Ultraprocessed Foods and Neuropsychiatric Outcomes: Putative Mechanisms
Abstract
:1. Introduction
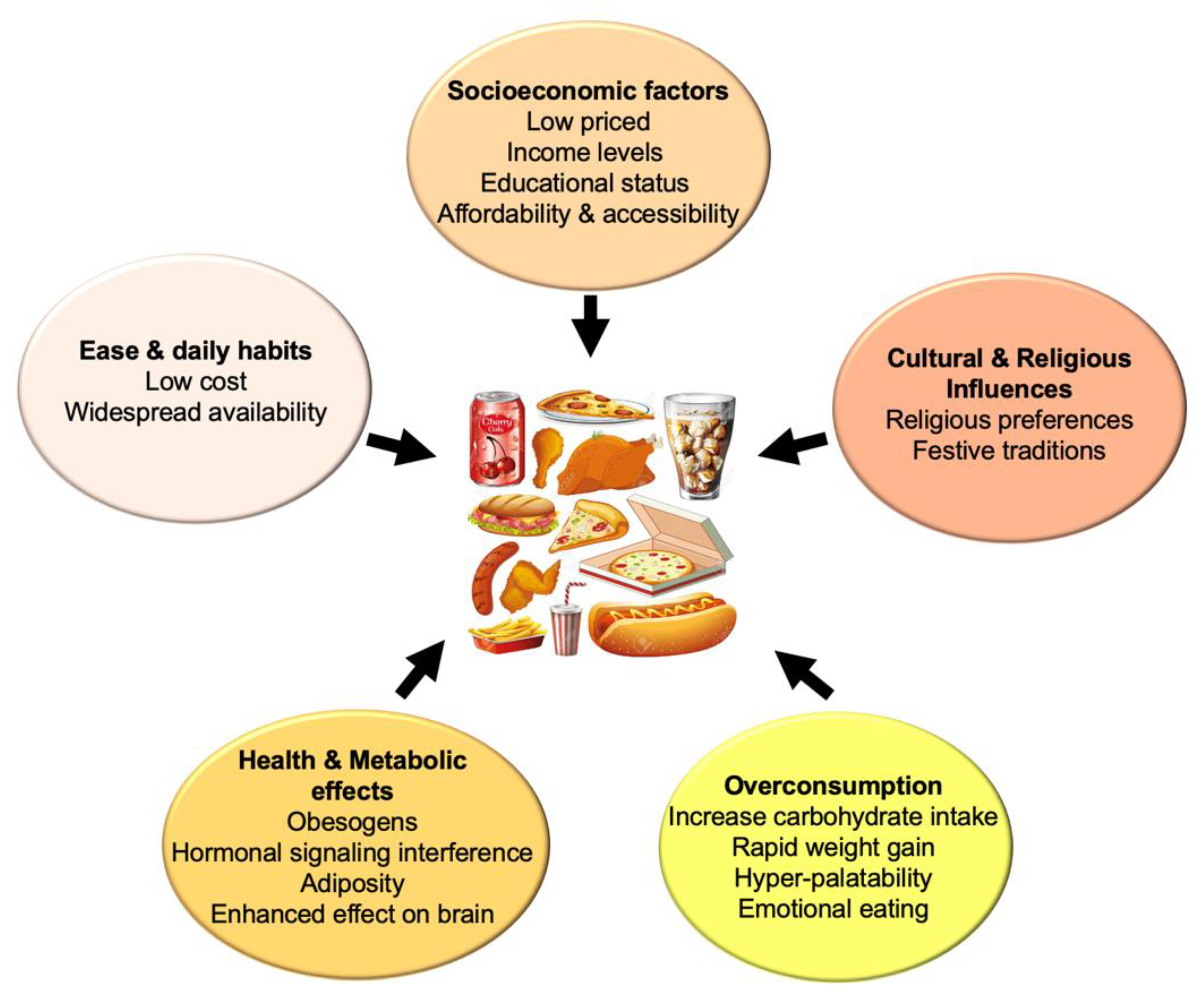
Ultraprocessed Foods
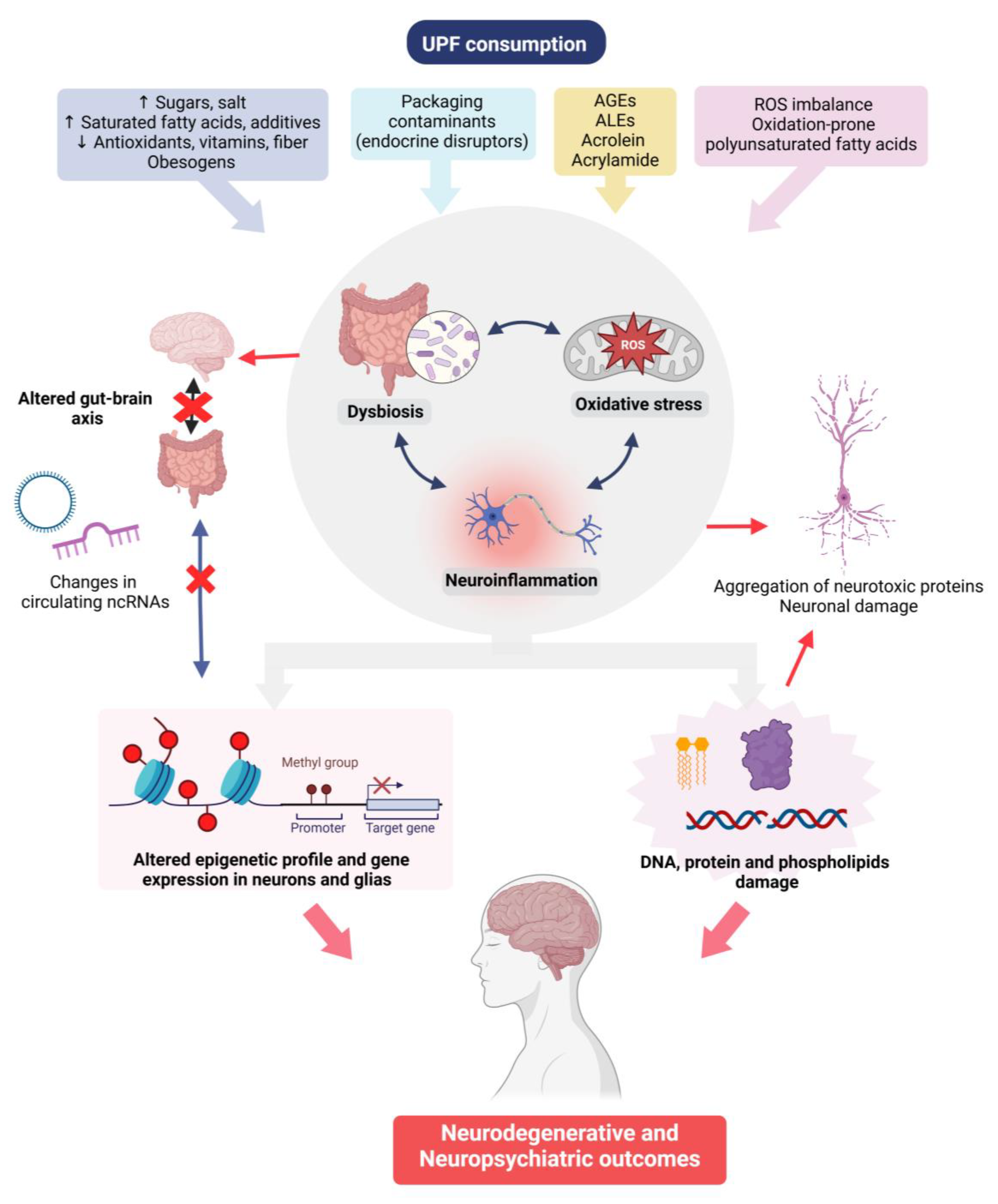
2. UPFs and Mental Health—Mechanisms Involved
2.1. Inflammation and Oxidative Stress
2.2. Thermal Treatment of Foods and Generation of Toxins
2.2.1. AGEs and ALEs
2.2.2. Acrolein and Acrylamide
2.3. Gut Microbiota
2.4. Epigenetic Mechanisms: Implications for Gene Regulation and Inheritance
3. Mental Health-Related Outcomes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | β-Amyloid |
| AD | Alzheimer’s disease |
| AGEs | Advanced glycation end-products |
| ALEs | Lipoxidation end-products |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| CRP | C-reactive protein |
| EWASs | Epigenome-wide association studies |
| NCDs | Non-communicable diseases |
| PUFAs | Polyunsaturated fatty acids |
| RAGEs | Receptor of advanced glycation end-products |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| UPFs | Ultraprocessed foods |
References
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Chowdhary, N.; Barbui, C.; Anstey, K.J.; Kivipelto, M.; Barbera, M.; Peters, R.; Zheng, L.; Kulmala, J.; Stephen, R.; Ferri, C.P.; et al. Reducing the risk of cognitive decline and dementia: WHO recommendations. Front. Neurol. 2022, 12, 765584. [Google Scholar] [CrossRef] [PubMed]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Liu, Y.H.; Gao, X.; Na, M.; Kris-Etherton, P.M.; Mitchell, D.C.; Jensen, G.L. Dietary pattern, diet quality, and dementia: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Alzheimer’s Dis. 2020, 78, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.; Machado, P.; Steele, E.M. Association between ultraprocessed food consumption and cognitive performance in US older adults: A cross-sectional analysis of the NHANES 2011–2014. Eur. J. Nutr. 2022, 61, 3975–3985. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Yang, H.; Zhang, Y.; Zhang, S.; Ma, Y.; Hou, Y.; Zhang, X.; Niu, K.; Borné, Y.; et al. Association of ultraprocessed food consumption with risk of dementia: A prospective cohort. Neurology 2022, 99, e1056e66. [Google Scholar] [CrossRef]
- Monteiro, C.; Cannon, G.; Levy, R.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Bueno, N.B.; DiFeliceantonio, A.G.; Roberto, C.A.; Jiménez-Murcia, S.; Fernandez-Aranda, F. Social, clinical, and policy implications of ultra-processed food addiction. BMJ 2023, 383, e075354. [Google Scholar] [CrossRef]
- Ravandi, R.; Ispirova, G.; Sebek, M.; Mehler, P.; Barabási, A.-L.; Menichetti, G. Prevalence of processed foods in major US grocery stores. Nat. Food 2025, 6, 296–308. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tzivaki, I.; Zafeiri, G.C.M.; Rigatou, A.; Daskalopoulou, S.; Stratigou, T.; Karampela, I.; Dalamaga, M. Ultra-processed foods and childhood obesity: Current evidence and perspectives. Curr. Nutr. Rep. 2025, 14, 5. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V. Processed diets cause excess calorie intake and weight gain: An inpatient Randomized Controlled Trial of ad libitum food intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Lustig, R.H.; Howard, S.; Corkey, B.E. Obesogens: A unifying theory for the global rise in obesity. Int. J. Obes. 2024, 48, 449–460. [Google Scholar] [CrossRef]
- DiFeliceantonio, A.G.; Coppin, G.; Rigoux, L.; Edwin Thanarajah, S.; Dagher, A.; Tittgemeyer, M.; Small, D.M. Supraadditive effects of combining fat and carbohydrate on food reward. Cell Metab. 2018, 28, 33–44.e3. [Google Scholar] [CrossRef]
- Kelly, A.L.; Baugh, M.E.; Oster, M.E.; Di Feliceantonio, A.G. The impact of caloric availability on eating behavior and ultra-processed food reward. Appetite 2022, 178, 106274. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Gold, S.; Delgado, A.; Kopsahelis, N.; Kachrimanidou, V.; Kaur, L.; Galli, F.; Rock, E. How can food processing achieve food and nutrition security? Sustain Develop. 2024, 32, 4172–4185. [Google Scholar] [CrossRef]
- Freudenberg, N.; Lee, K.; Buse, K.; Collin, J.; Crosbie, E.; Friel, S.; Klein, D.E.; Lima, J.M.; Marten, R.; Mialon, M. Defining priorities for action and research on the commercial determinants of health: A conceptual review. Am. J. Public Health 2021, 111, 2202–2211. [Google Scholar] [CrossRef]
- Leigh, S.J.; Lee, F.; Morris, M.J. The intersection of palatable food, cues, and reward pathways, stress, and cognition. Curr. Obes. Rep. 2018, 7, 362–373. [Google Scholar] [CrossRef]
- Stariolo, J.B.; Lemos, T.C.; Khandpur, N.; Pereira, M.G.; de Oliveira, L.; Mocaiber, I.; Ramos, T.C.; David, I.A. Addiction to ultra-processed foods as a mediator between psychological stress and emotional eating during COVID-19 pandemic. Psicol. Reflex. Crít. 2024, 37, 39. [Google Scholar] [CrossRef]
- Dicken, S.J.; Qamar, S.; Batterham, R.L. Who consumes ultra-processed food? A systematic review of sociodemographic determinants of ultra-processed food consumption from nationally representative samples. Nutr. Res. Rev. 2024, 37, 416–456. [Google Scholar] [CrossRef]
- Grant, W.B.; Blake, S.M. Diet’s role in modifying risk of Alzheimer’s disease: History and present understanding. J. Alzheimer’s Dis. 2023, 96, 1353–1382. [Google Scholar] [CrossRef]
- Vitale, M.; Costabile, G.; Testa, R.; D’Abbronzo, G.; Nettore, I.C.; Macchia, P.E.; Giacco, R. Ultra-Processed Foods and Human Health: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2024, 15, 100121. [Google Scholar] [CrossRef]
- Mendoza, K.; Smith-Warner, S.A.; Rossato, S.L.; Khandpur, N.; Manson, J.A.E.; Qi, L.; Mukamal, K.J.; Willett, W.C.; Wang, M.; Hu, F.B.; et al. Ultra-processed foods and cardiovascular disease: Analysis of three large US prospective cohorts and a systematic review and meta-analysis of prospective cohort studies. Lancet Reg. Health Am. 2024, 37, 100859. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, H.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef]
- Suksatan, W.; Moradi, S.; Naeini, F.; Mehrabani, S.; Hojjati Kermani, M.A.; Suzuki, K. Ultra-processed food consumption and adult mortality risk: A Systematic Review and Dose-Response Meta-Analysis of 207,291 participants. Nutrients 2021, 14, 174. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary patterns and risk of dementia: A Systematic Review and Meta-Analysis of cohort studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef]
- Fu, J.; Tan, L.J.; Lee, J.E.; Shin, S. Association between the Mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 946361. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Zhong, K.; Wang, C.; Xu, X. The associations between endocrine disrupting chemicals and markers of inflammation and immune responses: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2022, 234, 113382. [Google Scholar] [CrossRef]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, P.R.; Lo, Y.C.; Sun, C.W.; Pan, W.H.; Wang, S.L.; Huang, H.B. Food processing and phthalate exposure: The Nutrition and Health Survey in Taiwan (1993–1996 and 2005–2008). Front. Nutr. 2021, 8, 766992. [Google Scholar] [CrossRef]
- Dubeau, C.; Aker, A.; Caron-Beaudoin, É.; Ayotte, P.; Blanchette, C.; McHugh, N.G.; Lemire, M. Perfluoroalkyl acid and bisphenol-A exposure via food sources in four First Nation communities in Quebec, Canada. Public Health Nutr. 2023, 26, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Naspolini, N.F.; Machado, P.P.; Moreira, J.C.; Asmus, C.I.; Meyer, A. Maternal consumption of ultra-processed foods and newborn exposure to perfluoroalkyl substances (PFAS). Cad. Saude Publica 2021, 37, e00152021. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Khandpur, N.; da Costa Louzada, M.L.; Monteiro, C.A. Association between dietary contribution of ultraprocessed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS ONE 2020, 15, e0236738. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s disease: Current progress in molecular signaling and therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Reales-Moreno, M.; Fernández-Barrès, S.; Cimpean, A.; Arnoriaga-Rodríguez, M.; Puig, J.; Biarnés, C.; Motger-Albertí, A.; Cano, M.; Fernández-Real, J.M. Consumption of ultra-processed foods is associated with depression, mesocorticolimbic volume, and inflammation. J. Affect. Disord. 2023, 335, 340–348. [Google Scholar] [CrossRef]
- Laster, J.; Frame, L.A. Beyond the calories-Is the problem in the processing? Curr. Treat. Options Gastroenterol. 2019, 17, 577–586. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The bidirectional relationship of depression and inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef]
- Espinet, C.; Gonzalo, H.; Fleitas, C.; Menal, M.J.; Egea, J. Oxidative stress and neurodegenerative diseases: A neurotrophic approach. Curr. Drug Targets 2015, 16, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Ray, C.A. Control of Maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Walker, D.; Lue, L.F.; Paul, G.; Patel, A.; Sabbagh, M.N. Receptor for advanced glycation end product modulators: A new therapeutic target in Alzheimer’s disease. Expert Opin. Investig. Drugs 2015, 24, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; Vlassara, H. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: Association with increased AGER1 expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Sergi, D.; Lane, M.M.; Naumovski, N.; Gamage, E.; Rajendran, A.; Kouvari, M.; Gauci, S.; Dissanayka, T.; Marx, W.; et al. The effects of dietary advanced glycation end-products on neurocognitive and mental disorders. Nutrients 2022, 14, 2421. [Google Scholar] [CrossRef]
- Hellwig, M.; Diel, P.; Eisenbrand, G.; Grune, T.; Guth, S.; Henle, T.; Humpf, H.U.; Joost, H.G.; Marko, D.; Raupbach, J.; et al. Dietary glycation compounds—Implications for human health. Crit. Rev. Toxicol. 2024, 54, 485–617. [Google Scholar] [CrossRef]
- Deng, S.; He, R.; Yue, Z.; Li, B.; Li, F.; Xiao, Q.; Wang, X.; Li, Y.; Chen, R.; Rong, S. Association of Advanced Glycation End Products with cognitive function: HealthyDance Study. J. Alzheimer’s Dis. 2024, 100, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Berends, E.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Wrigglesworth, J.; Ward, P.; Harding, I.H.; Nilaweera, D.; Wu, Z.; Woods, R.L.; Ryan, J. Factors associated with brain ageing—A systematic review. BMC Neurol. 2021, 21, 312. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R. Advanced lipoxidation end-products. Chem. Biol. Interact. 2011, 192, 14–20. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Melnikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Uribarri, J. Healthy eating recommendations: Good for reducing dietary contribution to the body’s advanced glycation/lipoxidation end products pool? Nutr. Res. Rev. 2021, 34, 48–63. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Liu, F.; Zheng, J.; Zhao, D.; Ou, S. Origin and fate of acrolein in foods. Foods 2022, 11, 1976. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Vasanth, K. Food contaminants: Impact of food processing, challenges and mitigation strategies for food security. Food Res. Int. 2024, 191, 114739. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Tsou, H.H.; Hsu, W.C.; Fuh, J.L.; Chen, S.P.; Liu, T.Y.; Wang, H.T. Alterations in acrolein metabolism contribute to Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 571–580. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Y.; Zheng, B.; Chen, Y.; Xie, J.; Song, Y.; Ding, X.; Hu, X.; Hu, X.; Yu, Q. The role of acrolein in neurodegenerative diseases and its protective strategy. Foods 2022, 11, 3203. [Google Scholar] [CrossRef]
- Hamann, K.; Shi, R. Acrolein scavenging: A potential novel mechanism of attenuating oxidative stress following spinal cord injury. J. Neurochem. 2009, 111, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.A.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4- hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Rad. Biol. Med. 2010, 48, 1570–1576. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.S.; Markesbery, W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef]
- Gomes Gonçalves, N.; Vidal Ferreira, N.; Khandpur, N.; Martinez Steele, E.; Bertazzi Levy, R.; Andrade Lotufo, P.; Bensenor, I.M.; Caramelli, P.; de Matos, S.M.A.; Marchioni, D.M. Association between consumption of ultraprocessed foods and cognitive decline. JAMA Neurol. 2023, 80, 142–150. [Google Scholar] [CrossRef]
- Seidler, N.W.; Squire, T.J. Abeta-polyacrolein aggregates: Novel mechanism of plastic formation in senile plaques. Biochem. Biophys. Res. Commun. 2005, 335, 501–504. [Google Scholar] [CrossRef]
- Singh, M.; Nam, D.T.; Arseneault, M.; Ramassamy, C. Role of by-products of lipid oxidation in Alzheimer’s disease brain: A focus on acrolein. J. Alzheimer’s Dis. 2010, 21, 741–756. [Google Scholar] [CrossRef]
- Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Epigenetic aberrations in major psychiatric diseases related to diet and gut microbiome alterations. Genes 2023, 14, 1506. [Google Scholar] [CrossRef]
- Lim, J.S.; Lim, M.Y.; Choi, Y.; Ko, G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol. Brain 2017, 20, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pardo, P.; de Jong, E.M.; Broersen, L.M.; van Wijk, N.; Attali, A.; Garssen, J.; Kraneveld, A.D. Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 57. [Google Scholar] [CrossRef]
- Yang, J.; Liang, J.; Hu, N.; He, N.; Liu, B.; Liu, G.; Qin, Y. The gut microbiota modulates neuroinflammation in Alzheimer’s disease: Elucidating crucial factors and mechanistic underpinnings. CNS Neurosci. Ther. 2024, 30, e70091. [Google Scholar] [CrossRef]
- De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gut microbiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Jena, P.K.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Maezawa, I.; Wan, Y.Y. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J. 2018, 32, 2866–2877. [Google Scholar] [CrossRef]
- Martínez Leo, E.E.; Segura Campos, M.R. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition 2020, 71, 110609. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Lim, S.L.; Rodriguez-Ortiz, C.J.; Kitazawa, M. Infection, systemic inflammation, and Alzheimer’s disease. Microbes Infect. 2015, 17, 549–556. [Google Scholar] [CrossRef]
- Aguayo-Patrón, S.V.; Calderón de la Barca, A.M. Old fashioned vs. Ultra-processed-based current diets: Possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods 2017, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Roy-Sarkar, S.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Bekdash, R.A. Epigenetics, nutrition, and the brain: Improving mental health through diet. Int. J. Mol. Sci. 2024, 25, 4036. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Lin, Z.J.; Li, C.C.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Li, F.; Yuan, L.Q.; Li, Z.H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Parrillo, L.; Spinelli, R.; Nicolò, A.; Longo, M.; Mirra, P.; Raciti, G.A.; Miele, C.; Beguinot, F. Nutritional factors, DNA methylation, and risk of type 2 diabetes and obesity: Perspectives and challenges. Int. J. Mol. Sci. 2019, 20, 2983. [Google Scholar] [CrossRef]
- Long, Y.; Mao, C.; Liu, S.; Tao, Y.; Xiao, D. Epigenetic modifications in obesity-associated diseases. Med. Commun. 2020, 5, e496. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. MicroRNAs: The novel mediators for nutrient-modulating biological functions. Trends Food Sci. Technol. 2021, 114, 167–175. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Martínez-Ibarra, A.; Monroy, A.; Miranda-Ríos, J.; Cerbón, M. Effect of dietary fatty acids on microRNA expression related to metabolic disorders and inflammation in human and animal trials. Nutrients 2021, 13, 1830. [Google Scholar] [CrossRef]
- Murashov, A.K.; Pak, E.S.; Mar, J.; O’Brien, K.; Fisher-Wellman, K.; Bhat, K.M. Paternal Western diet causes transgenerational increase in food consumption in Drosophila with parallel alterations in the offspring brain proteome and microRNAs. FASEB J. 2023, 37, e22966. [Google Scholar] [CrossRef]
- Wu, X.; Shi, X.; Chen, X.; Yin, Z. Advanced glycation end products regulate the receptor of AGEs epigenetically. Front. Cell Dev. Biol. 2023, 11, 1062229. [Google Scholar] [CrossRef]
- Dufault, R.J.; Adler, K.M.; Carpenter, D.O.; Gilbert, S.G.; Crider, R.A. Nutritional epigenetics education improves diet and attitude of parents of children with autism or attention deficit/hyperactivity disorder. World J. Psychiatry 2024, 14, 159–178. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The role of oxidative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef] [PubMed]
- Sertorio, M.N.; César, H.; de Souza, E.A.; Mennitti, L.V.; Santamarina, A.B.; De Souza Mesquita, L.M.; Jucá, A.; Casagrande, B.P.; Estadella, D.; Aguiar, O., Jr.; et al. Parental high-fat high-sugar diet intake programming inflammatory and oxidative parameters of reproductive health in male offspring. Front. Cell Dev. Biol. 2022, 10, 867127. [Google Scholar] [CrossRef]
- Llauradó-Pont, J.; Stratakis, N.; Fiorito, G.; Handakas, E.; Neumann, A.; Barros, H.; Brantsæter, A.L.; Chang, K.; Chatzi, L.; Felix, J.F.; et al. A meta-analysis of epigenome-wide association studies of ultra-processed food consumption with DNA methylation in European children. Clin. Epigenet 2025, 17, 3. [Google Scholar] [CrossRef]
- Ott, R.; Stein, R.; Hauta-Alus, H.H.; Ronkainen, J.; Fernández-Barrés, S.; Spielau, U.; Kirsten, H.; Poulain, T.; Melton, P.E.; Küpers, L.K.; et al. Epigenome-wide meta-analysis reveals associations between dietary glycemic index and glycemic load and DNA methylation in children and adolescents of different body sizes. Diabetes Care 2023, 46, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Barragán, J.; Fernández-Sanlés, A.; Hernáez, Á.; Llauradó-Pont, J.; Marrugat, J.; Robinson, O.; Tzoulaki, I.; Elosua, R.; Lassale, C. Blood DNA methylation signature of diet quality and association with cardiometabolic traits. Eur. J. Prev. Cardiol. 2024, 31, 191–202. [Google Scholar] [CrossRef]
- Hibar, D.; Adams, H.; Jahanshad, N.; Chauhan, G.; Stein, J.L.; Hofer, E.; Renteria, M.E.; Bis, J.C.; Arias-Vasquez, A.; Ikram, M.K.; et al. Novel genetic loci associated with hippocampal volume. Nat. Commun. 2017, 8, 13624. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, N.; Wang, M.; Lu, L.; Luo, C.; Tang, B.; Sweeney, J.A.; Gong, Q.; Qiu, C.; Lui, S. Hippocampal subfield alterations in schizophrenia and major depressive disorder: A systematic review and network meta-analysis of anatomic MRI studies. J. Psychiatry Neurosci. 2023, 48, E34–E49. [Google Scholar] [CrossRef]
- Grau-Del Valle, C.; Fernández, J.; Solá, E.; Montoya-Castilla, I.; Morillas, C.; Bañuls, C. Association between gut microbiota and psychiatric disorders: A systematic review. Front. Psychol. 2023, 14, 1215674. [Google Scholar] [CrossRef]
- Akhatova, A.; Jones, C.; Coward, K.; Yeste, M. How do lifestyle and environmental factors influence the sperm epigenome? Effects on sperm fertilising ability, embryo development, and offspring health. Clin. Epigenet. 2025, 17, 7. [Google Scholar] [CrossRef]
- Manterola, M.; Palominos, M.F.; Calixto, A. The heritability of behaviors associated with the host gut microbiota. Front. Immunol. 2021, 12, 658551. [Google Scholar] [CrossRef]
- Adjibade, M.; Julia, C.; Allès, B.; Touvier, M.; Lemogne, C.; Srour, B.; Hercberg, S.; Galan, P.; Assmann, K.E.; Kesse-Guyot, E. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Santé cohort. BMC Med. 2019, 17, 78. [Google Scholar] [CrossRef]
- Gómez-Donoso, C.; Sánchez-Villegas, A.; Martínez-González, M.A.; Gea, A.; Mendonça, R.; Lahortiga-Ramos, F.; Bes-Rastrollo, M. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: The SUN project. Eur. J. Nutr. 2019, 59, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Hecht, E.M.; Rabil, A.; Martinez Steele, E.; Abrams, G.A.; Ware, D.; Landy, D.C.; Hennekens, C.H. Cross-sectional examination of ultra-processed food consumption and adverse mental health symptoms. Public Health Nutr. 2022, 25, 3225–3234. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghavami, A.; Zarpoosh, M.; Mohammadi, H.; Wong, A.; Nordvall, M.; Kermani, M.A.H.; et al. The association of ultra-processed food consumption with adult mental health disorders: A systematic review and dose-response meta-analysis of 260,385 participants. Nutr. Neurosci. 2023, 26, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-processed food consumption and mental health: A systematic review and meta-analysis of observational studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef]
- Dai, S.; Wellens, J.; Yang, N.; Li, D.; Wang, J.; Wang, L.; Yuan, S.; He, Y.; Song, P.; Munger, R.; et al. Ultra-processed foods and human health: An umbrella review and updated meta-analysis of observational evidence. Clin. Nutr. 2024, 43, 1386–1394. [Google Scholar] [CrossRef]
- Arshad, H.; Head, J.; Jacka, F.N.; Lane, M.M.; Kivimaki, M.; Akbaraly, T. Association between ultra-processed foods and recurrence of depressive symptoms: The Whitehall II cohort study. Nutr. Neurosci. 2024, 27, 42–54. [Google Scholar] [CrossRef]
- Leal, A.C.G.; Lopes, L.J.; Rezende-Alves, K.; Bressan, J.; Pimenta, A.M.; Hermsdorff, H.H.M. Ultra-processed food consumption is positively associated with the incidence of depression in Brazilian adults (CUME project). J. Affect. Disord. 2023, 328, 58–63. [Google Scholar] [CrossRef]
- Ghernati, L.; Tamim, H.; Chokor, F.A.Z.; Taktouk, M.; Assi, B.; Nasreddine, L.; Elbejjani, M. Processed and ultra-processed foods are associated with depression and anxiety symptoms in a cross-sectional sample of urban Lebanese adults. Nutr. Res. 2025, 133, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Liu, P.; Tu, H.; Zhang, R.; Zhang, Y.; Sun, N.; Zhang, K. Gut microbiota composition in depressive disorder: A systematic review, meta-analysis, and meta-regression. Transl. Psychiatry 2023, 13, 379. [Google Scholar] [CrossRef]
- Brushett, S.; Gacesa, R.; Vich Vila, A.; Brandao Gois, M.F.; Andreu-Sánchez, S.; Swarte, J.C.; Klaassen, M.A.Y.; Collij, V.; Sinha, T.; Bolte, L.A.; et al. Gut feelings: The relations between depression, anxiety, psychotropic drugs and the gut microbiome. Gut Microbes 2023, 15, 2281360. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Acceptability, tolerability, and estimates of putative treatment effects of probiotics as adjunctive treatment in patients with depression: A Randomized Clinical Trial. JAMA Psychiatry 2023, 80, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of inflammatory mechanisms in major depressive disorder: From etiology to potential pharmacological targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef]
- Dziedzic, A.; Maciak, K.; Bliźniewska-Kowalska, K.; Gałecka, M.; Kobierecka, W.; Saluk, J. The power of psychobiotics in depression: A modern approach through the microbiota–gut–brain axis: A literature review. Nutrients 2024, 16, 1054. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Wu, J.; Zhang, H.; Huang, Y.; Tan, X.; Zhou, X.; Xie, P.; Olasunkanmi, O.I.; Zhou, J.; et al. Changes of gut microbiota reflect the severity of major depressive disorder: A cross-sectional study. Transl. Psychiatry 2023, 13, 137. [Google Scholar] [CrossRef]
- Cocean, A.M.; Vodnar, D.C. Exploring the gut-brain Axis: Potential therapeutic impact of psychobiotics on mental health. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 134, 111073. [Google Scholar] [CrossRef]

| Results and Conclusions | Type of Study | N° Studies and Subjects (N) | Reference |
|---|---|---|---|
| ↑ intake of UPFs correlates with ↑ risk of depressive symptoms, as indicated by 2221 incident cases. | Longitudinal study, 5.4 years | N = 26,730 (20,380 women, 6350 men) without depressive symptoms | Adjibade et al., 2019 [103] |
| Individuals with the highest consumption of UPFs exhibited ↑ risk of depression, as demonstrated by 774 incident cases. | Longitudinal study, 10.3 years | N = 14,907 free of depression | Gómez-Donoso et al., 2019 [104] |
| ↑ consumption of UPFs is associated with mild depression, anxiety, and mental unhealth. | Cross-sectional study, 5 years | N = 10,359 | Hecht et al., 2022 [105] |
| UPFs are linked to ↑ risk of depression, with no similar association observed for anxiety. Notably, for every 10% ↑ in UPFs intake as a share of daily calories, there is an 11% ↑ in depression risk, indicating a dose–response relationship. | Systematic review and meta-analysis | 26 studies N = 260,385 | Mazloomi et al., 2023 [106] |
| ↑ consumption of UPFs is associated with ↑ odds of depressive and anxiety symptoms, and ↑ intake of these foods ↑ the risk of developing depression over time. | Systematic review and meta-analysis (15 cross-sectional, 2 prospective) | 17 studies N = 385,541 | Lane et al., 2022 [107] |
| UPFs consumption is associated with depression and 25 additional adverse health outcomes. | Umbrella review, meta-analysis | 39 studies, of which 4 were on depression | Dai et al., 2024 [108] |
| British people with high UPFs intake associate with recurrent depressive symptoms and has ↑ odds of experiencing these symptoms compared to those with low UPFs consumption. | Longitudinal study, 13 years | N = 4554 | Arshad et al., 2024 [109] |
| Participants in the highest quartile of UPFs consumption had ↑ risk of developing depressive disorders. | Longitudinal study, 2.96 years | N = 2572 | Leal et al., 2023 [110] |
| Increasing UPFs intake is associated with ↑ odds of depression. | Cross-sectional study | N = 188 | Ghernati et al., 2025 [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, M.; Arancibia, M.; Moran-Kneer, J.; Manterola, M. Ultraprocessed Foods and Neuropsychiatric Outcomes: Putative Mechanisms. Nutrients 2025, 17, 1215. https://doi.org/10.3390/nu17071215
Lutz M, Arancibia M, Moran-Kneer J, Manterola M. Ultraprocessed Foods and Neuropsychiatric Outcomes: Putative Mechanisms. Nutrients. 2025; 17(7):1215. https://doi.org/10.3390/nu17071215
Chicago/Turabian StyleLutz, Mariane, Marcelo Arancibia, Javier Moran-Kneer, and Marcia Manterola. 2025. "Ultraprocessed Foods and Neuropsychiatric Outcomes: Putative Mechanisms" Nutrients 17, no. 7: 1215. https://doi.org/10.3390/nu17071215
APA StyleLutz, M., Arancibia, M., Moran-Kneer, J., & Manterola, M. (2025). Ultraprocessed Foods and Neuropsychiatric Outcomes: Putative Mechanisms. Nutrients, 17(7), 1215. https://doi.org/10.3390/nu17071215






