Associations of Metabolites Related Salt Sensitivity of Blood Pressure and Essential Hypertension in Chinese Population: The EpiSS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Determination of Salt Sensitivity of Blood Pressure
2.3. Data Collection
2.4. Metabolomic Profiling and Quality Control
2.5. Statistical Methods
3. Results
3.1. Basic Characteristics of the Participants
3.2. Association Between Metabolites and SSBP
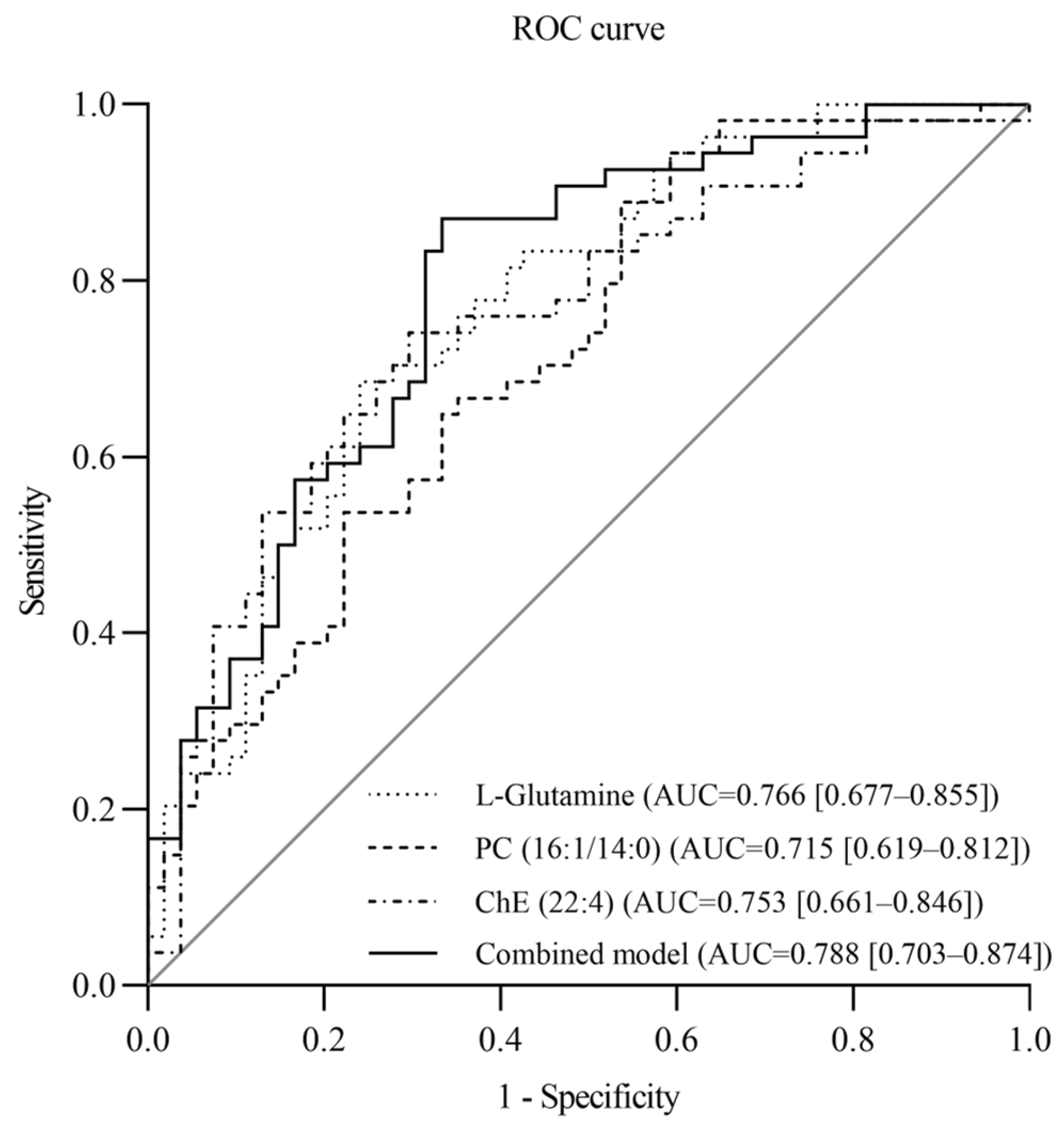
3.3. Evaluation of the Value of Metabolites in Diagnosing SSBP
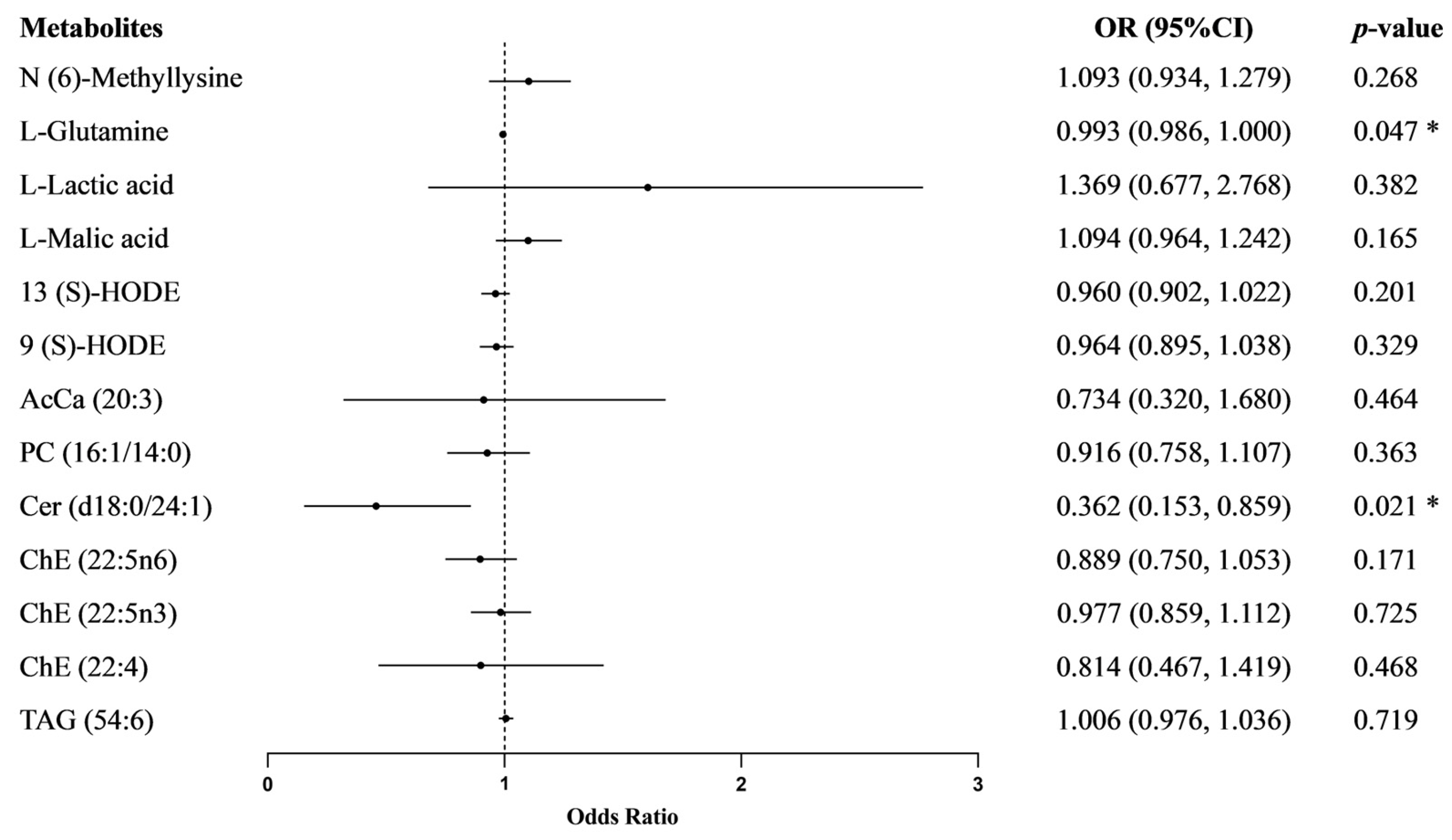
3.4. The Effect of Metabolites on Essential Hypertension
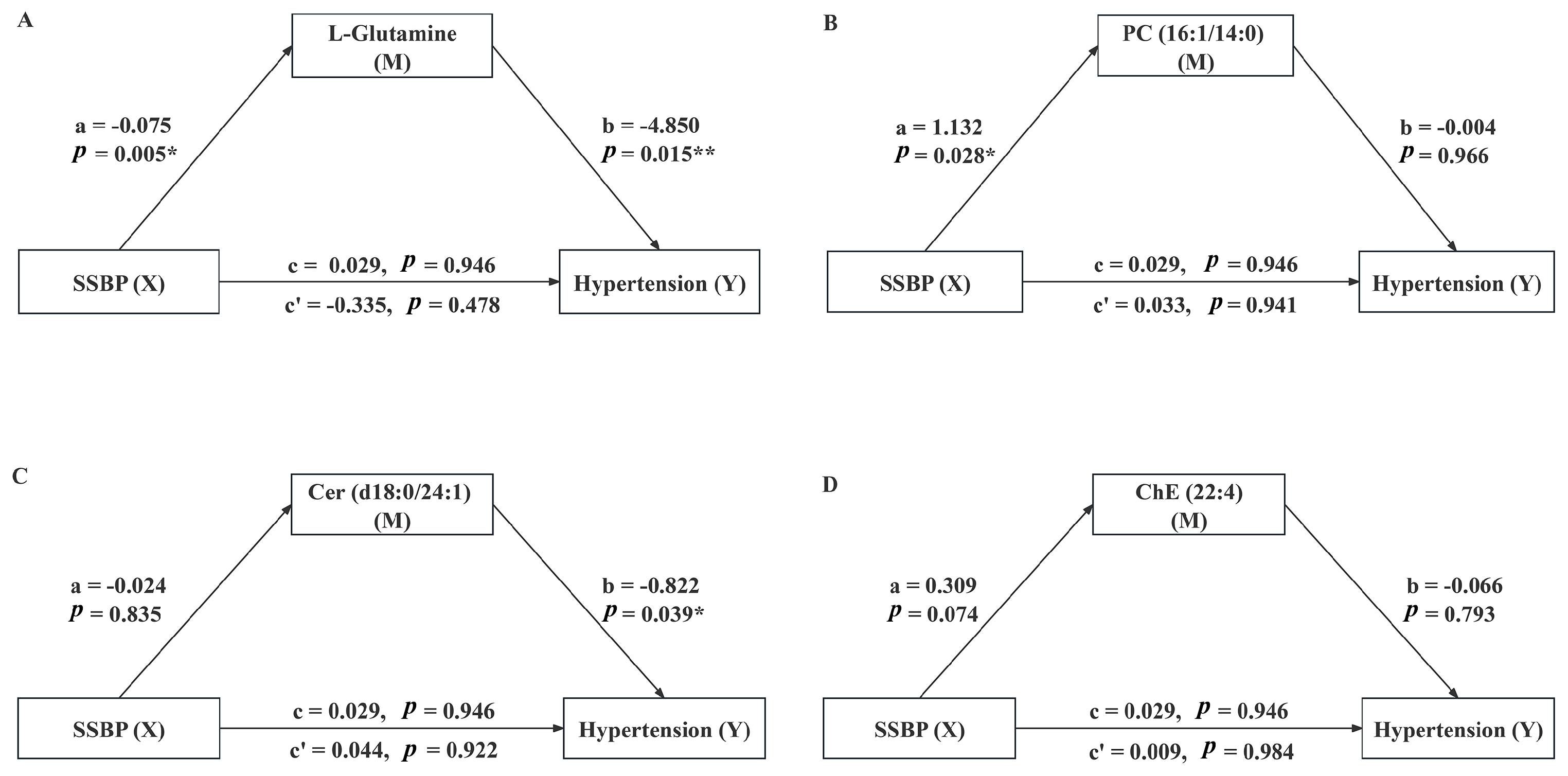
3.5. Mediating Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSBP | Salt sensitivity of blood pressure |
| SS | Salt-sensitive |
| SR | salt-resistant |
| SSH | Salt-sensitive hypertension |
| BP | Blood pressure |
| MAP | Mean arterial pressure |
| ΔMAP1 | Mean arterial pressure change during the acute saline load period |
| ΔMAP2 | Mean arterial pressure change during the diuresis shrinkage period |
| BMI | Body mass index |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| eGFR | estimated glomerular filtration rate |
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.; Thomopoulos, C.; Konstantinidis, D.; Siafi, E.; Tatakis, F.; Manta, E.; Drogkaris, S.; Polyzos, D.; Kyriazopoulos, K.; Grigoriou, K.; et al. DASH vs. Mediterranean diet on a salt restriction background in adults with high normal blood pressure or grade 1 hypertension: A randomized controlled trial. Clin. Nutr. 2023, 42, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L. Salt Sensitivity of Blood Pressure: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e7–e46. [Google Scholar] [CrossRef]
- Iatrino, R.; Manunta, P.; Zagato, L. Salt Sensitivity: Challenging and Controversial Phenotype of Primary Hypertension. Curr. Hypertens. Rep. 2016, 18, 70. [Google Scholar] [CrossRef]
- He, J.; Huang, J.F.; Li, C.; Chen, J.; Lu, X.; Chen, J.C.; He, H.; Li, J.X.; Cao, J.; Chen, C.S.; et al. Sodium Sensitivity, Sodium Resistance, and Incidence of Hypertension: A Longitudinal Follow-Up Study of Dietary Sodium Intervention. Hypertension 2021, 78, 155–164. [Google Scholar] [CrossRef]
- Qi, H.; Liu, B.; Guo, C.; Liu, Z.; Cao, H.; Liu, K.; Sun, W.; Zhang, L. Effects of environmental and genetic risk factors for salt sensitivity on blood pressure in northern China: The systemic epidemiology of salt sensitivity (EpiSS) cohort study. BMJ Open 2018, 8, e023042. [Google Scholar] [CrossRef]
- He, J.; Gu, D.; Chen, J.; Jaquish, C.E.; Rao, D.C.; Hixson, J.E.; Chen, J.C.; Duan, X.; Huang, J.F.; Chen, C.S.; et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J. Hypertens. 2009, 27, 48–54. [Google Scholar] [CrossRef]
- Afolabi, J.; Laffer, C.L.; Beasley, H.K.; Hinton, A.; Masenga, S.K.; Kirabo, A. Salt Sensitivity of Blood Pressure. Circ. Res. 2024, 134, 1234–1239. [Google Scholar] [CrossRef]
- Ussher, J.R.; Elmariah, S.; Gerszten, R.E.; Dyck, J.R. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2850–2870. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C. Metabolomics takes its place as latest up-and-coming “omic” science. J. Natl. Cancer Inst. 2004, 96, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; He, J.; Li, C.; Lu, X.; He, W.J.; Cao, J.; Chen, J.; Chen, J.C.; Bazzano, L.A.; Li, J.X.; et al. Metabolomics study of blood pressure salt-sensitivity and hypertension. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1681–1692. [Google Scholar] [CrossRef]
- Huang, C.C.; Huang, Y.L.; Lin, C.H.; Chen, J.W. Plasma metabolomic profiles associated with hypertension and blood pressure in response to thiazide diuretics. Hypertens. Res. 2022, 45, 464–473. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.; Yang, X.; Chen, M.; Dong, Y.; Li, J. A cross-sectional study identifying disparities in serum metabolic profiles among hypertensive patients with ISH, IDH and SDH subtypes. Front. Cardiovasc. Med. 2023, 10, 1102754. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, H.; Pan, X.; Xue, H.; Wan, Y.; Wang, T.; Tian, Z.; Hou, E.; Lanza, I.R.; Liu, P.; et al. Urinary Metabolites Associated with Blood Pressure on a Low- or High-Sodium Diet. Theranostics 2018, 8, 1468–1480. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.; Yang, X.; Peng, W.; Qi, H.; Li, B.; Wen, F.; Li, P.; Sun, Y.; Zhang, L. Association of Serum Metabolites and Salt Sensitivity of Blood Pressure in Chinese Population: The EpiSS Study. Nutrients 2023, 15, 690. [Google Scholar] [CrossRef]
- Peng, W.; Xie, Y.; Xia, J.; Li, B.; Zhang, F.; Wen, F.; Liu, K.; Cao, H.; Qi, H.; Zhang, L. Association of Circulating Biomarkers of lnc-IGSF3-1:1, SCOC-AS1, and SLC8A1-AS1 with Salt Sensitivity of Blood Pressure in Chinese Population. J. Cardiovasc. Transl. Res. 2022, 15, 906–917. [Google Scholar] [CrossRef]
- Gupta, D.K.; Lewis, C.E.; Varady, K.A.; Su, Y.R.; Madhur, M.S.; Lackland, D.T.; Reis, J.P.; Wang, T.J.; Lloyd-Jones, D.M.; Allen, N.B. Effect of Dietary Sodium on Blood Pressure: A Crossover Trial. JAMA 2023, 330, 2258–2266. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, H.; Liu, B.; Liu, K.; Wu, J.; Cao, H.; Zhang, J.; Yan, Y.; He, Y.; Zhang, L. Genetic susceptibility to salt-sensitive hypertension in a Han Chinese population: A validation study of candidate genes. Hypertens. Res. 2017, 40, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Joint Committee Guideline Revision. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension—A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J. Geriatr. Cardiol. 2019, 16, 182–241. [Google Scholar] [CrossRef]
- Son, A.; Kim, W.; Lee, W.; Park, J.; Kim, H. Applicability of selected reaction monitoring for precise screening tests. Expert. Rev. Proteom. 2024, 21, 237–246. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Fairchild, A.J.; Fritz, M.S. Mediation analysis. Annu. Rev. Psychol. 2007, 58, 593–614. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Krull, J.L.; Lockwood, C.M. Equivalence of the mediation, confounding and suppression effect. Prev. Sci. 2000, 1, 173–181. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Xi, P.; Jiang, Z.; Zheng, C.; Lin, Y.; Wu, G. Regulation of protein metabolism by glutamine: Implications for nutrition and health. Front. Biosci. (Landmark Ed) 2011, 16, 578–597. [Google Scholar] [CrossRef]
- Durante, W. The Emerging Role of l-Glutamine in Cardiovascular Health and Disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Qorbani, M.; Heshmat, R.; Larijani, B.; Hosseini, S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015, 31, 119–126. [Google Scholar] [CrossRef]
- Boelens, P.G.; van Leeuwen, P.A.; Dejong, C.H.; Deutz, N.E. Intestinal renal metabolism of L-citrulline and L-arginine following enteral or parenteral infusion of L-alanyl-L-[2,15N]glutamine or L-[2,15N]glutamine in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G679–G685. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Syme, C.; Czajkowski, S.; Shin, J.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Strug, L.; et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation 2016, 134, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Fretts, A.M.; Miao, G.; Malloy, K.M.; Zhang, Y.; Umans, J.G.; Cole, S.A.; Best, L.G.; Fiehn, O.; Zhao, J. Plasma lipidomic markers of diet quality are associated with incident coronary heart disease in American Indian adults: The Strong Heart Family Study. Am. J. Clin. Nutr. 2024, 119, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gray, A.; Xue, L.; Farb, M.G.; Ayalon, N.; Andersson, C.; Ko, D.; Benjamin, E.J.; Levy, D.; Vasan, R.S.; et al. Metabolomic Profiles, Ideal Cardiovascular Health, and Risk of Heart Failure and Atrial Fibrillation: Insights from the Framingham Heart Study. J. Am. Heart Assoc. 2023, 12, e028022. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Mayr, J.A. Choline-related-inherited metabolic diseases-A mini review. J. Inherit. Metab. Dis. 2019, 42, 237–242. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zhang, L.Y.; Jiang, X.M.; Xue, C.H.; Chi, N.; Zhang, T.T.; Wang, Y.M. Effects of dietary choline, betaine, and L-carnitine on the generation of trimethylamine-N-oxide in healthy mice. J. Food Sci. 2020, 85, 2207–2215. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Z.; Liu, B.; Cao, H.; Sun, W.; Yan, Y.; Zhang, L. micro-RNA screening and prediction model construction for diagnosis of salt-sensitive essential hypertension. Medicine 2017, 96, e6417. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.; Li, J.; Wang, H.; Sun, J.; Yu, Z.; Zhao, X.; Zhao, M.; Xi, B. The association of dietary glutamine supplementation with the development of high salt-induced hypertension in rats. Front. Nutr. 2022, 9, 1011739. [Google Scholar] [CrossRef]
- Egnatchik, R.A.; Brittain, E.L.; Shah, A.T.; Fares, W.H.; Ford, H.J.; Monahan, K.; Kang, C.J.; Kocurek, E.G.; Zhu, S.; Luong, T.; et al. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Fenger, M.; Linneberg, A.; Jørgensen, T.; Madsbad, S.; Søbye, K.; Eugen-Olsen, J.; Jeppesen, J. Genetics of the ceramide/sphingosine-1-phosphate rheostat in blood pressure regulation and hypertension. BMC Genet. 2011, 12, 44. [Google Scholar] [CrossRef]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, B.; Li, C.; Zhao, Z. Ceramide in cerebrovascular diseases. Front. Cell Neurosci. 2023, 17, 1191609. [Google Scholar] [CrossRef]
- Mantovani, A.; Bonapace, S.; Lunardi, G.; Salgarello, M.; Dugo, C.; Canali, G.; Byrne, C.D.; Gori, S.; Barbieri, E.; Targher, G. Association between plasma ceramides and inducible myocardial ischemia in patients with established or suspected coronary artery disease undergoing myocardial perfusion scintigraphy. Metabolism 2018, 85, 305–312. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma Ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef]
- Lee, T.H.; Cheng, C.N.; Chao, H.C.; Lee, C.H.; Kuo, C.H.; Tang, S.C.; Jeng, J.S. Plasma ceramides are associated with outcomes in acute ischemic stroke patients. J. Formos. Med. Assoc. 2022, 121, 43–50. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Liu, F.; Cao, J.; Chen, S.; Chen, J.; Huang, K.; Wang, Y.; Li, H.; Wang, Y.; et al. Metabolomics signature of blood pressure salt sensitivity and its link to cardiovascular disease: A dietary salt-intervention trial. Sci. China Life Sci. 2024, 67, 1666–1675. [Google Scholar] [CrossRef]
| Variables | Total (n = 108) | SS (n = 54) | SR (n = 54) | p |
|---|---|---|---|---|
| Age (years) | 58.85 ± 6.89 | 58.69 ± 7.44 | 59.02 ± 6.36 | 0.805 a |
| Female (n, %) | 60 (55.56) | 31 (57.41) | 29 (53.70) | 0.851 c |
| BMI (kg/m2) | 25.88 ± 3.53 | 25.64 ± 3.71 | 26.12 ± 3.36 | 0.503 a |
| WHR | 0.90 ± 0.06 | 0.90 ± 0.61 | 0.90 ± 0.06 | 0.541 a |
| SBP (mmHg) | 122.33 ± 18.39 | 118.17 ± 18.05 | 128.49 ± 17.94 | 0.014 a |
| DBP (mmHg) | 77.29 ± 10.11 | 74.49 ± 9.57 | 80.09 ± 9.95 | 0.002 a |
| MAP (mmHg) | 92.30 ± 11.60 | 89.05 ± 11.30 | 95.56 ± 11.07 | 0.001 a |
| ΔMAP1 (mmHg) | 3.15 ± 6.03 | 7.23 ± 4.15 | −0.94 ± 4.71 | <0.001 a |
| ΔMAP2 (mmHg) | −1.28 ± 6.76 | −3.27 ± 7.14 | 0.72 ± 5.76 | 0.005 a |
| FBG (mmol/L) | 6.09 ± 2.09 | 6.41 ± 2.49 | 5.77 ± 1.55 | 0.203 b |
| TC (mmol/L) | 5.13 ± 1.04 | 4.94 ± 1.09 | 5.31 ± 0.96 | 0.052 a |
| TG (mmol/L) | 2.04 ± 1.34 | 2.08 ± 1.52 | 2.01 ± 1.13 | 0.914 b |
| LDL-C (mmol/L) | 2.62 ± 0.69 | 2.48 ± 0.65 | 2.76 ± 0.71 | 0.023 a |
| HDL-C (mmol/L) | 1.30 ± 0.52 | 1.42 ± 0.69 | 1.18 ± 0.23 | 0.160 b |
| 24hUNa (g/day) | 3.24 ± 1.68 | 3.47 ± 1.61 | 3.01 ± 1.74 | 0.198 b |
| 24hUK (g/day) | 1.86 ± 1.06 | 2.06 ± 1.21 | 1.66 ± 0.84 | 0.051 b |
| Smoking (yes, %) | 26 (24.07) | 16 (29.63) | 10 (18.52) | 0.286 c |
| Drinking (yes, %) | 37 (34.26) | 23 (42.59) | 14 (25.93) | 0.078 c |
| Family history of hypertension (n, %) | 50 (46.30) | 24 (44.44) | 26 (48.15) | 0.845 c |
| Metabolites | SSBP | ΔMAP1 (mmHg) | ΔMAP2 (mmHg) |
|---|---|---|---|
| OR (95% CI) | β (95% CI) | β (95% CI) | |
| N(6)-Methyllysine | 1.020 (0.987, 1.053) | 0.259 (−0.089, 0.608) | −0.121 (−0.561, 0.319) |
| L-Glutamine | 0.998 (0.997, 0.999) ** | −0.010 (−0.024, 0.004) | 0.011 (−0.007, 0.028) |
| L-Lactic acid | 1.035 (0.897, 1.195) | 0.728 (−0.830, 2.287) | 0.001 (−1.958, 1.960) |
| L-Malic acid | 1.018 (0.992, 1.044) | −0.001 (−0.282, 0.280) | −0.213 (−0.562, 0.135) |
| 13(S)-HODE | 0.996 (0.983, 1.008) | 0.043 (−0.095, 0.180) | 0.163 (−0.006, 0.333) |
| 9(S)-HODE | 0.999 (0.984, 1.014) | 0.066 (−0.100, 0.233) | 0.146 (−0.061, 0.352) |
| AcCa (20:3) | 0.929 (0.778, 1.109) | −0.736 (−2.661, 1.189) | 0.856 (−1.553, 3.266) |
| PC (16:1/14:0) | 1.039 (1.003, 1.077) * | 0.168 (−0.227, 0.563) | −0.218 (−0.712, 0.276) |
| Cer (d18:0/24:1) | 0.973 (0.826, 1.146) | −0.650 (−2.425, 1.125) | 0.362 (−1.863, 2.588) |
| ChE (22:5n6) | 1.017 (0.982, 1.053) | 0.198 (−0.185, 0.582) | −0.113 (−0.595, 0.369) |
| ChE (22:5n3) | 1.010 (0.984, 1.036) | 0.096 (−0.184, 0.376) | −0.209 (−0.557, 0.140) |
| ChE (22:4) | 1.115 (1.002, 1.240) * | 0.537 (−0.642, 1.716) | −0.537 (−2.015, 0.940) |
| TAG (54:6) | 0.999 (0.994, 1.005) | −0.051 (−0.112, 0.010) | −0.003 (−0.080, 0.075) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, F.; Zhang, B.; Qi, H.; Xie, Y.; Peng, W.; Li, B.; Wen, F.; Li, P.; Sun, Y.; et al. Associations of Metabolites Related Salt Sensitivity of Blood Pressure and Essential Hypertension in Chinese Population: The EpiSS Study. Nutrients 2025, 17, 1289. https://doi.org/10.3390/nu17071289
Yang X, Zhang F, Zhang B, Qi H, Xie Y, Peng W, Li B, Wen F, Li P, Sun Y, et al. Associations of Metabolites Related Salt Sensitivity of Blood Pressure and Essential Hypertension in Chinese Population: The EpiSS Study. Nutrients. 2025; 17(7):1289. https://doi.org/10.3390/nu17071289
Chicago/Turabian StyleYang, Xiaojun, Fengxu Zhang, Bowen Zhang, Han Qi, Yunyi Xie, Wenjuan Peng, Bingxiao Li, Fuyuan Wen, Pandi Li, Yuan Sun, and et al. 2025. "Associations of Metabolites Related Salt Sensitivity of Blood Pressure and Essential Hypertension in Chinese Population: The EpiSS Study" Nutrients 17, no. 7: 1289. https://doi.org/10.3390/nu17071289
APA StyleYang, X., Zhang, F., Zhang, B., Qi, H., Xie, Y., Peng, W., Li, B., Wen, F., Li, P., Sun, Y., Qu, A., & Zhang, L. (2025). Associations of Metabolites Related Salt Sensitivity of Blood Pressure and Essential Hypertension in Chinese Population: The EpiSS Study. Nutrients, 17(7), 1289. https://doi.org/10.3390/nu17071289







