Preliminary Evidence Suggests That a 12-Week Treatment with Tirzepatide Plus Low-Energy Ketogenic Therapy Is More Effective than Its Combination with a Low-Calorie Diet in Preserving Fat-Free Mass, Muscle Strength, and Resting Metabolic Rate in Patients with Obesity
Abstract
1. Introduction
2. Materials and Methods
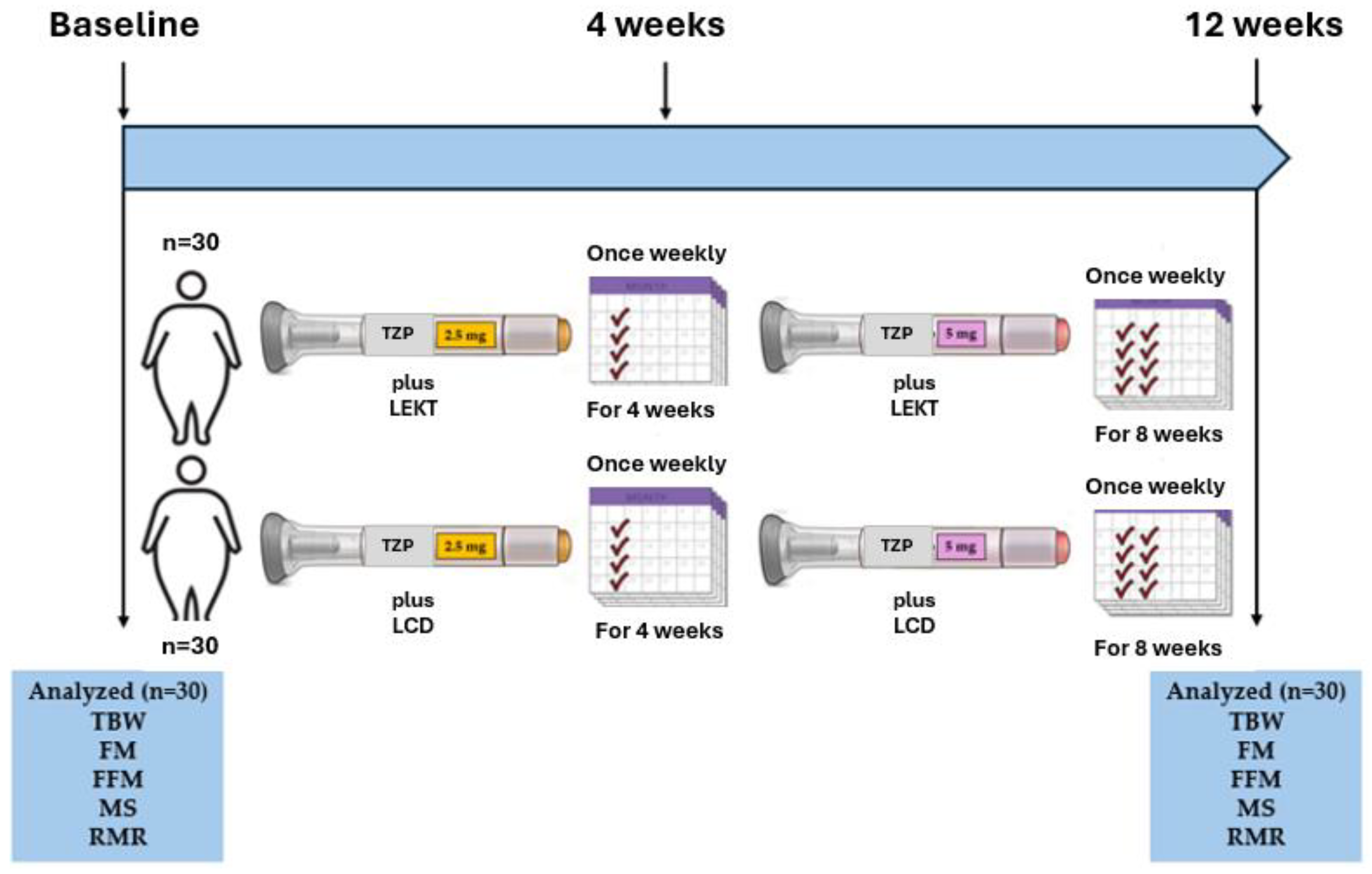
2.1. Study Design and Patient Selection
2.2. TZP Protocol and LEKT and LCD Characteristics
2.3. Assessment of BW, FM, FFM, MS, RMR, and Laboratory Parameters
2.4. Evaluation of Treatment Adherence and Side Effects
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Groups at Baseline
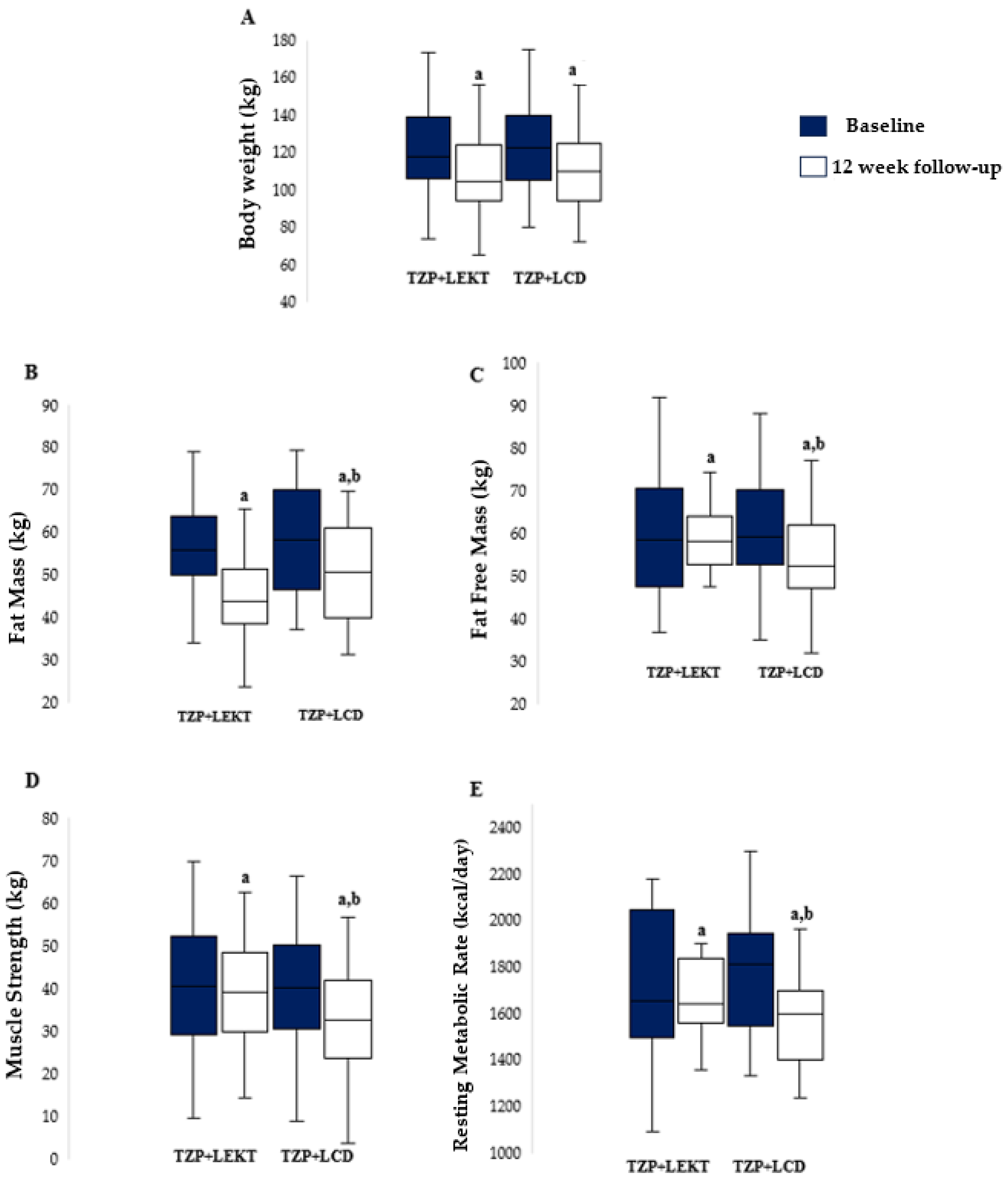
3.2. Impact of TZP+LEKT vs. TZP+LCD on BW, FM, FFM, MS, and RMR
3.3. Impact of TZP+LEKT vs. TZP+LCD on Patient’s Clinical Parameters
3.4. Impact of TZP+LEKT vs. TZP+LCD on Patient’s Appetite and Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2025).
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bray, G.A. Handbook of Obesity Treatment, 2nd ed.; Guilford Press: New York, NY, USA, 2018. [Google Scholar]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Stahl, J.M.; Malhotra, S. Obesity Surgery Indications and Contraindications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Fanshier, A.V.; Crews, B.K.; Garrett, M.C.; Johnson, J.L. Tirzepatide: A Novel Glucose-Dependent Insulinotropic Polypeptide/Glucagon-Like Peptide 1 Receptor Agonist for the Treatment of Type 2 Diabetes: The First Twincretin. Clin. Diabetes Publ. Am. Diabetes Assoc. 2023, 41, 367–377. [Google Scholar] [CrossRef]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Kaneko, S. Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. TouchREVIEWS Endocrinol. 2022, 18, 10–19. [Google Scholar] [CrossRef]
- Venniyoor, A. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 1433–1434. [Google Scholar] [PubMed]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [PubMed]
- Wadden, T.A.; Chao, A.M.; Machineni, S.; Kushner, R.; Ard, J.; Srivastava, G.; Halpern, B.; Zhang, S.; Chen, J.; Bunck, M.C.; et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nat. Med. 2023, 29, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment with Tirzepatide for Maintenance of Weight Reduction in Adults with Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Hall, K.D.; Klein, S. Is Weight Loss-Induced Muscle Mass Loss Clinically Relevant? JAMA 2024, 332, 9–10. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef]
- Ravussin, E.; Lillioja, S.; Knowler, W.C.; Christin, L.; Freymond, D.; Abbott, W.G.; Boyce, V.; Howard, B.V.; Bogardus, C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N. Engl. J. Med. 1988, 318, 467–472. [Google Scholar] [CrossRef]
- Schiavo, L.; Santella, B.; Paolini, B.; Rahimi, F.; Giglio, E.; Martinelli, B.; Boschetti, S.; Bertolani, L.; Gennai, K.; Arolfo, S.; et al. Adding Branched-Chain Amino Acids and Vitamin D to Whey Protein Is More Effective than Protein Alone in Preserving Fat Free Mass and Muscle Strength in the First Month after Sleeve Gastrectomy. Nutrients 2024, 16, 1448. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Iannelli, A.; Barbarisi, A. Preservation of Fat-Free Mass After Bariatric Surgery: Our Point of View. Obes. Surg. 2017, 27, 1071–1073. [Google Scholar]
- Rochira, V.; Greco, C.; Boni, S.; Costantino, F.; Dalla Valentina, L.; Zanni, E.; Itani, L.; El Ghoch, M. The Effect of Tirzepatide on Body Composition in People with Overweight and Obesity: A Systematic Review of Randomized, Controlled Studies. Diseases 2024, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Grassi, D.; Cicero, A.F.G.; Bagnato, C.; Paolini, B.; Muscogiuri, G. A New Nomenclature for the Very Low-Calorie Ketogenic Diet (VLCKD): Very Low-Energy Ketogenic Therapy (VLEKT). Ketodiets and Nutraceuticals Expert Panels: “KetoNut”, Italian Society of Nutraceuticals (SINut) and the Italian Association of Dietetics and Clinical Nutrition (ADI). Curr. Nutr. Rep. 2024, 13, 552–556. [Google Scholar] [PubMed]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Pilone, V.; Tramontano, S.; Renzulli, M.; Romano, M.; Cobellis, L.; Berselli, T.; Schiavo, L. Metabolic effects, safety, and acceptability of very low-calorie ketogenic dietetic scheme on candidates for bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2018, 14, 1013–1019. [Google Scholar] [CrossRef]
- Santella, B.; Mingo, M.; Papp, A.; Rice, M.; Chiappetta, S.; Calabrese, P.; Calenda, F.; Pilone, V.; Schiavo, L. Safety and Effectiveness of a 4-Week Diet on Low-Carb Ready-to-Eat Ketogenic Products as Preoperative Care Treatment in Patients Scheduled for Metabolic and Bariatric Surgery. Nutrients 2024, 16, 3875. [Google Scholar] [CrossRef]
- Schiavo, L.; Pierro, R.; Asteria, C.; Calabrese, P.; Di Biasio, A.; Coluzzi, I.; Severino, L.; Giovanelli, A.; Pilone, V.; Silecchia, G. Low-Calorie Ketogenic Diet with Continuous Positive Airway Pressure to Alleviate Severe Obstructive Sleep Apnea Syndrome in Patients with Obesity Scheduled for Bariatric/Metabolic Surgery: A Pilot, Prospective, Randomized Multicenter Comparative Study. Obes. Surg. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Schiavo, L.; Sarno, G.; Camajani, E.; Iannelli, A.; Caprio, M.; Pilone, V.; Colao, A.; Muscogiuri, G. Very Low-Calorie Ketogenic Diet (VLCKD) as Pre-Operative First-Line Dietary Therapy in Patients with Obesity Who Are Candidates for Bariatric Surgery. Nutrients 2023, 15, 1907. [Google Scholar] [CrossRef]
- Schiavo, L.; De Stefano, G.; Persico, F.; Gargiulo, S.; Di Spirito, F.; Griguolo, G.; Petrucciani, N.; Fontas, E.; Iannelli, A.; Pilone, V. A Randomized, Controlled Trial Comparing the Impact of a Low-Calorie Ketogenic vs. a Standard Low-Calorie Diet on Fat-Free Mass in Patients Receiving an Elipse™ Intragastric Balloon Treatment. Obes. Surg. 2021, 31, 1514–1523. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [PubMed]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [PubMed]
- Amanollahi, A.; Khazdouz, M.; Malekahmadi, M.; Klement, R.J.; Lee, D.; Khodabakhshi, A. Effect of Ketogenic Diets on Cardio-Metabolic Outcomes in Cancer Patients: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Nutr. Cancer 2023, 75, 95–111. [Google Scholar] [PubMed]
- Chen, S.; Su, X.; Feng, Y.; Li, R.; Liao, M.; Fan, L.; Liu, J.; Chen, S.; Zhang, S.; Cai, J.; et al. Ketogenic Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analysis. Nutrients 2023, 15, 4161. [Google Scholar] [CrossRef]
- Abbasi, M.M.; Jafari, A.; Mohtadi, M.; Shahabi, M.; Bakhshimoghaddam, F.; Abbasi, H.; Eslamian, G. Illuminating the Safety, Tolerability, and Efficacy of Different Ketogenic Diets for Individuals with Epilepsy: A Scoping Meta-Review. Seizure 2025, 125, 140–151. [Google Scholar]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G.; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, iii-173. [Google Scholar]
- Adachi, T.; Tsunekawa, Y.; Tanimura, D. Association between the Big Five personality traits and medication adherence in patients with cardiovascular disease: A cross-sectional study. PLoS ONE 2022, 17, e0278534. [Google Scholar]
- Schiavo, L.; Pilone, V.; Tramontano, S.; Rossetti, G.; Iannelli, A. May Bioelectrical Impedance Analysis Method Be Used in Alternative to the Dual-Energy X-Ray Absorptiometry in the Assessment of Fat Mass and Fat-Free Mass in Patients with Obesity? Pros, Cons, and Perspectives. Obes. Surg. 2020, 30, 3212–3215. [Google Scholar]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Quagliariello, V.; Iannelli, A.; Barbarisi, A. A Comparative Study Examining the Impact of a Protein-Enriched vs. Normal Protein Postoperative Diet on Body Composition and Resting Metabolic Rate in Obese Patients after Sleeve Gastrectomy. Obes. Surg. 2017, 27, 881–888. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Iannelli, A.; Barbarisi, A. Fat mass, fat-free mass, and resting metabolic rate in weight-stable sleeve gastrectomy patients compared with weight-stable nonoperated patients. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2017, 13, 1692–1699. [Google Scholar]
- Haugen, H.A.; Chan, L.N.; Li, F. Indirect calorimetry: A practical guide for clinicians. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2007, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Austin, M.D.; Benezra, L.; Pearce, S.; McInnis, T.; Unick, J.; Gross, S.J. Validation of Cosmed’s FitMate in measuring oxygen consumption and estimating resting metabolic rate. Res. Sports Med. 2006, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.A.; Melanson, E.L.; Tran, Z.V.; Kearney, J.T.; Hill, J.O. Variability of measured resting metabolic rate. Am. J. Clin. Nutr. 2003, 78, 1141–1145. [Google Scholar] [PubMed]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L.; Evidence Analysis Working Group. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Alba, D.L.; Wu, L.; Cawthon, P.M.; Mulligan, K.; Lang, T.; Patel, S.; King, N.J.; Carter, J.T.; Rogers, S.J.; Posselt, A.M.; et al. Changes in Lean Mass, Absolute and Relative Muscle Strength, and Physical Performance After Gastric Bypass Surgery. J. Clin. Endocrinol. Metab. 2019, 104, 711–720. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Gomez-Arbelaez, D.; Zulet, M.A.; Carreira, M.C.; Sajoux, I.; de Luis, D.; Castro, A.I.; Baltar, J.; Baamonde, I.; Sueiro, A.; et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: A marker of metabolic stress? Int. J. Obes. 2017, 41, 1570–1578. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- Sajoux, I.; Lorenzo, P.M.; Gomez-Arbelaez, D.; Zulet, M.A.; Abete, I.; Castro, A.I.; Baltar, J.; Portillo, M.P.; Tinahones, F.J.; Martinez, J.A.; et al. Effect of a Very-Low-Calorie Ketogenic Diet on Circulating Myokine Levels Compared with the Effect of Bariatric Surgery or a Low-Calorie Diet in Patients with Obesity. Nutrients 2019, 11, 2368. [Google Scholar] [CrossRef]
- Roekenes, J.; Martins, C. Ketogenic diets and appetite regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar]
- Ahmad, Y.; Seo, D.S.; Jang, Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. Int. J. Mol. Sci. 2024, 25, 7076. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The Ketogenic Diet and Sport: A Possible Marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benítez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef]
- Piaggi, P. Metabolic Determinants of Weight Gain in Humans. Obesity 2019, 27, 691–699. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.H. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol. Metab. 2020, 35, 1–6. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Palabiyik, A.A.; Palabiyik, E. Pharmacological approaches to enhance mitochondrial biogenesis: Focus on PGC-1A, AMPK, and SIRT1 in cellular health. Mol. Biol. Rep. 2025, 52, 270. [Google Scholar]
- Hass, D.T.; Barnstable, C.J. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D. Respiration uncoupling and metabolism in the control of energy expenditure. Proc. Nutr. Soc. 2005, 64, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Klonek, G.; Zydek, G.; Roczniok, R.; Panek, M.; Zając, A.; Michalczyk, M.M. Effects of a 12 Week Ketogenic Diet Intervention on Obese and Overweight Females with Glucose and Lipid Metabolism Disturbance. Nutrients 2024, 16, 4218. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Hussein, T.; Asfar, S.K.; Behbahani, A.; Khoursheed, M.A.; Al-Sayer, H.M.; Bo-Abbas, Y.Y.; Al-Zaid, N.S. Long-term effects of a ketogenic diet in obese patients. Exp. Clin. Cardiol. 2004, 9, 200–205. [Google Scholar]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar]
- Ciaffi, J.; Mancarella, L.; Pederzani, G.; Lisi, L.; Brusi, V.; Pignatti, F.; Ricci, S.; Vitali, G.; Faldini, C.; Ursini, F. Efficacy, Safety, and Tolerability of a Very Low-Calorie Ketogenic Diet in Women with Obesity and Symptomatic Knee Osteoarthritis: A Pilot Interventional Study. Nutrients 2024, 16, 3236. [Google Scholar] [CrossRef]
| LEKT (N = 30) | LCD (N = 30) | p (LEKT vs. LCD) | |
|---|---|---|---|
| Sex (male/female, n) | 14/16 | 13/17 | - |
| Body Weight (kg) | 121.9 ± 23.6 | 124.3 ± 23.8 | 0.70 |
| Body Mass Index (kg/m2) | 44.9 ± 6.28 | 45.5 ± 7.35 | 0.73 |
| Fat Mass (kg) | 56.4 ± 11.6 | 58.4 ± 13 | 0.53 |
| Fat Free Mass (kg) | 59.9 ± 14.3 | 60.6 ± 12.8 | 0.84 |
| Muscle Strength (kg) | 41 ± 15 | 40.5 ± 14.1 | 0.79 |
| Resting Metabolic Rate (kcal/day) | 1716 ± 317 | 1769 ± 235 | 0.46 |
| Clinical Parameters (Cut-Off) | Group | Baseline | 12-Week Follow-Up | p |
|---|---|---|---|---|
| Glucose (70–100 mg/dL) | TZP+LEKT | 87 ± 21 | 80 ± 18 | 0.17 |
| TZP+LCD | 92 ± 17 | 87 ± 13 | 0.21 | |
| Insulin (<25 mU/L) | TZP+LEKT | 8.5 ± 6.3 | 7.1 ± 5.2 | 0.35 |
| TZP+LCD | 7.7 ± 4.7 | 7.2 ± 3.1 | 0.63 | |
| HOMA Index (<2.5) | TZP+LEKT | 1.83 ± 0.8 | 1.40 ± 1.1 | 0.09 * |
| TZP+LCD | 1.75 ± 1.4 | 1.55 ± 0.96 | 0.52 | |
| Hemoglobin A1C (<6.1%) | TZP+LEKT | 4.7 ± 1.7 | 3.7 ± 1.1 | 0.01 * |
| TZP+LCD | 4.3 ± 0.77 | 4.0 ± 0.80 | 0.14 | |
| Creatine (0.55–1.02 mg/dL) | TZP+LEKT | 0.76 ± 0.19 | 0.81 ± 0.26 | 0.40 |
| TZP+LCD | 0.79 ± 0.17 | 0.83 ± 0.15 | 0.34 | |
| Iron (50–170 μg/dL) | TZP+LEKT | 59.3 ± 34 | 60 ± 24 | 0.93 |
| TZP+LCD | 68 ± 19 | 63.5 ± 21 | 0.39 | |
| Uric acid (3.0–7.0 mg/dL) | TZP+LEKT | 5.3 ± 1.2 | 5.7 ± 2.2 | 0.39 |
| TZP+LCD | 5.4 ± 1.4 | 5.1 ± 1.3 | 0.39 | |
| Total cholesterol (<200 mg/dL) | TZP+LEKT | 194 ± 42 | 162 ± 11 | <0.001 * |
| TZP+LCD | 176 ± 48 | 159 ± 23 | 0.09 | |
| HDL (>50 mg/dL) | TZP+LEKT | 47 ± 12 | 55.5 ± 18 | 0.03 |
| TZP+LCD | 49.9 ± 22 | 45.4 ± 10 | 0.31 | |
| Triglycerides (<150 mg/dL) | TZP+LEKT | 151 ± 75 | 118 ± 28.5 | 0.03 * |
| TZP+LCD | 116 ± 54 | 109 ± 18.2 | 0.50 | |
| GOT (<34 U/L) | TZP+LEKT | 23 ± 13.8 | 24 ± 8.2 | 0.73 |
| TZP+LCD | 26 ± 22.9 | 29 ± 9.5 | 0.51 | |
| GPT (<55 U/L) | TZP+LEKT | 29 ± 21.1 | 19 ± 3.7 | 0.01 |
| TZP+LCD | 35 ± 32.4 | 29 ± 8.7 | 0.33 | |
| GGT (<38 U/L) | TZP+LEKT | 32 ± 26.4 | 28 ± 6.9 | 0.42 |
| TZP+LCD | 31 ± 21.1 | 29 ± 9.2 | 0.64 | |
| Na2+ (13–146 mmol/L) | TZP+LEKT | 134 ± 33.7 | 132 ± 32.2 | 0.81 |
| TZP+LCD | 136 ± 32.3 | 135 ± 35.2 | 0.91 | |
| K+ (3.4–5.1 mmol/L) | TZP+LEKT | 4.1 ± 0.48 | 3.9 ± 0.4 | 0.08 |
| TZP+LCD | 4.2 ± 0.44 | 4 ± 0.7 | 0.19 | |
| Cl2− (101–110 mmol/L) | TZP+LEKT | 103 ± 6.3 | 101 ± 5.4 | 0.19 |
| TZP+LCD | 106 ± 2.7 | 105 ± 3.5 | 0.22 | |
| Ketonemia (<0.6 mmol/L) | TZP+LEKT | 0.3 ± 0.04 | 0.82 ± 0.49 | <0.001 |
| TZP+LCD | 0.25 ± 0.07 | 0.27 ± 0.05 | 0.21 |
| Symptoms | LEKT (N = 30) | LCD (N = 30) |
|---|---|---|
| Decreased appetite, (%) | 18 | 8 |
| Nausea, (%) | 15 | 17 |
| Vomiting, (%) | 6 | 7 |
| Constipation, (%) | 16 | 15 |
| Diarrhea, (%) | 4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavo, L.; Santella, B.; Mingo, M.; Rossetti, G.; Orio, M.; Cobellis, L.; Maurano, A.; Iannelli, A.; Pilone, V. Preliminary Evidence Suggests That a 12-Week Treatment with Tirzepatide Plus Low-Energy Ketogenic Therapy Is More Effective than Its Combination with a Low-Calorie Diet in Preserving Fat-Free Mass, Muscle Strength, and Resting Metabolic Rate in Patients with Obesity. Nutrients 2025, 17, 1216. https://doi.org/10.3390/nu17071216
Schiavo L, Santella B, Mingo M, Rossetti G, Orio M, Cobellis L, Maurano A, Iannelli A, Pilone V. Preliminary Evidence Suggests That a 12-Week Treatment with Tirzepatide Plus Low-Energy Ketogenic Therapy Is More Effective than Its Combination with a Low-Calorie Diet in Preserving Fat-Free Mass, Muscle Strength, and Resting Metabolic Rate in Patients with Obesity. Nutrients. 2025; 17(7):1216. https://doi.org/10.3390/nu17071216
Chicago/Turabian StyleSchiavo, Luigi, Biagio Santella, Monica Mingo, Gianluca Rossetti, Marcello Orio, Luigi Cobellis, Attilio Maurano, Antonio Iannelli, and Vincenzo Pilone. 2025. "Preliminary Evidence Suggests That a 12-Week Treatment with Tirzepatide Plus Low-Energy Ketogenic Therapy Is More Effective than Its Combination with a Low-Calorie Diet in Preserving Fat-Free Mass, Muscle Strength, and Resting Metabolic Rate in Patients with Obesity" Nutrients 17, no. 7: 1216. https://doi.org/10.3390/nu17071216
APA StyleSchiavo, L., Santella, B., Mingo, M., Rossetti, G., Orio, M., Cobellis, L., Maurano, A., Iannelli, A., & Pilone, V. (2025). Preliminary Evidence Suggests That a 12-Week Treatment with Tirzepatide Plus Low-Energy Ketogenic Therapy Is More Effective than Its Combination with a Low-Calorie Diet in Preserving Fat-Free Mass, Muscle Strength, and Resting Metabolic Rate in Patients with Obesity. Nutrients, 17(7), 1216. https://doi.org/10.3390/nu17071216








