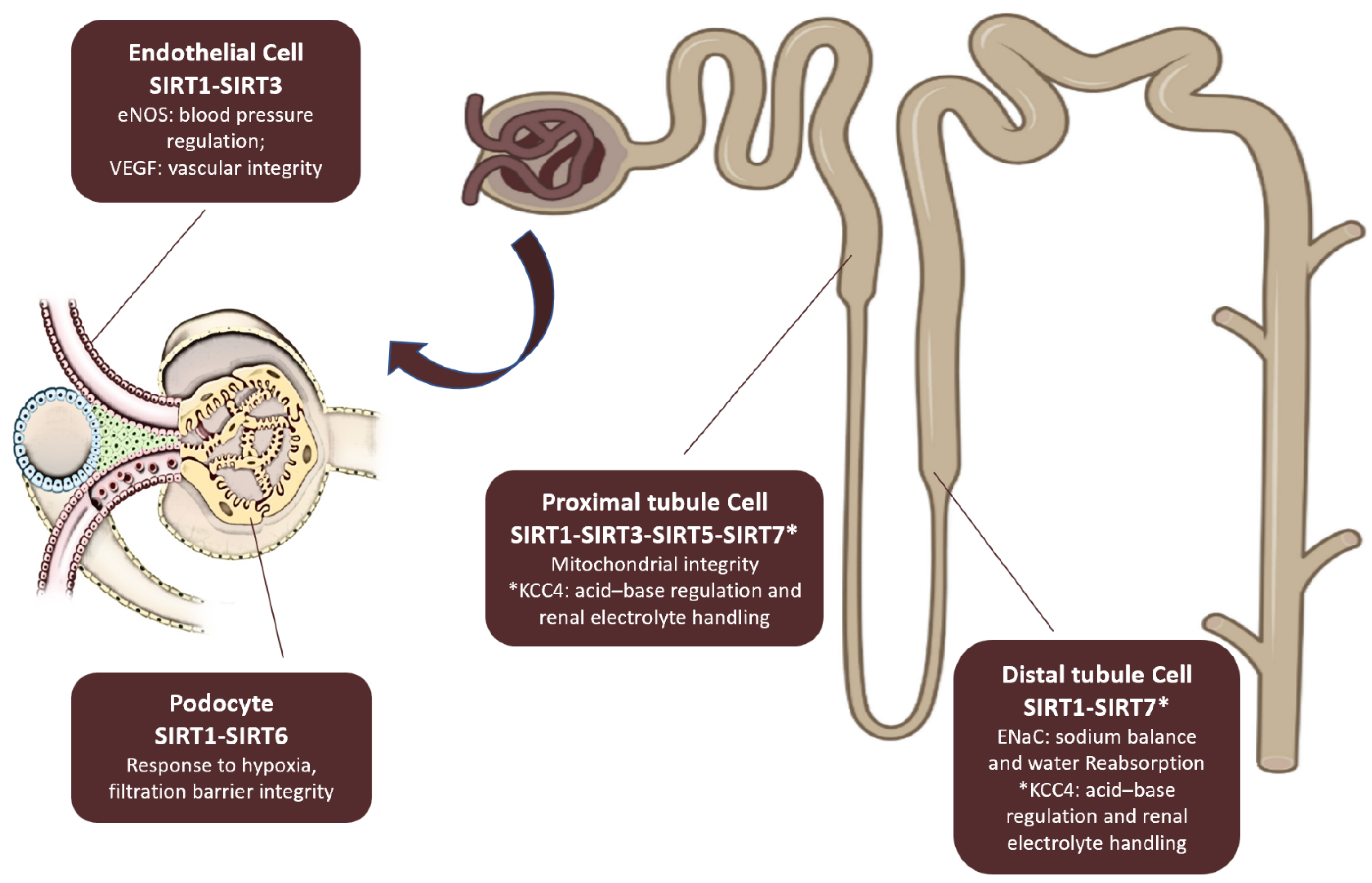
3. Sirtuins and Kidney Diseases
3.1. Sirtuins and Acute Kidney Injury
3.2. Sirtuins in Chronic Kidney Disease and Fibrosis
3.3. Sirtuins and Hypertensive Nephropathy
3.4. Sirtuins and Diabetic Kidney Disease
3.5. Sirtuins and Polycystic Kidney Disease
4. Sirtuins and Cardiovascular Diseases
5. Sirtuins and Cardio-Renal Diseases
6. Resveratrol and Cardio-Renal Diseases
7. Sirtuins Agonists in Cardiorenal Disease
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aliberti, S.M.; De Caro, F.; Funk, R.H.W.; Schiavo, L.; Gonnella, J.; Boccia, G.; Capunzo, M. Extreme Longevity: Analysis of the Direct or Indirect Influence of Environmental Factors on Old, Nonagenarians, and Centenarians in Cilento, Italy. Int. J. Environ. Res. Public Health 2022, 19, 1589. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009, 20, 325–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Auwerx, J. The role of sirtuins in the control of metabolic homeostasis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E10–E19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Y.; Wang, X.; Yang, D.; Lu, Y.; Wei, G.; Yu, W.; Liu, X.; Zheng, Q.; Ying, J.; Hua, F. Relieving Cellular Energy Stress in Aging, Neurodegenerative, and Metabolic Diseases, SIRT1 as a Therapeutic and Promising Node. Front. Aging Neurosci. 2021, 13, 738686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McReynolds, M.R.; Chellappa, K.; Baur, J.A. Age-related NAD+ decline. Exp. Gerontol. 2020, 134, 110888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Tan, J.; Jin, Z.; Jiang, T.; Wu, J.; Yu, X. Research progress on Sirtuins (SIRTs) family modulators. Biomed. Pharmacother. 2024, 174, 116481. [Google Scholar] [CrossRef] [PubMed]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and non conserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar]
- Hao, W.; Jialong, Z.; Jiuzhi, Y.; Yang, Y.; Chongning, L.; Jincai, L. ADP-ribosylation, a multifaceted modification: Functions and mechanisms in aging and aging-related diseases. Ageing Res. Rev. 2024, 98, 102347. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T. Screening and profiling assays for HDACs and sirtuins, Drug Discovery Today: Technologies, Screening and profiling assays for HDACs and sirtuins. Drug Discov. Today Technol. 2015, 18, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lombard, D.B. Functions of the sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 311–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poltronieri, P.; Celetti, A.; Palazzo, L. Mono(ADP-ribosyl)ation Enzymes and NAD+ Metabolism: A Focus on Diseases and Therapeutic Perspectives. Cells 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carollo, C.; Firenze, A.; Caimi, G. Sirtuins and Aging: Is there a Role for Resveratrol? Int. J. Adv. Nutr. Health Sci. 2016, 4, 203–211. [Google Scholar]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peasley, K.; Chiba, T.; Goetzman, E.; Sims-Lucas, S. Sirtuins play critical and diverse roles in acute kidney injury. Pediatr. Nephrol. 2021, 36, 3539–3546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mortuza, R.; Chen, S.; Feng, B.; Sen, S.; Chakrabarti, S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE 2013, 8, e54514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, C.-M.; Volker, H.H. Sirtuins and their relevance to the kidney. J. Am. Soc. Nephrol. 2010, 21, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Kitada, M.; Koya, D. Sirtuins and Renal Oxidative Stress. Antioxidants 2021, 10, 1198. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pezzotta, A.; Perico, L.; Corna, D.; Morigi, M.; Remuzzi, G.; Benigni, A.; Imberti, B. Sirt3 deficiency promotes endothelial dysfunction and aggravates renal injury. PLoS ONE 2023, 18, e0291909. [Google Scholar] [CrossRef]
- Perico, L.; Morigi, M.; Pezzotta, A.; Corna, D.; Brizi, V.; Conti, S.; Zanchi, C.; Sangalli, F.; Trionfini, P.; Buttò, S.; et al. Post-translational modifications by SIRT3 de-2-hydroxyisobutyrylase activity regulate glycolysis and enable nephrogenesis. Sci. Rep. 2021, 11, 23580. [Google Scholar] [CrossRef] [PubMed]
- Haschler, T.N.; Horsley, H.; Balys, M.; Anderson, G.; Taanman, J.-W.; Unwin, R.J.; Norman, J.T. Sirtuin 5 depletion impairs mitochondrial function in human proximal tubular epithelial cells. Sci. Rep. 2021, 11, 15510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Cruz, P.; Kone, B.C. Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription collecting duct. J. Biol. Chem. 2009, 284, 20917–20926. [Google Scholar] [CrossRef]
- Noriega, L.G.; Melo, Z.; Rajaram, R.D.; Mercado, A.; Tovar, A.R.; A Velazquez-Villegas, L.; Castañeda-Bueno, M.; Reyes-López, Y.; Ryu, D.; Rojas-Vega, L.; et al. SIRT7 modulates the stability and activity of the renal K-Cl cotransporter KCC4 through deacetylation. EMBO Rep. 2021, 22, e50766. [Google Scholar] [CrossRef]
- Fan, H.; Yang, H.-C.; You, L.; Wang, Y.-Y.; He, W.-J.; Hao, C.-M. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013, 83, 404–413. [Google Scholar] [PubMed]
- Shi, S.; Lei, S.; Tang, C.; Wang, K.; Xia, Z. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci. Rep. 2019, 39, BSR20181614. [Google Scholar]
- Hansen, L.W.; Khader, A.; Yang, W.-L.; Prince, J.M.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Sirtuin 1 activator SRT1720 protects against organ injury induced by intestinal ischemia-reperfusion. Shock 2016, 45, 359–366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, J.S.-C.; Huang, L.; Belousova, T.; Lu, L.; Yang, Y.; Reddel, R.; Chang, A.; Ju, H.; DiMattia, G.; Tong, Q.; et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J. Am. Soc. Nephrol. 2015, 26, 364–378. [Google Scholar] [CrossRef]
- Clark, A.J.; Parikh, S.M. Targeting energy pathways in kidney disease: The roles of sirtuins, AMPK, and PGC1α. Kidney Int. 2021, 99, 828–840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiba, T.; Peasley, K.D.; Cargill, K.R.; Maringer, K.V.; Bharathi, S.S.; Mukherjee, E.; Zhang, Y.; Holtz, A.; Basisty, N.; Yagobian, S.D.; et al. Sirtuin 5 Regulates Proximal Tubule Fatty Acid Oxidation to Protect against AKI. J. Am. Soc. Nephrol. 2019, 30, 2384–2398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Z.; Chen, X.; Fan, Y.; Zhu, K.; Shi, M.; Ding, G. Sirt6 attenuates hypoxia-induced tubular epithelial cell injury via targeting G2/M phase arrest. J. Cell Physiol. 2020, 235, 3463–3473. [Google Scholar]
- You, J.; Li, Y.; Chong, W. The role and therapeutic potential of SIRTs in sepsis. Front. Immunol. 2024, 15, 1394925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, Y.A.; Bae, S.Y.; Ahn, S.Y.; Kim, J.; Kwon, Y.J.; Jung, W.Y.; Ko, G.J. Resveratrol Ameliorates Contrast Induced Nephropathy Through the Activation of SIRT1-PGC-1α-Foxo1 Signaling in Mice. Kidney Blood Press. Res. 2017, 42, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Li, N.; Zhang, J.; Yang, J.; Bu, P. Sirtuin 3 deficiency aggravates contrast-induced acute kidney injury. J. Transl. Med. 2018, 16, 313, Erratum in J. Transl. Med. 2022, 20, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2024. Kidney Int. 2024, 105 (Suppl. 4S), S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Abdel Kader, K. Symptoms with or because of Kidney Failure? Clin. J. Am. Soc. Nephrol. 2022, 17, 475–477. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2023. [Google Scholar]
- Nitta, K.; Okada, K.; Yanai, M.; Takahashi, S. Aging and chronic kidney disease. Kidney Blood Press Res. 2013, 38, 109–120. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Larsson, T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Lerman, L.O. Cellular Senescence: A New Player in Kidney Injury. Hypertension 2020, 76, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.; Huang, L.; Lu, J.; Cheng, L.; Wu, D.; Li, L.; Zhang, S.; Lai, X.; Xu, L. The relationship between telomere length and aging-related diseases. Clin. Exp. Med. 2025, 25, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, H.J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11, 565023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung Anthony, K.L. PARPs. Curr. Biol. 2017, 27, R1256–R1258. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, C. Cross-talk of renal cells through WNT signal transduction in the development of fibrotic kidneys. Front. Cell Dev. Biol. 2025, 12, 1517181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, B.; Phan, S.H. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol. Res. 2016, 108, 57–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopes-Gonçalves, G.; Costa-Pessoa, J.M.; Pimenta, R.; Tostes, A.F.; da Silva, E.M.; Ledesma, F.L.; Malheiros, D.M.A.C.; Zatz, R.; Thieme, K.; Câmara, N.O.S.; et al. Evaluation of glomerular sirtuin-1 and claudin-1 in the pathophysiology of nondiabetic focal segmental glomerulosclerosis. Sci. Rep. 2023, 13, 22685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, W.; Wang, Y.; Zhang, M.-Z.; You, L.; Davis, L.S.; Fan, H.; Yang, H.-C.; Fogo, A.B.; Zent, R.; Harris, R.C.; et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Investig. 2010, 120, 1056–1068. [Google Scholar] [CrossRef]
- Zhang, Y.; Connelly, K.A.; Thai, K.; Wu, X.; Kapus, A.; Kepecs, D.; Gilbert, R.E. Sirtuin 1 activation reduces transforming growth factor-β1-induced fibrogenesis and afords organ protection in a model of progressive, experimental kidney and associated cardiac disease. Am. J. Pathol. 2017, 187, 80–90. [Google Scholar] [CrossRef]
- Huang, X.Z.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C.M. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J. Cell Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Y.; Qin, X.; Chen, K.; Wang, R.; Yuan, L.; Chen, X.; Hao, C.; Huang, X. SIRT1 attenuates renal fibrosis by repressing HIF-2α. Cell Death Discov. 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, J.; Liu, Z.; Huang, X.; Shu, S.; Hu, X.; Zheng, M.; Tang, C.; Liu, Y.; Chen, G.; Sun, L.; et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 2020, 97, 106–118, Erratum in Kidney Int. 2022, 101, 422. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Zhang, Z.; Wang, W.; Huang, H. SIRT6 overexpression retards renal interstitial fibrosis through targeting HIPK2 in chronic Kidney Disease. Front. Pharmacol. 2022, 13, 1007168. [Google Scholar] [CrossRef]
- Cai, J.; Wang, T.; Zhou, Y.; Tang, C.; Liu, Y.; Dong, Z. Phosphorylation by GSK-3β increases the stability of SIRT6 to alleviate TGF-β-induced fibrotic response in renal tubular cells. Life Sci. 2022, 308, 120914. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, X.; Jian, Y.; Liu, J.; Ke, X.; Chen, S.; Yang, D.; Yang, D. SIRT3 deficiency exacerbates early-stage fibrosis after ischaemia-reperfusion-induced AKI. Cell Signal. 2022, 93, 110284. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, J.; Yan, X.; Zhang, C.; Liu, H.; Shan, X.; Li, J.; Yang, Y.; Huang, C.; Zhang, P.; et al. SIRT3-KLF15 signaling ameliorates kidney injury induced by hypertension. Oncotarget 2017, 8, 39592–39604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xi, J.; Chen, Y.; Jing, J.; Zhang, Y.; Liang, C.; Hao, Z.; Zhang, L. Sirtuin 3 suppresses the formation of renal calcium oxalate crystals through promoting M2 polarization of macrophages. J. Cell Physiol. 2019, 234, 11463–11473. [Google Scholar] [CrossRef]
- Chen, H.-H.; Zhang, Y.-X.; Lv, J.-L.; Liu, Y.-Y.; Guo, J.-Y.; Zhao, L.; Nan, Y.-X.; Wu, Q.-J.; Zhao, Y.-H. Role of sirtuins in metabolic disease-related renal injury. Biomed. Pharmacother. 2023, 161, 114417. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Ren, L.; Juin, S.K.; Majumder, S.; Kulkarni, R.; Sen, U. Methylation-dependent antioxidant-redox imbalance regulates hypertensive kidney injury in aging. Redox Biol. 2020, 37, 101754. [Google Scholar] [CrossRef]
- Jain, S.; Rana, A.; Jain, K.; Perla, S.K.; Puri, N.; Kumar, A. Age-Related Expression of Human AT1R Variants and Associated Renal Dysfunction in Transgenic Mice. Am. J. Hypertens. 2018, 31, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Su, H.; He, X.; Chen, J.-X.; Zeng, H. SIRT3 Deficiency Sensitizes Angiotensin-II-Induced Renal Fibrosis. Cells 2020, 9, 2510. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Wu, J.; Liu, M.; Li, M.; Sun, Y.; Huang, W.; Li, Y.; Zhang, Y.; Tang, W.; et al. Endothelial SIRT6 Is Vital to Prevent Hypertension and Associated Cardiorenal Injury Through Targeting Nkx3.2-GATA5 Signaling. Circ. Res. 2019, 124, 1448–1461. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, J.; Yang, Q.; Feng, J.; Hu, J.; Ren, Z.; Yang, H.; Yang, D.; Ding, G. Sirt6-mediated Nrf2/HO-1 activation alleviates angiotensin II-induced DNA DSBs and apoptosis in podocytes. Food Funct. 2021, 12, 7867–7882. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision Medicine in Diabetes: A Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1617–1635. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Koya, D. Role of sirtuins in kidney disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 75–79. [Google Scholar] [CrossRef]
- Zhong, Y.; Lee, K.; He, J.C. SIRT1 is a potential drug target for treatment of diabetic kidney disease. Front. Endocrinol. 2018, 9, 624. [Google Scholar] [CrossRef]
- Dong, Y.J.; Liu, N.; Xiao, Z.; Sun, T.; Wu, S.H.; Sun, W.X.; Xu, Z.G.; Yuan, H. Renal protective effect of Sirtuin 1. J. Diabetes Res. 2014, 2014, 843786. [Google Scholar] [CrossRef]
- Yasuda, I.; Hasegawa, K.; Sakamaki, Y.; Muraoka, H.; Kawaguchi, T.; Kusahana, E.; Ono, T.; Kanda, T.; Tokuyama, H.; Wakino, S.; et al. Pre-emptive Short-term Nicotinamide Mononucleotide Treatment in a Mouse Model of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1355–1370, Erratum in J. Am. Soc. Nephrol. 2021, 32, 2683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Z.; Liu, H.; Song, N.; Liang, Y.; Zhu, J.; Chen, J.; Ning, Y.; Hu, J.; Fang, Y.; Teng, J.; et al. METTL14 aggravates podocyte injury and glomerulopathy progression through N6-methyladenosine-dependent downregulating of Sirt1. Cell Death Dis. 2021, 12, 881. [Google Scholar]
- Qi, W.; Hu, C.; Zhao, D.; Li, X. SIRT1-SIRT7 in Diabetic Kidney Disease: Biological Functions and Molecular Mechanisms. Front. Endocrinol. 2022, 13, 801303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, J.-X.; Qin, H. Glucagon-like peptide-1 protects mouse podocytes against high glucose-induced apoptosis, and suppresses reactive oxygen species production and proinflammatory cytokine secretion, through sirtuin 1 activation in vitro. Mol. Med. Rep. 2018, 18, 1789–1797. [Google Scholar]
- Wang, Y.; Zhang, X.; Wang, P.; Shen, Y.; Yuan, K.; Li, M.; Liang, W.; Que, H. Sirt3 overexpression alleviates hyperglycemia-induced vascular inflammation through regulating redox balance, cell survival, and AMPK-mediated mitochondrial homeostasis. J. Recept. Signal Transduct. 2019, 39, 341–349. [Google Scholar]
- Xu, X.; Zhang, L.; Hua, F.; Zhang, C.; Zhang, C.; Mi, X.; Qin, N.; Wang, J.; Zhu, A.; Qin, Z.; et al. FOXM1-activated SIRT4 inhibits NF-κB signaling and NLRP3 inflammasome to alleviate kidney injury and podocyte pyroptosis in diabetic nephropathy. Exp. Cell Res. 2021, 408, 112863. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, B.; Nie, L.; Li, P. microRNA-20b contributes to high glucose-induced podocyte apoptosis by targeting SIRT7. Mol. Med. Rep. 2017, 16, 5667–5674. [Google Scholar] [CrossRef]
- Liu, S.; Gao, X.; Fan, Z.; Wang, Q. SIRT2 affects cell proliferation and apoptosis by suppressing the level of autophagy in renal podocytes. Dis. Markers 2022, 2022, 4586198. [Google Scholar]
- Bian, C.; Gao, J.; Wang, Y.; Li, J.; Luan, Z.; Lu, H.; Ren, H. Association of SIRT6 circulating levels with urinary and glycometabolic markers in pre-diabetes and diabetes. Acta Diabetol. 2021, 58, 1551–1562. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, L.X.; Sweeney WEJr Denu, J.M.; Avner, E.D.; Li, X. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Investig. 2013, 123, 3084–3098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Ters, M.; Zhou, X.; Lepping, R.J.; Lu, P.; Karcher, R.T.; Mahnken, J.D.; Brooks, W.M.; Winklhofer, F.T.; Li, X.; Yu, A.S.L. Biological Efficacy and Safety of Niacinamide in Patients With ADPKD. Kidney Int. Rep. 2020, 5, 1271–1279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurtgoz, P.O.; Karakose, S.; Cetinkaya, C.D.; Erkus, E.; Guney, I. Evaluation of sirtuin 1 (SIRT1) levels in autosomal dominant polycystic kidney disease. Int. Urol. Nephrol. 2022, 54, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Hong, Y.; Lu, X.; Zhang, J.; Chen, H.; Li, Y.; Ma, Y.; Ying, W. SIRT2 mediates oxidative stress-induced apoptosis of differentiated PC12 cells. NeuroReport 2014, 25, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, C.P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Porter, G.A.; Urciuoli, W.R.; Brookes, P.S.; Nadtochiy, S.M. SIRT3 deficiency exacerbates ischemia-reperfusion injury: Implication for aged hearts. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1602–H1609. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, X.L.; Tong, M.M.; Gan, L.; Chen, H.; Wu, S.S.; Chen, J.X.; Li, R.L.; Wu, Y.; Zhang, H.Y.; et al. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms. Basic Res. Cardiol. 2016, 111, 13. [Google Scholar] [CrossRef]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef]
- Lynn, E.G.; McLeod, C.J.; Gordon, J.P.; Bao, J.; Sack, M.N. SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett. 2008, 582, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Wen, R.; Liu, C.-F.; Zhang, T.-N.; Yang, N. Cellular and molecular biology of sirtuins in cardiovascular disease. Biomed. Pharmacother. 2023, 164, 114931. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Xie, Y.; Bu, J.; Yuan, R.; Zhang, X. Exploring Sirtuins: New Frontiers in Managing Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2024, 25, 7740. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 523. [Google Scholar]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Potenta, S.E.; Sugimoto, H.; Zeisberg, M.; Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008, 19, 2282–2287. [Google Scholar] [CrossRef]
- Kizu, A.; Medici, D.; Kalluri, R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am. J. Pathol. 2009, 175, 1371–1373. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Dijke, M.C.G.-P.P.T. TGF-beta-Induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Zha, S.; Cao, Q.; Sheng, J.; Chen, S. SIRT1 inhibits TGF-β-induced endothelial-mesenchymal transition in human endothelial cells with Smad4 deacetylation. J. Cell Physiol. 2018, 233, 9007–9014. [Google Scholar] [CrossRef]
- Lin, J.R.; Zheng, Y.J.; Zhang, Z.B.; Shen, W.L.; Li, X.D.; Wei, T.; Ruan, C.C.; Chen, X.H.; Zhu, D.L.; Gao, P.J. Suppression of Endothelial-to-Mesenchymal Transition by SIRT (Sirtuin) 3 Alleviated the Development of Hypertensive Renal Injury. Hypertension 2018, 72, 350–360. [Google Scholar] [PubMed]
- Delgado-Valero, B.; Cachofeiro, V.; Martínez-Martínez, E. Fibrosis, the Bad Actor in Cardiorenal Syndromes: Mechanisms Involved. Cells 2021, 10, 1824. [Google Scholar] [CrossRef]
- Shestakova, M.V.; Iarek-Martynova, I.R.; Ivanishina, N.S.; Aleksandrov, A.A.; Dedov, I.I. Cardiorenal syndrome in type 1 diabetes mellitus: The role of endothelial dysfunction]. Kardiologiia 2005, 45, 35–41. (In Russian) [Google Scholar] [PubMed]
- Shestakova, M.V.; Jarek-Martynowa, I.R.; Ivanishina, N.S.; Kuharenko, S.S.; Yadrihinskaya, M.N.; Aleksandrov, A.A.; Dedov, I.I. Role of endothelial dysfunction in the development of cardiorenal syndrome in patients with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2005, 68 (Suppl. S1), S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Hatamizadeh, P.; Fonarow, G.C.; Budoff, M.J.; Darabian, S.; Kovesdy, C.P.; Kalantar-Zadeh, K. Cardiorenal syndrome: Pathophysiology and potential targets for clinical management. Nat. Rev. Nephrol. 2013, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lumpuy-Castillo, J.; Amador-Martínez, I.; Díaz-Rojas, M.; Lorenzo, O.; Pedraza-Chaverri, J.; Sánchez- Lozada, L.G.; Aparicio-Trejo, O.E. Role of mitochondria in reno-cardiac diseases: A study of bioenergetics, biogenesis, and GSH signaling in disease transition. Redox Biol. 2024, 76, 103340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iside, C. Sirtuin 1 activation by natural phytochemicals: An overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, H.; Jiang, X.; Chen, N. Resveratrol combats chronic diseases through enhancing mitochondrial quality. Food Sci. Hum. Wellness 2024, 13, 597–610. [Google Scholar]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res. Clin. Pract. 2020, 39, 244–258. [Google Scholar]
- Liu, Z.-H.; Zhang, Y.; Wang, X.; Fan, X.-F.; Zhang, Y.; Li, X.; Gong, Y.-S.; Han, L.-P. SIRT1 activation attenuates cardiac fibrosis by endothelial-to-mesenchymal transition. Biomed. Pharmacother. 2019, 118, 109227. [Google Scholar]
- US National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?intr=Resveratrol&limit=100&page=1&viewType=Table (accessed on 3 February 2025).
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health--a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Du, C.; Shi, Y.; Wei, J.; Wu, H.; Cui, H. The Sirt 1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 2017, 39, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, F.; Fabbrizi, E.; Mai, A.; Rotili, D. Activation and inhibition of sirtuins: From bench to bedside. Med. Res. Rev. 2025, 45, 484–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, N.; Li, J.; Lin, S.; Zhang, J.; Zeng, W.; Ma, G.; Wang, Y. Emerging roles of SIRT1 activator, SRT2104, in disease treatment. Sci. Rep. 2024, 14, 5521. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.M.; White, D.S.; Donu, D.; Cen, Y. Human Sirtuin Regulators: The “Success” Stories. Front. Physiol. 2021, 12, 752117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeon, J.Y.; Choi, S.E.; Ha, E.S.; Lee, H.B.; Kim, T.H.; Han, S.J.; Kim, H.J.; Kim, D.J.; Kang, Y.; Lee, K.W. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. Int. J. Mol. Med. 2019, 44, 1161–1171. [Google Scholar]
- Li, J.; Liu, H.; Takagi, S.; Nitta, K.; Kitada, M.; Srivastava, S.P.; Takagaki, Y.; Kanasaki, K.; Koya, D. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 2020, 5, e129034. [Google Scholar] [CrossRef]
- Osataphan, S.; Macchi, C.; Singhal, G.; Chimene-Weiss, J.; Sales, V.; Kozuka, C.; Dreyfuss, J.M.; Pan, H.; Tangcharoenpaisan, Y.; Morningstar, J. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 2019, 4, e123130. [Google Scholar]
- Packer, M. Cardioprotective effects of sirtuin-1 and its downstream effectors: Potential role in mediating the heart failure benefits of SGLT2 (Sodium-Glucose Cotransporter 2) inhibitors. Circ. Heart Fail. 2020, 13, e007197. [Google Scholar] [CrossRef]
- Sharma, A.; Mukherjee, M.; Kumar, A.; Sharma, G.; Tabassum, F.; Akhtar, S.; Imam, M.T.; Saeed Almalki, Z.S. Preliminary investigation on impact of intergenerational treatment of resveratrol endorses the development of ‘super-pups’. Life Sci. 2023, 314, 121322. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Rahbarinejad, P.; Asghari, G.; Azizi, F. Dietary Intakes of Total Polyphenol and Its Subclasses in Association with the Incidence of Chronic Kidney Diseases: A Prospective Population-based Cohort Study. BMC Nephrol. 2021, 22, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Peng, W.; Hu, F.; Li, G. Association between dietary intake of flavonoid and chronic kidney disease in US adults: Evidence from NHANES 2007–2008, 2009–2010, and 2017–2018. PLoS ONE 2024, 19, e0309026. [Google Scholar] [CrossRef] [PubMed]
- Arisi, T.O.P.; da Silva, D.S.; Stein, E.; Weschenfelder, C.; de Oliveira, P.C.; Marcadenti, A.; Lehnen, A.M.; Waclawovsky, G. Effects of cocoa consumption on cardiometabolic risk markers: Protocol for a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0309824. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Turner, C.G.; Wong, B.J.; Feresin, R.G. Berry-Derived Polyphenols in Cardiovascular Pathologies: Mechanisms of Disease and the Role of Diet and Sex. Nutrients 2021, 13, 387. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, C.; Sorce, A.; Cirafici, E.; Mulè, G.; Caimi, G. Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential. Nutrients 2025, 17, 1212. https://doi.org/10.3390/nu17071212
Carollo C, Sorce A, Cirafici E, Mulè G, Caimi G. Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential. Nutrients. 2025; 17(7):1212. https://doi.org/10.3390/nu17071212
Chicago/Turabian StyleCarollo, Caterina, Alessandra Sorce, Emanuele Cirafici, Giuseppe Mulè, and Gregorio Caimi. 2025. "Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential" Nutrients 17, no. 7: 1212. https://doi.org/10.3390/nu17071212
APA StyleCarollo, C., Sorce, A., Cirafici, E., Mulè, G., & Caimi, G. (2025). Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential. Nutrients, 17(7), 1212. https://doi.org/10.3390/nu17071212






