The Interplay Between Iron Metabolism and Insulin Resistance: A Key Factor in Optimizing Obesity Management in Children and Adolescents
Abstract
:1. Introduction
2. Methods
3. Iron Deficiency and Insulin Resistance in Pediatric Obesity
3.1. Iron Deficiency in Pediatric Obesity
3.2. Insulin Resistance in Pediatric Obesity
3.3. Association Between Iron Deficiency and Insulin Resistance in Pediatric Obesity
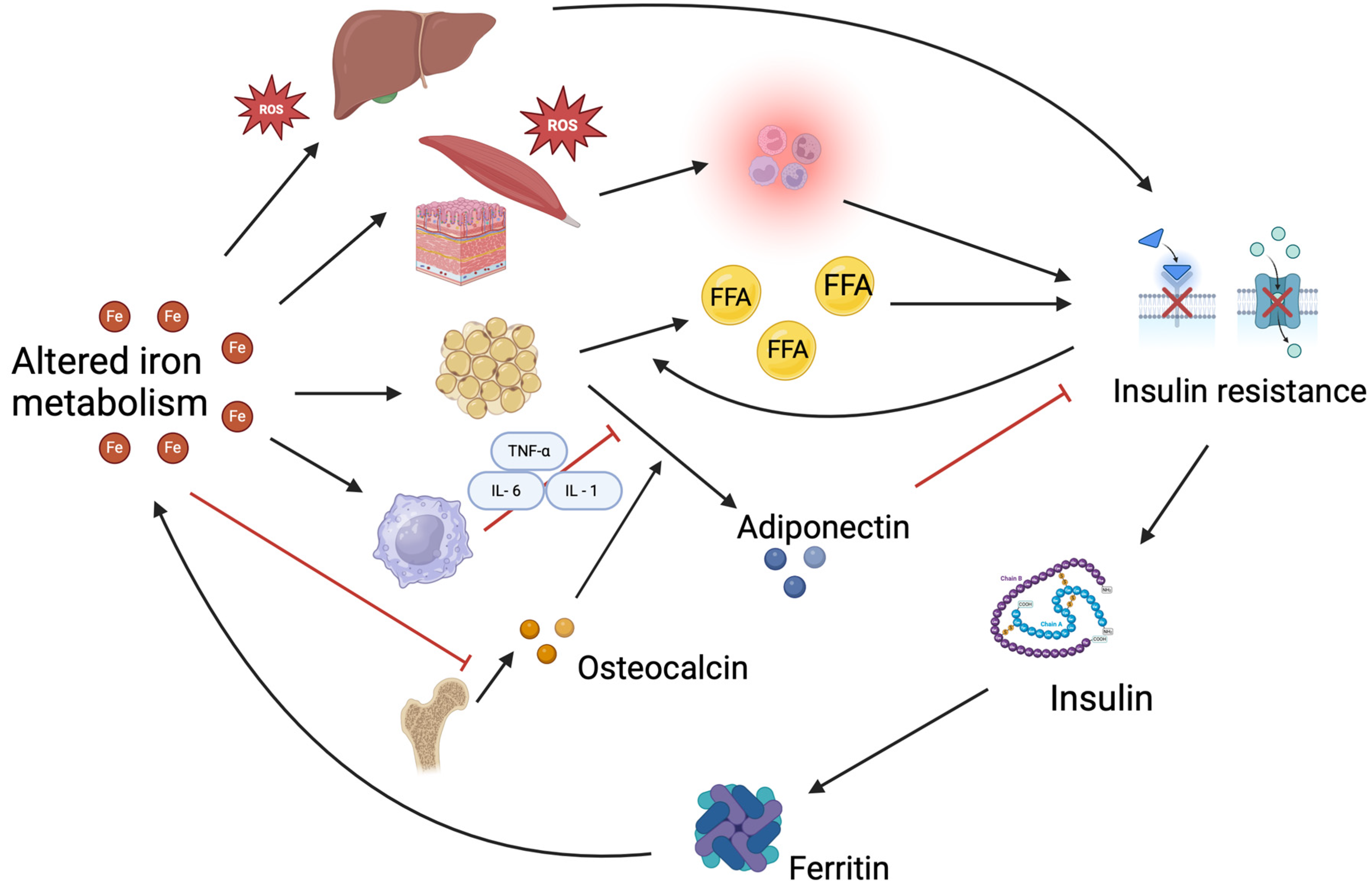
4. Interaction Between Iron Metabolism and Insulin Resistance
5. Iron Requirement and Intake in the Pediatric Population
6. Iron Intake in Children with Obesity
7. Nutritional and Lifestyle Strategies to Improve Iron Status in Children with Obesity
7.1. Effect of Weight Loss Through Balanced Diet and Physical Activity on Iron Status
7.2. Role of Breakfast
8. Limitations
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef]
- Thomas-Eapen, N. Childhood Obesity. Prim. Care Clin. Off. Pract. 2021, 48, 505–515. [Google Scholar] [CrossRef]
- Weihrauch-Blüher, S.; Wiegand, S. Risk Factors and Implications of Childhood Obesity. Curr. Obes. Rep. 2018, 7, 254–259. [Google Scholar] [CrossRef]
- Shalitin, S.; Kiess, W. Putative Effects of Obesity on Linear Growth and Puberty. Horm. Res. Paediatr. 2017, 88, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Danielsson, P.; Hagman, E. Pediatric obesity—Long-term consequences and effect of weight loss. J. Intern. Med. 2022, 292, 870–891. [Google Scholar] [CrossRef]
- Gunaratne, N.; Deplewski, D. Metabolic Consequences of Pediatric Obesity: A Review of Pathophysiology, Screening, and Treatment. Pediatr. Ann. 2023, 52, E60–E65. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Twig, G. The Impact of Childhood and Adolescent Obesity on Cardiovascular Risk in Adulthood: A Systematic Review. Curr. Diabetes Rep. 2018, 18, 91. [Google Scholar] [CrossRef]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Mittal, M.; Jain, V. Management of Obesity and Its Complications in Children and Adolescents. Indian J. Pediatr. 2021, 88, 1222–1234. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Milanta, C.; Agostinelli, M.; Todisco, C.F.; Bona, F.; Dolor, J.; La Mendola, A.; Tosi, M.; Zuccotti, G. Micronutrient Deficiency in Children and Adolescents with Obesity—A Narrative Review. Children 2023, 10, 695. [Google Scholar] [CrossRef]
- Berton, P.F.; Gambero, A. Hepcidin and inflammation associated with iron deficiency in childhood obesity—A systematic review. J. Pediatr. 2024, 100, 124–131. [Google Scholar] [CrossRef]
- Malden, S.; Gillespie, J.; Hughes, A.; Gibson, A.-M.; Farooq, A.; Martin, A.; Summerbell, C.; Reilly, J.J. Obesity in young children and its relationship with diagnosis of asthma, vitamin D deficiency, iron deficiency, specific allergies and flat-footedness: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22, e13129. [Google Scholar]
- Sachdeva, M.; Malik, M.; Purohit, A.; Jain, L.; Kaur, K.; Pradhan, P.; Mathew, J.L. Association of iron deficiency and anemia with obesity among children: A systematic review and meta-analysis. Obes. Rev. 2025, e13892. [Google Scholar] [CrossRef]
- Pujadas, G.C.; Ortiz-Marrón, H.; Ortiz-Pinto, M.A.; Gscheidle, A.G.; Tejerina, P.d.l.C.; Donoso-Navarro, E.; Gavín, M.O.; Galán, I. Changes in obesity and iron deficiency between 4 and 9 years of age. Longitudinal study of childhood obesity (ELOIN). Int. J. Obes. 2022, 46, 1992–1999. [Google Scholar] [CrossRef]
- Sobieska, K.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. Iron homeostasis and insulin sensitivity: Unraveling the complex interactions. Rev. Endocr. Metab. Disord. 2024, 25, 925–939. [Google Scholar] [CrossRef]
- Hutchinson, C. A review of iron studies in overweight and obese children and adolescents: A double burden in the young? Eur. J. Nutr. 2016, 55, 2179–2197. [Google Scholar] [CrossRef]
- Tan, X.; Tan, P.Y.; Gong, Y.Y.; Moore, J.B. Overnutrition is a risk factor for iron, but not for zinc or vitamin A deficiency in children and young people: A systematic review and meta-analysis. BMJ Glob. Health 2024, 9, e015135. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Shen, Y.; Fang, X.; Wang, Y.; Wang, F. Obesity and iron deficiency: A quantitative meta-analysis. Obes. Rev. 2015, 16, 1081–1093. [Google Scholar] [CrossRef]
- Aka, S.; Kilercik, M.; Arapoglu, M.; Semiz, S. The Hepcidin and 25-OH-Vitamin D Levels in Obese Children as a Potential Mediator of the Iron Status. Clin. Lab. 2021, 67, 1154–1162. [Google Scholar] [CrossRef]
- Panichsillaphakit, E.; Suteerojntrakool, O.; Pancharoen, C.; Nuchprayoon, I.; Chomtho, S. The Association between Hepcidin and Iron Status in Children and Adolescents with Obesity. J. Nutr. Metab. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Tok, T.-S.; Lu, C.-L.; Ko, H.-C.; Chen, M.-Y.; Chen, S.C.-C. Relationship Between being Overweight and Iron Deficiency in Adolescents. Pediatr. Neonatol. 2015, 56, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Doğan, G.; Andiran, N.; Celik, N.; Uysal, S. Iron parameters, pro-hepcidin and soluble transferrin receptor levels in obese children. Minerva Pediatr. 2020, 72, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Siyaram, D.; Bhatia, P.; Dayal, D.; Bhalla, A.K.; Marathe, R. Hypoferremic State in Overweight and Obese Children. Indian Pediatr. 2018, 55, 72–73. [Google Scholar] [PubMed]
- Nazif, H.K.; El-Shaheed, A.A.; El-Shamy, K.A.I.; Mohsen, M.A.; Fadl, N.N.; Moustafa, R.S.I. Study of Serum Hepcidin as a Potential Mediator of the Disrupted Iron Metabolism in Obese Adolescents. Int. J. Health Sci. 2015, 9, 172–178. [Google Scholar] [CrossRef]
- Grandone, A.; Marzuillo, P.; Perrone, L.; Del Giudice, E.M. Iron Metabolism Dysregulation and Cognitive Dysfunction in Pediatric Obesity: Is There a Connection? Nutrients 2015, 7, 9163–9170. [Google Scholar] [CrossRef]
- Frelut, M.-L.; Girardet, J.-P.; Bocquet, A.; Briend, A.; Chouraqui, J.-P.; Darmaun, D.; Dupont, C.; Feillet, F.; Hankard, R.; Rozé, J.-C.; et al. Impact of obesity on biomarkers of iron and vitamin D status in children and adolescents: The risk of misinterpretation. Arch. Pediatr. 2018, 25, 3–5. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, H.K.; Hwang, J.S. HbA1c and glucose intolerance in obese children and adolescents. Diabet. Med. 2012, 29, e102–e105. [Google Scholar] [CrossRef]
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef]
- Thota, P.; Perez-Lopez, F.R.; Benites-Zapata, V.A.; Pasupuleti, V.; Hernandez, A.V. Obesity-related insulin resistance in adolescents: A systematic review and meta-analysis of observational studies. Gynecol. Endocrinol. 2017, 33, 179–184. [Google Scholar] [CrossRef]
- Yan, W.; Wu, S.; Liu, Q.; Zheng, Q.; Gu, W.; Li, X. The link between obesity and insulin resistance among children: Effects of key metabolites. J. Diabetes 2023, 15, 1020–1028. [Google Scholar] [CrossRef]
- Daneshzad, E.; Rostami, S.; Aghamahdi, F.; Mahdavi-Gorabi, A.; Qorbani, M. Association of cardiometabolic risk factors with insulin resistance in overweight and obese children. BMC Endocr. Disord. 2022, 22, 320. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Correa-Rodríguez, M.; Calderón-González, J.C.; Dávila-Grisales, A.; González-Ruíz, K.; Correa-Bautista, J.E.; Izquierdo, M. The association between insulin resistance and cytokines in adolescents with excess of adiposity. Curr. Probl. Cardiol. 2024, 50, 102925. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- García, O.P.; Ronquillo, D.; del Carmen Caamaño, M.; Martínez, G.; Camacho, M.; López, V.; Rosado, J.L. Zinc, Iron and Vitamins A, C and E Are Associated with Obesity, Inflammation, Lipid Profile and Insulin Resistance in Mexican School-Aged Children. Nutrients 2013, 5, 5012–5030. [Google Scholar] [CrossRef]
- Ferrari, M.; Cuenca-García, M.; Valtueña, J.; Moreno, L.A.; Censi, L.; González-Gross, M.; Androutsos, O.; Gilbert, C.C.; Huybrechts, I.; Dallongeville, J.; et al. Inflammation profile in overweight/obese adolescents in Europe: An analysis in relation to iron status. Eur. J. Clin. Nutr. 2014, 69, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight Children and Adolescents: A Risk Group for Iron Deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar] [CrossRef] [PubMed]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini–Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased Adipose Tissue Expression of Hepcidin in Severe Obesity Is Independent from Diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef]
- Klisic, A.; Kavaric, N.; Kotur, J.; Ninic, A. Serum soluble transferrin receptor levels are independently associated with homeostasis model assessment of insulin resistance in adolescent girls. Arch. Med. Sci. 2021, 19, 987–994. [Google Scholar] [CrossRef]
- Suárez-Ortegón, M.; Blanco, E.; McLachlan, S.; Fernandez-Real, J.; Burrows, R.; Wild, S.; Lozoff, B.; Gahagan, S. Ferritin levels throughout childhood and metabolic syndrome in adolescent stage. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 268–278. [Google Scholar] [CrossRef]
- Moschonis, G.; Chrousos, G.P.; Lionis, C.; Mougios, V.; Manios, Y.; Healthy Growth Study Group. Association of total body and visceral fat mass with iron deficiency in preadolescents: The Healthy Growth Study. Br. J. Nutr. 2011, 108, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Chiarelli, F. Obesity and insulin resistance in children. Curr. Opin. Pediatr. 2020, 32, 582–588. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Millán-Martínez, M.; Domínguez-Riscart, J.; Mateos, R.M.; Lechuga-Sancho, A.M.; González-Domínguez, R. Altered Metal Homeostasis Associates with Inflammation, Oxidative Stress, Impaired Glucose Metabolism, and Dyslipidemia in the Crosstalk between Childhood Obesity and Insulin Resistance. Antioxidants 2022, 11, 2439. [Google Scholar] [CrossRef]
- Ortiz-Marrón, H.; Cabañas Pujadas, G.; Donoso Navarro, E.; Burreros García, M.; Herreros Álvaro, M.I.; Mejía Fernández De Velasco, A.M.; Gutiérrez, A.C.; Galán, I. Association between biomarkers of iron status and cardiometabolic risk in Spanish children aged 9–10 years. The ELOIN study. Eur. J. Pediatr. 2023, 182, 5649–5659. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jang, H.B.; Park, J.E.; Park, K.-H.; Kang, J.H.; Park, S.I.; Song, J. Relationship between Serum Levels of Body Iron Parameters and Insulin Resistance and Metabolic Syndrome in Korean Children. Osong Public Health Res. Perspect. 2014, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Podmore, C.; Meidtner, K.; Schulze, M.B.; Scott, R.A.; Ramond, A.; Butterworth, A.S.; Di Angelantonio, E.; Danesh, J.; Arriola, L.; Barricarte, A.; et al. Association of Multiple Biomarkers of Iron Metabolism and Type 2 Diabetes: The EPIC-InterAct Study. Diabetes Care 2016, 39, 572–581. [Google Scholar] [CrossRef]
- Wei, J.; Luo, X.; Zhou, S.; He, X.; Zheng, J.; Sun, X.; Cui, W. Associations between iron status and insulin resistance in Chinese children and adolescents: Findings from the China Health and Nutrition Survey. Asia Pac. J. Clin. Nutr. 2019, 28, 819–825. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Wang, F. Pleiotropic actions of iron balance in diabetes mellitus. Rev. Endocr. Metab. Disord. 2015, 16, 15–23. [Google Scholar] [CrossRef]
- McClain, D.A.; Sharma, N.K.; Jain, S.; Harrison, A.; Salaye, L.N.; Comeau, M.E.; Langefeld, C.D.; Lorenzo, F.R.; Das, S.K. Adipose Tissue Transferrin and Insulin Resistance. J. Clin. Endocrinol. Metab. 2018, 103, 4197–4208. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Q.; Lv, Y.; Song, X.; Qu, H.; Chen, Y. The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Nutr. Diabetes 2020, 10, 17. [Google Scholar] [CrossRef]
- Miranda, M.A.; Lawson, H.A. Ironing out the Details: Untangling Dietary Iron and Genetic Background in Diabetes. Nutrients 2018, 10, 1437. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; McClain, D.; Manco, M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care 2015, 38, 2169–2176. [Google Scholar] [CrossRef]
- Szklarz, M.; Gontarz-Nowak, K.; Matuszewski, W.; Bandurska-Stankiewicz, E. “Ferrocrinology”—Iron Is an Important Factor Involved in Gluco- and Lipocrinology. Nutrients 2022, 14, 4693. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Ambachew, S.; Biadgo, B. Hepcidin in Iron Homeostasis: Diagnostic and Therapeutic Implications in Type 2 Diabetes Mellitus Patients. Acta Haematol. 2017, 138, 183–193. [Google Scholar] [CrossRef]
- Harrison, A.V.; Lorenzo, F.R.; McClain, D.A. Iron and the Pathophysiology of Diabetes. Annu. Rev. Physiol. 2023, 85, 339–362. [Google Scholar] [PubMed]
- Vaquero, M.P.; Martínez-Maqueda, D.; Gallego-Narbón, A.; Zapatera, B.; Pérez-Jiménez, J. Relationship between iron status markers and insulin resistance: An exploratory study in subjects with excess body weight. PeerJ 2020, 8, e9528. [Google Scholar] [CrossRef] [PubMed]
- Sachinidis, A.; Doumas, M.; Imprialos, K.; Stavropoulos, K.; Katsimardou, A.; Athyros, V.G. Dysmetabolic Iron Overload in Metabolic Syndrome. Curr. Pharm. Des. 2020, 26, 1019–1024. [Google Scholar] [CrossRef]
- Huang, J.; Karnchanasorn, R.; Ou, H.-Y.; Feng, W.; Chuang, L.-M.; Chiu, K.C.; Samoa, R. Association of insulin resistance with serum ferritin and aminotransferases-iron hypothesis. World J. Exp. Med. 2015, 5, 232–243. [Google Scholar] [CrossRef]
- Wlazlo, N.; Van Greevenbroek, M.M.J.; Ferreira, I.; Jansen, E.H.J.M.; Feskens, E.J.M.; Van Der Kallen, C.J.H.; Schalkwijk, C.G.; Bravenboer, B.; Stehouwer, C.D.A. Iron metabolism is prospectively associated with insulin resistance and glucose intolerance over a 7-year follow-up period: The CODAM study. Acta Diabetol. 2015, 52, 337–348. [Google Scholar] [CrossRef]
- Abu AlSel, B.T.; Mahmoud, A.A.; Hamed, E.O.; Hakim, N.A.; Sindi, A.A.A.; Jawad, N.M.M.; Gusti, A.M.T.; Fawzy, M.S.; El-Fadeal, N.M.A. Iron Homeostasis-Related Parameters and Hepcidin/Ferritin Ratio: Emerging Sex-Specific Predictive Markers for Metabolic Syndrome. Metabolites 2024, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.; Yang, Y.; Ma, L. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.-C.; Huang, S.-Y.; Hsieh, C.-H.; Hsu, M.-I.; Hsu, C.-S. Serum ferritin levels and polycystic ovary syndrome in obese and nonobese women. Taiwan J. Obstet. Gynecol. 2015, 54, 403–407. [Google Scholar] [CrossRef]
- Pluta, D.; Franik, G.; Blukacz, L.; Kowalczyk, K.; Witkowska, A.; Wysocka, M.; Madej, P. The correlation between the concentration of hepcidin in serum and the occurrence of insulin resistance and hyperandrogenemia in women with polycystic ovary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7379–7384. [Google Scholar]
- Bahaaeldin, A.M.; Hussein, M.S.; Hashem, S.S.; Saleh, A.M.M. Study of the Relationship Between Insulin Resistance, Iron Status Markers, and Body Weight in a Sample of Egyptian Population. Curr. Diabetes Rev. 2024, 20, e170823219896. [Google Scholar] [PubMed]
- Huth, C.; Beuerle, S.; Zierer, A.; Heier, M.; Herder, C.; Kaiser, T.; Koenig, W.; Kronenberg, F.; Oexle, K.; Rathmann, W.; et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: The KORA F4 study. Eur. J. Endocrinol. 2015, 173, 643–653. [Google Scholar] [CrossRef]
- Krisai, P.; Leib, S.; Aeschbacher, S.; Kofler, T.; Assadian, M.; Maseli, A.; Todd, J.; Estis, J.; Risch, M.; Risch, L.; et al. Relationships of iron metabolism with insulin resistance and glucose levels in young and healthy adults. Eur. J. Intern. Med. 2016, 32, 31–37. [Google Scholar] [CrossRef]
- Shalitin, S.; Deutsch, V.; Tauman, R. Hepcidin, soluble transferrin receptor and IL-6 levels in obese children and adolescents with and without type 2 diabetes mellitus/impaired glucose tolerance and their association with obstructive sleep apnea. J. Endocrinol. Investig. 2018, 41, 969–975. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004.
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar]
- Società Italiana di Nutrizione Umana. LARN: Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana, 5th ed.; Biomedia: Milan, Italy, 2024. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for iron. EFSA J. 2015, 13, 4254. [Google Scholar]
- Mistura, L.; Le Donne, C.; D’Addezio, L.; Ferrari, M.; Comendador, F.J.; Piccinelli, R.; Martone, D.; Sette, S.; Catasta, G.; Turrini, A. The Italian IV SCAI dietary survey: Main results on food consumption. Nutr. Metab. Cardiovasc. Dis. 2025, 103863. [Google Scholar] [CrossRef]
- Cepeda-Lopez, A.C.; Osendarp, S.J.; Melse-Boonstra, A.; Aeberli, I.; Gonzalez-Salazar, F.; Feskens, E.; Villalpando, S.; Zimmermann, M.B. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am. J. Clin. Nutr. 2011, 93, 975–983. [Google Scholar] [CrossRef]
- Suteerojntrakool, O.; Khongcharoensombat, T.; Chomtho, S.; Bongsebandhu-Phubhakdi, C.; Tempark, T.; Fewtrell, M. Anthropometric Markers and Iron Status of 6–12-Year-Old Thai Children: Associations and Predictors. J. Nutr. Metab. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Ranjan, R.; Kratasyuk, V.A. Is Body Mass Index a potential biomarker for anemia in obese adolescents? J. Nutr. Intermed. Metab. 2019, 15, 1–2. [Google Scholar] [CrossRef]
- Aeberli, I.; Hurrell, R.F.; Zimmermann, M.B. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int. J. Obes. 2009, 33, 1111–1117. [Google Scholar] [CrossRef]
- Kirti, K.; Singh, S.K. Obesogenic diet and metabolic syndrome among adolescents in India: Data-driven cluster analysis. BMC Cardiovasc. Disord. 2023, 23, 1–13. [Google Scholar] [CrossRef]
- Queiroz, J.C.D.L.S.; Rey, L.C.; Ataide, T.D.R.; Florêncio, T.M.D.M.T.; Silva-Neto, L.G.R. Consumption of ultra-processed foods is associated with dietary iron availability, anemia, and excess weight in socially vulnerable children. Clin. Nutr. ESPEN 2025, 65, 461–468. [Google Scholar] [CrossRef]
- Coimbra, S.; Catarino, C.; Nascimento, H.; Alves, A.I.; Medeiros, A.F.; Bronze-Da-Rocha, E.; Costa, E.; Rocha-Pereira, P.; Aires, L.; Seabra, A.; et al. Physical exercise intervention at school improved hepcidin, inflammation, and iron metabolism in overweight and obese children and adolescents. Pediatr. Res. 2017, 82, 781–788. [Google Scholar] [CrossRef]
- Were, J.M.; Stranges, S.; Wilk, P.; Ali, S.; Sharma, I.; Vargas-Gonzalez, J.C.; Campbell, M.K. The double burden of malnutrition among women of reproductive age and preschool children in low- and middle-income countries: A scoping review and thematic analysis of literature. Nutrition 2023, 111, 112053. [Google Scholar] [CrossRef]
- Moscheo, C.; Licciardello, M.; Samperi, P.; La Spina, M.; Di Cataldo, A.; Russo, G. New Insights into Iron Deficiency Anemia in Children: A Practical Review. Metabolites 2022, 12, 289. [Google Scholar] [CrossRef]
- Alshwaiyat, N.M.; Ahmad, A.; Hassan, W.M.R.W.; Al-Jamal, H.A.N. Association between obesity and iron deficiency (Review). Exp. Ther. Med. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Sanad, M.; Osman, M.; Gharib, A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: A case control study. Ital. J. Pediatr. 2011, 37, 34. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. 2008, 32, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Smuts, C.M.; Aeberli, I.; Malan, L.; Tjalsma, H.; Zimmermann, M.B. Overweight impairs efficacy of iron supplementation in iron-deficient South African children: A randomized controlled intervention. Int. J. Obes. 2013, 37, 24–30. [Google Scholar] [CrossRef]
- Dorsey, A.F.; Penny, M.E.; Thompson, A.L. Adiposity and pathogen exposure: An investigation of response to iron supplementation and hypothesized predictors in anemic pre-school-aged children living in a dual burden environment. Am. J. Phys. Anthr. 2021, 176, 54–65. [Google Scholar] [CrossRef]
- Amato, A.; Santoro, N.; Calabrò, P.; Grandone, A.; Swinkels, D.W.; Perrone, L.; del Giudice, E.M. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int. J. Obes. 2010, 34, 1772–1774. [Google Scholar] [CrossRef]
- Gong, L.; Yuan, F.; Teng, J.; Li, X.; Zheng, S.; Lin, L.; Deng, H.; Ma, G.; Sun, C.; Li, Y. Weight Loss, Inflammatory Markers, and Improvements of Iron Status in Overweight and Obese Children. J. Pediatr. 2014, 164, 795–800.e2. [Google Scholar] [CrossRef]
- Ozcelik-Ersu, D.; Kızıltan, G. Association of the relationship between nutritional status and certain biochemical parameters in obese children. Prog Nutr. 2021, 23, e2021237. [Google Scholar]
- Yıldırım, Ö.; Demircan, T.; Tüfekçi, Ö.; Kızılca, Ö.; Kuyum, P.; Kır, M.; Abacı, A.; Ünal, N.; Arslan, N.; Böber, E.; et al. Anemia and Its Effect on Cardiovascular Findings in Obese Adolescents. Turk. J. Hematol. 2018, 35, 192–196. [Google Scholar] [CrossRef]
- Ibrahim, L.; Allehdan, S.; Alassaf, A.; Tayyem, R. Iron deficiency and obesity in pre-school children. Nutr. Food Sci. 2018, 48, 418–432. [Google Scholar] [CrossRef]
- Barham, R.; Tayyem, R.; Al-Majali, L.; Al-Khatib, B.; Al Jawaldeh, A. Evaluation of micronutrient and nutritional status among preschool children in Jordan: Results from a Nationwide survey. Front. Nutr. 2024, 11, 1423904. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, C.; Matalas, A.-L. Breakfast intake is associated with nutritional status, Mediterranean diet adherence, serum iron and fasting glucose: The CYFamilies study. Public Health Nutr. 2015, 18, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Chan, D.F.Y.; Lee, C.K.; Tsoi, W.C.; Lau, C.W.; Leung, J.N.S.; So, J.C.C.; Wong, C.L.P.; Tsang, S.T.Y.; Chu, Y.Y.L.; et al. Iron Deficiency among School-Aged Adolescents in Hong Kong: Prevalence, Predictors, and Effects on Health-Related Quality of Life. Int. J. Environ. Res. Public Health 2023, 20, 2578. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Aigner, E.; Weghuber, D.; Paulmichl, K. The Potential Role of Iron and Copper in Pediatric Obesity and Nonalcoholic Fatty Liver Disease. BioMed Res. Int. 2015, 2015, 287401. [Google Scholar] [CrossRef]
- Zheng, H.; Long, W.; Tan, W.; Yang, C.; Cao, M.; Zhu, Y. Anaemia, iron deficiency, iron-deficiency anaemia and their associations with obesity among schoolchildren in Guangzhou, China. Public Health Nutr. 2020, 23, 1693–1702. [Google Scholar] [CrossRef]

| First Author’s Name | Type of Study | Country | Sample | Main Results |
|---|---|---|---|---|
| Garcìa O. P. et al. [34] | Cross-sectional study | Mexico | 197, aged 6–10.5 years | Low iron concentrations in children with overweight and obesity are associated with increased levels of lipids, inflammation, and insulin resistance |
| H. Lee H. et al. [45] | Cross-sectional study | Korea | 1350, aged 7–13 years | Iron-related factors may involve insulin resistance. |
| Ferrari M. et al. [35] | Cross-sectional study | Europe | 876, aged 12.5–17.5 years | The level of adiposity in European adolescents was sufficient to induce chronic inflammation but did not reach the threshold necessary to compromise iron status or lead to iron deficiency. |
| Wei J. et al. [47] | Cross-sectional study | China | 689, aged 6–18 years | Serum transferrin and sTfR were statistically significantly associated with glucose parameters. |
| Suàrez Ortegòn M.F. et al. [40] | Cohort study | Chile | 1892, aged 4 months | Ferritin levels in infancy are positively and longitudinally associated with cardiometabolic risk at adolescent stage. |
| Klisic A. et al. [39] | Case–control study | Montenegro | 60, aged 16–19 years | sTfR levels independently correlate with HOMA-IR; elevated ferritin and adipokines are linked to higher HOMA-IR. |
| Gonzàlez Dominguez A. et al. [43] | Case–control study | Spain | 72, aged 6–10 years | Metal-related abnormalities were sharpened in subjects presenting IR compared to children with metabolically healthy obesity. |
| Ortiz Marròn H. et al. [44] | Cross-sectional study | Spain | 1954, aged 9–10 years | The glycemic profile is better in children who have high concentrations of Is and transferrin saturation. |
| Female | LARN | 7–12 months | 1–3 years | 4–6 years | 7–10 years | 11–14 years | 15–17 years | |
| 7 (AR), 11 (PRI) | 4 (AR), 8 (PRI) | 5 (AR), 11 (PRI) | 5 (AR), 13 (PRI) | 7 (AR) 10 (PRI) * | 10 (AR) 18 (PRI) | |||
| EFSA | 7–11 months | 1–3 years | 4–6 years | 7–11 years | 12–17 years | |||
| 8 (AR), 11 (PRI) | 5 (AR), 7 (PRI) | 5 (AR), 7 (PRI) | 8 (AR), 11 (PRI) | 7 (AR), 13 (PRI) | ||||
| FAO/WHO (for a dietary iron bioavailability of 15%) | 5–12 months | 1–3 years | 4–6 years | 7–10 years | 11–14 years | 15–17 years | ||
| 6.2 (PRI) | 3.9 (PRI) | 4.2 (PRI) | 5.9 (PRI) | 9.3 (PRI) ** | 20.7 (PRI) | |||
| Male | LARN | 7–12 months | 1–3 years | 4–6 years | 7–10 years | 11–14 years | 15–17 years | |
| 7 (AR), 11 (PRI) | 4 (AR), 8 (PRI) | 5 (AR), 11 (PRI) | 5 (AR), 13 (PRI) | 7 (AR) 10 (PRI) | 9 (AR) 13 (PRI) | |||
| EFSA | 7–11 months | 1–3 years | 4–6 years | 7–11 years | 12–17 years | |||
| 8 (AR), 11 (PRI) | 5 (AR), 7 (PRI) | 5 (AR), 7 (PRI) | 8 (AR), 11 (PRI) | 8 (AR), 11 (PRI) | ||||
| FAO/WHO (for a dietary iron bioavailability of 15%) | 7–12 months | 1–3 years | 4–6 years | 7–10 years | 11–14 years | 15–17 years | ||
| 6.2 (PRI) | 3.9 (PRI) | 4.2 (PRI) | 5.9 (PRI) | 9.7 (PRI) | 12.5 (PRI) | |||
| First Author’s Name | Type of Study | Sample | Objectives | Main Results |
|---|---|---|---|---|
| Association of iron deficiency with dietary parameters or habits | ||||
| Ferrari M. et al. [35] | Cross-sectional study | 876 adolescents aged 12.5–17.5 years | To investigate the association among obesity, inflammation, and iron status. To assess the intake of relevant nutrients and their association with BMI z-score, FM, and FFM. | No significant (p > 0.05) differences between BMI and the intake of:
|
| Kirti K. et al. [78] | Cross-sectional study | 12,318 adolescents aged 10–19 years | To identify clusters based on adolescents’ dietary patterns; to correlate clusters with obesity prevalence, lipid anomalies, hypertension, and micronutrient deficiencies |
|
| Ozcelik-Ersu D. et al. [90] | Cross-sectional study | 93 children and adolescents with obesity aged 10–17 years | To examine the relationship between nutritional status and biochemical parameters |
|
| Queiroz J. et al. [79] | Cross-sectional study | 443 children aged 6–59 months | To assess iron availability and the presence of anemia and excess body weight and to determine their association with ultra-processed food (UPF) consumption. | The highest relative share of UPF in total calorie consumption is inversely associated with iron availability (β quartile 4 versus quartile 1: −0.12; 95% CI: −0.23; −0.01; p = 0.037); and directly associated with excess body weight (OR quartile 4 versus quartile 1: 2.16; 95% CI 1.05; 4.46; p = 0.038) and anemia (OR quartile 4 versus quartile 1: 2.45; 95% CI: 1.26; 4.78; p = 0.009). |
| Yıldırım O. et al. [91] | Cross-sectional study | Adolescents with obesity aged 12–19 years, divided into 2 groups: N = 29 anemics, N = 33 non-anemics; there was also a third control group of 33 healthy individuals without obesity | To assess the effect of anemia (defined as Hb ≤ 12 g/dL in women and ≤13 g/dL in men) on cardiovascular findings in adolescents with obesity |
|
| Nutritional and lifestyle strategy | ||||
| Cheung Y.T. et al. [95] | Cross-sectional study | 523 adolescents aged 16–19 years | To determine the prevalence of ID and IDA. To identify the dietary predictors of iron status. To evaluate the association between iron status and functional outcomes (HRQoL and fatigue). |
|
| Coimbra S. et al. [80] | Longitudinal intervention study | 73 children and adolescents aged 5–17 years; intervention group: N = 44, control group: N = 29 | To evaluate the impact of an 8-month school-based physical exercise program on hepcidin levels, inflammation markers, and iron metabolism. | The PE group showed a decrease in BMI z-score (p = 0.003), body fat mass (p = 0.012), CRP (p = 0.002), IL-6 (p = 0.048), ferritin (p = 0.013), hepcidin (p = 0.040), and sTfR (p = 0.010), as well as an increase in iron concentration (p = 0.002). |
| Dorsey A.F. et al. [87] | Intervention study | 50 children aged 2–5 years | To test 1-month iron supplementation (15 mg/day) in children with anemia (Hb < 11 g/dL), also testing the following conditions:
|
|
| Lazarou C. et al. [94] | Cross-sectional study | 83 children aged 6–12 years | To assess the association between breakfast intake and Mediterranean diet adherence (evaluated with modified KIDMED score), physical activity levels, obesity, selected cardiovascular risk markers, and iron status. |
|
| Reviews | ||||
| Alshwaiyat N. et al. [83] | Review | Experimental article on the relationship between ID and obesity conducted from January 2015 to January 2021 focusing on individuals with overweight and obesity (children/adolescents and adults) | To discuss the evidence on the relationship between obesity and iron deficiency | Obesity can disrupt iron balance, leading to IDA, potentially due to inflammation-driven increases in hepcidin levels. Weight loss helps reduce inflammation and hepcidin, thereby enhancing iron absorption and improving iron status. |
| Berton P.F. et al. [11] | Systematic review | 2543 children and adolescents, aged 3–21 years, from 16 articles. | To study iron deficiency in children and adolescents with obesity and its association with inflammation (interleukins) and hepcidin. | Obesity’s chronic inflammation leads to the production of IL-6, which stimulates hepcidin synthesis, resulting in ID. ID is common in children and adolescents with obesity, who respond inadequately to iron supplementation but respond adequately to interventions against chronic inflammation, such as weight loss and physical activities. |
| Calcaterra V. et al. [10] | Narrative review | 45 articles published from 2008 to 2023 focusing on micronutrient deficiencies in childhood obesity | To analyze and summarize main deficiencies associated with obesity, their clinical consequences, and evidence regarding possible supplementation | Iron is one of the most common deficient microelements (together with vitamins A, B, C, D, and E; folic acid; zinc; and copper). Relationship between obesity and micronutrient deficiencies remains unclear. Weight loss was linked to improved iron and inflammatory status. |
| Feldman A. et al. [96] | Narrative review | Findings from pediatric studies. Relevant data from adult studies are included when applicable for clinical extrapolation | To summarize available data about iron and copper in the context of obesity and NAFLD in children. | Perturbations of iron homeostasis shown to contribute to the pathogenesis of NAFLD (not sufficiently examined in pediatric cohorts). Iron supplementation is less effective in children with overweight. Weight reduction leads to a decrease in hepcidin and leptin and to an increase in iron absorption and an improvement of iron status. |
| Grandone A. et al. [25] | Narrative Review | Studies on children; Studies on adults to support pathophysiological aspects | To study ID in children with obesity and the role of hepcidin, as well as iron status and its consequences on health, particularly regarding cognitive function and and obesity | The best treatment for obesity-related ID may be weight loss, alone or in combination with iron supplementation. |
| Hutchinson C. [16] | Narrative Review | 48 observational studies, case reports, and interventional studies published until December 2015 and conducted on children and adolescents. | To evaluate the relationship between iron and overweight and obesity in children and adolescents, with an emphasis on iron status, oral iron response, dietary intake, and inflammatory markers. | ID (or risk of ID) is more prevalent among children and adolescents with overweight and obesity; chronic inflammation is a plausible explanation, rather than dietary factors. Weight loss improves inflammatory status and indicators of iron status. Children and adolescents with overweight/obesity have reduced response to oral iron. |
| Ibrahim L. et al. [92] | Literature narrative review | Data from children under the age of 5 years | To review the association between ID and obesity in toddlers and preschool children. | Conflicting results, but most articles agree that ID is significantly associated with overweight and obesity in children; systemic inflammatory reaction seems to be the major cause through hepcidin, which decreases the duodenal absorption of iron, in addition to other causes, including dietary and genetic factors. Unbalanced diet, either in excess or shortage, may affect serum iron. Dietary interventions aimed at promoting a balanced diet and limiting the consumption of calorie-dense, low-nutrient foods may be beneficial. |
| Malden S. [12] | Systematic review and meta-analysis | 9381 children aged 2–19 years (from 10 different studies focusing on iron deficiency) | To study the association between different medical conditions and comorbidities and obesity in young children. | Having obesity doubled the odds of iron deficiency diagnosis (OR 2.1; 95% CI 1.4–3.2). The condition remains associated with obesity even when controlling for diet as a covariate. |
| Pande S. et al. [76] | Narrative Review | Data from studies on children and adolescents with obesity. Some animal experiments and data from adult studies are also cited. | To establish the role hepcidin plays in obesity and its relation with anemia. To endorse BMI as a biomarker for anemia in adolescents with obesity. | Adolescents with obesity were twice as likely to be anemic than normal-weight adolescents; hepcidin mediates the anemia through obesity-induced inflammation. Screening for iron status among adolescents with elevated BMI is advisable. Weight reduction can be useful to reduce inflammation and improve iron absorption. |
| Sachdeva M. et al. [13] | Systematic review and meta-analysis | 49,206 children and adolescents aged <18 years, from 42 studies | To examine the association between obesity and ID, IDA, and various hematological parameters. | Pooled OR (95% CI) for ID = 1.64 (1.22, 2.21; p = 0.001), and pooled prevalence of ID = 20.07% (14.98, 25.16) among children living with obesity. Pooled OR (95% CI) for IDA = 0.78 (0.43, 1.43, p = 0.43). |
| Were J. et al. [81] | Scoping review | 720 studies on the coexistence of undernutrition and overnutrition among women of reproductive age (15–49 y) and preschool children (≤5 y) in low- and middle-income countries | To map the literature on the DBM, providing an understanding of how the DBM construct has been defined in the current literature. To elucidate plausible mechanisms underlying DBM development and its common risk factor. | The understanding of the DBM in the literature is ambiguous. The predominant mechanism that emerged with regard to overweight/obesity and ID DBM was chronic low-grade inflammation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Cena, H.; Bolpagni, F.; Taranto, S.; Vincenti, A.; Madini, N.; Diotti, M.; Quatrale, A.; Zuccotti, G. The Interplay Between Iron Metabolism and Insulin Resistance: A Key Factor in Optimizing Obesity Management in Children and Adolescents. Nutrients 2025, 17, 1211. https://doi.org/10.3390/nu17071211
Calcaterra V, Cena H, Bolpagni F, Taranto S, Vincenti A, Madini N, Diotti M, Quatrale A, Zuccotti G. The Interplay Between Iron Metabolism and Insulin Resistance: A Key Factor in Optimizing Obesity Management in Children and Adolescents. Nutrients. 2025; 17(7):1211. https://doi.org/10.3390/nu17071211
Chicago/Turabian StyleCalcaterra, Valeria, Hellas Cena, Federica Bolpagni, Silvia Taranto, Alessandra Vincenti, Nagaia Madini, Marianna Diotti, Antonia Quatrale, and Gianvincenzo Zuccotti. 2025. "The Interplay Between Iron Metabolism and Insulin Resistance: A Key Factor in Optimizing Obesity Management in Children and Adolescents" Nutrients 17, no. 7: 1211. https://doi.org/10.3390/nu17071211
APA StyleCalcaterra, V., Cena, H., Bolpagni, F., Taranto, S., Vincenti, A., Madini, N., Diotti, M., Quatrale, A., & Zuccotti, G. (2025). The Interplay Between Iron Metabolism and Insulin Resistance: A Key Factor in Optimizing Obesity Management in Children and Adolescents. Nutrients, 17(7), 1211. https://doi.org/10.3390/nu17071211









